Apoyo conductual adicional como complemento a la farmacoterapia para el abandono del hábito de fumar
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Setting: community health centre, USA Recruitment: African‐American light smokers recruited from the clinic and using various routes of advertisement | |
| Participants | 755 smokers of ≤ 10 cigarettes per day; the characteristics of 378 participants in the relevant arm were as follows: 66.1% to 68.3% female; average age 43.5 to 45.2; average cigarettes per day 7.5 to 7.8 Therapists: trained counsellors who followed semi‐structured scripts | |
| Interventions | Pharmacotherapy: NRT; 2 mg nicotine gum for 8 weeks including weaning period. Dose depended on the number of cigarettes smoked per day 1. Motivational interviewing: 3 sessions in person and 3 sessions by telephone, each lasting 20 minutes 2. Health education: 3 sessions in person and 3 sessions by telephone, each lasting 20 minutes | |
| Outcomes | 7‐day point prevalence abstinence at weeks 1, 3, 6, 8, 16 and 6 months Validation: cotinine‐verification (≤ 20 ng/mL), expired carbone monoxide ≤ 10 ppm | |
| Source of Funding/CoI | National Cancer Institute at the National Institutes of Health (R01CA091912) Glaxo‐SmithKline provided study medication. No declarations of interest | |
| Notes | New for 2019 update. Previously excluded. Reason: Conselling conditions had same number of contacts and duration. Compared Motivational Interviewing and Health Education (HE) in a factorial trial with nicotine gum or placebo (results favoured HE (control) condition). Included in Lindson‐Hawley 2015 Cochrane review of motivational interviewing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Low risk | Sealed envelope with pre‐assigned randomisation numbers |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 11.1% to 16.9% lost to follow‐up at 6 months |
| Methods | Setting: rheumatology clinic (single centre), Christchurch, New Zealand Recruitment: smokers with rheumatoid arthritis. No mention of intended selection for motivation but the authors mentioned that the study population was likely to have been highly motivated | |
| Participants | 39 smokers; 55% female; average age 56.5; average cigarettes per day 16.5 Therapists: community‐based arthritis educators trained in smoking cessation | |
| Interventions | Pharmacotherapy: NRT for 8 weeks 1. usual care (brief advice and subsidised NRT) for 3 months 2. usual care + rheumatoid arthritis‐specific programme for 3 months via face‐to‐face, telephone and email; 4 sessions at week 0, 1, 4, 8 | |
| Outcomes | Continuous abstinence at 3 and 6 months Validation: none | |
| Source of Funding/CoI | New Zealand Health Research Council, Arthritis New Zealand and University of Otago Research Fund. Authors declared receipt of consultant fees, speaking fees, and/or honoraria from AbbVie and Janssen (less than $10,000 each). | |
| Notes | New for 2019 update One participant was excluded from analysis after intervention and follow‐up when found not to have rheumatoid arthritis. Did not contribute to analysis 1.2 or analysis 1.3 as duration of control group contact not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by a biostatistician using an Excel spreadsheet in 6 blocks x 8 allocations |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only |
| Incomplete outcome data (attrition bias) | Low risk | 0‐15.8% lost to follow‐up at 6 months |
| Methods | Setting: cessation clinic, USA | |
| Participants | 240 smokers of > 1 pack/day; 45% to 54% female, average age 40, average cpd 27 | |
| Interventions | Pharmacotherapy: NRT; 21 mg patch for 8 weeks (including weaning period) | |
| Outcomes | Abstinence at 1 year | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | 3 versus 1 in main analysis. Quit rates significantly lower in 2 than 1 or 3. 35/160 quit when 2 & 3 combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Urn technique" |
| Allocation concealment (selection bias) | Unclear risk | No details given. Allocation took place after baseline session common to all conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Small and similar rate lost to follow‐up in each group (approx 7%). "Intent to treat" analyses reported in the paper excluded 2 deaths and 2 who did not provide cotinine samples |
| Methods | Setting: 26 general practices (primary care clinics), UK | |
| Participants | 925 smokers; 51% F, av. age 43, 50% smoked 11 to 20 cpd | |
| Interventions | Pharmacotherapy: NRT; 16 mg patch for 8 weeks | |
| Outcomes | Abstinence at 12 months (sustained at 1, 4, 12, 26 weeks) | |
| Source of Funding/CoI | Cancer Research UK. Authors declared interests. | |
| Notes | Therapists were not full‐time specialist counsellors. Difference between support conditions relatively small | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Over 30% lost to follow‐up but similar percentage followed up in both groups (69% intervention vs 68% control, no evidence of differential attrition) |
| Methods | Setting: high schools in San Francisco, USA Recruitment: adolescent smokers were recruited over a period of 3 years on a non‐rolling basis, with a new cohort participating each academic school year. Selected for motivation to quit | |
| Participants | 143 smokers; 37.6% female; average age 16.9; average cigarettes smoked per week 97.1 Therapists: research intervention staff with Bachelor's degree or higher. Supervised by the project director (clinical psychologist) | |
| Interventions | Pharmacotherapy: NRT (nicotine patch); 9 weeks (dosage and titration schedule determined by number of cigarettes smoked per day) 1. Group based cognitive behavioural therapy and skills training (10 weeks) 2. Group based cognitive behavioural therapy and skills training (10 weeks) + extended face‐to‐face group sessions (9 sessions over 14 weeks) | |
| Outcomes | 7‐day point prevalence abstinence at 6 months Validation: expired carbon monoxide using Bedfont Smokerlyzers | |
| Source of Funding/CoI | National Cancer Institute at the National Institutes of Health (R01 CA 118035 to JDK). No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up rate: extended group 16.7%; other 16.9% |
| Methods | Setting: Centre for Brain and Mental health Research, University of Newcastle, New South Wales; School of Public Health, University of New South Wales, Sydney; Monash Alfred Psychiatry Research Centre, Monash University and the Alfred, Melbourne, Australia Recruitment: smokers recruited from three sites in Newcastle, Sydney and Melbourne, Australia. Referral sources included health services, media advertisement and other research programmes or registers. | |
| Participants | 235 smokers; 41.3% female; average age 41.6; average cigarettes smoked per day 28.6 Therapists: psychologists guided by intervention manuals | |
| Interventions | Pharmacotherapy: NRT; 24 weeks’ supply of NRT delivered at weeks 1, 4 and 8 and thereafter by arrangement. Participants smoking ≥ 30 cigarettes per day were eligible to receive double patching in addition to up to 12 x 2 mg lozenges per day, with NRT tapering occurring in the last month of delivery. 1. Predominantly telephone‐based (17 sessions; 290 minutes in total) 2. Face‐to‐face healthy lifestyle therapy (17 sessions; 1050 minutes in total) | |
| Outcomes | 7‐day point prevalence abstinence at week 15 and months 12, 18, 24, 30, 36 Validation; carbon monoxide ≤ 10 ppm | |
| Source of Funding/CoI | Australian National Health and Medical Research Council and the Commonwealth Department of Health and Aging. NRT was provided free of charge by GlaxoSmithKline. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Permuted block randomisation approach mentioned but no further detail |
| Allocation concealment (selection bias) | Low risk | Sealed randomisation envelope by an independent person displaying a participant identification code. Participants opened the envelope at the end of the initial intervention session. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 39.8 to 45.9% lost to follow‐up at 3 years |
| Methods | Setting: USA Recruitment: participants identified from electronic medical records. Eligibility assessment in person and selected for motivation to quit | |
| Participants | 471 smoker; 8.5% female, average age 59; heaviness of smoking index mean 2.8 Therapists: masters‐level counsellors with training | |
| Interventions | Pharmacotherapy: NRT; inhaler, patch, spray and/or bupropion (regimen and dosage dependent on the number of cigarettes smoked per day and tobacco cessation anxiety) 1. Usual care: 5 telephone sessions every 3‐4 weeks; each session lasting 20 minutes 2. Family‐supported 5 telephone sessions every 3‐4 weeks; each session lasting 20 minutes | |
| Outcomes | 7‐day point prevalence abstinence at 5 and 12 months Validation: attempted verification by mailing saliva‐sampling kits to test for cotinine level but the return rates were low (50.5%) | |
