Tratamiento combinado de primera línea versus monoterapia de primera línea para la hipertensión primaria
Appendices
Appendix 1. Hypertension Group Specialised Register search strategy
Database: Hypertension Group Specialised Register
Search Date: 18 February 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 ((combination* or combined or dual or polytherap* or versus or vs)) AND ((monotherap* or single )) (3824)
#2 hypertens* (30884)
#3 RCT:DE (22196)
#4 (Review OR Meta‐Analysis):MISC2 (1073)
#5 (#3 OR #4) (23269)
#6 #1 AND #2 AND #5 (2073)
#7 (#6) AND (_>_15/1/2015:CRSCREATED) (54)
Appendix 2. CENTRAL search strategy
Database: Cochrane Central Register of Controlled Trials on Wiley <Issue 2, 2016>
Search Date: 24 February 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 MESH DESCRIPTOR Thiazides EXPLODE ALL TREES 2247
#2 MESH DESCRIPTOR Sodium Chloride Symporter Inhibitors EXPLODE ALL TREES 2703
#3 MESH DESCRIPTOR Sodium Potassium Chloride Symporter Inhibitors EXPLODE ALL TREES 967
#4 (loop or ceiling) next diuretic* 430
#5 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*) 5995
#6 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide) 1047
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 7024
#8 MESH DESCRIPTOR Angiotensin‐Converting Enzyme Inhibitors EXPLODE ALL TREES 5527
#9 angiotensin converting enzyme inhibit* 5257
#10 ace near3 inhibit* 2876
#11 acei 527
#12 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril*) 8166
#13 #8 OR #10 OR #11 OR #12 9839
#14 MESH DESCRIPTOR Renin EXPLODE ALL TREES WITH QUALIFIERS AI 117
#15 (aliskiren or ciprokiren or ditekiren or enalkiren or remikiren or rasilez or tekturna or terlakiren or zankiren) 402
#16 renin inhibit* 233
#17 #14 OR #15 OR #16 484
#18 MESH DESCRIPTOR Angiotensin Receptor Antagonists EXPLODE ALL TREES 2422
#19 angiotensin near3 (receptor antagon* or receptor block*) 2999
#20 arb* 4936
#21 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan) 4796
#22 #18 OR #19 OR #20 OR #21 10295
#23 MESH DESCRIPTOR Calcium Channel Blockers EXPLODE ALL TREES 7947
#24 (amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil) 11977
#25 calcium near2 (antagonist* or block* or inhibit*) 6289
#26 #23 OR #24 OR #25 14839
#27 MESH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL TREES 9426
#28 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) 14878
#29 beta near2 (adrenergic* or antagonist* or block* or receptor*) 12586
#30 #27 OR #28 OR #29 20454
#31 MESH DESCRIPTOR Adrenergic alpha‐Antagonists EXPLODE ALL TREES 2967
#32 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin) 2151
#33 adrenergic near2 (alpha or antagonist*) 6322
#34 (adrenergic or alpha or receptor*) near2 block* 9598
#35 #31 OR #32 OR #33 OR #34 16896
#36 (#7 and #13) or (#7 and #17) or (#7 and #22) or (#7 and #26) or (#7 and #30) or (#7 and #35) or (#13 and #17) or (#13 and #22) or (#13 and #26) or (#13 and #30) or (#13 and #35) or (#17 and #22) or (#17 and #26) or (#17 and #30) or (#17 and #35) or (#22 and #26) or (#22 and #30) or (#22 and #35) or (#26 and #30) or (#26 and #35) or (#30 and #35) 18270
#37 MESH DESCRIPTOR Drug Therapy, Combination EXPLODE ALL TREES 37231
#38 (add* or combin* or multipl* or plus or polytherap* or versus) 340456
#39 #37 OR #38 340830
#40 hypertens* 38052
#41 (elevat* blood pressure) OR (high blood pressure) 1237
#42 #40 OR #41 38384
#43 #36 AND #39 AND #42 5921
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update
Search Date: 17 February 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp thiazides/ (14707)
2 exp sodium chloride symporter inhibitors/ (13491)
3 exp sodium potassium chloride symporter inhibitors/ (12903)
4 ((loop or ceiling) adj diuretic?).tw. (2260)
5 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide? or torasemide or torsemide).tw. (31142)
