Vacunación oral contra Haemophilus influenzae para la prevención de las exacerbaciones agudas de la bronquitis crónica y la enfermedad pulmonar obstructiva crónica
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Double‐blind, placebo‐controlled, prospective RCT over a 3‐month winter period in 1983 | |
| Participants | 50 patients from Royal Newcastle Hospital with COPD not taking corticosteroids or immunosuppressants Mean age of all participants: 65.5 | |
| Interventions | NTHi vaccine and 2 placebo arms (enteric‐coated glucose tablets or 25 mg sodium tauroglycocholate). 3 courses of tablets were taken at 0, 28, 56 days. Each course was 2 tablets taken before breakfast on 3 consecutive days. | |
| Outcomes |
| |
| Notes | Many participants were taking antibiotics and bronchodilator agents but were not taking steroids or immunosuppressants. Ciba‐Geigy (Australia) was cited for financial assistance in the Discussion. Trial was conducted at the Royal Newcastle Hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was completed independently by a "Dr Smith" who kept the trial code allocations privately; how randomisation was performed was not disclosed. Whilst Dr Smith is not one of the trial authors, the exact nature of their relationship with the study is unknown. Randomisation in 1 arm had a very uneven male‐to‐female ratio |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | For each participant with an acute upper and lower respiratory infection, an infection questionnaire was completed by doctors who were not involved with the study and had no knowledge of the participant's test group |
| Blinding of outcome assessment (detection bias) | Low risk |
|
| Incomplete outcome data (attrition bias) | Low risk | All participants were accounted for. 2 participants out of the 50 originally enrolled died during the study |
| Selective reporting (reporting bias) | Low risk | Nil |
| Other bias | Low risk | Nil |
| Methods | Single‐blind RCT | |
| Participants | 6‐month study on Australians recruited during winter 1986 through radio station advertisement Mean age of 47.4 in the vaccine arm and 46.3 in the placebo arm | |
| Interventions | Oral vaccination with killed NTHi and placebo with glucose; both were enteric‐coated | |
| Outcomes |
| |
| Notes | Participants were assessed on admission to trial and at 3 and 6 months during the trial. Most participants had previously unrecognised smoking‐related chronic lung disease. 72% smokers and 58% had chronic bronchitis. Participant admission criteria were > 3 episodes of acute bronchitis (cough productive with sputum) over previous 2 years and an absence of chronic lung disease determined at clinical interview Trial was conducted as joint research at the Royal Newcastle Hospital and Macquarie University There was no disclosure of financial assistance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were coded and randomised by an independent third party into 2 groups. 40 participants were admitted to study; 37 completed the 6‐month trial. Randomisation gave well‐matched treatment groups, which were equal at baseline measurement. There was no significant reduction in the number of participants suffering from an episode of acute bronchitis |
| Allocation concealment (selection bias) | High risk | Participants were allocated to the intervention group based on periods of acute bronchitis that had been assessed by a nurse practitioner using an infection questionnaire, which may have room for subjectivity |
| Blinding of participants and personnel (performance bias) | High risk | Medication had the same regimen and administration for both groups. Participants in the treatment arm were given the active preparation, each tablet containing 10 x killed NTHi. Participants in the placebo arm were given a preparation containing glucose. Both were given in the form of 2 enteric‐coated tablets before breakfast on each of 3 consecutive days and repeated at 28 and 56 days. However, only participants were blinded in this study (single‐blinded) |
| Blinding of outcome assessment (detection bias) | Low risk |
|
| Incomplete outcome data (attrition bias) | Low risk | All participants were accounted for. 3 participants dropped out from the study: 2 from the placebo arm, 1 from the active arm due to poor compliance |
| Selective reporting (reporting bias) | Low risk | Nil |
| Other bias | Low risk | Nil |
| Methods | Double‐blind, placebo‐controlled, prospective study for 9 months over the Australian winter of 2011 | |
| Participants | 320 moderate‐severe COPD participants with FEV1 < 60% predicted were recruited from 21 sites across Australia Mean age of participants in the vaccine arm was 71.2 and in the placebo arm was 67.9 | |
