Tacrólimus tópico para la dermatitis atópica
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | Adults with moderate to severe AD | |
| Interventions | Tacrolimus ointment 0.1% (12 participants) compared with 17‐butyrate‐hydrocortisone ointment 0.1% (12 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were given a run‐in number on enrolment; the randomisation list was created using Random Allocation Software |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were not blinded, but there was no interference with the results, as the participant's assessment was not evaluated. In the same way, physicians who gave the drug were not blinded, but they were not the people evaluating the outcomes, thus, also not interfering with the results. (We received this information after contacting the study author) |
| Blinding of outcome assessment (detection bias) | Low risk | The physician that evaluated SCORAD was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | There were 3 losses out of 24 (12.5%) participants; there was no ITT analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (randomised, double‐blind, multicentre, comparative study) | |
| Participants | Paediatric patients (2 to 15 years) with history of moderate to severe AD and a severe flare (IGA > = 4) | |
| Interventions | Tacrolimus ointment 0.03% BID (136 participants) compared with methylprednisolone aceponate 0.1% in the evening and no active treatment ointment in the morning (129 participants) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in blocks to achieve balanced randomisation overall and within each centre |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) | Low risk | The trial used identical tubes (morning and evening) |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was investigator blinded |
| Incomplete outcome data (attrition bias) | Low risk | There were 8 losses out of 265 (3%) participants. ITT analysis was done, and the 'last observation carried forward' principle was applied to impute missing values |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (double‐blind, randomised, multicentre trial) | |
| Participants | Paediatric patients with moderate to severe AD | |
| Interventions | Tacrolimus ointment 0.03% (43 participants) compared with tacrolimus ointment 0.1% (49 participants) versus tacrolimus ointment 0.3% (44 participants) compared with vehicle (44 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated within each centre by using permuted blocks of size 8 by means of a centralised computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | The interventions were identical in their appearance (identical coded tubes) |
| Blinding of outcome assessment (detection bias) | Low risk | The interventions were identical in their appearance (identical coded tubes) (blinded to all investigators, participants, and the sponsor) |
| Incomplete outcome data (attrition bias) | Low risk | There were 11 out of 180 (6.1%) losses. ITT analysis was done with no reference to the method for imputing missing data |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT | |
| Participants | Adults with moderate to severe AD | |
| Interventions | Tacrolimus ointment 0.1% (10 participants) compared with hydrocortisone butyrate 0.1% ointment (10 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding was not described |
| Incomplete outcome data (attrition bias) | High risk | There were 4 losses out of 20 (20%) participants in both groups. There was no ITT analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (double‐blind, randomised, non‐inferiority, multicentre trial) | |
| Participants | Paediatric patients (2 to 15 years) with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) and insufficient response to topical corticosteroids | |
| Interventions | Tacrolimus ointment 0.03% (240 participants) compared with fluticasone 0.005% ointment (239 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | Moderate‐potency corticosteroids (fluticasone 0.005% ointment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1: the Data Operations Department, Astellas Pharma, generated the list. Randomisation occurred in the order that the participants passed selection criteria |
| Allocation concealment (selection bias) | Low risk | Each participant received a unique treatment number, which was printed on sealed boxes containing the ointment tubes |
| Blinding of participants and personnel (performance bias) | Low risk | All interventions had the same appearance |
| Blinding of outcome assessment (detection bias) | Low risk | The study was investigator blinded |
| Incomplete outcome data (attrition bias) | Low risk | There were 41 losses out of 479 (8.6%) participants. ITT analysis was done with the last observation carried forward (LOCF) rule |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (randomised, multicentre, double‐blind clinical trial) | |
| Participants | Adults with moderate to severe AD | |
| Interventions | Tacrolimus 0.03% ointment (67 participants) compared with tacrolimus 0.1% ointment (68 participants) compared with vehicle ointment (67 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | Children were also evaluated, but the comparison was tacrolimus 0.03% ointment only with placebo (with no active treatment); therefore, we did not include them in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised using aleatory distribution |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was double‐blind, with no description of the method |
| Blinding of outcome assessment (detection bias) | Unclear risk | The study was double‐blind, with no description of the method |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete outcome data were not described |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (randomised, investigator‐blind, parallel group, multicentre trial) | |
| Participants | Adults with moderate to severe AD | |
| Interventions | Tacrolimus 0.1% ointment (19 participants) compared with pimecrolimus 1% cream (18 participants) (BID) for 13 days | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drug assignment was computer‐generated. (We obtained this information after contacting the author) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were not blinded because the intervention was an ointment and the control was a cream, but there was no interference with the results, as the participant's assessment was not evaluated |
| Blinding of outcome assessment (detection bias) | Low risk | The trial investigators were blinded |
| Incomplete outcome data (attrition bias) | Low risk | The study lost no participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (prospective, randomised, investigator‐blinded, multicentre, comparative trial) | |
| Participants | Adults (> = 16 years) with moderate to severe AD | |
| Interventions | Tacrolimus 0.1% ointment (141 participants) compared with pimecrolimus 1% cream (140 participants) (BID) for 6 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A specialised company conducted centralised randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation was by phone |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were not blinded because the intervention was an ointment and the control was a cream, but there was no interference with the results, as the participant's assessment was not evaluated |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome investigator was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | There were 64 losses out of 281 (22.8%) participants. ITT analysis was done with last observation carried forward analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (2 randomised, double‐blind, multicentre studies) | |
| Participants | Adults (> = 16 years) with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) | |
| Interventions | Tacrolimus 0.03% ointment (211 participants) compared with tacrolimus 0.1% ointment (209 participants) compared with vehicle (ointment base) (212 participants) (BID) for 12 weeks | |
| Outcomes |
| |