6 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. (2104)
7 or/1‐6 [Diur] (45728)
8 exp angiotensin‐converting enzyme inhibitors/ (39581)
9 angiotensin converting enzyme inhibit$.tw. (16370)
10 (ace adj2 inhibit$).tw. (16651)
11 acei.tw. (2456)
12 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. (24274)
13 or/8‐12 [ACEI] (53483)
14 renin/ai (1792)
15 (aliskiren or ciprokiren or ditekiren or enalkiren or remikiren or rasilez or tekturna or terlakiren or zankiren).mp. (1050)
16 renin inhibit$.tw. (1579)
17 or/14‐16 [RI] (2521)
18 exp Angiotensin Receptor Antagonists/ (19071)
19 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. (10291)
20 arb?.tw. (4369)
21 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan).tw. (13495)
22 or/18‐21 [ARB] (26662)
23 exp calcium channel blockers/ (75276)
24 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. (56584)
25 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. (35051)
26 or/23‐25 [CCB] (100261)
27 exp adrenergic beta‐antagonists/ (78362)
28 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. (57503)
29 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (88743)
30 or/27‐29 [BB] (143122)
31 exp adrenergic alpha antagonists/ (47646)
32 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. (12950)
33 (adrenergic adj2 (alpha or antagonist?)).tw. (18615)
34 ((adrenergic or alpha or receptor?) adj2 block$).tw. (51746)
35 or/31‐34 [AB] (104786)
36 (7 and 13) or (7 and 17) or (7 and 22) or (7 and 26) or (7 and 30) or (7 and 35) or (13 and 17) or (13 and 22) or (13 and 26) or (13 and 30) or (13 and 35) or (17 and 22) or (17 and 26) or (17 and 30) or (17 and 35) or (22 and 26) or (22 and 30) or (22 and 35) or (26 and 30) or (26 and 35) or (30 and 35) (72944)
37 drug therapy, combination/ (145056)
38 (add$ or combin$ or multipl$ or plus or polytherap$ or versus).tw. (4293782)
39 or/37‐38 (4359134)
40 hypertension/ (206653)
41 hypertens$.tw. (322164)
42 (elevat$ blood pressure or high blood pressure).tw. (15326)
43 or/40‐42 (377727)
44 randomized controlled trial.pt. (406353)
45 controlled clinical trial.pt. (90060)
46 randomized.ab. (303578)
47 placebo.ab. (155055)
48 clinical trials as topic/ (174893)
49 randomly.ab. (214986)
50 trial.ti. (131809)
51 or/44‐50 (928118)
52 animals/ not (humans/ and animals/) (4155531)
53 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ (812029)
54 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. (12640)
55 51 not (52 or 53 or 54) (818364)
56 36 and 39 and 43 and 55 (5067)
Appendix 4. Embase search strategy
Database: Embase <1980 to 2016 February 17>
Search Date: 17 February 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp thiazide diuretic agent/ (46226)
2 exp loop diuretic agent/ (56565)
3 ((loop or ceiling) adj diuretic?).tw. (3459)
4 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. (37810)
5 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. (3263)
6 or/1‐5 [Diur] (106881)
7 exp dipeptidyl carboxypeptidase inhibitor/ (146186)
8 angiotensin converting enzyme inhibit$.tw. (21331)
9 (ace adj2 inhibit$).tw. (25520)
10 acei.tw. (5240)
11 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. (33968)
12 or/7‐11 [ACEI] (154071)
13 exp renin inhibitor/ (4859)
14 (aliskiren or ciprokiren or ditekiren or enalkiren or remikiren or rasilez or tekturna or terlakiren or zankiren).tw. (1813)
15 renin inhibit$.tw. (2287)
16 or/13‐15 [RI] (5298)
17 exp angiotensin receptor antagonist/ (69189)
18 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. (16085)
19 arb?.tw. (9548)
20 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan).tw. (21598)
21 or/17‐20 [ARB] (74927)
22 calcium channel blocking agent/ (52169)
23 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. (73579)