| Interventions | HI‐164 oral vaccine enteric‐coated tablets (2 per day) each of which contained 45 mg active substance of formalin‐inactivated NTHi (HI‐164) | |
| Outcomes |
| |
| Notes | This was a multicentre trial conducted over various health districts across Australia The trial does not cite any financial acknowledgement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not disclose method. Randomisation mentioned but technique not specified |
| Allocation concealment (selection bias) | Unclear risk | Does not disclose method. Participants assumed to be blinded to the allocation process and to whether treatment or placebo received |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded study with same administration of medication for both treatment and placebo arms. The treatment consisted of 3 courses of tablets; each course was 2 tablets daily (before breakfast) for 3 consecutive days, with courses repeated at day 28 and day 56. Following randomisation, participants attended site visits at weeks 4, 8, 12 and thereafter at 4‐week intervals until week 36. The placebo group had the same regimen, except with matched placebo tablets |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded study where acute episodes and background symptoms were recorded by a questionnaire at all visits. To document change in day‐to‐day symptoms, all participants used a diary and the St George's Respiratory Questionnaire for COPD patients (SGRQ‐C) (version 1.1; 11‐12008) was administered at visit 2 (baseline), visit 5 (week 12), visit 6 (week 24), and visit 7 (week 36) |
| Incomplete outcome data (attrition bias) | Low risk | 320 participants were the ITT population, with a 10% dropout rate anticipated to ensure that greater than 270 completed the study as required by the power analysis. Specifically, adverse event rates were similar in both groups: serious adverse events (placebo 33.5%; active 38.5%) with 4 deaths in the active group and 2 in the placebo group. None of these events was attributed to treatment modality |
| Selective reporting (reporting bias) | Low risk | Nil |
| Other bias | Low risk | Nil |
| Methods | RCT of 12 months' duration, double‐blind, placebo‐controlled | |
| Participants | Adults identified as suffering from chronic lung disease 62 participants included Recruitment: nominated Inclusion: productive cough fitting the time criteria for chronic lung disease Mean age of participants in the vaccine arm was 52.6 and in the placebo arm was 53.7 | |
| Interventions | Oral inactivated vaccine containing 10 Haemophilus influenzae Control: placebo, not specified Duration: 2 tablets in the morning for 3 consecutive days at monthly intervals for 3 consecutive months | |
| Outcomes |
| |
| Notes | An acute exacerbation is defined as an increase in the volume and purulence of sputum with no evidence of respiratory distress, with or without chest pain or fever. This definition was consolidated with clinical examination, respiratory questionnaire in Melasian pigeon English, spirometry, and sputum samples Conducted at PNG Institute of Medical Research. Auspharm International Ltd cited in acknowledgements for setting up study and ongoing support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe appropriate random sequence generation using a randomisation code for the courses of vaccine and placebo tablets |
| Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment, as randomisation was performed by the third party "Auspharm International Ltd." in New South Wales, Australia, accounting for the concealment of allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial. Blinding of participants and key study personnel ensured and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind trial. The outcomes assessed in both groups were measured using the same questionnaire and a medical examination. Methods of ensuring blinding of outcome assessment were not reported |
| Incomplete outcome data (attrition bias) | High risk | Concluded from the trial report, there is no mention of ITT for participants lost to follow‐up (8 from vaccine group and 3 from placebo group). This is substantial considering the small group sizes; it is possible that it had an effect on the outcome |
| Selective reporting (reporting bias) | Low risk | Study report fails to include results for a key outcome (prescription rate of antibiotics) that would be expected to have been reported for such a study, although the protocol is not available. However, all primary outcomes are reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | 6‐month RCT, double‐blind, placebo‐controlled | |
