Tacrólimus tópico para la dermatitis atópica
Appendices
Appendix 1. Validated scores and classification criteria
Validated scores, scales, and diagnosis and severity criteria (Charman 2000; Rehal 2011)
‐ Hanifin and Rajka criteria
"The diagnosis of atopic dermatitis using the Hanifin and Rajka criteria requires that patients have at least 3 of the 4 major criteria and 3 of the 23 minor criteria.
Major criteria:
-
Pruritus
-
Dermatitis affecting flexural surfaces in adults and the face and extensors in infants
-
Chronic or relapsing dermatitis
-
Personal or family history of cutaneous or respiratory atopy
Minor criteria can be divided into four categories:
-
Facial features: facial pallor, facial erythema, hypopigmented patches, infraorbital darkening, infraorbital folds (Dennie‐Morgan folds), cheilitis, recurrent conjunctivitis, anterior neck folds
-
Triggers: foods, emotional factors, environmental factors, skin irritants
-
Complications: susceptibility to cutaneous infections, impaired cell‐mediated immunity, immediate skin‐test reactivity, elevated IgE, keratoconus, anterior subcapsular cataracts
-
Other: early age of onset, dry skin, ichthyosis, hyperlinear palms, keratosis pilaris, hand and foot dermatitis, nipple eczema, white dermatographism, perifollicular accentuation" (Hanifin 1980).
‐ The Rajka and Langeland Scoring System
"It is a simple scale measuring clinical course, intensity, and extent of atopic eczema. It is probably most suitable for baseline categorization of patients rather than to monitor severity changes in trials. The scale involves an assessment of body surface area involvement, albeit into 1 of 3 categories only" (Rajka 1989).
‐ Severity Scoring of Atopic Dermatitis (SCORAD)
On this score, the disease extent is assessed by the rule of nines* and disease severity is evaluated based on five clinical characteristics: one ‐ erythema, two ‐ edema, three ‐ oozing/crusts, four ‐ excoriation, and five ‐ lichenification. It still evaluates pruritus and sleep loss (subjective symptoms) with Visual Analogue Scales. The combination of the points given to those 3 aspects (extention and severity of disease and subjective symptoms) give a maximum score of 103.
*The rule of 9 is used to calculate the affected area: head and neck representing 9%; each upper limb representing 9%; each lower limb representing 18%; anterior trunk representing 18%; the back representing 18%; genitals, each palm, and the back of each hand representing 1% each.
‐ Eczema Area and Severity Index (EASI) and modified EASI (mEASI)
On this score, four clinical characteristics (erythema, induration, excoriation, and lichenification) are evaluated on a scale of zero (absent) to three (severe), together with disease extension measured at four body sites (head and neck, upper limbs, trunk, lower limbs). EASI give a maximum score of 72.
mEASI represents a variation of EASI, with the inclusion of the assessment of pruritus, not included on the EASI score (Hanifin 2001b).
‐ Investigator's Global Assessment (IGA)
"This score uses a 6‐point severity scale from clear to very severe disease (0 = clear, 1 = almost clear, 2 = mild disease, 3 = moderate disease, 4 = severe disease, and 5 = very severe disease). IGA uses clinical characteristics of erythema, infiltration, papulation, oozing and crusting as guidelines for the overall severity assessment" (Rehal 2011).
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 (eczema or neurodermatitis or dermatitis):ti,ab,kw
#2 MeSH descriptor Eczema explode all trees
#3 MeSH descriptor Dermatitis explode all trees
#4 MeSH descriptor Neurodermatitis explode all trees
#5 MeSH descriptor Dermatitis, Atopic explode all trees
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 (tacrolimus or protopic or fk506 or "fk 506"):ti,ab,kw
#8 MeSH descriptor Tacrolimus explode all trees
#9 (#7 OR #8)
#10 (#6 AND #9)
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Eczema/ or eczema.mp.
2. exp Dermatitis, Atopic/
3. neurodermatitis.mp. or exp Neurodermatitis/
4. exp Dermatitis/ or dermatitis.mp.
5. or/1‐4
6. exp Tacrolimus/
7. topical tacrolimus.mp.
8. Protopic.mp.
9. (tacrolimus adj3 topical$).mp.
10. (tacrolimus adj3 ointment).mp.
11. (fk 506 or fk506).mp.
12. or/6‐11
13. randomized controlled trial.pt.
14. controlled clinical trial.pt.
15. randomized.ab.
16. placebo.ab.
17. clinical trials as topic.sh.
18. randomly.ab.
19. trial.ti.
20. 13 or 14 or 15 or 16 or 17 or 18 or 19
21. (animals not (humans and animals)).sh.
22. 20 not 21
23. 5 and 12 and 22
[13‐22: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)].
Appendix 4. EMBASE (Ovid) search strategy
1. eczema.mp. or exp ECZEMA/
2. exp DERMATITIS/ or dermatitis.mp.
3. exp atopic dermatitis/
4. neurodermatitis.mp. or exp NEURODERMATITIS/
5. or/1‐4
6. exp tacrolimus/
7. topical tacrolimus.ti,ab.
8. Protopic.ti,ab.
9. (tacrolimus adj3 ointment).ti,ab.
10. (tacrolimus adj3 topical).ti,ab.
11. (fk506 or fk 506).ti,ab.
12. or/6‐11
13. random$.mp.
14. factorial$.mp.
15. (crossover$ or cross‐over$).mp.
16. placebo$.mp. or PLACEBO/
17. (doubl$ adj blind$).mp.