24 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. (44698)
25 or/22‐24 [CCB] (131470)
26 exp beta adrenergic receptor blocking agent/ (236463)
27 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. (68222)
28 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (103274)
29 or/26‐28 [BBs] (287177)
30 exp alpha adrenergic receptor blocking agent/ (115277)
31 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. (15697)
32 (andrenergic adj2 (alpha or antagonist?)).tw. (6)
33 ((andrenergic or alpha or receptor?) adj2 block$).tw. (55368)
34 or/30‐33 [ABs] (162951)
35 (6 and 12) or (6 and 16) or (6 and 21) or (6 and 25) or (6 and 29) or (6 and 34) or (12 and 16) or (12 and 21) or (12 and 25) or (12 and 29) or (12 and 34) or (16 and 21) or (16 and 25) or (16 and 29) or (16 and 34) or (21 and 25) or (21 and 29) or (21 and 34) or (25 and 29) or (25 and 34) or (29 and 34) (174303)
36 drug combination/ (54909)
37 (add$ or combin$ or multipl$ or plus or polytherap$ or versus).tw. (5913215)
38 or/36‐37 (5945339)
39 exp hypertension/ (542792)
40 (hypertens$ or antihypertens$).tw. (491524)
41 (elevat$ blood pressure or high blood pressure).tw. (22308)
42 or/39‐41 (707892)
43 randomized controlled trial/ (392740)
44 crossover procedure/ (46085)
45 double‐blind procedure/ (126172)
46 (randomi$ed or randomly).tw. (308240)
47 (crossover$ or cross‐over$).tw. (77955)
48 placebo.ab. (219015)
49 (doubl$ adj blind$).tw. (158957)
50 assign$.ab. (270427)
51 allocat$.ab. (97040)
52 or/43‐51 (1023296)
53 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5434986)
54 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ (581628)
55 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. (18196)
56 52 not (53 or 54 or 55) (865566)
57 35 and 38 and 42 and 56 (6223)
Appendix 5. Search strategies for other databases
Database: ClinicalTrials.gov
Search Date: 18 February 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Search terms: combination AND randomized AND (versus OR vs)
Study type: Interventional Studies
Conditions: hypertension
Outcome: blood pressure (81)
***************************
Database: WHO International Clinical Trials Registry Platform
Search Date: 18 February 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 hypertens* AND combination AND randomized AND versus (46)
#2 hypertens* AND combination AND randomized AND vs (20)
#3 #1 OR #2 (60)
****************************
Database: LILACS
Search Date: 03 March 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(tw:(monoter* OR combinac*)) AND (tw:(hipertens*)) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials" OR "systematic_reviews"))
Appendix 6. Pharmaceutical companies checked
| Company | Website |
| Abbot | www.abbott.com/citizenship/disclosures/clinical‐study‐results.htm |
| Abbvie | |
| AstraZeneca | |
| Bayer | pharma.bayer.com/en/research‐and‐development/clinical‐trials/trial‐finder/index.php |
| Boehringer Ingelheim | |
| Bristol‐Myers Squibb | |
| Daiichi Sankyo | www.daiichisankyo.com/rd/our_approach/clinical_studies/index.html |
| GlaxoSmithKline | |
| Menarini | |
| Merck | |
| Mylan | |
| Novartis | www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp |
| Pfizer | www.pfizer.com/research/research_clinical_trials/trial_results_research_progress |
| Recordati | |
| Sanofi Aventis | en.sanofi.com/rd/clinical_trials/our_commitments/clinical_study_results.aspx |
| Servier | |
| Takeda | |
| Teva | |
| UCB |
Appendix 7. Reviews and guidelines checked
Hilleman 1999; Ruzicka 2001; Law 2003a; Law 2009; Wald 2009; Sood 2010; Gradman 2010; Lv 2010; NICE 2011; ESH‐ESC 2013; Liu 2013; Makani 2013; Povoa 2014; Bakris 2014; CHEP 2015; JNC 8 2014.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Combination therapy versus monotherapy, Outcome 1 Total mortality.