| Participants | Adults with history of chronic bronchitis with recurrent respiratory tract infections (RTIs) Mean age vaccine group: 73.1 years; placebo group: 71.1 years Settings: Chest Clinic, Western Australia | |
| Interventions | The oral vaccine contained 10 killed H influenzae Control: placebo was a lactose substitute for bacteria Duration: 2 tablets in the morning for 3 consecutive days monthly for 3 consecutive months (day 0, 28, 56) | |
| Outcomes |
| |
| Notes | Acute exacerbation defined as: increase in volume and purulence of sputum usually associated with an increase in breathlessness or fever, or both requiring treatment with antibiotics Exacerbation assessed by the trial authors using the following: physical exam, respiratory questionnaire (ATS‐DLD‐78), lung function via spirometry, and sputum samples Auspharm cited for providing Bronchostat and placebo tablets. Conducted at the repatriation hospital Western Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomisation methodology in the sequence generation process, i.e. a randomisation chart |
| Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because pharmacy‐controlled central allocation was used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Blinding of participants and key study personnel ensured and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Low risk | Method of ensuring blinding of outcome assessors is not reported. Respiratory questionnaire was used to collect data for primary outcomes |
| Incomplete outcome data (attrition bias) | Low risk | 10 participants lost to follow‐up (3 in vaccine group and 7 in placebo group) were analysed by intention‐to‐treat, which showed no significant differences. For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | 4‐month double‐blind, placebo‐controlled RCT carried out in winter in 4 centres in Western Australia | |
| Participants | People with severe COPD defined by FEV1 < 50% or > 2 acute exacerbations per year for 2 consecutive years Mean age of participants in the vaccine arm was 69.5 and in the placebo arm was 67.3 | |
| Interventions | HI‐1640V: each tablet contained 45 mg approximately 10 bacteria of formalin‐inactivated NTHi provided as enteric‐coated tablets Control: enteric‐coated placebo tablets containing excipients only Duration: protocol stated that participants took 3 courses of 2 tablets in the morning for 3 consecutive days with courses repeated at 28 and 56 days | |
| Outcomes |
| |
| Notes | Multicentre trial. Most authors disclosed contributions from Hunter Immunology Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were reportedly randomised, although the method of randomisation was not discussed. Baseline characteristics suggest that randomisation was successful |
| Allocation concealment (selection bias) | Unclear risk | No information was provided about the procedure for protecting the randomisation process so that the treatment to be allocated was not known before the patient was entered into the study |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial |
| Blinding of outcome assessment (detection bias) | Low risk | Primary outcome was measured using a respiratory questionnaire. These subjective data are prone to recall bias. Secondary outcomes were objectively measured using bacterial colonisations and antibody titres |
| Incomplete outcome data (attrition bias) | Low risk | Participants were followed up for 4 months after completing the 3 courses. No participants were lost to follow‐up. Data surrounding withdrawal and discontinuation from the study were well described |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Other bias | Low risk | No other sources of biases were identified |
ATS‐DLD‐78: American Thoracic Society Division of Lung Disease questionnaire
COAD: chronic obstructive airways disease
COPD: chronic obstructive pulmonary disease
FEV1: forced expiratory volume in one second
ITT: intention‐to‐treat
NTHi: non‐typeable Haemophilus influenzae
PNG: Papua New Guinea
RCT: randomised controlled trial
RTI: respiratory tract infection
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| The participants and outcome measures of this study did not match this review's protocol. Participants were smokers with no clearly defined respiratory disease. Outcome measures were limited to physiological markers as opposed to the clinical outcomes of this review |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Participants will be randomly allocated to active tablets each containing 45 mg HI‐1‐164‐AS (inactivated non‐typeable Haemophilus influenzae). Study medication (2 tablets) will be taken on days 1, 2, 3, 29, 30, 31, 57, 58, 59. The live phase of the study will be of 8 months duration (March to October) |
