| Haemophilus influenzae oral vaccination for prevention of acute exacerbations of chronic bronchitis and COPD |
| Patient or population: adults (> 18 years of age) with either COPD or recurrent acute exacerbations of chronic bronchitis Settings: community and outpatients Intervention: oral monobacterial vaccination with killed NTHi Comparison: placebo |
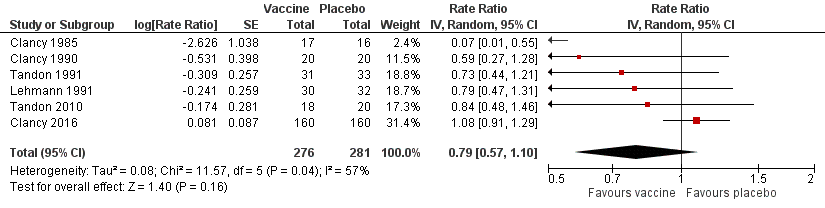
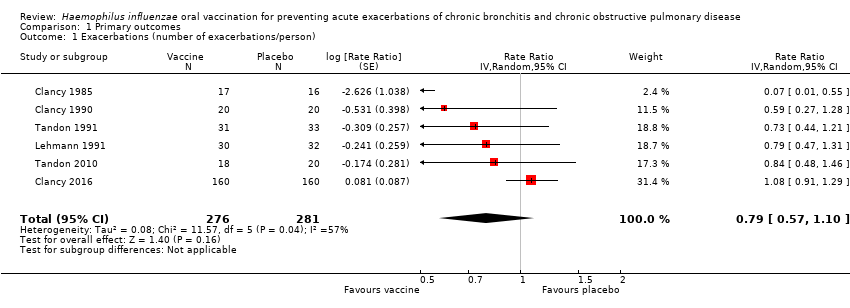
| Acute exacerbations (number of exacerbations/person/year) | 2.111 exacerbations per person/year | 1.668 exacerbations
per person/year | RR 0.79 (0.57 to 1.10) | 557
(6) | ⊕⊕⊝⊝
low1 | Despite an absolute estimated decrease in the rate of exacerbations in the vaccinated group, the result is negligible (95% CI crosses 1.00) and not statistically significant (P = 0.16) |
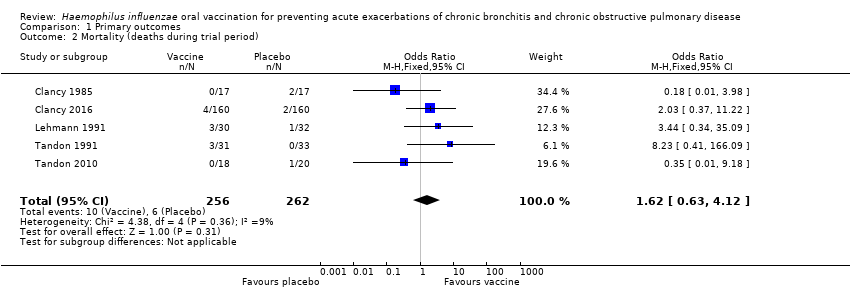
| Mortality (deaths during trial period) | 23 per 1000 | 37 per 1000
(15 to 88) | OR 1.62 (0.63 to 4.12) | 518
(5) | ⊕⊝⊝⊝
very low2 | Despite more absolute deaths occurring in the vaccinated group, the result is negligible (95% CI crosses 1.00) and not statistically significant (P = 0.31). Deaths were not necessarily attributed to the use of the vaccine |
| Carriage of NTHi Not meta‐analysed | N/A | N/A | N/A | N/A | ⊕⊝⊝⊝
very low6 | We were unable to meta‐analyse the carriage levels of NTHi in participants as each trial reported this result using different units and tools of measurement. 4 trials showed no significant difference in carriage levels, while 2 trials showed a significant decrease in carriage levels in the vaccinated group compared with the placebo group |
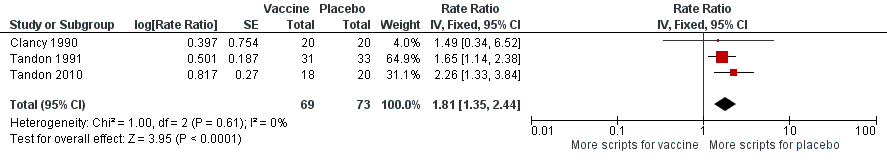
| Antibiotic prescriptions (number of courses/person/year) **Corticosteroids not meta‐analysed | 5.723
prescriptions
per person/year | 3.162
prescriptions
per person/year | RR 1.81 (1.35 to 2.44) | 142
(3) | ⊕⊕⊝⊝
low3 | Courses of antibiotics were found to be prescribed in the placebo group at a rate approximately 80% greater than in the vaccinated group (P < 0.001) (Note that a RR > 1.0 here indicates more antibiotics being prescribed to participants in the placebo group, i.e. RR 1.81 corresponds to an approximately 80% increased rate of antibiotic prescriptions when not receiving the vaccine. The placebo group is being compared to the vaccine group in this instance to attempt to demonstrate how many more antibiotics are required in those not vaccinated) **2studies reported corticosteroid use, however due to differences in units of measurement, these results could not be meta‐analysed |
| Hospital admissions (number of participants hospitalised during trial period) | N/A | N/A | N/A | N/A | ⊕⊕⊕⊝
moderate4 | Hospital admissions was not meta‐analysed due to differing units of measurement by the 2 trials that reported this finding. Notwithstanding that pooling the data for the 2 trials would have yielded high heterogeneity. Hospital admissions were not necessarily attributable to the vaccine |
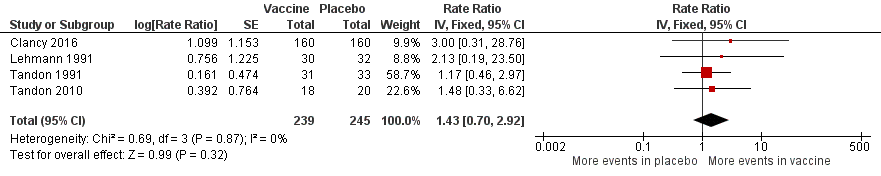
| Adverse events (number of adverse events/person/year) | 0.319
adverse events
per person/year | 0.456
adverse events
per person/year | RR 1.43 (0.70 to 2.92) | 484
(5) | ⊕⊕⊝⊝
low5 | Despite an estimated absolute increased rate of adverse events in the vaccinated group, the result is negligible (95% CI crosses 1.00) and not statistically significant (P = 0.61). Adverse events were not necessarily attributable to the vaccine |
| Quality of life Not meta‐analysed | N/A | N/A | N/A | N/A | ⊕⊝⊝⊝
very low6 | Quality of life was not meta‐analysed due to differing units of measurement, but was reported in 2 trials, which showed an improvement at 6 months in the vaccine group (scoring at least 2 points better than the placebo group; significance unknown) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval (CI)) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; NTHi: non‐typeable Haemophilus influenzae; OR: odds ratio; RR: risk ratio |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. |