| Notes | Adverse events were reported in an additional paper (Soter 2001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised 1:1:1 within each centre; the method was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial was double blind (but there was no description of the blinding methods) |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial was double blind (but there was no description of the blinding methods) |
| Incomplete outcome data (attrition bias) | Low risk | There was 1 loss out of 632 (0.16%) participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (randomised, parallel, open‐label, single centre study) | |
| Participants | 9‐month‐old to 33‐year‐old participants with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) | |
| Interventions | Tacrolimus 0.03% ointment alone compared with tacrolimus 0.03% ointment and fusidic acid 2% cream compared with fluticasone propionate 0.05% cream alone compared with fluticasone propionate 0.05% cream and fusidic acid 2% cream (15 participants randomised in each group) (BID) for 8 weeks | |
| Outcomes |
| |
| Notes | Moderate‐potency glucocorticoid (fluticasone propionate 0.05% cream) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the method was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were not blinded because of the different treatment appearance, but there was no interference with the results, as the participant's assessment was not evaluated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment was not described |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (multicentre, randomised, investigator‐blind, parallel group study) | |
| Participants | Paediatric patients (2 to 17 years) with moderate AD (IGA) | |
| Interventions | Tacrolimus 0.03% ointment (70 participants) compared with pimecrolimus 1% cream (71 participants) (BID) for 6 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial was randomised using a validated telephone system that automates the random assignment of treatment groups to randomisation numbers. A block size of 4 was used |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were not blinded because the intervention was an ointment and the control was a cream, but there was no interference with the results, as the participant's assessment was not evaluated |
| Blinding of outcome assessment (detection bias) | Low risk | The trial investigators were blinded |
| Incomplete outcome data (attrition bias) | Low risk | There were 16 losses out of 141 (11.3%) participants. ITT analysis was done with last observation carried forward analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (randomised, double‐blind, multicentre study) | |
| Participants | Paediatric patients (2 to 15 years) with moderate to severe AD (Rajka and Langeland (Rajka 1989)) | |
| Interventions | Tacrolimus 0.03% ointment (72 participants) compared with tacrolimus 0.1% ointment (70 participants) compared with vehicle ointment (71 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done through random assignment with a keycode |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial was double blind, but there was no description of the blinding methods |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial was double blind, but there was no description of the blinding methods |
| Incomplete outcome data (attrition bias) | Low risk | There were 8 losses out of 221 (3.6%) participants. ITT analysis was done with no reference to the method for imputing missing data |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (single centre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel group study) | |
| Participants | 13‐year‐old to 45‐year‐old patients with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) | |
| Interventions | Tacrolimus 0.1% ointment BID and placebo tablets (15 participants) compared with ciclosporin 3 mg/kg daily and placebo ointment (15 participants) for 6 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but there was no description of the method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Both groups received tablets and ointment |
| Blinding of outcome assessment (detection bias) | Low risk | Treatments were administered by a person who was unaware of who was participating in the study |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (randomised, double‐blind, multicentre trial) | |
| Participants | Children (2 to 15 years) with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) | |
| Interventions | Tacrolimus 0.03% ointment (117 participants) compared with tacrolimus 0.1% ointment (118 participants) compared with vehicle (ointment base) (116 participants) (BID) for 12 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified by age and randomised 1:1:1 within each centre. The method was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | The investigator, participants, guardian, and study co‐ordinator were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | The investigator, participants, guardian, and study co‐ordinator were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | There were 40 losses out of 235 (17.0%) participants. ITT analysis was done, but the method was not described |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (multicentre, randomised, investigator‐blinded study) | |
| Participants | Paediatric patients (2 to 15 years) with moderate to severe AD (IGADA) | |
| Interventions | Tacrolimus 0.1% ointment (112 participants) compared with pimecrolimus 1% cream (113 participants) (BID) for 6 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was 1:1; numbers were assigned sequentially and stratified by age. A controlled randomisation system at the EMMES Corporation (Rockville, Md) conducted randomisation and stratification |
| Allocation concealment (selection bias) | Low risk | A study co‐ordinator, independently of the examining physician, placed a call to a centralised randomisation centre to obtain the next sequential participant number and drug assignment |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were not blinded because the intervention was an ointment and the control was a cream, but there was no interference with the results, as the participant's assessment was not evaluated |
| Blinding of outcome assessment (detection bias) | Low risk | The study was investigator blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | There were 274 losses out of 1065 (25.7%) participants. ITT analysis was done, but the method was not described |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (multicentre, randomised, double‐blind, parallel group study) | |
| Participants | Adults (16 to 70 years) with moderate to severe AD | |
| Interventions | Tacrolimus 0.03% ointment (193 participants) compared with tacrolimus 0.1% ointment (191 participants) compared with hydrocortisone butyrate 0.1% ointment (186 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred in the order that the participants passed the selection criteria (parallel groups: 1:1:1). The sponsor supplied each centre a unique block of sequentially ordered participant numbers from a randomisation list |
| Allocation concealment (selection bias) | Low risk | For treatment allocation, an ointment supply box bearing a unique participant number was dispensed |
| Blinding of participants and personnel (performance bias) | Low risk | Identical tubes were used with no information |
| Blinding of outcome assessment (detection bias) | Low risk | Identical tubes were used with no information |
| Incomplete outcome data (attrition bias) | Low risk | There were 61 losses out of 570 (10.7%) participants. ITT analysis was done, but the method was not described |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (multicentre, randomised, double‐blind, parallel group study) | |
| Participants | Paediatric patients (2 to 15 years) with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) | |
| Interventions | Tacrolimus 0.03% ointment (189 participants) compared with tacrolimus 0.1% ointment (186 participants) compared with hydrocortisone acetate 1% ointment (185 participants) (BID) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred in the order that the participants passed the selection criteria (parallel groups: 1:1:1), stratified by centre and age. The sponsor supplied each centre with a unique block of sequentially ordered participant numbers from a randomisation list |