18. (singl$ adj blind$).mp.
19. (assign$ or allocat$).mp.
20. volunteer$.mp. or VOLUNTEER/
21. Crossover Procedure/
22. Double Blind Procedure/
23. Randomized Controlled Trial/
24. Single Blind Procedure/
25. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26. 5 and 12 and 25
Appendix 5. LILACS search strategy
(eczema or eccema or dermatitis or neurodermatitis) and (tacrolimus or protopic or fk506 or "fk 506")
[Searched using the Controlled clinical trials topic‐specific query filter].
Appendix 6. MEDLINE (Ovid) adverse effects search strategy
1. exp product surveillance, postmarketing/ or exp adverse drug reaction reporting systems/ or exp clinical trials, phase iv/
2. ((adverse or undesirable or harm$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab.
3. exp hypersensitivity/ or exp drug hypersensitivity/ or exp drug eruptions/ or exp hypersensitivity, delayed/ or exp hypersensitivity, immediate/
4. exp anaphylaxis/ or exp conjunctivitis, allergic/ or exp dermatitis, atopic/ or exp food hypersensitivity/ or exp respiratory hypersensitivity/ or exp urticaria/
5. side effect$.ti,ab.
6. exp Poisoning/
7. exp hepatitis, toxic/ or exp hepatitis, chronic, drug‐induced/
8. exp Substance‐Related Disorders/
9. exp Drug Toxicity/
10. exp Abnormalities, Drug‐Induced/
11. exp Teratogens/
12. exp Mutagens/
13. exp Carcinogens/
14. metabolite$.ti,ab.
15. exp dermatitis, contact/ or exp dermatitis, allergic contact/ or exp dermatitis, irritant/ or exp dermatitis, phototoxic/
16. photoallergic reaction$.ti,ab.
17. exp dermatitis, allergic contact/ or exp dermatitis, photoallergic/
18. phototoxicit$.ti,ab.
19. (sensitization or sensitisation).ti,ab.
20. exp Burning Mouth Syndrome/
21. stinging.ti,ab.
22. burning.ti,ab.
23. fetal abnormalit$.ti,ab.
24. exp Drug Monitoring/
25. drug effect$.ti,ab.
26. Sleep Apnea, Obstructive/
27. ARRHYTHMIA/
28. (safe or safety).ti,ab.
29. toxicity.ti,ab.
30. noxious.ti,ab.
31. complication$.ti,ab.
32. treatment emergent.ti,ab.
33. tolerability.ti,ab.
34. rebound.ti,ab.
35. Hypercalcemia/ci [Chemically Induced]
36. Urinary Calculi/ci [Chemically Induced]
37. Tachyphylaxis/ci, de [Chemically Induced, Drug Effects]
38. Substance Withdrawal Syndrome/ci, de [Chemically Induced, Drug Effects]
39. ATROPHY/ci [Chemically Induced]
40. TELANGIECTASIS/ci [Chemically Induced]
41. skin thinning.ti,ab.
42. Liver Diseases/ci [Chemically Induced]
43. Kidney Diseases/ci [Chemically Induced]
44. Disseminated Intravascular Coagulation/ci [Chemically Induced]
45. Multiple Organ Failure/ci [Chemically Induced]
46. Stevens‐Johnson Syndrome/ci [Chemically Induced]
47. Epidermal Necrolysis, Toxic/ci [Chemically Induced]
48. Heart Block/ci [Chemically Induced]
49. COMA/ci [Chemically Induced]
50. PARALYSIS/ci [Chemically Induced]
51. exp Nausea/
52. exp Vomiting/
53. benign intracranial hypertension.ti,ab. or exp Pseudotumor Cerebri/
54. exp Pigmentation Disorders/ or pigmentation.ti,ab. or exp Pigmentation/
55. lupus induced hepatitis.ti,ab.
56. or/1‐55
57. ae.fs.
58. to.fs.
59. co.fs.
60. po.fs.
61. or/57‐60
62. exp Tacrolimus/
63. tacrolimus.ti,ab.
64. protopic.ti,ab.
65. (fk506 or fk 506).ti,ab.
66. 62 or 63 or 64 or 65
67. exp Ointments/
68. ointment$.ti,ab.
69. exp Skin Cream/
70. (cream or creams).ti,ab.
71. (lotion or lotions).ti,ab.
72. topical$.ti,ab.
73. epicutaneous.ti,ab.
74. skin.ti,ab.
75. 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74
76. 66 and 75
77. 56 and 76
78. 61 and 76
79. 77 or 78
Appendix 7. EMBASE (Ovid) adverse effects search strategy
1. side effect$.ti,ab.
2. metabolite$.ti,ab.
3. photoallergic reaction$.ti,ab.
4. phototoxicit$.ti,ab.
5. (sensitization or sensitisation).ti,ab.
6. stinging.ti,ab.
7. burning.ti,ab.
8. fetal abnormalit$.ti,ab.
9. (toxic effect$ or drug effect$).ti,ab.
10. (safe or safety).ti,ab.
11. toxicity.ti,ab.
12. noxious.ti,ab.
13. complication$.ti,ab.
14. tolerability.ti,ab.
15. treatment emergent.ti,ab.
16. tolerability.ti,ab.
17. ((adverse or undesirable or harm$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab.
18. rebound.ti,ab.
19. skin thinning.ti,ab.
20. lupus induced hepatitis.ti,ab.