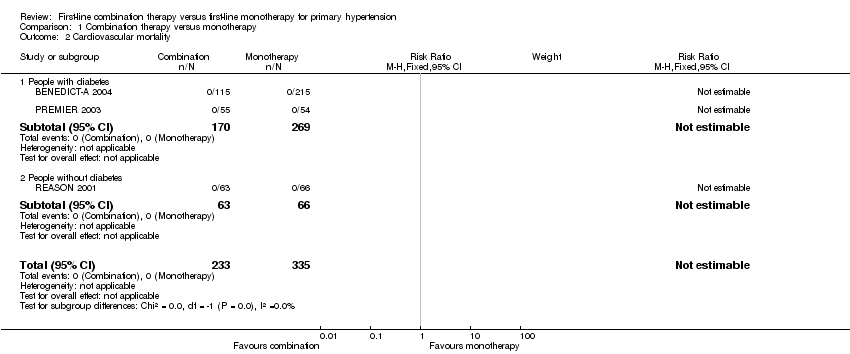
Comparison 1 Combination therapy versus monotherapy, Outcome 2 Cardiovascular mortality.

Comparison 1 Combination therapy versus monotherapy, Outcome 3 Cardiovascular events.
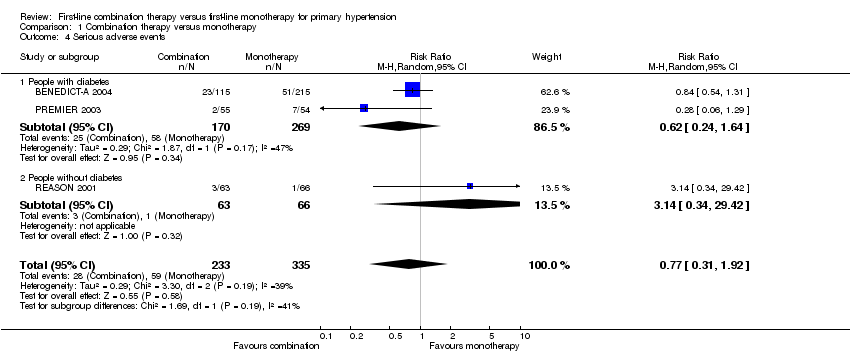
Comparison 1 Combination therapy versus monotherapy, Outcome 4 Serious adverse events.

Comparison 1 Combination therapy versus monotherapy, Outcome 5 Withdrawals due to adverse effects.

Comparison 1 Combination therapy versus monotherapy, Outcome 6 Reaching target blood pressure at 1 year.

Comparison 1 Combination therapy versus monotherapy, Outcome 7 Systolic blood pressure change from baseline at end of 1 year.
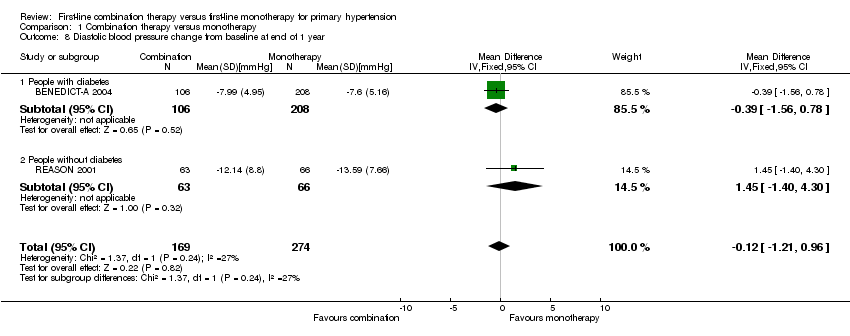
Comparison 1 Combination therapy versus monotherapy, Outcome 8 Diastolic blood pressure change from baseline at end of 1 year.

Comparison 2 Combination therapy versus monotherapy (men versus women), Outcome 1 Serious adverse events.

Comparison 2 Combination therapy versus monotherapy (men versus women), Outcome 2 Withdrawals due to adverse effects.

Comparison 2 Combination therapy versus monotherapy (men versus women), Outcome 3 Systolic blood pressure change from baseline at end of 1 year.