| Allocation concealment (selection bias) | Low risk | For treatment allocation, an ointment supply box bearing a unique participant number was dispensed |
| Blinding of participants and personnel (performance bias) | Low risk | Identical tubes were used with no information |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was investigator blinded |
| Incomplete outcome data (attrition bias) | Low risk | There were 54 losses out of 560 (9.6%) participants. ITT analysis was done, but the method was not described |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (randomised, double‐blind, multicentre, comparative study) | |
| Participants | Paediatric patients (2 to 15 years) with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) | |
| Interventions | Tacrolimus ointment 0.03% once daily (207 participants) compared with tacrolimus ointment 0.03% BID (210 participants) compared with hydrocortisone acetate ointment 1% BID (207 participants) for 3 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial had 1:1:1 stratification by centre and age. The sponsor supplied each centre with a unique block of sequentially ordered participant numbers from a randomisation list. Randomisation occurred in the order that the participants passed the selection criteria |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | 2 sets of identical tubes (morning and evening) were used for all of the groups |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was investigator blinded |
| Incomplete outcome data (attrition bias) | Low risk | There were 88 losses out of 624 (14.1%) participants. ITT analysis was done, but the method was not described |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (double‐blind, randomised, comparative, phase III study) | |
| Participants | Adults with moderate to severe AD | |
| Interventions | Tacrolimus ointment 0.1% (487 participants) compared with hydrocortisone butyrate ointment 0.1% on the trunk and extremities and hydrocortisone acetate ointment 1% on the face and neck (485 participants) (BID) for 6 months | |
| Outcomes |
| |
| Notes | In an additional paper (Mandelin 2010), a subgroup of 80 participants were analysed Poole 2010 reported others outcomes of the study, quality of life, and health‐related utility analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study sponsor generated the list, and participants were allocated by an investigator, using 1:1 stratification by centre, in the order that participants passed the selection criteria |
| Allocation concealment (selection bias) | Low risk | Participants were allocated by the investigator, using 1:1, stratified by centre, in the order that the participants passed the selection criteria |
| Blinding of participants and personnel (performance bias) | Low risk | The trial used identical tubes: 5 tubes for the trunk and extremities and 2 tubes for the head and neck for both groups |
| Blinding of outcome assessment (detection bias) | Low risk | The trial used identical tubes: 5 tubes for the trunk and extremities and 2 tubes for the head and neck for both groups |
| Incomplete outcome data (attrition bias) | High risk | There were 328 losses out of 972 participants. ITT analysis was done, but the method was not described |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
| Methods | RCT (comparative, multicentre, open, randomised, parallel group study) | |
| Participants | Older children (7 to 15 years) with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) | |
| Interventions | Tacrolimus 0.03% ointment BID (15 participants) compared with clobetasone butyrate 0.05% cream BID (15 participants) compared with clobetasone butyrate 0.05% cream in the morning and tacrolimus 0.03% ointment in the evening (15 participants) for 4 weeks | |
| Outcomes |
| |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified by age and disease severity and randomised in parallel groups (1:1:1) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were not blind because the intervention was an ointment and the control was a cream, but there was no interference with the results, as the participant's assessment was not evaluated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding was unclear |
| Incomplete outcome data (attrition bias) | Low risk | There were no participant losses |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were described |
| Other bias | Low risk | This trial was free of other bias |
AD: atopic dermatitis.
BID: twice a day.
BSA: Body Surface Area.
DLQI: Dermatology Life Quality Index.
EASI: Eczema Area and Severity Index.
IGA: Investigators' Global Assessment.
IGADA: Investigator's Global Atopic Dermatitis Assessment.
ITT: intention‐to‐treat.
LOCF: last observation carried forward.
mEASI: modified Eczema Area and Severity Index.
RCT: randomised controlled trial.
SCORAD: SCORing Atopic Dermatitis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This was not randomised | |
| This study compared the intervention only with placebo. (There was no comparison with other active treatments) | |
| Participants in the study were treated and assessed only on a selected area of skin; whereas, this review sought studies where the whole person was treated and evaluated | |
| This study evaluated only facial eczema | |
| This study analysed only a selected area for treatment (only lesions on the forearms); whereas, this review sought studies where the whole person was treated and evaluated | |
| The study did not divide the different severity groups and analyse the global data; participants included mild cases, which were not of interest to this review | |
| This study compared the intervention only with placebo. (There was no comparison with other active treatments) | |
| The study did not divide the different severity groups and analyse the global data, including the mild cases | |
| The study was not a RCT. It was based on a RCT's data and used surveys after the treatment period to compare both groups | |
| The study compared tacrolimus in the different formulations only to placebo. No comparison between the different formulations was made | |
| The study evaluated only the head and neck area | |
| The study did not divide the different severity groups and analyse the global data, including the mild cases | |
| The study compared the intervention only to placebo (no comparison with other active treatments) | |
| We excluded this study because of an ineligible intervention | |
| This was an open‐labelled pilot study on patient vehicle (ointment versus cream) preference | |
| The study compared the intervention only to placebo. (There was no comparison with other active treatments) | |
| The study classified atopic dermatitis based only on body surface area and not on severity scores. We could not make classification as mild, moderate, or severe | |
| The study analysed only a selected area for treatment (200 to 1000 cm²) | |
| The study compared the intervention only with placebo. (There was no comparison with other active treatments) | |
| This study used 'maintenance therapy', which was not of interest to this review. This review only investigated studies with active treatment | |
| This study did not classify disease severity | |
| This was a non‐comparative study | |
| Participants in this study were treated and assessed only on a selected area of skin; whereas, this review sought studies where the whole person was treated and evaluated |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT (3 randomised, double‐blind, multicentre studies) |
| Participants | Adults and children with moderate to severe AD (Rajka and Langeland criteria (Rajka 1989)) |
| Interventions | Tacrolimus 0.03% ointment versus tacrolimus 0.1% ointment versus vehicle (ointment base) (BID) |
| Outcomes |
|
| Notes | We contacted the author to identify the 3 original trials |
AD: atopic dermatitis.
BID: twice a day.
DLQI: Dermatology Life Quality Index.
CDLQI: Children's Dermatology Life Quality Index.