| Source of Funding/CoI | Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Health Services Research and Development. Authors declared a consultancy to Gilead Sciences and Watermark Research Partners. | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Low rates of return when biochemical validation was attempted, but contact‐matched so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) | Low risk | 21.7 to 28.4% lost to follow‐up rate |
| Methods | Setting: NHS Stop Smoking clinic, UK Recruitment: smokers recruited from the participating general practices and Stop Smoking services. Selected for motivation to quit | |
| Participants | 119 smokers; 69% female; average age 44.8; average cigarettes smoked per day 20.8 Therapists: trained research nurses and Stop Smoking advisors | |
| Interventions | Pharmacotherapy: NRT; 21 mg per 24 hour nicotine patches for 8 to 12 weeks 1. 7 weekly sessions of withdrawal support, of which 5 sessions included placebo training (16 minutes each) starting one week prior to quit date 2. 7 weekly sessions of withdrawal support, of which 5 sessions included attentional retraining (16 minutes each) starting one week prior to quit date | |
| Outcomes | Prolonged abstinence at weeks 4, 8, and at 6 months Validation: exhaled carbon monoxide < 10 ppm | |
| Source of Funding/CoI | National Institute for Health Research. Authors declared research and consultancy for manufacturers of smoking cessation medication, including consultancy for GlaxoSmithKline Consumer Healthcare and research‐initiated project grant funding from Pfizer. | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated simple randomisation scheme |
| Allocation concealment (selection bias) | Low risk | An independent programmer entered the sequence onto a dedicated online database which was accessed by study staff in clinics |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 30.0% to 40.7% lost to follow‐up at 6 months |
| Methods | Setting: cardiac wards, Netherlands Recruitment: inpatients by ward nurses, at the bedside | |
| Participants | 372 in relevant arms, excl 7 deaths (5 TC, 2 FC) 73% M, av age 56, av cpd 21 Therapists: face‐to‐face counselling (FC) provided by recently trained cardiac nurses, telephone counselling (TC) provided by experienced telephone counsellors | |
| Interventions | Pharmacotherapy: NRT; patches (21 mg/day or 14 mg/day (10 to 20 cpd) for 8 weeks incl weaning) 1. UC (control): brief quit advice from ward nurses + brochure; no NRT (historical control, before wards assigned to interventions, not used in review) 2. TC: usual care + 7 x 15‐min telephone sessions, weekly for 5 weeks, week 7, week 12 3. FC: usual care + 6 x 45‐min + 1 x 15 min face‐to‐face sessions, same schedule as TC | |
| Outcomes | Abstinence at 6 months (90 day PP since last counselling session) Validation: none | |
| Source of Funding/CoI | ZonMw, the Dutch Organization for Health Research and Development. Authors declared no conflicts of interest. | |
| Notes | 3 vs 2 in analyses, patch use was similar across TC & FC groups Intraclass correlation coefficient assessed; "intraclass correlations were small and not statistically different from zero" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation with sequential cross‐over design. Method of randomising wards to begin with FC or TC not described |
| Allocation concealment (selection bias) | High risk | Nurses knew assignment when recruiting patients. "Although not reported by the nurses, they may have been selective in their recruitment because patients in the intervention groups appeared more motivated in their drive to quit smoking". However, this probably had greater impact on comparison with usual care, not used in this review. |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only |
| Incomplete outcome data (attrition bias) | Low risk | Approximately 20% lost to follow up in each group (TC = 22%, FC = 21%) |
| Methods | Setting: research fitness facility, USA Recruitment: smokers recruited from newspaper and radio advertisements | |
| Participants | 61 smokers; 63.3% to 67.7% female; average age 47; average cigarettes smoked per day 19.4 to 20.3 Therapists: aerobic exercise sessions were supervised by exercise physiologist. Unclear who provided health education sessions | |
| Interventions | Pharmacotherapy: NRT; 8 weeks of transdermal nicotine patch (21 mg for weeks 5 to 8, 14 mg for weeks 9 to 10, 7 mg for weeks 11 to 12) 1. 8 sessions of telephone counselling (20 minutes each) + 12 weekly group health education sessions (60 minutes each) 2. 8 sessions of telephone counselling (20 minutes each) + 12 weekly sessions of group aerobic exercise (20 to 40 minutes) + 12 weekly cognitive behavioural sessions just before the exercise sessions (20 minutes each) | |
| Outcomes | Continuous abstinence at months 3, 6, 12 Validation: expired carbon monoxide < 10 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | New for 2019 update Authors confirmed a typographical error in the abstinence rate in Bloom 2017 paper and stated the figures in Abrantes 2014 are correct. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 10.0‐12.9% lost to follow‐up at 12 months |
| Methods | Setting: 3 primary care clinics, New England, USA Recruitment: smokers identified by clinic personnel during registration. Research assistants screened interested individuals | |
| Participants | 846 smokers 69% F, av age: 40, av cpd not described Therapists: smoking cessation specialists | |
| Interventions | Pharmacotherapy: NRT; patch for 8 weeks 1. Standard care: brief physician advice, patch education 2. Motivational enhancement treatment: standard care + 45‐min individual counselling session & 2 counselling calls either on quit day & 2 weeks later or at 2 & 4 weeks after 1st session | |
| Outcomes | Abstinence at 12 months (7‐day PP) Validation: carbon monoxide < 5 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse. Authors declared no conflicts of interest. | |
| Notes | Data previously confirmed with authors for another review; 48/406 vs 58/440 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomised |
| Allocation concealment (selection bias) | Low risk | Research assistants enrolled prior to computer randomisation |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | High risk | Dropout over 50%; 58.6% in SC 238/440) and 52.7% in ME (232/406) |
| Methods | Setting: Health Maintenance Organization, USA | |
| Participants | 1329 HMO members; 58% F, av age 47, 66% smoked > pack/day | |
| Interventions | Pharmacotherapy: all participants had filled a prescription. Almost 95% used; ˜51% only bupropion, 26% only NRT, remainder both | |
| Outcomes | Abstinence at 12 months (repeated 7‐day PP at 3 months & 12 months) | |
| Source of Funding/CoI | Robert Wood Johnson Foundation Addressing Tobacco in Managed Care Program. No declarations of interest | |
| Notes | 49% of intervention group reached, 36% of those declined, 31% of total accepted counselling. Average N of calls 5. There was no evidence of a greater relative effect in those reached or those accepting counselling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, stratified by presence of chronic disease. Method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only |
| Incomplete outcome data (attrition bias) | Low risk | Over 30% lost to follow‐up but similar percentage followed up in both groups (66% intervention vs 65% control, no evidence of differential attrition) |
| Methods | Setting: Quitline in South Carolina, USA Recruitment: recruited uninsured callers to the South Carolina State Quitline | |
| Participants | 121 smokers; 69% female; average age 39.1; 65% smoked more than half pack per day Therapists: counsellors were Bachelors or Masters level providers with at least 3 years of general counselling experience | |
| Interventions | Pharmacotherapy: NRT; 2‐week course of nicotine patch or gum (participant’s choice) 1. 5 sessions of cognitive behavioural therapy telephone intervention (1st call 30 minutes and each subsequent call 15 minutes) 2. 5 sessions of acceptance and commitment therapy telephone intervention (1st call 30 minutes and each subsequent call 15 minutes) | |
| Outcomes | 30‐day point prevalence abstinence at 6 months Validation: none | |
| Source of Funding/CoI | National Institute on Drug Abuse, National Cancer Institute. Authors declared consultancy for Pfizer | |
| Notes | New for 2019 update. Previously excluded. Reason: both the control and the intervention received equal amounts behavioural counselling; telephone‐delivered acceptance and commitment therapy (ACT) versus cognitive behavioral therapy (CBT) for smoking cessation was being assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details given |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐report only but contact matched in both groups so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) | Low risk | 27.1‐38.7% lost to follow‐up at 6 months |