| Participants | Both males and females, greater than or equal to 18 years of age, with moderate to severe airway disease Total number of participants is 124; randomised to either active or placebo group |
| Interventions | HI‐1‐164‐AS (inactivated non‐typeable Haemophilus influenzae) oral vaccine tablet |
| Outcomes | Primary outcomes: number of episodes of acute bronchitis during the study; proportion of participants experiencing an episode of acute bronchitis during the study; duration of episodes of acute bronchitis during the study; number of courses of antibiotics taken for treatment of acute episodes of bronchitis during the study Secondary outcomes: NTHi‐specific antibody; pharyngeal colonisation with H influenzae; presence of H influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, and Pseudomonas species in sputum; severity of episodes of acute bronchitis |
| Notes | It was planned in this trial that the analysis of the severity of episodes of acute bronchitis was to be based on the respiratory questionnaires completed by the participants at the time of each episode. However, insufficient respiratory questionnaires were completed during the study to allow for analysis of the data collected. In accordance with recent draft guidance for industry for developing drugs for treatment of COPD issued by the FDA in November 2007, assessment of modification or prevention of exacerbations of disease can include severity of exacerbations as a primary efficacy endpoint. This can be based on worsening of symptoms requiring changes in treatment or requiring urgent treatment or hospitalisation. On a post hoc basis, rates of hospitalisation, corticosteroid use, and a review of the medications used to treat the episodes of acute bronchitis were all analysed as measures of severity of episodes |
| Methods | Participants will be randomly allocated to active tablets each containing 45 mg HI‐1‐164‐AS (inactivated non‐typeable Haemophilus influenzae). Study medication (2 tablets) will be taken on days 1, 2, 3, 29, 30, 31, 57, 58, 59. The live phase of the study will be of 8 months duration (March to October) |
| Participants | Both males and females, greater than or equal to 18 years of age, with mild to severe airway disease Total number of participants is 124; randomised to either active or placebo group |
| Interventions | HI‐1‐164‐AS (inactivated non‐typeable Haemophilus influenzae) oral vaccine tablet |
| Outcomes | Primary outcomes: number of episodes of acute bronchitis during the study; proportion of participants experiencing an episode of acute bronchitis during the study; duration of episodes of acute bronchitis during the study; number of courses of antibiotics taken for treatment of acute episodes of bronchitis during the study Secondary outcomes: NTHi‐specific antibody; pharyngeal colonisation with H influenzae; presence of H influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, and Pseudomonas species in sputum; severity of episodes of acute bronchiti |
| Notes | This trial is synonymous to ACTRN12606000074594, with the only difference to be found in the inclusion criteria of participants; this trial accepts patients with mild to severe airway disease (versus moderate to severe). However, there is no further specification or discussion on how the investigators discern the extent of airway disease in their study participants. Both studies enrolled participants on 7 March 2006 and were conducted simultaneously |
COPD: chronic obstructive pulmonary disease
FDA: US Food and Drug Administration
NTHi: non‐typeable Haemophilus influenzae
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations (number of exacerbations/person) Show forest plot | 6 | 557 | Rate Ratio (Random, 95% CI) | 0.79 [0.57, 1.10] |
| Analysis 1.1 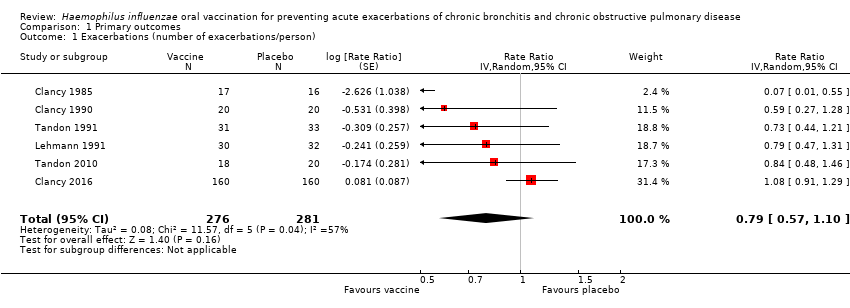 Comparison 1 Primary outcomes, Outcome 1 Exacerbations (number of exacerbations/person). | ||||
| 2 Mortality (deaths during trial period) Show forest plot | 5 | 518 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.63, 4.12] |
| Analysis 1.2 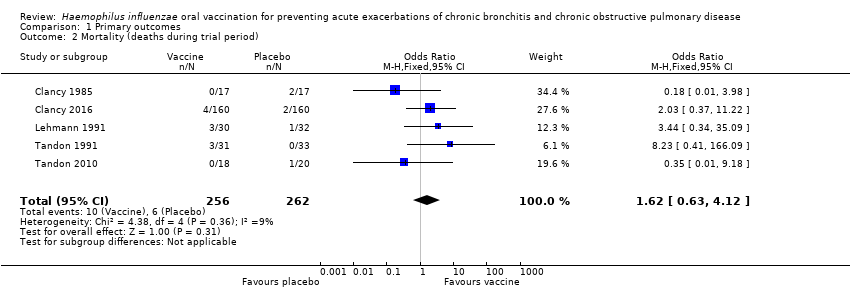 Comparison 1 Primary outcomes, Outcome 2 Mortality (deaths during trial period). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prescriptions (number of courses/person/year) Show forest plot | 3 | 142 | Rate Ratio (Fixed, 95% CI) | 1.81 [1.35, 2.44] |
| Analysis 2.1  Comparison 2 Secondary outcomes, Outcome 1 Prescriptions (number of courses/person/year). | ||||
| 2 Adverse events (number of adverse events/person) Show forest plot | 4 | 484 | Rate Ratio (Fixed, 95% CI) | 1.43 [0.70, 2.92] |
| Analysis 2.2  Comparison 2 Secondary outcomes, Outcome 2 Adverse events (number of adverse events/person). | ||||

Inclusion of trials flow diagram.