21. exp postmarketing surveillance/
22. exp drug surveillance program/
23. exp drug hypersensitivity/ or exp hypersensitivity reaction/ or exp delayed hypersensitivity/ or exp hypersensitivity/ or exp immediate type hypersensitivity/
24. exp drug eruption/
25. exp anaphylaxis/
26. exp allergic conjunctivitis/
27. exp atopic dermatitis/
28. exp food allergy/
29. exp respiratory tract allergy/
30. exp urticaria/
31. exp intoxication/
32. exp toxic hepatitis/
33. exp addiction/
34. exp drug toxicity/
35. exp teratogenic agent/
36. exp mutagenic agent/
37. exp carcinogen/
38. exp contact dermatitis/
39. exp skin allergy/
40. exp irritant dermatitis/
41. exp phototoxicity/
42. exp photodermatosis/ or exp photoallergy/
43. exp burning mouth syndrome/
44. exp drug monitoring/
45. exp sleep apnea syndrome/
46. exp heart arrhythmia/
47. hypercalcemia/
48. urolithiasis/
49. tachyphylaxis/
50. withdrawal syndrome/
51. atrophy/
52. telangiectasia/
53. liver disease/
54. kidney disease/
55. disseminated intravascular clotting/
56. multiple organ failure/
57. Stevens Johnson syndrome/
58. toxic epidermal necrolysis/
59. heart block/
60. coma/
61. paralysis/
62. nausea/
63. vomiting/
64. benign intracranial hypertension.ti,ab. or exp brain pseudotumor/
65. exp pigment disorder/
66. exp pigmentation/
67. pigmentation.ti,ab.
68. exp adverse drug reaction/
69. exp drug safety/
70. exp phase 4 clinical trial/
71. (ae or to).fs.
72. or/1‐70
73. exp tacrolimus/
74. tacrolimus.ti,ab.
75. protopic.ti,ab.
76. (fk506 or fk 506).ti,ab.
77. or/73‐76
78. exp ointment/
79. ointment$.ti,ab.
80. exp skin cream/
81. (cream or creams).ti,ab.
82. exp lotion/
83. (lotion or lotions).ti,ab.
84. exp topical drug administration/
85. topical$.ti,ab.
86. epicutaneous.ti,ab.
87. skin.ti,ab.
88. or/78‐87
89. 77 and 88
90. 72 and 89
91. 71 and 89
92. 90 or 91
Appendix 8. Study selection form
Study eligibility ‐ topical tacrolimus for atopic dermatitis
| First author | Journal/Conference proceedings, etc. | Year |
| RCT | Relevant participants People with atopic dermatitis who have been diagnosed by a physician | Relevant interventions Topical tacrolimus | Relevant outcomes Physician's overall evaluation; patient's self‐assessment; rates of improvement as defined in the trial report; improvement in atopic dermatitis severity grade; incidence and severity of adverse effects; dropout rates |
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No*/Unclear |