Comparison 2 Combination therapy versus monotherapy (men versus women), Outcome 4 Diastolic blood pressure change from baseline at end of 1 year.
| Combination therapy versus monotherapy for primary hypertension | ||||||
| Patient or population: people with primary hypertension | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Monotherapy | Combination therapy | |||||
| Total mortality | 3 per 1000 | 4 per 1000 | RR 1.35 | 568 | ⊕⊝⊝⊝ | ‐ |
| Cardiovascular mortality | See footnote4 | See footnote4 | Not estimable | 568 | ⊕⊝⊝⊝ | ‐ |
| Cardiovascular events | 9 per 1000 | 9 per 1000 | RR 0.98 | 568 | ⊕⊝⊝⊝ | ‐ |
| Serious adverse events | 176 per 1000 | 136 per 1000 | RR 0.77 | 568 | ⊕⊝⊝⊝ | ‐ |
| Withdrawals due to adverse effects | 128 per 1000 | 109 per 1000 | RR 0.85 | 568 | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed is the mean monotherapy group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the combination group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All data come from subgroups of participants not predefined in the original studies. Outcomes of our review were not the primary outcome in any included trial. | ||||||
| Characteristic | Treatment | Mean (standard deviation) | ||
| Number of participants | Combination | 115 | 55 | 63 |
| Monotherapy | 215 | 54 | 66 | |
| Total participants included in the trial (%) | Combination | 38.08% | 22.78% | 28.09% |
| Monotherapy | 35.54% | 22.54% | 25.82% | |
| Age (years) | Combination | 60.98 (7.62) | 57.27 (8.53) | 52.49 (12.68) |
| Monotherapy | 60.62 (8.36) | 59.93 (8.75) | 50.38 (10.57) | |
| Sex (% men) | Combination | 67.83% | 74.55% | 71.43% |
| Monotherapy | 69.30% | 77.78% | 62.12% | |
| Ethnicity (% white people) | Combination | 100.00% | 96.36% | 98.41% |
| Monotherapy | 100.00% | 88.89% | 93.94% | |
| Body mass index (kg/m2) | Combination | 28.68 (5.19) | 28.23 (3.18) | 26.85 (3.11) |
| Monotherapy | 28.34 (4.42) | 29.22 (3.51) | 26.99 (2.38) | |
| Systolic blood pressure (mm Hg) | Combination | 151.61 (9.70) | 154.56 (9.86) | 162.56 (11.24) |
| Monotherapy | 152.11 (11.57) | 154.04 (11.67) | 158.74 (12.84) | |
| Diastolic blood pressure (mm Hg) | Combination | 88.72 (7.17) | 90.98 (8.43) | 97.65 (6.89) |
| Monotherapy | 89.54 (6.32) | 91.00 (8.26) | 98.94 (5.07) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total mortality Show forest plot | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| 1.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| 1.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Cardiovascular mortality Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cardiovascular events Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.22, 4.41] |
| 3.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.10, 3.95] |
| 3.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.13, 75.69] |
| 4 Serious adverse events Show forest plot | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.31, 1.92] |
| 4.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.24, 1.64] |
| 4.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.34, 29.42] |
| 5 Withdrawals due to adverse effects Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| 5.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.49, 1.35] |
| 5.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.45] |
| 6 Reaching target blood pressure at 1 year Show forest plot | 3 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.52, 2.54] |
| 6.1 People with diabetes, target ≤ 120/80 mmHg | 1 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.18] |
| 6.2 People with diabetes, target ≤ 140/90 mmHg | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [1.24, 3.22] |
| 6.3 People without diabetes, target ≤ 140/90 mmHg | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.62, 1.28] |
| 7 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 3 | 548 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐5.39, 1.27] |
| 7.1 People with diabetes | 2 | 419 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐8.27, 3.19] |
| 7.2 People without diabetes | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐7.28, 2.62] |
| 8 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 2 | 443 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.21, 0.96] |
| 8.1 People with diabetes | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.56, 0.78] |
| 8.2 People without diabetes | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.45 [‐1.40, 4.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.52, 3.00] |
| 1.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 2 Withdrawals due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.43, 3.73] |
| 2.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.66] |
| 3 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [‐2.10, 5.58] |
| 3.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐3.25, 1.19] |
| 4 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐1.96, 2.90] |
| 4.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐2.08, 0.54] |