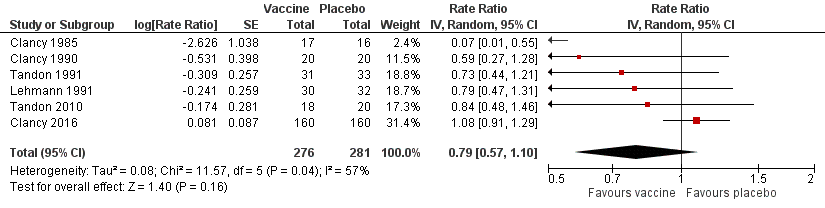
Forest plot of comparison: 1 Primary outcomes, outcome: 1.1 Exacerbations (number of exacerbations/person/year).
Refer to Table 1 for Overall rate estimates of acute exacerbations across included studies.

Forest plot of comparison: 1 Primary outcomes, outcome: 1.2 Mortality (deaths during trial period).
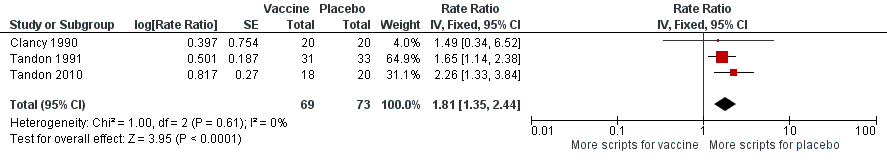
Forest plot of comparison: 2 Secondary outcomes, outcome: 2.1 Prescriptions (number of courses/person/year).
Refer to Table 2 for Overall rate estimates of antibiotic prescriptions across included studies.
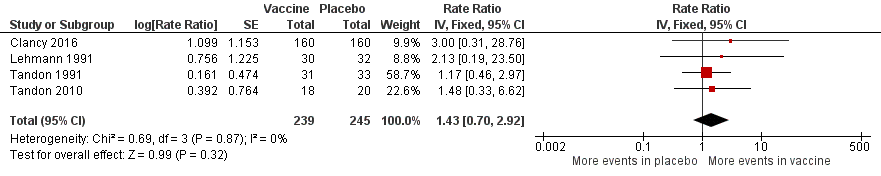
Forest plot of comparison: 2 Secondary outcomes, outcome: 2.3 Adverse events (number of adverse events/person/year).
Refer to Table 3 for Overall rate estimates of adverse events across included studies.

Comparison 1 Primary outcomes, Outcome 1 Exacerbations (number of exacerbations/person).

Comparison 1 Primary outcomes, Outcome 2 Mortality (deaths during trial period).

Comparison 2 Secondary outcomes, Outcome 1 Prescriptions (number of courses/person/year).

Comparison 2 Secondary outcomes, Outcome 2 Adverse events (number of adverse events/person).
| Haemophilus influenzae oral vaccination for prevention of acute exacerbations of chronic bronchitis and COPD | ||||||
| Patient or population: adults (> 18 years of age) with either COPD or recurrent acute exacerbations of chronic bronchitis Settings: community and outpatients Intervention: oral monobacterial vaccination with killed NTHi Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk Not vaccinated | Corresponding risk NTHi oral vaccinated | |||||
| Acute exacerbations (number of exacerbations/person/year) | 2.111 exacerbations per person/year | 1.668 exacerbations | RR 0.79 (0.57 to 1.10) | 557 | ⊕⊕⊝⊝ | Despite an absolute estimated decrease in the rate of exacerbations in the vaccinated group, the result is negligible (95% CI crosses 1.00) and not statistically significant (P = 0.16) |
| Mortality (deaths during trial period) | 23 per 1000 | 37 per 1000 | OR 1.62 (0.63 to 4.12) | 518 | ⊕⊝⊝⊝ | Despite more absolute deaths occurring in the vaccinated group, the result is negligible (95% CI crosses 1.00) and not statistically significant (P = 0.31). Deaths were not necessarily attributed to the use of the vaccine |
| Carriage of NTHi Not meta‐analysed | N/A | N/A | N/A | N/A | ⊕⊝⊝⊝ | We were unable to meta‐analyse the carriage levels of NTHi in participants as each trial reported this result using different units and tools of measurement. 4 trials showed no significant difference in carriage levels, while 2 trials showed a significant decrease in carriage levels in the vaccinated group compared with the placebo group |
| Antibiotic prescriptions (number of courses/person/year) **Corticosteroids not meta‐analysed | 5.723 | 3.162 | RR 1.81 (1.35 to 2.44) | 142 | ⊕⊕⊝⊝ | Courses of antibiotics were found to be prescribed in the placebo group at a rate approximately 80% greater than in the vaccinated group (P < 0.001) (Note that a RR > 1.0 here indicates more antibiotics being prescribed to participants in the placebo group, i.e. RR 1.81 corresponds to an approximately 80% increased rate of antibiotic prescriptions when not receiving the vaccine. The placebo group is being compared to the vaccine group in this instance to attempt to demonstrate how many more antibiotics are required in those not vaccinated) **2studies reported corticosteroid use, however due to differences in units of measurement, these results could not be meta‐analysed |