| Methods | Setting: Veterans Affairs Los Angeles Healthcare System, USA Recruitment: smokers recruited via flyer advertisement from the smoking and schizophrenia treatment programmes. Selected for motivation | |
| Participants | 42 smokers; 100% male; average age 56.3 to 57.5 years; average cigarettes smoked per day 18.5 to 19.6 Therapists: cognitive behavioural therapy by a psychologist; home visits by the study investigators | |
| Interventions | Pharmacotherapy: NRT; ‐ combination extended treatment groups (with or without home visit): combination of three medications (bupropion, nicotine patch, nicotine lozenge) for 6 months ‐ usual care: single smoking cessation medication (patch, bupropion or varenicline) typically for at least 2 to 4 weeks 1. combination extended treatment without home visit: 12 weekly sessions of cognitive behavioural therapy (60 minutes each) 2. combination extended treatment plus home visit: 12 weekly sessions of cognitive behavioural therapy (60 minutes each) + biweekly home visits (20 to 30 minutes each) 3. usual care (excluded from our meta‐analyses due to different pharmacotherapy to the other groups) | |
| Outcomes | 7‐day point prevalence abstinence at week 12 and at 6 months Validation: exhaled carbon monoxide ≤ 3 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse, Department of Veterans Affairs Office of Research and Development and Tobacco‐Related Disease Research Program. No declarations of interest | |
| Notes | New for 2019 update Lost to follow‐up numbers were obtained from the authors via email correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up rates were 21.4% to 28.6% in combination extended treatment groups and 7.1% in usual care group at 6 months. |
| Methods | Setting: community, USA Recruited: community volunteers who had “previous difficulty quitting for even short periods of time." Selected if motivated to quit | |
| Participants | N = 49; dropouts: 7 49% F, av age: standard = 48.30; distress tolerance = 47.19, av. cpd standard = 22; distress = 21 Therapists: doctoral‐level psychologists or trainees (psychology interns/postdoctoral fellows) delivered the treatment. | |
| Interventions | Pharmacotherapy: “8 weeks of nicotine replacement therapy in the form of the nicotine patch (Nicoderm CQ) beginning on quit day, including 4 weeks of the 21 mg patch, 2 weeks of 14 mg, and 2 weeks of 7 mg.” 1. standard smoking cessation treatment 2. distress tolerance treatment = incorporated elements of exposure‐based therapies and Acceptance and Commitment Therapy | |
| Outcomes | Abstinence at 26 weeks (7‐day PP) Validation: expired carbon monoxide (CO, 5 ppm or less) + cotinine verification (cotinine, 10 ng/mL or less) | |
| Source of Funding/CoI | National Institute on Drug Abuse. Authors declared no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants treated in groups, 3 groups for each condition; “each treatment assignment was randomly selected from the fixed pool of possible assignments” |
| Allocation concealment (selection bias) | High risk | Type of treatment allocated for next group likely to have been known before participant recruitment |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Overall 14% lost; standard 9% (2/22), distress 19% (5/27) |
| Methods | Setting: Miriam and Rhode Island Hospitals in Providence, USA Recruitment: inpatient cardiac units at the Miriam and Rhode Island Hospitals in Providence | |
| Participants | 64 smokers; 27.1% female; average age 55.6; average cigarettes smoked per day 16.4 Therapists: research team members (licenced clinical psychologist and clinical psychology post‐doctoral fellow) | |
| Interventions | Pharmacotherapy: NRT; 8 weeks of nicotine patches starting on 21 mg patch for those smoking > 10 cigarettes per day and on 14 mg for those starting ≤ 10 cigarettes per day 1. Usual care: one in‐hospital counselling session (50 minutes) + 5 mailings of print materials + 5 brief “check‐in” calls from a health educator following each mailing (5 to 10 minutes each) 2. One in‐hospital counselling session + > 5 post‐discharge contacts at 1, 3, 6, 9 and 12 weeks. Sessions 1 & 2 (50 minutes each) in‐person at a research clinic or in the participant’s home; Sessions 3 to 6 (30 minutes each) by phone | |
| Outcomes | 7‐day point prevalence abstinence at weeks 12 and 24 post‐discharge from the hospital Validation: carbon monoxide < 10 ppm | |
| Source of Funding/CoI | National Heart, Lung, and Blood Institute of the National Institutes of Health. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | The study statistician provided sequenced randomisation envelopes. The randomisation envelopes were opened by counsellors following the completion of each in‐hospital smoking cessation session. Counsellors then immediately informed the participant of their treatment condition. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 21.2 to 22.6% lost to follow‐up at 24 weeks post‐discharge |
| Methods | Setting: community with large military population, USA | |
| Participants | 314 military and civilian smokers, excluded 198 people, assignment NS, who did not attend any sessions after randomisation. 44% F, age and smoking not described | |
| Interventions | All participants offered free NRT (in group 2 conditional on attending 75% classes) | |
| Outcomes | Abstinence at 6 months (PP) | |
| Source of Funding/CoI | ||
| Notes | Early benefit of VUMC lost at 6 months. No observed effect in active duty participants at any time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", method not stated, stratified by military or civilian |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Unclear risk | 198 (out of 512 randomised) did not participate, group not stated, not clear if participants knew what group they were assigned to before attending first session. |
| Methods | Setting: university student body, USA Recruitment: advertised through flyers in campus halls, newsletters, email, and during presentations in classes Smoking cessation counsellors enrolled participants | |
| Participants | 509 smokers (≥ 1 cpd) 53% F, av age 24.5, 39% smoked 11‐20 cpd Therapists: counsellors trained specifically in behaviour change/cigarette counselling | |
| Interventions | Pharmacotherapy: NRT; patch offered to participants smoking ≥ 5 cpd 1. Self‐help written material, ≤ 5 mins minimal counselling, and no persuasive communication or assistance to participants 2. In‐person motivational counselling with health feedback, 2 x 60 to 120 mins over 3 months, and access to 5 web‐based booster sessions | |
| Outcomes | Abstinence at 12 m (30‐day PP) Validation: 46 of 79 who reported abstinence provided a salivary cotinine value ≤ 5 ng/mL. | |
| Source of Funding/CoI | National Cancer Institute. Authors declared no conflicts of interest. | |
| Notes | Coded as validated, however not all self‐reported quitters were validated due to problems with sample collection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistical software package generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer occurred after enrolment. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | High risk | Dropout high and differential; intervention 43% (120/278), control 66% (153/231) |
| Methods | Setting: primary care clinics, USA Recruitment: adult smokers recruited during primary care visits who were willing to reduce their smoking but not quit | |
| Participants | 517 smokers; 63.4% female, average age 47.0; average number of cigarettes smoked per day 17.5 Therapists: no details given | |
| Interventions | Pharmacotherapy: NRT; 14 mg patches daily for 6 weeks and/or 2 mg gum for 6 weeks (≥ 9 per day, 1 piece for 1 to 2 hours) 1. behavioural reduction: an initial 20 minute in‐person counselling session followed by 6 weekly 10‐minute counselling calls; 7 sessions in total 2. motivational interviewing: an initial 20‐minute in‐person counselling session followed by three biweekly, 10‐minute counselling calls over 6 weeks; 4 sessions in total | |
| Outcomes | 7‐day point prevalence abstinence at week 12 and at 6 months Validation: none | |
| Source of Funding/CoI | National Cancer Institute, the Wisconsin Partnership Program. Authors supported by National Research Service Award from the Health Resources and Services Administration, NSF grant, NIH grants, Merit Review Award from the US Department of Veterans Affairs. No declarations of interest | |
| Notes | New for 2019 update Abstinence data received from authors via email correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | “staff were blinded to randomisation until eligibility was confirmed but not beyond that point; participants were blinded until consent was provided”. |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow up n = 66; withdrawn n = 17 |
| Methods | Setting: community corrections offices, USA Recruitment: smokers under community corrections supervision were recruited via flyers posted at the community corrections offices | |