| *Do not proceed if any of the above answers are 'No'. If the study is to be included in the 'Excluded studies' section of the review, record below the information to be inserted into the 'Characteristics of excluded studies' tables |
|
|
References to trial
Check other references identified in the searches. If there are further references to this trial, link the papers now and list them below. All references to a trial should be linked under one Study ID in Review Manager.
| Code each paper | Author(s) | Journal/Conference proceedings, etc. | Year |
| A | The paper listed above |
|
|
| B | Further papers |
|
|
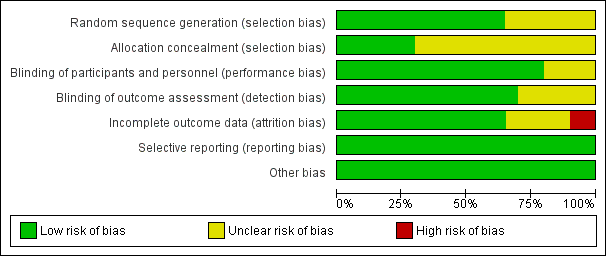
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

Forest plot of comparison: 2 Tacrolimus 0.1% versus pimecrolimus 1%, outcome: 2.1 Physician's assessment of global response of improvement, clear or excellent
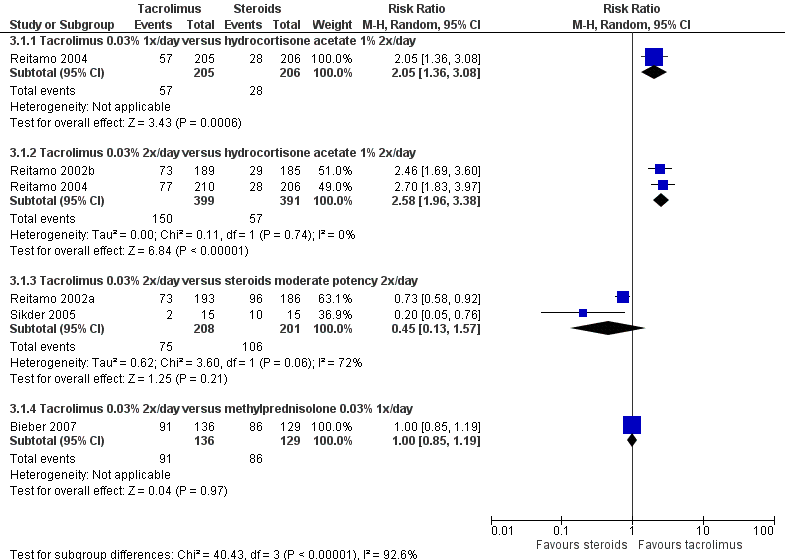
Forest plot of comparison: 3 Tacrolimus 0.03% versus corticosteroids, outcome: 3.1 Physician's assessment of global response of improvement, clear or excellent
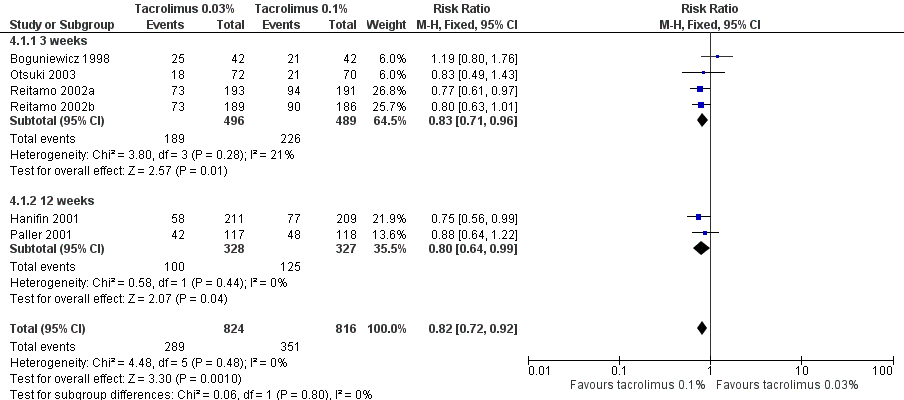
Forest plot of comparison: 4 Tacrolimus 0.03% versus tacrolimus 0.1%, outcome: 4.1 Physician's assessment of global response of improvement, clear or excellent

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
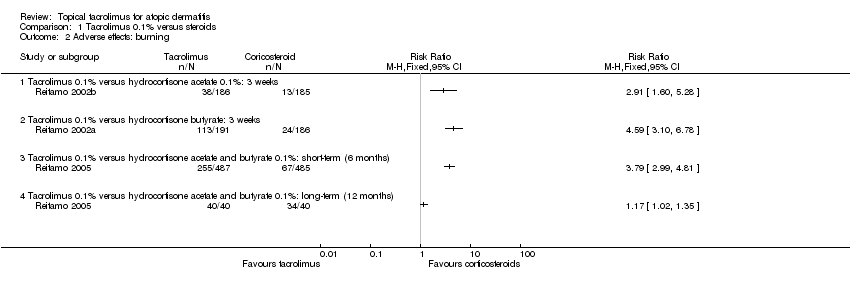
Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 2 Adverse effects: burning.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 3 Adverse effects: pruritus.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 4 Adverse effects: skin infection.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 5 SCORAD: 3 weeks.

Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
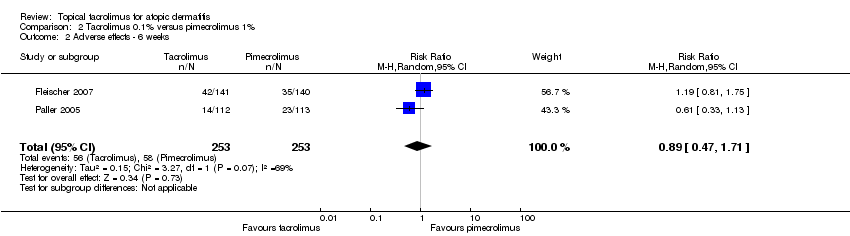
Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 2 Adverse effects ‐ 6 weeks.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
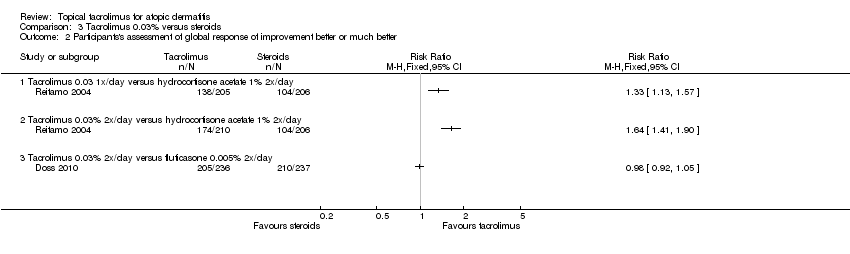
Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 2 Participants's assessment of global response of improvement better or much better.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 3 Adverse effects: burning.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 4 Adverse effects: pruritus.
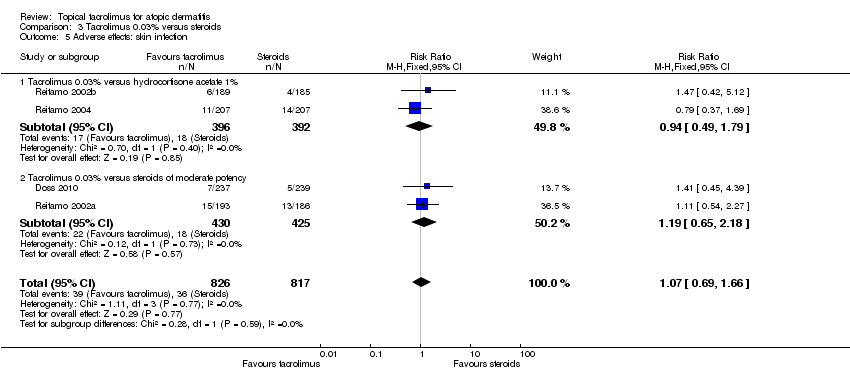
Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 5 Adverse effects: skin infection.