| Hospital admissions (number of participants hospitalised during trial period) | N/A | N/A | N/A | N/A | ⊕⊕⊕⊝ | Hospital admissions was not meta‐analysed due to differing units of measurement by the 2 trials that reported this finding. Notwithstanding that pooling the data for the 2 trials would have yielded high heterogeneity. Hospital admissions were not necessarily attributable to the vaccine |
| Adverse events (number of adverse events/person/year) | 0.319 | 0.456 | RR 1.43 (0.70 to 2.92) | 484 | ⊕⊕⊝⊝ | Despite an estimated absolute increased rate of adverse events in the vaccinated group, the result is negligible (95% CI crosses 1.00) and not statistically significant (P = 0.61). Adverse events were not necessarily attributable to the vaccine |
| Quality of life Not meta‐analysed | N/A | N/A | N/A | N/A | ⊕⊝⊝⊝ | Quality of life was not meta‐analysed due to differing units of measurement, but was reported in 2 trials, which showed an improvement at 6 months in the vaccine group (scoring at least 2 points better than the placebo group; significance unknown) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval (CI)) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; NTHi: non‐typeable Haemophilus influenzae; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One study had marked heterogeneity; most studies had a low number of participants; one study had significant attrition. | ||||||
| Study | Vaccinated | Placebo | *Absolute rate difference |
| Clancy 1985 | 0.256 | 0.272 | 0.016 (‐) |
| Clancy 1990 | 1.000 | 1.700 | 0.700 (‐) |
| Clancy 2016 | 0.717 | 0.767 | 0.050 (‐) |
| Lehmann 1991 | 0.800 | 1.210 | 0.410 (‐) |
| Tandon 1991 | 3.355 | 4.364 | 1.009 (‐) |
| Tandon 2010 | 3.667 | 4.350 | 0.683 (‐) |
| Overall mean | 1.633 | 2.111 | 0.478 (‐) |
| *Estimated rate of exacerbation calculated as number of exacerbations per person per year. Refer to Analysis 1.1: Forest plot comparison and rate ratios for exacerbations. | |||
| Study | Vaccinated | Placebo | *Absolute rate difference |
| Clancy 1990 | 0.500 | 1.200 | 0.700 (‐) |
| Tandon 1991 | 5.806 | 10.194 | 4.388 (‐) |
| Tandon 2010 | 3.180 | 7.200 | 4.020 (‐) |
| Overall mean | 3.162 | 6.198 | 3.036 (‐) |
| *Estimated rate of antibiotic prescriptions calculated as number of antibiotic courses per person per year. Refer to Analysis 2.1: Forest plot comparison and rate ratios for antibiotic prescriptions. | |||
| Study | Vaccinated | Placebo | *Absolute rate difference |
| Clancy 2016 | 0.008 | 0.025 | 0.017 (‐) |
| Lehmann 1991 | 0.067 | 0.031 | 0.036 (+) |
| Tandon 1991 | 1.032 | 1.212 | 0.180 (‐) |
| Tandon 2010 | 0.167 | 0.450 | 0.283 (‐) |
| Overall mean | 0.319 | 0.430 | 0.111 (‐) |
| *Estimated rate of adverse events calculated as number of adverse events per person per year. Refer to Analysis 2.2: Forest plot comparison and rate ratios for adverse events. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations (number of exacerbations/person) Show forest plot | 6 | 557 | Rate Ratio (Random, 95% CI) | 0.79 [0.57, 1.10] |
| 2 Mortality (deaths during trial period) Show forest plot | 5 | 518 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.63, 4.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prescriptions (number of courses/person/year) Show forest plot | 3 | 142 | Rate Ratio (Fixed, 95% CI) | 1.81 [1.35, 2.44] |
| 2 Adverse events (number of adverse events/person) Show forest plot | 4 | 484 | Rate Ratio (Fixed, 95% CI) | 1.43 [0.70, 2.92] |