| Participants | 500 smokers; 33.0% female, average age 37.4; average number of cigarettes smoked per day 17.9 Therapists: counsellors were doctoral or masters level clinical psychologists who had been trained in smoking cessation counselling | |
| Interventions | Pharmacotherapy: NRT; 12 weeks’ supply of bupropion 1. one session of face‐to‐face brief advice 2. four weekly sessions of face‐to‐face brief advice and intensive counselling, each lasting 20 to 30 minutes | |
| Outcomes | Abstinence (carbon monoxide level ≤ 3 ppm) at all study visits (weeks 8, 12 and months 6, 9, 12) Validation: carbon monoxide level (≤ 3 ppm) measured using the Vitalograph Breath Carbone Monoxide monitor | |
| Source of Funding/CoI | The National Cancer Institute and the National Institute of Heatlh. No declarations of interest | |
| Notes | New for 2019 update Data on the number of abstinent participants received from the authors via email correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomisation scheme was blocked on race…”. No further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up rates at 12 months: no counselling arm 25.8%; counselling arm 23.4% |
| Methods | Settng: primary care patients, 50 rural practices, Kansas, USA | |
| Participants | 750 smokers of > 10 cpd, 59% F, av age 47, av cpd 24, 61% contemplation, 30% preparation | |
| Interventions | All participants mailed an offer of free pharmacotherapy every 6 months, 4 times in total. Nicotine patch 21 mg for 6 weeks or bupropion SR (150 mg twice daily) for 7 weeks 1. Control. No other contact 2. Moderate intensity disease management: up to 2 calls from counsellor in each cycle encouraging uptake of pharmacotherapy, newsletter mailings & periodic progress reports with counselling suggestions faxed to physician 3. High‐intensity disease management, up to 6 calls at approx 1, 3, 6, 9, 12 weeks from start of each cycle | |
| Outcomes | Abstinence at 24 months (PP). Study also reported analysis based on combination of effects at all follow‐up points. Sustained abstinence not a suitable outcome since no quit date and repeated intervention Validation: attempted saliva cotinine (< 15 ng/mL) by mail at 12 and 24 months. Proxy report used at 24 months for non‐returners. Rate of validation similar across groups | |
| Source of Funding/CoI | National Cancer Institute. Medication provided by GlaxoSmithKline, "The funding sources were not involved in the design, conduct or analysis of this study or the decision to submit the study for publication". No declarations of interest | |
| Notes | Participants could have multiple courses of pharmacotherapy; 23%, 33%, 23%, 12%, and 9% of participants requested 0, 1, 2, 3, or 4 courses, Disease management conditions increased use in first cycle and reduced it later. 41% of cycles used bupropion & 59% patch. Over 24 months, average number of calls 3.6 in 2. and 8.2 in 3. Fewer calls in later cycles No evidence of effect based on PP, but some evidence of benefit when all follow‐ups taken into account High intensity vs control in main comparison. Moderate intensity quit almost identical (35/238 14.7%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random numbers table was utilized to generate allocation cards in blocks of 24 with allocation equally distributed across treatment groups". |
| Allocation concealment (selection bias) | Low risk | Quote: "cards were placed in sequentially numbered, opaque, sealed envelopes". |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated and proxy report used for non‐returners; rate of non‐return similar across groups |
| Incomplete outcome data (attrition bias) | Low risk | Less than 20% lost to follow‐up, similar distribution amongst groups (11% control, 16% in both moderate‐ and high‐intensity intervention arms) |
| Methods | Setting: National quitline, Engalnd Recruitment: non‐pregnant smokers aged ≥ 16 years, residing in England who called the quitline and agreed to set a quit date | |
| Participants | 2591 smokers in total (1295 in the relevant arms); 52.3% female, average age 38; average number of cigarettes smoked per day: 497 in ≤ 10 cpd category; 1226 in 11 to 20 cpd category; 547 in 21 to 30 cpd category; 230 in ≥ 31 cpd category Therapists: trained advisors from two helpline centres | |
| Interventions | Pharmacotherapy: NRT; no cost vouchers for 21 days’ supply of 15 mg per 16 hour transdermal nicotine patches which were redeemed by a telephone call. A second 21 days’ supply could be redeemed in the same way three weeks after the initial batch. 1. usual care (support materials by email, letter or text message before, on and after quit date + proactive telephone contact + brief motivational messages) 2. 6 sessions of more intensive, proactive support by telephone | |
| Outcomes | Prolonged abstinence at months 1 and 6 Validation: a minority of participants (255 out of 2591 had face‐to‐face follow‐up for validation of abstinence by carbon monoxide (cut‐off of < 10 ppm)) | |
| Source of Funding/CoI | The English Department of Health, the UK Centre for Tobacco Control Studies. No declarations of interest | |
| Notes | New for 2019 update Previously excluded. Contact amount not known so excluded from analyses 1.2 and 1.3. Use of pharmacotherapy was low; only 42.9% of those offered NRT reported receiving any and of those only 51.3% used every day. There was also little difference between number of calls completed between proactive and standard telephone counselling conditions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up rate: NRT + usual care arm 44.7%; NRT + proactive support arm 45.5% |
| Methods | Setting: primary care patients, 16 clinics, USA | |
| Participants | 961 smokers of ≥ 10 cpd. (a further 908 were allowed to select treatment, not included in review. Demographic details based on 1869); 58% F, av age 40, av cpd 22 | |
| Interventions | Pharmacotherapy: NRT (patch, 22 mg, 8 weeks including tapering) 1. NRT alone | |
| Outcomes | Continuous abstinence at 1 year (no relapse lasting 7 days), also PP | |
| Source of Funding/CoI | National Cancer Institute. SmithKline Beecham provided nicotine patches and access to the CQ program, but did not participate in any aspect of study design or data analysis. | |
| Notes | 3 versus 1 used in primary analysis. 3 & 2 versus 1 was more conservative since 2 had lower quit rates than 1. Use of PP outcome did not alter findings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | People who did not pick up patches were excluded from analyses, similar distribution amongst groups (17% control, 16% in intervention arm 1, 14% intervention arm 2). No reported loss to follow‐up for remaining participants |
| Methods | Setting: academic research centre, USA | |
| Participants | 260 light smokers (6 ‐ 15 cpd), 57% F, av age ˜43, av cpd 11, approx 1/3 smoked < 10 cpd, approx 50% had history of smoking 20 cpd Therapists: cessation counsellors | |
| Interventions | 2 x 2 double‐blind double‐dummy. Participants randomised to either nicotine patch (21 mg/day or 14 mg/day (< 10 cpd) for 8 wks incl weaning) or bupropion (9 wks) 1. Pharmacotherapy & medication management, 4 x 5‐10 min visits over 6 wks 2. Pharmacotherapy & counselling, 10 weekly individual 10‐15 min sessions | |
| Outcomes | Abstinence at 1 yr, sustained with no relapse of over 7 days smoking (study primary outcome was PP abstinence) Validation: CO ≤ 9 ppm & cotinine (NicAlert) ≤ 200 ng/mL or cotinine < 50 ng/mL | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | NRT & bupropion conditions not reported separately by counselling condition, so 2 vs 1 entered in NRT or bupropion section. Favoured NRT but no significant difference at any follow‐up. More evidence of effect on sustained than PP rates at 1 yr, but substituting PP in MA did not affect findings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated "urn" randomisation by independent data analyst |
| Allocation concealment (selection bias) | Low risk | Randomisation after enrolment, not predictable |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 108 (84%) intervention and 108 (82%) control reached at 1 yr |
| Methods | Setting: academic research centre, USA Recruitment: community volunteers | |
| Participants | 303 smokers with at least 1 quit attempt in past 2 years 58.7% F, av age: 45.99, av cpd 24 Therapists: abuse therapist + clinical psychology doctoral students | |
| Interventions | Pharmacotherapy: bupropion for 10 weeks. 1. Control; 1 hr of "medication instruction group presenting the rationale for bupropion" 2. Bupropion plus functional analytic psychotherapy (FAP) and acceptance and commitment therapy (ACT), 20 sessions, 1 group & 1 individual session per wk for 10 wks | |
| Outcomes | Abstinence at 1 yr (7‐day PP). Continuous abstinence also reported but denominators not clear Validation: CO ≤ 10 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | Numbers quit calculated from percentages. Included in brief intervention subgroup 1.1.1, sensitivity analysis in dose‐response did not alter estimates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using generator www.randomizer.org |