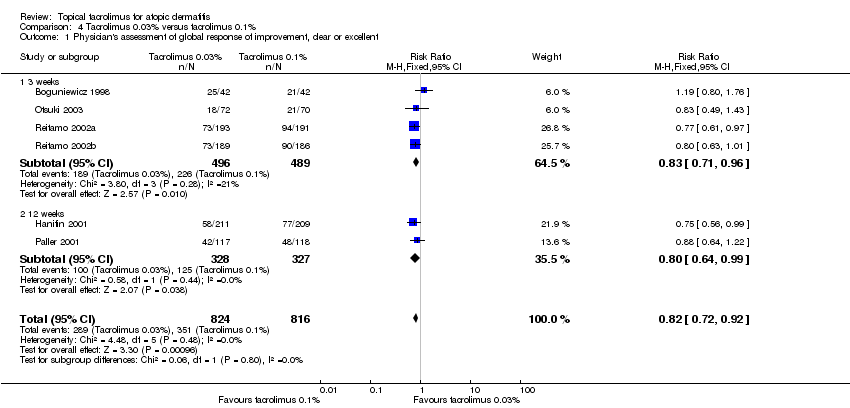
Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
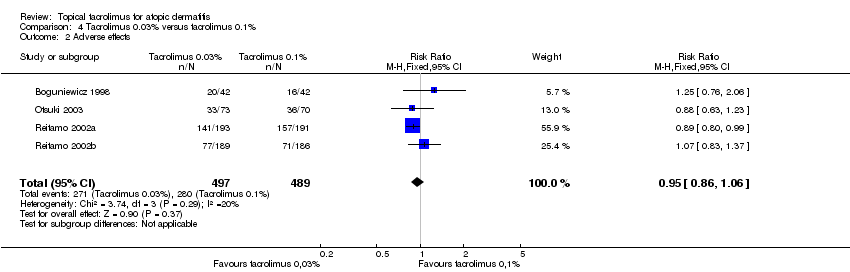
Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 2 Adverse effects.

Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement.
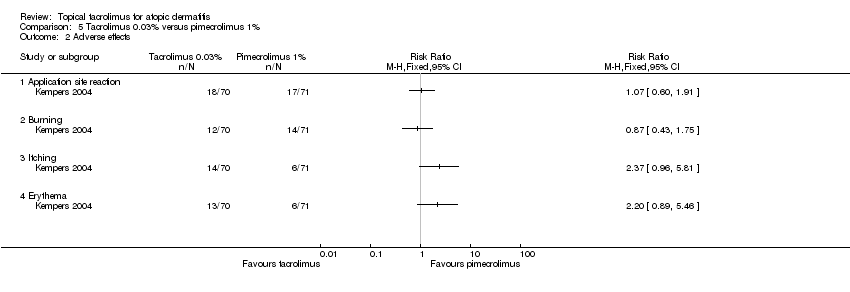
Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 2 Adverse effects.

Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 1 Adverse effects.

Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 2 SCORAD.
| Tacrolimus 0.1% compared with corticosteroids for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis Intervention: tacrolimus 0.1% | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Tacrolimus 0.1% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | Study population | RR 3.09 | 371 | ⊕⊕⊕⊝ | ‐ | |
| 157 per 1000 | 484 per 1000 | |||||
| Moderate | ||||||
| 157 per 1000 | 485 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | Study population | RR 0.95 | 377 | ⊕⊕⊝⊝ | ‐ | |
| 516 per 1000 | 490 per 1000 | |||||
| Moderate | ||||||
| 516 per 1000 | 490 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short term (6 months) | Study population | RR 1.32 | 972 | ⊕⊕⊝⊝ | ‐ | |
| 464 per 1000 | 612 per 1000 | |||||
| Moderate | ||||||
| 464 per 1000 | 612 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | Study population | RR 2.91 | 371 | ⊕⊕⊕⊝ | ‐ | |
| 70 per 1000 | 204 per 1000 | |||||
| Moderate | ||||||
| 70 per 1000 | 204 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | Study population | RR 4.59 | 377 | ⊕⊕⊕⊝ | ‐ | |
| 129 per 1000 | 592 per 1000 | |||||
| Moderate | ||||||
| 129 per 1000 | 592 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: 6 months Follow‐up: 6 months | Study population | RR 3.79 (2.99 to 4.81) | 972 (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 138 per 1000 | 524 per 1000 (413 to 664) | |||||
| Moderate | ||||||
| 138 per 1000 | 524 per 1000 (413 to 664) | |||||
| Participant's self‐assessment of global response of improvement Follow‐up: mean 6 months | Study population | RR 1.21 (1.13 to 1.29) | 974 (1 study) | ⊕⊕⊝⊝ | ‐ | |
| 718 per 1000 | 868 per 1000 (811 to 926) | |||||
| Moderate | ||||||
| 718 per 1000 | 869 per 1000 (811 to 926) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only one study was identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.1% compared with pimecrolimus 1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pimecrolimus 1% | Tacrolimus 0.1% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ 6 weeks | Study population | RR 1.8 | 506 | ⊕⊕⊕⊝ | ‐ | |
| 202 per 1000 | 363 per 1000 | |||||
| Moderate | ||||||
| 199 per 1000 | 358 per 1000 | |||||
| Adverse effects ‐ 6 weeks | Study population | RR 0.89 | 506 | ⊕⊝⊝⊝ | ‐ | |
| 229 per 1000 | 204 per 1000 | |||||
| Moderate | ||||||
| 227 per 1000 | 202 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only one study was identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.03% compared with corticosteroids for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Ilustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Tacrolimus 0.03% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 1x/day versus hydrocortisone acetate 1% 2x/day | Study population | RR 2.05 | 411 | ⊕⊕⊕⊝ | ‐ | |
| 136 per 1000 | 279 per 1000 | |||||
| Moderate | ||||||
| 136 per 1000 | 279 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | Study population | RR 2.58 | 790 | ⊕⊕⊕⊝ | ‐ | |
| 146 per 1000 | 376 per 1000 | |||||
| Moderate | ||||||
| 146 per 1000 | 377 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus corticosteroids moderate‐potency 2x/day | Study population | RR 0.45 | 409 | ⊕⊝⊝⊝ | ‐ | |
| 527 per 1000 | 237 per 1000 | |||||
| Moderate | ||||||
| 591 per 1000 | 266 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus methylprednisolone 0.03% 1x/day | Study population | RR 1 | 265 | ⊕⊕⊝⊝ | ‐ | |
| 667 per 1000 | 667 per 1000 | |||||
| Moderate | ||||||
| 667 per 1000 | 667 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.03% versus steroids | Study population | RR2.48 | 1883 | ⊕⊕⊕⊕ | ‐ | |
| 89 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 70 per 1000 | 174 per 1000 | |||||
| Participant's self‐assessment of global response of improvement: tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day Follow‐up: 3 weeks | Study population | RR 1.64 (1.41 to 1.90) | 416 (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 505 per 1000 | 828 per 1000 (712 to 959) | |||||
| Moderate | ||||||
| 505 per 1000 | 828 per 1000 (712 to 959) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only very small number of studies were identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.03% compared with tacrolimus 0.1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tacrolimus 0.1% | Tacrolimus 0.03% | |||||
| Physician's assessment of global response of improvement, clear or excellent | Study population | RR 0.82 | 1640 | ⊕⊕⊕⊕ | ‐ | |