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | APPLES: A Prospective Pediatric Longitudinal Evaluation to Assess the Long‐Term Safety of Tacrolimus Ointment for the Treatment of Atopic Dermatitis |
| Methods | Observational, prospective, cohort study |
| Participants | People who first used tacrolimus 0.03% or 0.1% before they were 16 years of age and were treated for at least 6 weeks for the treatment of atopic dermatitis |
| Interventions | Topical tacrolimus 0.03% or 0.1% |
| Outcomes |
|
| Starting date | May 2005 |
| Contact information | ‐ |
| Notes | Each participant will be followed for 10 years in this study. ClinicalTrials.gov identifier: NCT00475605 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent. | ||||
| 1.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects: burning Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2 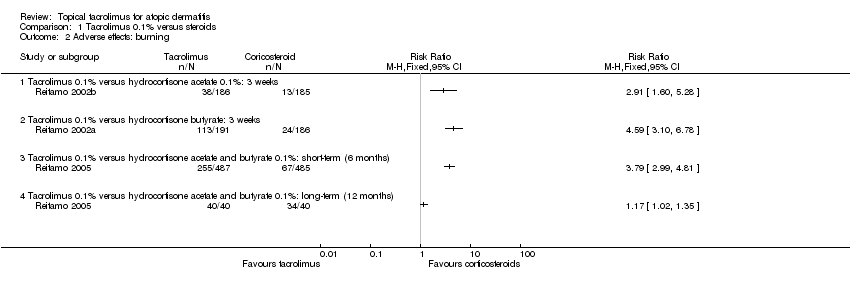 Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 2 Adverse effects: burning. | ||||
| 2.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: pruritus Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 3 Adverse effects: pruritus. | ||||
| 3.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: skin infection Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 4 Adverse effects: skin infection. | ||||
| 4.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 SCORAD: 3 weeks Show forest plot | 2 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐15.36, ‐2.27] |
| Analysis 1.5  Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 5 SCORAD: 3 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.35, 2.42] |
| Analysis 2.1  Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent. | ||||
| 1.1 13 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.19, 19.13] |
| 1.2 6 weeks | 2 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.34, 2.42] |
| 2 Adverse effects ‐ 6 weeks Show forest plot | 2 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.47, 1.71] |
| Analysis 2.2 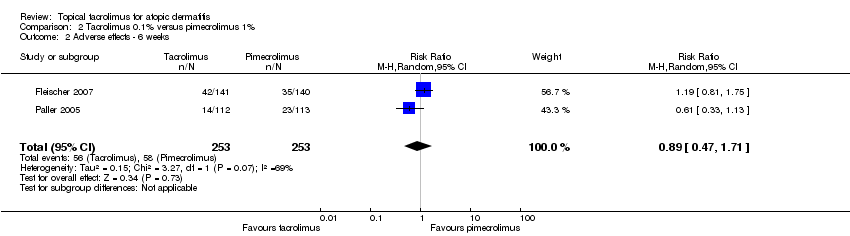 Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 2 Adverse effects ‐ 6 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent. | ||||
| 1.1 Tacrolimus 0.03% 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.36, 3.08] |
| 1.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 2 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [1.96, 3.38] |
| 1.3 Tacrolimus 0.03% 2x/day versus steroids moderate potency 2x/day | 2 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.13, 1.57] |
| 1.4 Tacrolimus 0.03% 2x/day versus methylprednisolone 0.03% 1x/day | 1 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.19] |
| 2 Participants's assessment of global response of improvement better or much better Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2 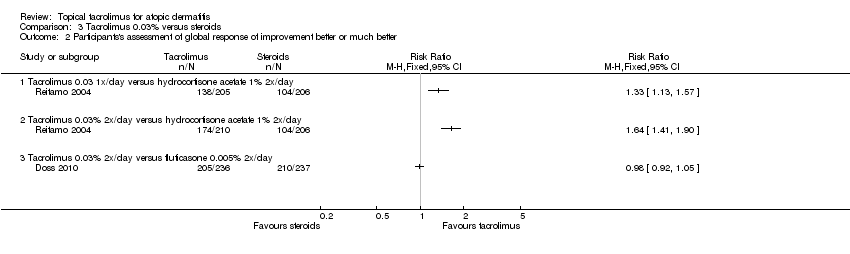 Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 2 Participants's assessment of global response of improvement better or much better. | ||||
| 2.1 Tacrolimus 0.03 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.03% 2x/day versus fluticasone 0.005% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: burning Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.96, 3.14] |
| Analysis 3.3  Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 3 Adverse effects: burning. | ||||
| 3.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.36, 2.57] |
| 3.2 Tacrolimus 0.03% versus steroids moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [2.45, 5.06] |