| Allocation concealment (selection bias) | Low risk | Randomisation did not occur until after enrolment. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | High risk | 38% intervention & 67% control lost to follow‐up, including 10 intervention & 2 control dropouts before treatment |
| Methods | Setting: academic research centre, USA | |
| Participants | 99 smokers with an acquaintance willing to participate as a support partner; 54% F, av age 38, av cpd 26 | |
| Interventions | Pharmacotherapy: nicotine gum, 2 mg, duration not specified 1. Instruction for gum use & educational materials, 2 brief sessions over 2 weeks | |
| Outcomes | Abstinence at 52 weeks (not clear if abstinence required at prior assessment at wks 4, 12, 26) | |
| Source of Funding/CoI | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to 3‐6 member groups in order of entrance into treatment within time constraints. Treatment for each group was randomly selected ...". |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 9 participants lost to follow‐up counted as smokers. 1 participant who died excluded from analyses |
| Methods | Setting: clinic, USA | |
| Participants | 84 smokers in relevant arms; 53% M, av age 38, av cpd 30.5 | |
| Interventions | Pharmacotherapy: NRT; gum (2 mg, available for 6 months) 1. Intensive behavioural treatment (incl relapse prevention skill training, relaxation, 30 seconds aversive smoking of 3 cigs). 14 x 75 min sessions over 8 weeks 3. Intensive behavioural, no gum. Not included in this review | |
| Outcomes | Abstinence at 52 weeks (assume PP) | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned within time constraints." Author clarification: "There were two or more treatment conditions available within any time block, and participants were randomly assigned to conditions within that time block". |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 3 dropouts from group 2 and 3 from group 3. Assumed to be included in denominator for reported % abstinent used to derive numbers quit |
| Methods | Setting: clinic, USA | |
| Participants | 139 smokers; 53% M, av age 39, av cpd 30 (71 in relevant arms) | |
| Interventions | Pharmacotherapy: NRT (gum). Placebo arms of factorial trial not used in review | |
| Outcomes | Abstinence at 52 wks (assume PP) | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 2 dropouts in 1 & 2 in 2 included in ITT analyses. "Differences between conditions were not statistically significant." |
| Methods | Country: USA | |
| Participants | 149 smokers (> 10 cpd) | |
| Interventions | Pharmacotherapy: NRT (gum, 2 mg for up to 12 wks, tapering from wk 4) 2. Standard group therapy. 5 x 90 min sessions over 8 wks. Information and group support for planning and implementing individual strategies | |
| Outcomes | Continuous abstinence at 52 wks (confirmed quit at all prior assessments and no smoking in previous wk) | |
| Source of Funding/CoI | National Institute on Drug Abuse. Merrell Dow Pharmaceuticals Inc. provided drugs. No declarations of interest. | |
| Notes | Both behavioural interventions were relatively intensive. Positive effect reported for subgroup with history of major depression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts included as smokers, but numbers not specified |
| Methods | Setting: cessation clinic, USA | |
| Participants | 199 smokers of ≥ 10 cpd; 55% F, av age 40, av cpd 21‐25; 33% had history of MDD | |
| Interventions | Pharmacotherapy: nortriptyline (titrated to therapeutic levels ‐ usually 75‐100 mg/day for 12 wks). Placebo arms of factorial trial not used in review 2. Standard group therapy control. 5 x 90 min sessions over 8 wks (see Hall 1994 for description of each intervention) | |
| Outcomes | Abstinence at 64 wks (1 yr post‐treatment). Continuous abstinence rates not reported by psychological treatment group | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | Both behavioural interventions were relatively intensive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer, after stratification on history of MDD and number of cigs smoked |
| Allocation concealment (selection bias) | Low risk | Computer randomisation after data collection |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 16% lost to follow‐up at 1 yr, no difference by group, included in denominators for MA |
| Methods | Country: USA | |
| Participants | 220 smokers (146 in relevant arms); ≥ 10 cpd; 40%‐47% F, av age 37‐43, av cpd 20‐23; 33% had history of MDD | |
| Interventions | Pharmacotherapy: bupropion (300 mg for 12 wks) or nortriptyline (titrated to therapeutic levels, typically 75 or 100 mg/d). Factorial 3 x 2 design, placebo arms not used in this review | |
| Outcomes | PP abstinence at 1 yr (47 wks post‐quit date). Continuous abstinence not reported by subgroup | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | Bupropion PI vs MM & nortriptyline PI vs MM used in relevant subgroups. Trial also contributed to review of combined interventions Stead 2016, using different combination of arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not specified, "double blind" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 19% lost to follow‐up at 12 m, similar numbers across groups |
| Methods | Setting: cessation clinic, USA Recruitment: community volunteers | |
| Participants | 402 smokers (≥ 10 cpd) aged ≥ 50; 40% F, av age 57, av cpd 21 | |
| Interventions | Pharmacotherapy: NRT (gum, 10 weeks, 2 or 4 mg) & bupropion (12 weeks). 2 arms had extended access to gum 1. "Standard treatment"; 5 group sessions over 8 weeks, 'Clear Horizons' manual 2. Extended CBT; 11 individual 20 to 40 min sessions from week 10 to week 52, schedule front‐loaded. Incl motivation, mood management, weight control, social support, coping with withdrawal 3. Extended NRT. nicotine gum available until week 52, no additional behavioural support 4. Extended combined, CBT & NRT; 3 & 4 | |
| Outcomes | Abstinence at 104 weeks (one year after end of all treatment) (PP) Validation: CO ≤ 10 ppm and urine anatabine/anabasine ≤ 2 mg/mL | |
| Source of Funding/CoI | National Institute on Drug Abuse. No declarations of interest | |
| Notes | Meta‐analysis comparison was 2 & 4 vs 1 & 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised at end of initial treatment, computerised allocation list by statistician who had no contact with participants. Stratified on gender, history of MDD, current cigarette abstinence status |
| Allocation concealment (selection bias) | Low risk | "The assignment of individual participants by subject number was then transmitted electronically to clinical staff." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Less than 20% lost to follow‐up in each group, denominator excluded participants who died during the study but counted all others lost to follow‐up as smokers. |
| Methods | Setting: North Shore Medical Center in Salem, USA Recruitment: smokers admitted with a cardiac or pulmonary illness were electronically identified | |
| Participants | 122 smokers in total (81 in the relevant arms); 39.5% female, average age 54.4 to 55.3; average number of cigarettes smoked per day 20.5 to 21.2 Therapists: no details given | |
| Interventions | Pharmacotherapy: NRT; free one‐month supply of nicotine patches with the initial dose based on the number of cigarettes they smoked prior to hospitalisation. Also given nicotine gum or lozenges to administer as needed 1. one intensive in‐hospital counselling (30 minutes) + five telephone calls with additional counselling at 1, 2, 4, 8 and 12 weeks post‐discharge (15 minutes each) 2. one intensive in‐hospital counselling (30 minutes) + five telephone calls with additional counselling at 1, 2, 4, 8 and 12 weeks post‐discharge (15 minutes each) + one in‐person hypnotherapy session within 1 to 2 weeks of hospital discharge (90 minutes) | |
| Outcomes | 7‐day point prevalence abstinence at week 12 and at 6 months Validation: urinary cotinine levels (< 15 mg/mL). In case of no urine sample returned, abstinence was confirmed by contacting a household proxy. | |
| Source of Funding/CoI | The Norman H. Read CharitableTrust Foundation. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “permuted blocks of three (1:1:1)”. No further details given |
| Allocation concealment (selection bias) | Low risk | “Assignments sequentially numbered and schedule was maintained independent of the study by the project coordinator. Randomised assignments were concealed from both patients and research staff until patients had signed the informed consent document and were enrolled in the study”. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up rates: Counselling arm 29.3%; counselling + hypnotherapy arm 32.5% |
| Methods | Setting: community‐based telephone quitline programme, Oregon, USA | |