| 430 per 1000 | 353 per 1000 | |||||
| Moderate | ||||||
| 445 per 1000 | 365 per 1000 | |||||
| Adverse effects | Study population | RR 0.95 | 986 | ⊕⊕⊕⊝ | ‐ | |
| 573 per 1000 | 544 per 1000 | |||||
| Moderate | ||||||
| 448 per 1000 | 426 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to imprecision: sample size is below the optimal information size. | ||||||
| Tacrolimus 0.03% versus pimecrolimus 1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tacrolimus 0.03% versus pimecrolimus 1% | |||||
| Physician's assessment of global response of improvement | Study population | RR 1.42 | 139 | ⊕⊕⊝⊝ | ‐ | |
| 429 per 1000 | 609 per 1000 | |||||
| Moderate | ||||||
| 429 per 1000 | 609 per 1000 | |||||
| Adverse effects ‐ application site reaction | Study population | RR 1.07 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 239 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 256 per 1000 | |||||
| Adverse effects ‐ burning | Study population | RR 0.87 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 197 per 1000 | 172 per 1000 | |||||
| Moderate | ||||||
| 197 per 1000 | 171 per 1000 | |||||
| Adverse effects ‐ itching | Study population | RR 2.37 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 85 per 1000 | 200 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 201 per 1000 | |||||
| Adverse effects ‐ erythema | Study population | RR 2.2 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 85 per 1000 | 186 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 187 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to imprecision: sample size is smaller than the optimal information size. | ||||||
| Tacrolimus 0.1% versus ciclosporin for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tacrolimus 0.1% versus ciclosporin | |||||
| Adverse effects | Study population | RR 1 | 30 | ⊕⊝⊝⊝ | ‐ | |
| 267 per 1000 | 267 per 1000 | |||||
| Moderate | ||||||
| 267 per 1000 | 267 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to risk of bias: randomisation and allocation concealment procedures were unclear. | ||||||
| Study | Number of participants (n = 5885) | Age | Intervention | Follow up | Classification of AD |
| 24 | Adults (21 to 65 years) | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (SCORAD) | |
| 265 | Children (2 to 15 years) | Tacrolimus 0.03% ointment (BID) vs methylprednisolone aceponate 0.1% ointment (evening) and vehicle ointment (morning) | 2 to 3 weeks | Severe flare (IGA > 4) history of moderate to severe AD | |
| 169 | Older children (7 to 16 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs tacrolimus 0.3% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 16 | Adults | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (SCORAD) | |
| 473 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs fluticasone 0.005% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) and with prior inadequate response to topical corticosteroids | |
| 202 | Adults (> 18 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe | |
| 37 | Adults | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 2 weeks | Moderate to severe (IGA) | |
| 281 | Adults (> = 16 years) | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate to severe (IGA) | |
| 632 | Adults (> = 16 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs vehicle ointment (BID) | 3 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 60 | Adults and children (9 months to 33 years) | Tacrolimus 0.03% ointment (BID) alone or with fusidic acid 2% cream vs fluticasone propionate 0.05% cream (BID) alone or with fusidic acid 2% cream | 6 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 141 (for safety) 139 (for efficacy) | Children (2 to 17 years) | Tacrolimus 0.03% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate (IGA) | |
| 213 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 30 | Adults and children (13 to 45 years) | Tacrolimus 0.1% ointment (BID) vs ciclosporin 3 mg/kg orally | 6 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 351 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 225 | Children (2 to 15 years) | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate to severe (IGA) | |
| 570 | Adults (16 to 70 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 560 | Children (2 to 15 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs hydrocortisone acetate 1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 621 | Children (2 to 15 years) | Tacrolimus 0.03% ointment (OD) vs tacrolimus 0.03% ointment (BID) vs hydrocortisone acetate 1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 972 | Adults (> = 18 years) | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (on trunk and extremities) and hydrocortisone acetate 1% ointment (on face and neck) (BID) | Up to 6 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 45 | Older children (7 to 15 years) | Tacrolimus 0.03% ointment (BID) vs clobetasone butyrate 0.05% cream (BID) vs clobetasone butyrate 0.05% cream (morning) and tacrolimus 0.03% ointment (evening) | 4 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| AD: atopic dermatitis. | |||||