| 4 Adverse effects: pruritus Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| Analysis 3.4  Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 4 Adverse effects: pruritus. | ||||
| 4.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.00, 1.88] |
| 4.2 Tacrolimus 0.03% versus steroids of moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.18, 2.80] |
| 5 Adverse effects: skin infection Show forest plot | 4 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.66] |
| Analysis 3.5 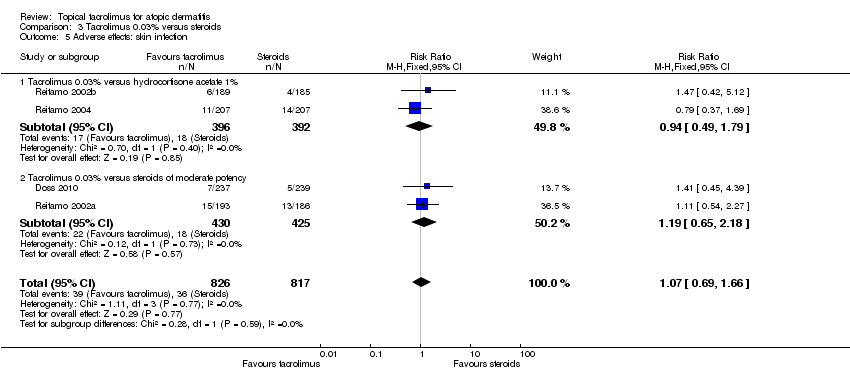 Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 5 Adverse effects: skin infection. | ||||
| 5.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.49, 1.79] |
| 5.2 Tacrolimus 0.03% versus steroids of moderate potency | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 6 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.92] |
| Analysis 4.1 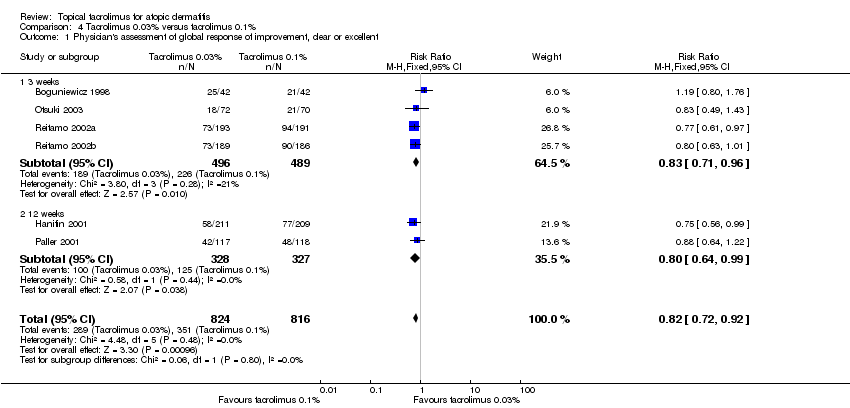 Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent. | ||||
| 1.1 3 weeks | 4 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.96] |
| 1.2 12 weeks | 2 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 0.99] |
| 2 Adverse effects Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Analysis 4.2 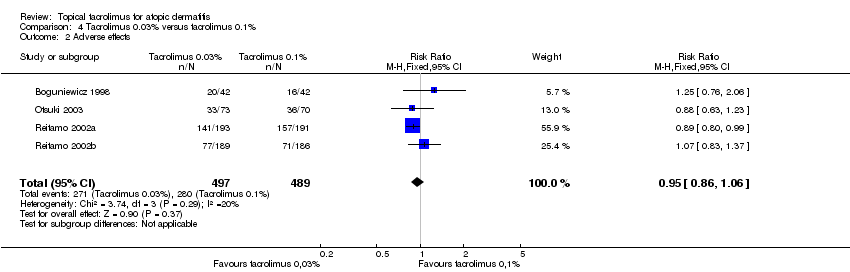 Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 2 Adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement. | ||||
| 2 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2 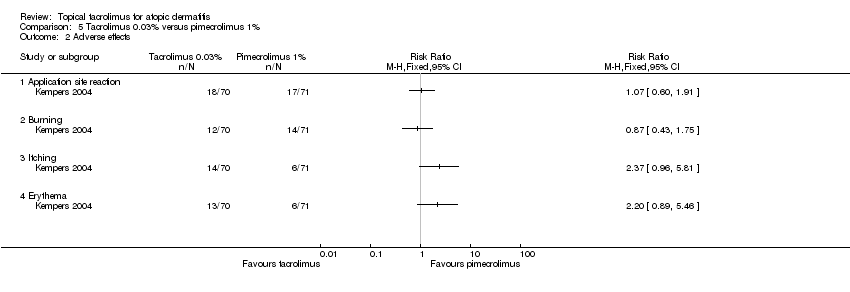 Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 2 Adverse effects. | ||||
| 2.1 Application site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Burning | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 1 Adverse effects. | ||||
| 2 SCORAD Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 2 SCORAD. | ||||
| 2.1 14 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 21 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 28 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 35 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 42 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
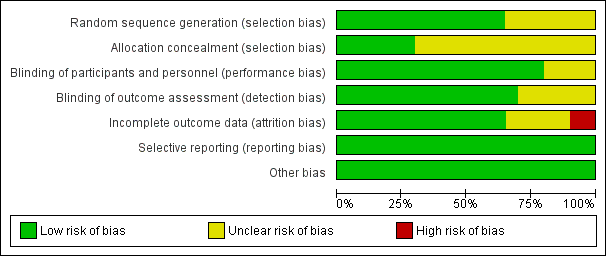
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

Forest plot of comparison: 2 Tacrolimus 0.1% versus pimecrolimus 1%, outcome: 2.1 Physician's assessment of global response of improvement, clear or excellent
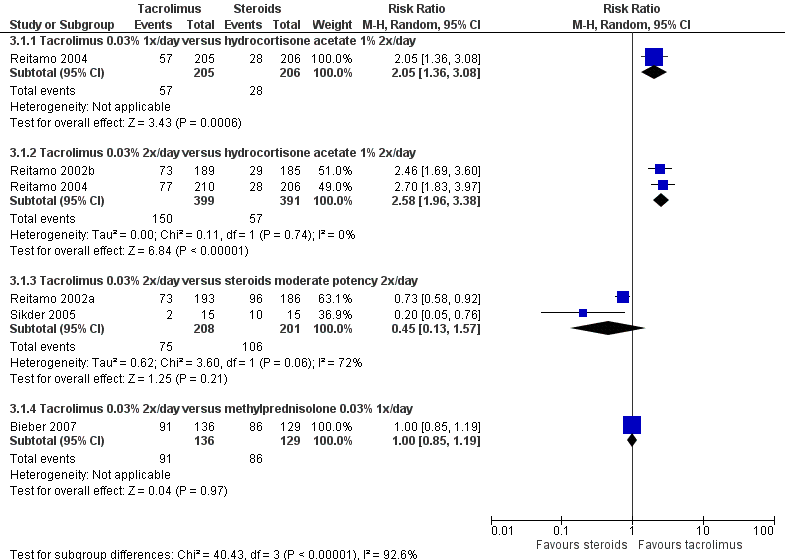
Forest plot of comparison: 3 Tacrolimus 0.03% versus corticosteroids, outcome: 3.1 Physician's assessment of global response of improvement, clear or excellent
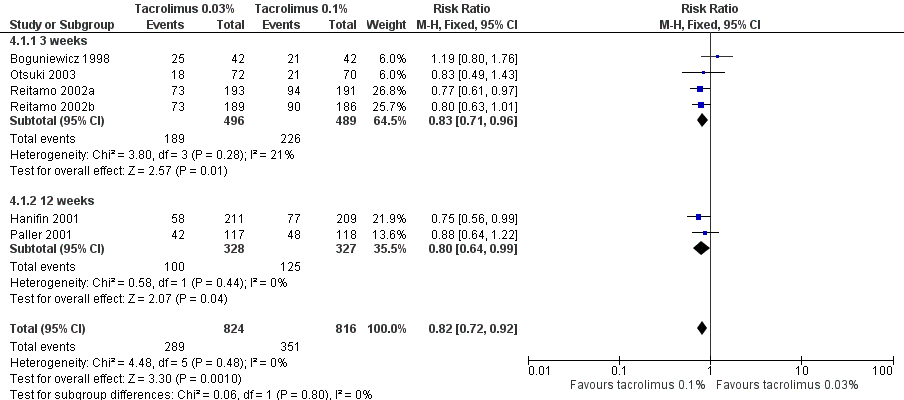
Forest plot of comparison: 4 Tacrolimus 0.03% versus tacrolimus 0.1%, outcome: 4.1 Physician's assessment of global response of improvement, clear or excellent

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 2 Adverse effects: burning.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 3 Adverse effects: pruritus.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 4 Adverse effects: skin infection.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 5 SCORAD: 3 weeks.

Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.

Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 2 Adverse effects ‐ 6 weeks.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 2 Participants's assessment of global response of improvement better or much better.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 3 Adverse effects: burning.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 4 Adverse effects: pruritus.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 5 Adverse effects: skin infection.

Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.

Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 2 Adverse effects.

Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement.

Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 2 Adverse effects.

Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 1 Adverse effects.

Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 2 SCORAD.
| Tacrolimus 0.1% compared with corticosteroids for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis Intervention: tacrolimus 0.1% | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Tacrolimus 0.1% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | Study population | RR 3.09 | 371 | ⊕⊕⊕⊝ | ‐ | |
| 157 per 1000 | 484 per 1000 | |||||
| Moderate | ||||||
| 157 per 1000 | 485 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | Study population | RR 0.95 | 377 | ⊕⊕⊝⊝ | ‐ | |
| 516 per 1000 | 490 per 1000 | |||||
| Moderate | ||||||
| 516 per 1000 | 490 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short term (6 months) | Study population | RR 1.32 | 972 | ⊕⊕⊝⊝ | ‐ | |
| 464 per 1000 | 612 per 1000 | |||||
| Moderate | ||||||
| 464 per 1000 | 612 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | Study population | RR 2.91 | 371 | ⊕⊕⊕⊝ | ‐ | |
| 70 per 1000 | 204 per 1000 | |||||
| Moderate | ||||||
| 70 per 1000 | 204 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | Study population | RR 4.59 | 377 | ⊕⊕⊕⊝ | ‐ | |
| 129 per 1000 | 592 per 1000 | |||||
| Moderate | ||||||
| 129 per 1000 | 592 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: 6 months Follow‐up: 6 months | Study population | RR 3.79 (2.99 to 4.81) | 972 (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 138 per 1000 | 524 per 1000 (413 to 664) | |||||
| Moderate | ||||||
| 138 per 1000 | 524 per 1000 (413 to 664) | |||||
| Participant's self‐assessment of global response of improvement Follow‐up: mean 6 months | Study population | RR 1.21 (1.13 to 1.29) | 974 (1 study) | ⊕⊕⊝⊝ | ‐ | |
| 718 per 1000 | 868 per 1000 (811 to 926) | |||||
| Moderate | ||||||
| 718 per 1000 | 869 per 1000 (811 to 926) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only one study was identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.1% compared with pimecrolimus 1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pimecrolimus 1% | Tacrolimus 0.1% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ 6 weeks | Study population | RR 1.8 | 506 | ⊕⊕⊕⊝ | ‐ | |
| 202 per 1000 | 363 per 1000 | |||||
| Moderate | ||||||
| 199 per 1000 | 358 per 1000 | |||||
| Adverse effects ‐ 6 weeks | Study population | RR 0.89 | 506 | ⊕⊝⊝⊝ | ‐ | |
| 229 per 1000 | 204 per 1000 | |||||
| Moderate | ||||||
| 227 per 1000 | 202 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only one study was identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.03% compared with corticosteroids for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Ilustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Tacrolimus 0.03% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 1x/day versus hydrocortisone acetate 1% 2x/day | Study population | RR 2.05 | 411 | ⊕⊕⊕⊝ | ‐ | |
| 136 per 1000 | 279 per 1000 | |||||
| Moderate | ||||||
| 136 per 1000 | 279 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | Study population | RR 2.58 | 790 | ⊕⊕⊕⊝ | ‐ | |
| 146 per 1000 | 376 per 1000 | |||||
| Moderate | ||||||
| 146 per 1000 | 377 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus corticosteroids moderate‐potency 2x/day | Study population | RR 0.45 | 409 | ⊕⊝⊝⊝ | ‐ | |
| 527 per 1000 | 237 per 1000 | |||||
| Moderate | ||||||
| 591 per 1000 | 266 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus methylprednisolone 0.03% 1x/day | Study population | RR 1 | 265 | ⊕⊕⊝⊝ | ‐ | |
| 667 per 1000 | 667 per 1000 | |||||
| Moderate | ||||||
| 667 per 1000 | 667 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.03% versus steroids | Study population | RR2.48 | 1883 | ⊕⊕⊕⊕ | ‐ | |
| 89 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 70 per 1000 | 174 per 1000 | |||||
| Participant's self‐assessment of global response of improvement: tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day Follow‐up: 3 weeks | Study population | RR 1.64 (1.41 to 1.90) | 416 (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 505 per 1000 | 828 per 1000 (712 to 959) | |||||
| Moderate | ||||||
| 505 per 1000 | 828 per 1000 (712 to 959) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only very small number of studies were identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.03% compared with tacrolimus 0.1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tacrolimus 0.1% | Tacrolimus 0.03% | |||||
| Physician's assessment of global response of improvement, clear or excellent | Study population | RR 0.82 | 1640 | ⊕⊕⊕⊕ | ‐ | |
| 430 per 1000 | 353 per 1000 | |||||
| Moderate | ||||||
| 445 per 1000 | 365 per 1000 | |||||
| Adverse effects | Study population | RR 0.95 | 986 | ⊕⊕⊕⊝ | ‐ | |
| 573 per 1000 | 544 per 1000 | |||||
| Moderate | ||||||
| 448 per 1000 | 426 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to imprecision: sample size is below the optimal information size. | ||||||
| Tacrolimus 0.03% versus pimecrolimus 1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tacrolimus 0.03% versus pimecrolimus 1% | |||||
| Physician's assessment of global response of improvement | Study population | RR 1.42 | 139 | ⊕⊕⊝⊝ | ‐ | |
| 429 per 1000 | 609 per 1000 | |||||
| Moderate | ||||||
| 429 per 1000 | 609 per 1000 | |||||
| Adverse effects ‐ application site reaction | Study population | RR 1.07 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 239 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 256 per 1000 | |||||
| Adverse effects ‐ burning | Study population | RR 0.87 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 197 per 1000 | 172 per 1000 | |||||
| Moderate | ||||||
| 197 per 1000 | 171 per 1000 | |||||
| Adverse effects ‐ itching | Study population | RR 2.37 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 85 per 1000 | 200 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 201 per 1000 | |||||
| Adverse effects ‐ erythema | Study population | RR 2.2 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 85 per 1000 | 186 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 187 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to imprecision: sample size is smaller than the optimal information size. | ||||||