| Participants | 4614 smokers randomised to: brief counselling (872, no NRT; 868, with NRT); moderate counselling (718, no NRT; 715, with NRT); intensive counselling (720, no NRT; 721, with NRT) 40% M, av age 41, 90% white, av cpd 21 | |
| Interventions | Factorial design; arms that were offered free NRT (patches, initial 5‐wk supply, 3 more wks available) contributed to this review | |
| Outcomes | 30‐day PPA at 6 and 12 months | |
| Source of Funding/CoI | National Cancer Institute. GlaxoSmithKline supplied nicotine patches. Two authors employed by Free & Clear, Inc, a for‐profit company providing telephone counselling services | |
| Notes | 3 vs 1 in main comparison. Actual contact in 3; mean 2.9 sessions, 60.6‐min contact Also contributed to review of combined interventions Stead 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a computer algorithm randomly assigned participants". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and different amounts of contact between arms |
| Incomplete outcome data (attrition bias) | Low risk | Moderate level of attrition but balanced between groups, and participants lost to follow‐up counted as smokers (72% followed up in groups 1 and 2, 68% followed up in group 3) |
| Methods | Setting: academic research centre, Germany | |
| Participants | 225 smokers (102 in relevant arms); 55% F, av age 38, av cpd 28 | |
| Interventions | Pharmacotherapy: nicotine gum, 2 or 4 mg 1. 5 x 90‐min weekly meetings. Included contracting, reinforcement, relaxation, skills training 4. Wait‐list control for 6 m. Not included in this review | |
| Outcomes | PP abstinence at 12 m | |
| Source of Funding/CoI | Not specified. No declarations of interest | |
| Notes | Control and intervention fell into same categories for number and duration of sessions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 31 people attending 2 or fewer meetings not included in analysis. Said to be evenly distributed. Later dropouts included as smokers; 90% of those receiving therapy (excluded wait‐list group 4, who were also excluded from this review) followed up at 12 m. |
| Methods | Setting: HIV clinics, USA Health Care Recruitment: HIV clinic patients, volunteering for study | |
| Participants | 209 smokers 82% M, av age 45, av cpd 20 Therapists: clinicians specialising in smoking cessation/social work/psychology | |
| Interventions | Pharmacotherapy: NRT; patch or gum for 10 weeks, available to those who smoked ≥ 5 cpd, number not eligible, not specified 1. Self help: "How to Quit Smoking"; brief meeting with study staff who reviewed guide and recommended establishing a quit date 2. Individual counselling: 6 x 40 to 60‐min sessions of CBT targeted towards needs of HIV positive smokers, weeks 1, 2, 3, 4, 8 & 12 3. Computer‐based: each component structured into a “step” roughly corresponding to the first 5 sessions of the counselling intervention. Individuals were directed to complete self‐assessment exercises and homework assignments. | |
| Outcomes | Abstinence at 52 weeks (7‐day PP) Validation: CO ≤ 10 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse. California Tobacco‐Related Disease Research Program. Authors declared no conflicts of interest. | |
| Notes | Individual counselling compared to self‐help in main MA, added computer‐based arm in sensitivity analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Smokers stratified based on N cpd, gender, history of depression and then within each stratum randomised via computer algorithm to 1 of 3 conditions in 1:1:1 fashion into a parallel‐group design |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment & stratification. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 19% overall loss to follow‐up |
| Methods | Country: 2 academic research sites, USA | |
| Participants | 504 smokers (≥ 15 cpd); ˜53% F, av age 44, av cpd 26‐29 | |
| Interventions | Compared 22 mg vs 44 mg nicotine patch and 3 types of adjuvant treatment. Patch groups collapsed. All participants had 8 weekly assessments by research staff | |
| Outcomes | 7‐day PP abstinence at 26 wks | |
| Source of Funding/CoI | Elan Pharmaceutical Research Corporation. Authors declared potential conflicts of interest. | |
| Notes | No significant difference in dose‐related outcome and no dose‐counselling interaction at 26 weeks reported. Patch arms collapsed in analysis. 3 vs 1 used in primary comparison, RR 0.99 (95% CI 0.69 to 1.42). RRs for other comparisons: 2 & 3 vs 1 = 1.10 (95% CI 0.81 to 1.49), 2 vs 1 = 1.21 (95% CI 0.86 to 1.70), 3 vs 2 = 0.82 (95% CI 0.58 to 1.15) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, method not stated |
| Allocation concealment (selection bias) | Unclear risk | "In a double blind manner" for NRT, but not specified for counselling |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Losses not specified by group, relatively low rate lost to follow‐up overall (16.3%), Counted as smokers in report & MA |
| Methods | Setting: community, USA Recruitment: smokers recruited through advertisements on multiple media | |
| Participants | 77 smokers; 50% female, average age 47.4 to 44.5; average number of cigarettes smoked per day 18.7 to 18.8 Therapists: six female doctoral level counsellors with prior experience in behavioural health counselling | |
| Interventions | Pharmacotherapy: NRT; 8 weeks of nicotine patches beginning on their scheduled quit date, which coincided with the third session (2 weeks after the initiation of treatment). Dosage dependent on the number of cigarettes smoked per day 1. Usual care: six sessions (five weekly and a final session that occurred 2 weeks later); session 1 lasted 60 minutes and the later sessions 30 minutes; 30 minutes of the session 1 and 20 minutes of the subsequent sessions were dedicated to teaching progressive muscle relaxation. 2. Positive psychotherapy: same as the usual care in terms of the number and duration of the sessions but 30 minutes of the session 1 and 20 minutes of the subsequent sessions were dedicated to positive psychotherapy‐specific content. | |
| Outcomes | Continous abstinence at weeks 8, 16, 26 Validation: alveolar carbon monoxide (≤ 8 ppm) using a Bedfont Scientific Smokelyzer breath carbon monoxide monitor; saliva cotinine (≤15 ng/mL) radioimmune assay analysis | |
| Source of Funding/CoI | The National Cancer Institute. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | “data for the randomisation were sent by research assistant to the project coordinator who conducted the computer‐based urn randomisation and informed the treatment provider of treatment assignment”. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up rates: Usual care arm 31.6%; positive psychotherapy arm 25.6% |
| Methods | Setting: community cessation clinic, USA Recruitment: community volunteers | |
| Participants | 301 smokers (≥ 10 cpd or 3.5 packs/wk) (excluded 3 participants who received wrong treatment); 40% F, av age ˜46, av cpd ˜20 | |
| Interventions | Pharmacotherapy: bupropion (300 mg, 9 wks) & NRT (21 mg patch, 8 wks incl tapering) Common behavioural therapy: 6 x 30‐min individual CBT sessions at baseline, TQD, 1, 2, 4, 6 wks 1. Extended therapy: 4 x 30‐min sessions at 8, 12, 16, 20 wk, & weekly check‐in calls to automated system; report of relapse or craving prompted proactive calls. 2. Control: 5‐min general support calls at 8, 12, 16, 20 wks | |
| Outcomes | Abstinence at 52 wks (7‐day abstinence at both 20 & 52 wks) (continuous abstinence also reported but not used in MA as could underestimate any effect on recycling) Validation: CO < 10 ppm (11 self‐reported quitters no longer living in study area accepted as quitters without validation) | |
| Source of Funding/CoI | National Institute on Drug Abuse. Authors declared no conflicts of interest. | |
| Notes | Tested extended duration therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a permuted block method (block size = 4), stratified on gender |
| Allocation concealment (selection bias) | Low risk | Participants assigned to next available ID number in corresponding gender. Researchers & participants were blinded to extended treatment assignment to the end of the open‐label phase. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 89% followed up in standard‐care group, 90% followed up intervention group. |
| Methods | Setting: community centre or office, USA Recruitment: smokers recruited via advertisements in Korean newspapers. Selected for motivation to quit | |
| Participants | 30 smokers; 23.3% female, average age 46.5; average number of cigarettes smoked per day 19.0 Therapists: two Korean bilingual clinicians | |
| Interventions | Pharmacotherapy: NRT; 8 weeks’ supply of nicotine patches 1. Eight weekly sessions of face‐to‐face individualised counselling focusing on medication management, each lasting 10 minutes 2. Eight weekly sessions of face‐to‐face individualised and culturally tailored cognitive behavioural therapy, each lasting 40 minutes | |