| Malignancy | Age (years) | Application site | Occurence site | Comment | Exposure to onset (days) |
| B‐cell lymphoma, EBV‐associated, and primary lung carcinoma | 49 | Face | Kidney | ‐ | 730 |
| Cutaneous Kaposi sarcoma | 28 | Axilla, groin | Axilla, groin | HIV patient on HAART, treated for inverse psoriasis, developed KS at these sites, which metastasised, and the patient died | 30 |
| Hepatoblastoma | 5 | ‐ | Liver | Considered unrelated | 455 |
| Lymphadenopathy – possible | 40 | Application site | Application site | Pre‐existing lymphoma lesions 'looked like' lymphoma and resolved spontaneously* | ‐ |
| Lymphoma or Sézary syndrome | 16 | Face | Lymph nodes | Participant also had been on systemic ciclosporin | 730 |
| Metastatic angiosarcoma | 16 | Face/neck | Clavicle | Present before treatment but increased rapidly in size | 105 |
| Metastatic melanoma | 39 | ‐ | Generalised | Metastatic disease newly detected from primary 3 years early | 21 to 28 |
| Metastatic sweat gland carcinoma | 43 | Not axilla | Axilla | ‐ | 4 years |
| Nodular follicular lymphoma | 60 | Lower limbs, face | ‐ | May be associated with EBV | 504 |
| Non‐Hodgkin lymphoma | 52 | ‐ | ‐ | Used tacrolimus for 6 months. Insufficient evidence | 365 |
| Non‐Hodgkin lymphoma | 54 | ‐ | ‐ | Used tacrolimus on extensive areas: 50% of body. Died from lymphoma. Insufficient evidence | ‐ |
| Oesophageal cancer with metastases | 49 | ‐ | Oesophagus | ‐ | 122 |
| Panniculitis‐like T‐cell lymphoma | 53 | Trunk, limbs | Trunk, limbs | Also used pimecrolimus | 240 |
| Squamous cell carcinoma | 34 | Face | Face | UV therapy, outdoor sports | ‐ |
| Squamous cell carcinoma | 57 | Penis | Penis | Treated for balanitis considered to be lichen sclerosus et atrophicus; non‐specific biopsy | 70 |
| Squamous cell carcinoma | 51 | ‐ | Mouth | Long history of pipe smoking | ‐ |
| Squamous cell carcinoma recurrence | 75 | Vulva | Vulva | Treated for lichen sclerosus et atrophicus | 42 |
| T‐cell lymphoma, anaplastic large cell | 50 | Right hip | Right hip | Insufficient evidence | ‐ |
| EBV: Epstein–Barr virus. | |||||
| Study | Study population | Follow‐up | Comparisons | Results related to lymphoma risks |
| 294 cases/293,000 controls | ‐ | TCIs and TCS in participants with AD | ‐ Increased risk in AD participants (related to severity) ‐ No evidence of increased risk with any of the topical treatments | |
| > 3,000,000 (cohort) | 1992 to 2006 | AD, treatment with topical immunosuppressants, or both | ‐ Increase risk in AD participants (related to severity) ‐ Increased risk with topical corticosteroids (related to potency) ‐ Insufficient data to assess TCI‐related risks | |
| 953,064 (cohort) (96% unexposed, 4% exposed) | Median 2.4 years | AD or eczema participants exposed or not to TCI | ‐ Increased risk in the exposed group** | |
| ‐ 118,863 for pimecrolimus ‐ 38,757 for tacrolimus ‐ 1,043,025 mid to potent corticosteroid ‐ 118,825 untreated dermatitis ‐ 118,863 for general population | 2002 to 2006 (median 1.3 years) | See study population | ‐ Increased risk compared with general population* ‐ No risk differences between the 3 treatments | |
| *pre‐existing lymphomas misdiagnosed as AD. | ||||
| Study | Study population | Follow up | Comparisons | Results related to skin cancer risks |
| 953,064 (cohort) (96% unexposed, 4% exposed) | Median 2.4 years | AD participants exposed or not to TCI | ‐ Similar risks for NMSC ‐ Lower risks for MM | |
| 875 cases 1946 controls | ‐ | Dermatitis participants (AD, seborrhoeic dermatitis, rosacea, other dermatitis) with or without use of TCI | ‐ No increased risk of NMSC in TCI‐treated participants ‐ MM risk not evaluated | |
| 9813 tacrolimus‐treated participants | 3 months to 4 years | AD participants with tacrolimus use compared with an aged cohort in the US | ‐ No increased risk of NMSC in tacrolimus treated participants ‐ MM risk not evaluated | |
| AD: atopic dermatitis. | ||||
| Study | 1. Population 2. Age group 3. Follow‐up | Tacrolimus formulation | Common local effects | Systemic effects | Laboratory values | Malignancies | Others (number of events) | Detectable blood concentration |
| 1. n = 174 2. Paediatric | 0.03% | ‐ Burning ‐ Pruritus | ‐ | ‐ | ‐ | ‐ Asthma (2) ‐ Pneumonia (2) ‐ Pyodermitis (1) | ‐ | |
| 1. n = 7923 2. Adult/paediatric 3. Median: 210 days | 0.1% (92.7%) 0.03% (7.3%) | ‐ Burning ‐ Pruritus | ‐ Flu‐like symptoms ‐ Headache (frequency similar to that expected of the general population) | ‐ | ‐ 13 cases of NMSC (no risk with calculated incidence) | ‐ Alcohol intolerance 3.7% | ‐ | |