| Tacrolimus 0.1% versus ciclosporin for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tacrolimus 0.1% versus ciclosporin | |||||
| Adverse effects | Study population | RR 1 | 30 | ⊕⊝⊝⊝ | ‐ | |
| 267 per 1000 | 267 per 1000 | |||||
| Moderate | ||||||
| 267 per 1000 | 267 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to risk of bias: randomisation and allocation concealment procedures were unclear. | ||||||
| Study | Number of participants (n = 5885) | Age | Intervention | Follow up | Classification of AD |
| 24 | Adults (21 to 65 years) | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (SCORAD) | |
| 265 | Children (2 to 15 years) | Tacrolimus 0.03% ointment (BID) vs methylprednisolone aceponate 0.1% ointment (evening) and vehicle ointment (morning) | 2 to 3 weeks | Severe flare (IGA > 4) history of moderate to severe AD | |
| 169 | Older children (7 to 16 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs tacrolimus 0.3% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 16 | Adults | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (SCORAD) | |
| 473 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs fluticasone 0.005% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) and with prior inadequate response to topical corticosteroids | |
| 202 | Adults (> 18 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe | |
| 37 | Adults | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 2 weeks | Moderate to severe (IGA) | |
| 281 | Adults (> = 16 years) | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate to severe (IGA) | |
| 632 | Adults (> = 16 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs vehicle ointment (BID) | 3 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 60 | Adults and children (9 months to 33 years) | Tacrolimus 0.03% ointment (BID) alone or with fusidic acid 2% cream vs fluticasone propionate 0.05% cream (BID) alone or with fusidic acid 2% cream | 6 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 141 (for safety) 139 (for efficacy) | Children (2 to 17 years) | Tacrolimus 0.03% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate (IGA) | |
| 213 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 30 | Adults and children (13 to 45 years) | Tacrolimus 0.1% ointment (BID) vs ciclosporin 3 mg/kg orally | 6 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 351 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 225 | Children (2 to 15 years) | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate to severe (IGA) | |
| 570 | Adults (16 to 70 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 560 | Children (2 to 15 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs hydrocortisone acetate 1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 621 | Children (2 to 15 years) | Tacrolimus 0.03% ointment (OD) vs tacrolimus 0.03% ointment (BID) vs hydrocortisone acetate 1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 972 | Adults (> = 18 years) | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (on trunk and extremities) and hydrocortisone acetate 1% ointment (on face and neck) (BID) | Up to 6 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 45 | Older children (7 to 15 years) | Tacrolimus 0.03% ointment (BID) vs clobetasone butyrate 0.05% cream (BID) vs clobetasone butyrate 0.05% cream (morning) and tacrolimus 0.03% ointment (evening) | 4 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| AD: atopic dermatitis. | |||||
| Malignancy | Age (years) | Application site | Occurence site | Comment | Exposure to onset (days) |
| B‐cell lymphoma, EBV‐associated, and primary lung carcinoma | 49 | Face | Kidney | ‐ | 730 |
| Cutaneous Kaposi sarcoma | 28 | Axilla, groin | Axilla, groin | HIV patient on HAART, treated for inverse psoriasis, developed KS at these sites, which metastasised, and the patient died | 30 |
| Hepatoblastoma | 5 | ‐ | Liver | Considered unrelated | 455 |
| Lymphadenopathy – possible | 40 | Application site | Application site | Pre‐existing lymphoma lesions 'looked like' lymphoma and resolved spontaneously* | ‐ |
| Lymphoma or Sézary syndrome | 16 | Face | Lymph nodes | Participant also had been on systemic ciclosporin | 730 |
| Metastatic angiosarcoma | 16 | Face/neck | Clavicle | Present before treatment but increased rapidly in size | 105 |
| Metastatic melanoma | 39 | ‐ | Generalised | Metastatic disease newly detected from primary 3 years early | 21 to 28 |
| Metastatic sweat gland carcinoma | 43 | Not axilla | Axilla | ‐ | 4 years |
| Nodular follicular lymphoma | 60 | Lower limbs, face | ‐ | May be associated with EBV | 504 |
| Non‐Hodgkin lymphoma | 52 | ‐ | ‐ | Used tacrolimus for 6 months. Insufficient evidence | 365 |
| Non‐Hodgkin lymphoma | 54 | ‐ | ‐ | Used tacrolimus on extensive areas: 50% of body. Died from lymphoma. Insufficient evidence | ‐ |
| Oesophageal cancer with metastases | 49 | ‐ | Oesophagus | ‐ | 122 |
| Panniculitis‐like T‐cell lymphoma | 53 | Trunk, limbs | Trunk, limbs | Also used pimecrolimus | 240 |
| Squamous cell carcinoma | 34 | Face | Face | UV therapy, outdoor sports | ‐ |
| Squamous cell carcinoma | 57 | Penis | Penis | Treated for balanitis considered to be lichen sclerosus et atrophicus; non‐specific biopsy | 70 |
| Squamous cell carcinoma | 51 | ‐ | Mouth | Long history of pipe smoking | ‐ |
| Squamous cell carcinoma recurrence | 75 | Vulva | Vulva | Treated for lichen sclerosus et atrophicus | 42 |
| T‐cell lymphoma, anaplastic large cell | 50 | Right hip | Right hip | Insufficient evidence | ‐ |
| EBV: Epstein–Barr virus. | |||||
| Study | Study population | Follow‐up | Comparisons | Results related to lymphoma risks |
| 294 cases/293,000 controls | ‐ | TCIs and TCS in participants with AD | ‐ Increased risk in AD participants (related to severity) ‐ No evidence of increased risk with any of the topical treatments | |
| > 3,000,000 (cohort) | 1992 to 2006 | AD, treatment with topical immunosuppressants, or both | ‐ Increase risk in AD participants (related to severity) ‐ Increased risk with topical corticosteroids (related to potency) ‐ Insufficient data to assess TCI‐related risks | |
| 953,064 (cohort) (96% unexposed, 4% exposed) | Median 2.4 years | AD or eczema participants exposed or not to TCI | ‐ Increased risk in the exposed group** | |
| ‐ 118,863 for pimecrolimus ‐ 38,757 for tacrolimus ‐ 1,043,025 mid to potent corticosteroid ‐ 118,825 untreated dermatitis ‐ 118,863 for general population | 2002 to 2006 (median 1.3 years) | See study population | ‐ Increased risk compared with general population* ‐ No risk differences between the 3 treatments | |