| Outcomes | Continuous abstinence at weeks 1, 4 and months 3, 6 Validation: carbon monoxide (< 6 ppm) measured by a Micro+ Smokerlyzer Carbone Monoxide monitor; saliva cotinine (≤ 1 ng/mL) assessed by the NicAlert test | |
| Source of Funding/CoI | National Institute of Drug Abuse. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up rates: Control 25.0%; intervention 21.4% |
| Methods | Setting: USA (no further detail reported) Recruitment: from the community via newspaper and television advertisement | |
| Participants | 49 participants, 32.7% female, average age: 42.8 ± 11.2, average cigs/day: 18.2 ± 5.2 Therapists: five therapists; a licensed psychologist, a master's level clinician, an intern, and two bachelor's level therapists | |
| Interventions | Pharmacotherapy: 8 weeks of transdermal nicotine replacement therapy Intervention: behavioural couples treatment. Total contact time: 60 minutes each x 7 = 420 minutes Control: individual standard treatment. Total contact time: 60 minutes each x 7 = 420 minutes | |
| Outcomes | 7‐day point prevalence abstinence at 3 and 6 months Validation: CO ≤ 8 ppm, or urinary cotinine | |
| Source of Funding/CoI | Funding: National Institute on Durg Abuse and National Heart Lung and Blood Institute at the National Institutes of Health, and the Department of Veterans Affairs No declaration of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Similar drop‐out rates (BCT‐S: 23.0%; ST: 21.7%) |
| Methods | Setting: Health Maintenance Organization, USA | |
| Participants | 509 smokers of > 20 cpd, motivated to quit; 56% F, av age 42, av cpd 28 | |
| Interventions | All participants received prescriptions for free nicotine patch (Prostep), 22 mg for a maximum of 6 weeks plus 11 mg for 2 wks. All attended 90‐min group orientation session describing study, use of patch, behavioural information, set quit date. Standard written materials with patch included description of a toll‐free telephone help line. | |
| Outcomes | Abstinence at 12 months (from quit date) | |
| Source of Funding/CoI | Lederle Laboratories. No declarations of interest | |
| Notes | Also contributed to Cochrane review of telephone counselling (Matkin 2019) Effect of counselling compared to contact & quitline alone (1 & 2 combined since fewer than 1% called quitline and no difference between quit rates). Participants who did not return questionnaires at 2, 5, 8, 12 weeks were called by telephone. Cluster‐randomised trial: analysis reported stated that it was adjusted for clustering effects via a mixed model, but these results were not reported except that group comparisons did not "approach statistical significance". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation by orientation session attended; participants did not know condition in advance so risk of selection bias probably low |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 82% response rate at 12 m, no difference between groups, missing treated as smoking |
| Methods | Setting: substance abuse outpatient facility, USA | |
| Participants | 69 smokers; 61% F, av age 39, av cpd 25 | |
| Interventions | Pharmacotherapy: nicotine patch (24‐hr, 10‐wk tapered dose) | |
| Outcomes | Abstinence at 12 months, 1‐week PP | |
| Source of Funding/CoI | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size 10) |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Low rate of attrition (though breakdown by group not provided): 12 administrative dropouts/exclusions not included in analyses |
| Methods | Setting: 6 outpatient HIV clinics & 2 primary care clinics, USA | |
| Participants | 444 HIV+ smokers; 37% F, av age 42, av cpd 18 | |
| Interventions | Pharmacotherapy: nicotine patch for up to 8 weeks if willing to set quit date 1. 2 brief counselling sessions, biweekly patch collection without counselling contact 2. 4 x 30‐min sessions plus quit day call, using motivational interviewing approach | |
| Outcomes | 7‐day point prevalence abstinence at 12 months Validation: carbon monoxide < 10 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse. Authors declared no conflicts of interest. | |
| Notes | 72% used patch at some point during study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised, stratified by gender and motivation to quit |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 75% intervention, 71% control followed up at 6 m. ITT and available‐case analyses reported |
| Methods | Setting: community, Australia | |
| Participants | 854 smokers interested in quitting; 51% F, av age 42, av cpd 24 | |
| Interventions | All participants received a free 2‐wk supply of nicotine patch by mail, instructed to purchase further supply; 14 or 21 mg depending on body weight 1. No further intervention | |
| Outcomes | Abstinence at 6 m (90‐day continuous) | |
| Source of Funding/CoI | GlaxoSmithKline funded study and all authors were employed by GSK. "The conduct of the study was independently monitored and the data verified by Datapharm Australia. GlaxoSmithKline took part in discussions about study design, but had no direct role in the analysis or interpretation of the results or preparation of the report for publication." | |
| Notes | Also contributed to Cochrane review of telephone counselling (Matkin 2019). No face‐to‐face contact | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized" by shuffling folders each day after participants to be included were listed. Since there was no personal contact with participants, risk of bias judged to be low |
| Allocation concealment (selection bias) | Low risk | Potential for bias since allocation sequence not fixed in advance; however, baseline characteristics similar across groups so no evidence of selection bias |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and differential levels of support |
| Incomplete outcome data (attrition bias) | Low risk | No significant difference in loss to follow‐up, 17% in NRT only, 15% in NRT+ at 6 m |
| Methods | Setting: USA (no further detail reported) Recruitment: using radio, web‐based, and newspaper advertisements | |
| Participants | 68 participants, 48.6% female, average age: intervention: 45 ± 12.2, control: 42.6 ± 11.5, average cigs/day: intervention: 18.8 ± 7.1, control: 17.3 ± 8.1 Therapists: two therapists with clinical psychology doctoral degrees and three therapists who were clinical psychology doctoral students | |
| Interventions | Pharmacotherapy: NRT; nicotine patches from quit date with an initial dose of 21 mg for 4 weeks, followed by 2 weeks of 14 mg, and 2 weeks of 7 mg. Participants who smoked on average 10 to 12 cigarettes per day started with 14 mg for the first 6 weeks. Intervention: 8 weekly sessions of behavioural activation treatment. Total contact time: 60 minutes each x 8 = 480 minutes Control: 8 weekly sessions of standard treatment. Total contact time: 60 minutes each x 8 = 480 minutes | |
| Outcomes | Abstinence: continuous abstinence at 1, 4, 16 weeks, and 6 months Validation: carbon monoxide ≤ 10 ppm, cotinine ≤ 5 ng/mL | |
| Source of Funding/CoI | Funding: National Institute on Drug Abuse No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | High risk | High losses to follow‐up in both arms: control: 63.6%; intervention: 57.1% |
| Methods | Setting: USA, LGBT health centres Recruitment: from the community | |
| Participants | 345 participants, 20.3% to 22.8% female, average age: 38.6‐39.4, average cigs/day: 12.1 to 13.8 Therapists: a professional and a lay counsellor who identified as lesbian, gay, bisexual or transgender facilitated each group. | |
| Interventions | Pharmacotherapy: NRT; nicotine patches for 8 weeks (dose regimen dependent on the number of cigarettes) Intervention: 6 weekly culturally tailored smoking cessation therapy sessions commencing two weeks before the quit date Control: 6 weekly standard smoking cessation therapy sessions commencing two weeks before the quit date | |
| Outcomes | Abstinence: 7‐day point prevalence at 1, 3, 6, and 12 months Validation: carbon monoxide at 1 and 3‐month follow‐up | |
| Source of Funding/CoI | Funding: National Institute on Drug Abuse, National Ceneter for Advancing Translational Sciences, National Institutes of Health, and National Cancer Institute Declarations of interest: one of the authors consulted with the Respiratory Health Association and served on a Health Advisory Board for Pfizer Inc. | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study statistician conducts the permuted‐block randomization using a software program developed by programmers at UIC." |
| Allocation concealment (selection bias) | Low risk | "The study statistician place the results of the assignments in sealed, solid envelopes. All study participants are blinded and retain no knowledge of CTQ or CTQ‐CT group". |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Similar attrition between groups: intervention: 33.7%; control: 34.1% |
| Methods | Setting: clinic, USA | |
| Participants | 463 smokers; 50% F, av age 36 to 41 across arms, av cpd 22 | |
| Interventions | Factorial trial of bupropion or placebo pharmacotherapy and counselling versus support | |