| 1. n = 50 2. Paediatric (< 2 years) 3. 2 years | 0.03% | ‐ Pruritus ‐ Local infection | ‐ Non‐serious respiratory infection and gastroenteritis | ‐ | ‐ | ‐ | < 1 ng/ml (in 98%) | |
| 1. n = 316 2. Adults 3. 6 to 12 months | 0.1% | ‐ Burning ‐ Pruritus ‐ Erythema | ‐ | Normal (only 1 transient increase in liver enzymes) | ‐ | ‐ Alcohol intolerance 5 serious events: ‐ Eczema herpeticum (1) ‐ Cellulitis (1) ‐ Varicella (1) ‐ AD flare‐up (1) ‐ Staphylococcus aureus superinfection (1) | Minimal < 1 ng/dl in 76% of participants | |
| 1. n = 672 2. Adults 3. 2 years | 0.1% | ‐ Burning ‐ Pruritus | ‐ | ‐ | ‐ 2 cases (Bowen and prostate carcinoma) not related ‐ Benign neoplasm (7) | ‐ Herpes (7%) (expected in AD participants) ‐ Eczema herpeticum (1) ‐ Erythroderma (1) ‐ AD exacerbation (1) | ‐ | |
| 1. n = 782 2. Adult/paediatric 3. 4 years (median: 1422 days) | 0.1% | ‐ Burning ‐ Pruritus ‐ Skin infection | ‐ Flu‐like symptoms (more in children) | ‐ | 6 cases ‐ Cervical carcinoma (1) ‐ Acute leukaemia (1) ‐ Chronic leukaemia (1) ‐ Basal cell carcinoma (2 to 3 on the same participant) ‐ 34 benign neoplasms | ‐ | ‐ | |
| 1. n = 466 2. Paediatric 3. 29.5 months (mean: 16.3 months) | 0.03% 0.1% | ‐ Burning ‐ Pruritus | ‐ Seasonal infection (flu‐syndrome) ‐ No growth retardation | Normal | ‐ | ‐ Leukopenia (1)* ‐ Herpes (4.9%)/eczema herpeticum (0.9%) ‐ Molluscum 3%) ‐ Warts (3.6%) | ‐ | |
| 1. n = 125 2. 12 to 69 years 3. 5 weeks | 0.03% | ‐ Burning ‐ Pruritus ‐ Erythema | ‐ | Normal | ‐ | ‐ | ‐ | |
| 1. n = 18 2. Adult/paediatric 3. 4 weeks | 0.03% | ‐ Burning ‐ Pruritus | ‐ | Normal | ‐ | Serious events (3): ‐ Flu‐syndrome (1) ‐ Severe skin rash (1) ‐ Eczema herpeticum (1) | ‐ | |
| 1. n = 30 2. Adult/paediatric 3. 4 weeks | 0.1% adults 0.03% paediatric | ‐ Burning ‐ Pruritus | ‐ | Normal | ‐ | ‐ | 2 participants < 5 ng/ml | |
| * 6‐year‐old participant, at month 6, resolution after withdrawn. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects: burning Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: pruritus Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: skin infection Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 SCORAD: 3 weeks Show forest plot | 2 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐15.36, ‐2.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.35, 2.42] |
| 1.1 13 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.19, 19.13] |
| 1.2 6 weeks | 2 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.34, 2.42] |
| 2 Adverse effects ‐ 6 weeks Show forest plot | 2 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.47, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Tacrolimus 0.03% 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.36, 3.08] |
| 1.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 2 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [1.96, 3.38] |
| 1.3 Tacrolimus 0.03% 2x/day versus steroids moderate potency 2x/day | 2 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.13, 1.57] |
| 1.4 Tacrolimus 0.03% 2x/day versus methylprednisolone 0.03% 1x/day | 1 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.19] |
| 2 Participants's assessment of global response of improvement better or much better Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Tacrolimus 0.03 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.03% 2x/day versus fluticasone 0.005% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: burning Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.96, 3.14] |
| 3.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.36, 2.57] |
| 3.2 Tacrolimus 0.03% versus steroids moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [2.45, 5.06] |
| 4 Adverse effects: pruritus Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| 4.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.00, 1.88] |
| 4.2 Tacrolimus 0.03% versus steroids of moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.18, 2.80] |
| 5 Adverse effects: skin infection Show forest plot | 4 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.66] |
| 5.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.49, 1.79] |
| 5.2 Tacrolimus 0.03% versus steroids of moderate potency | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 6 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.92] |
| 1.1 3 weeks | 4 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.96] |
| 1.2 12 weeks | 2 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 0.99] |
| 2 Adverse effects Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Application site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Burning | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 SCORAD Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 14 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 21 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 28 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 35 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 42 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