| *pre‐existing lymphomas misdiagnosed as AD. | ||||
| Study | Study population | Follow up | Comparisons | Results related to skin cancer risks |
| 953,064 (cohort) (96% unexposed, 4% exposed) | Median 2.4 years | AD participants exposed or not to TCI | ‐ Similar risks for NMSC ‐ Lower risks for MM | |
| 875 cases 1946 controls | ‐ | Dermatitis participants (AD, seborrhoeic dermatitis, rosacea, other dermatitis) with or without use of TCI | ‐ No increased risk of NMSC in TCI‐treated participants ‐ MM risk not evaluated | |
| 9813 tacrolimus‐treated participants | 3 months to 4 years | AD participants with tacrolimus use compared with an aged cohort in the US | ‐ No increased risk of NMSC in tacrolimus treated participants ‐ MM risk not evaluated | |
| AD: atopic dermatitis. | ||||
| Study | 1. Population 2. Age group 3. Follow‐up | Tacrolimus formulation | Common local effects | Systemic effects | Laboratory values | Malignancies | Others (number of events) | Detectable blood concentration |
| 1. n = 174 2. Paediatric | 0.03% | ‐ Burning ‐ Pruritus | ‐ | ‐ | ‐ | ‐ Asthma (2) ‐ Pneumonia (2) ‐ Pyodermitis (1) | ‐ | |
| 1. n = 7923 2. Adult/paediatric 3. Median: 210 days | 0.1% (92.7%) 0.03% (7.3%) | ‐ Burning ‐ Pruritus | ‐ Flu‐like symptoms ‐ Headache (frequency similar to that expected of the general population) | ‐ | ‐ 13 cases of NMSC (no risk with calculated incidence) | ‐ Alcohol intolerance 3.7% | ‐ | |
| 1. n = 50 2. Paediatric (< 2 years) 3. 2 years | 0.03% | ‐ Pruritus ‐ Local infection | ‐ Non‐serious respiratory infection and gastroenteritis | ‐ | ‐ | ‐ | < 1 ng/ml (in 98%) | |
| 1. n = 316 2. Adults 3. 6 to 12 months | 0.1% | ‐ Burning ‐ Pruritus ‐ Erythema | ‐ | Normal (only 1 transient increase in liver enzymes) | ‐ | ‐ Alcohol intolerance 5 serious events: ‐ Eczema herpeticum (1) ‐ Cellulitis (1) ‐ Varicella (1) ‐ AD flare‐up (1) ‐ Staphylococcus aureus superinfection (1) | Minimal < 1 ng/dl in 76% of participants | |
| 1. n = 672 2. Adults 3. 2 years | 0.1% | ‐ Burning ‐ Pruritus | ‐ | ‐ | ‐ 2 cases (Bowen and prostate carcinoma) not related ‐ Benign neoplasm (7) | ‐ Herpes (7%) (expected in AD participants) ‐ Eczema herpeticum (1) ‐ Erythroderma (1) ‐ AD exacerbation (1) | ‐ | |
| 1. n = 782 2. Adult/paediatric 3. 4 years (median: 1422 days) | 0.1% | ‐ Burning ‐ Pruritus ‐ Skin infection | ‐ Flu‐like symptoms (more in children) | ‐ | 6 cases ‐ Cervical carcinoma (1) ‐ Acute leukaemia (1) ‐ Chronic leukaemia (1) ‐ Basal cell carcinoma (2 to 3 on the same participant) ‐ 34 benign neoplasms | ‐ | ‐ | |
| 1. n = 466 2. Paediatric 3. 29.5 months (mean: 16.3 months) | 0.03% 0.1% | ‐ Burning ‐ Pruritus | ‐ Seasonal infection (flu‐syndrome) ‐ No growth retardation | Normal | ‐ | ‐ Leukopenia (1)* ‐ Herpes (4.9%)/eczema herpeticum (0.9%) ‐ Molluscum 3%) ‐ Warts (3.6%) | ‐ | |
| 1. n = 125 2. 12 to 69 years 3. 5 weeks | 0.03% | ‐ Burning ‐ Pruritus ‐ Erythema | ‐ | Normal | ‐ | ‐ | ‐ | |
| 1. n = 18 2. Adult/paediatric 3. 4 weeks | 0.03% | ‐ Burning ‐ Pruritus | ‐ | Normal | ‐ | Serious events (3): ‐ Flu‐syndrome (1) ‐ Severe skin rash (1) ‐ Eczema herpeticum (1) | ‐ | |
| 1. n = 30 2. Adult/paediatric 3. 4 weeks | 0.1% adults 0.03% paediatric | ‐ Burning ‐ Pruritus | ‐ | Normal | ‐ | ‐ | 2 participants < 5 ng/ml | |
| * 6‐year‐old participant, at month 6, resolution after withdrawn. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects: burning Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: pruritus Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: skin infection Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 SCORAD: 3 weeks Show forest plot | 2 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐15.36, ‐2.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.35, 2.42] |
| 1.1 13 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.19, 19.13] |
| 1.2 6 weeks | 2 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.34, 2.42] |
| 2 Adverse effects ‐ 6 weeks Show forest plot | 2 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.47, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Tacrolimus 0.03% 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.36, 3.08] |
| 1.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 2 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [1.96, 3.38] |
| 1.3 Tacrolimus 0.03% 2x/day versus steroids moderate potency 2x/day | 2 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.13, 1.57] |
| 1.4 Tacrolimus 0.03% 2x/day versus methylprednisolone 0.03% 1x/day | 1 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.19] |
| 2 Participants's assessment of global response of improvement better or much better Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Tacrolimus 0.03 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.03% 2x/day versus fluticasone 0.005% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: burning Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.96, 3.14] |
| 3.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.36, 2.57] |
| 3.2 Tacrolimus 0.03% versus steroids moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [2.45, 5.06] |
| 4 Adverse effects: pruritus Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| 4.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.00, 1.88] |
| 4.2 Tacrolimus 0.03% versus steroids of moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.18, 2.80] |
| 5 Adverse effects: skin infection Show forest plot | 4 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.66] |
| 5.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.49, 1.79] |
| 5.2 Tacrolimus 0.03% versus steroids of moderate potency | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 6 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.92] |
| 1.1 3 weeks | 4 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.96] |
| 1.2 12 weeks | 2 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 0.99] |
| 2 Adverse effects Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Application site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Burning | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 SCORAD Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 14 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 21 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 28 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 35 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 42 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