| Outcomes | 7‐day PP abstinence at 12 months (prolonged abstinence reported but not verified so PP used in MA) | |
| Source of Funding/CoI | National Institute on Drug Abuse & National Cancer Institute. GlaxoSmithKline provided complimentary active and placebo medication used in this study. "GlaxoSmithKline was not involved in the design, data collection, analysis, or reporting of this study." Authors declared potential conflicts of interest. | |
| Notes | 1 vs 2 used as test of adjunct behavioural support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Staff who screened and enrolled participants were unaware of the experimental condition to be assigned. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 63% reached at 12 m, but attrition rates did not differ by condition at any point |
| Methods | Setting: University of Connecticut Health Center, USA Recruitment: smokers with self‐reported desire to stop smoking | |
| Participants | 203 smokers Therapists: no details given | |
| Interventions | Pharmacotherapy: NRT; varenicline 1 tablet 0.5 mg once a day for three days followed by 1 tablet 0.5 mg twice a day for four days and then 1 tablet 1 mg twice a day for 11 weeks 1. Brief smoking cessation counselling weekly for five weeks 2. Brief smoking cessation counselling weekly for five weeks + behavioural therapy for weeks 2 to 5; ≥ 9 sessions in total | |
| Outcomes | Abstinence at 12 months Validation: carbon monoxide and cotinine levels | |
| Source of Funding/CoI | Unpublished study | |
| Notes | New for 2019 update Contacted Professor White (Co‐principal investigator) by email who informed us that the results were comparable for the two groups with quit rates about 50% in each group at 6 months. The results have not yet been published so we were only able to report this study narratively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unpublished study |
| Allocation concealment (selection bias) | Unclear risk | Unpublished study |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Unclear risk | Unpublished study |
| Methods | Setting: HIV primary care clinics, USA Recruitment: providers told potentially eligible patients about the study and offered them study coordinator's contact details. Selected for motivation to quit | |
| Participants | 53 smokers; 15.1% female; average age 49.7‐51.2; average number of cigarettes smoked per day 14.4 Therapists: intervention was provided by doctoral level clinical psychology interns and postdoctoral fellows supervised by the first author. The control group sessions were conducted by the study coordinator or research associate. | |
| Interventions | Pharmacotherapy: transdermal nicotine replacement therapy provided on the quit day 1. Psychoeducation session before randomisation (60 minutes) + face‐to‐face hybrid treatment that targeted smoking cessation, anxiety and depression simultaneously (60 minutes x 9 sessions) 2. Psychoeducation session before randomisation (60 minutes) + post‐quit sessions in person (10 minutes x 4 sessions) | |
| Outcomes | 7‐day point prevalence abstinence at 1, 2, 4, 6 months Validation: carbon monoxide level ≤ 4 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse. Authors declared potential conflicts of interest. | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation in blocks of 4 conducted by the study coordinator |
| Allocation concealment (selection bias) | Low risk | "Before the study's start, a randomisation chart was created, corresponding to each study identification number. The chart was secured on a password‐protected document accessible only by the study coordinator and the principal investigator. Assignment to study condition was concealed from participants and study clinicians until the end of session 1". |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up rate: intervention group 50.0%; control group 22.2% |
| Methods | Setting: primary care clinics, USA | |
| Participants | 380 smokers in relevant arms (excluded deaths and some who did not receive intervention); of 1223 smokers in study; 57% F, av age 35, av cpd 23 | |
| Interventions | Pharmacotherapy: nicotine gum; offer of free gum 2 x 3 factorial design, physician intervention ± follow‐up | |
| Outcomes | Abstinence at 6 m (7‐day); (3 m sustained abstinence rates not given by condition) | |
| Source of Funding/CoI | ||
| Notes | Marginal to include since relatively low use of pharmacotherapy; in intervention condition; of those reached, 33% refused use and 18% tried for 2 days or less 12 m abstinence rates reported in Ockene 1994 but not given by follow‐up condition. Also contributed to review of combined interventions (Stead 2016) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocated prior to physician encounter |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and differential support between arms |
| Incomplete outcome data (attrition bias) | Low risk | 19% lost to follow‐up, higher in telephone follow‐up group. All included as smokers in analysis |
| Methods | Setting: homeless shelters, Minnesota, USA community Recruitment: homeless adults willing to use nicotine patch | |
| Participants | 430 smokers (≥ 1 cpd for last 7 days) 25.3% female, av age 44.4, av cpd 19.3 Therapists: trained counsellors | |
| Interventions | Pharmacotherapy: NRT; 21 mg patch for 8 weeks 1. Single session 10 to 15‐min brief advice 2. Motivational interviewing, 6 x 15 to 20‐min sessions, baseline, 1, 2, 4. 6 & 8 weeks Focus on encouraging cessation and NRT adherence | |
| Outcomes | Abstinence at 26 weeks (7‐day PP) Validation: CO < 5 ppm | |
| Source of Funding/CoI | National Heart Lung and Blood Institute. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At the baseline visit, pre‐assigned randomization numbers prepared by the study statistician determined which study arm the participant would be enrolled." |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Overall 25% lost to follow‐up, not significantly different across groups |
| Methods | Setting: Brazil | |
| Participants | 1199 smokers (included 254 non‐attenders); 63% F, av age 42, 46% smoked > 20 cpd | |
| Interventions | Factorial design with NRT (21 mg or 14 mg patch for 8 weeks including tapering) or no NRT and 5 levels of behavioural support collapsed into 3 for analysis. Arms without NRT did not contribute to this review. | |
| Outcomes | Abstinence at 12 months (7‐day PP) | |
| Source of Funding/CoI | ||
| Notes | 3 vs 1 in patch condition only in primary analysis. Also contributed to review of combined interventions (Stead 2016) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, stratified by age & sex, by independent specialist |
| Allocation concealment (selection bias) | Low risk | Trial administrators blind |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and differential levels of support between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow‐up not provided. Non‐participants and losses to follow‐up included as smokers |
| Methods | Setting: USA, YMCA and worksite fitness centres Recruitment: by provider referrals and flyers posted in the clinics, and radio and newspaper advertisements. Willing to quit | |
| Participants | 30 participants, 100% female, average age: control: 38.0 ± 11.0; intervention: 37.0 ± 10.0, average cigs/day: ≥ 10 Therapists: certified wellness coaches with a master's degree in clinical psychology or bachelor's degree in health education | |
| Interventions | Pharmacotherapy: 4‐week supply of nicotine patches at weeks 2 and 6 Intervention: exercise counselling delivered while the participant was engaged in exercise. The individual‐based counselling included social cognitive theory–based assessment and problem‐solving of exercise barriers, reinforcement (shaping) of exercise, and methods to enhance exercise self‐efficacy, using a motivational interviewing counselling style. Total contact time: 36 X 30‐ to 40‐minute sessions = 1080 minutes Control: health education. Individual‐based sessions, lectures, handouts, films, and discussions covered various women's health and lifestyle issues. Total contact time: 36 X 30‐ to 40‐minute sessions = 1080 minutes | |
| Outcomes | Abstinence: 7‐day point prevalence at 12 weeks and 6 months Validation: saliva cotinine (abstinent if < 10 ng/mL) | |
| Source of Funding/CoI | Funding: National Center for Advancing Translational Sciences of the National Institutes of Health No declarations of interest | |
| Notes | New for 2019 update A small number of participants attended all 36 sessions (n = 3 for intervention and n = 1 for control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | "[A]llocation to treatment conditions was unknown to the study staff or investigators prior to assignment and participants completed baseline assessments prior to being informed of their allocation to treatment condition. A study coordinator blinded to allocation group conducted all follow‐ups in‐person". However, no description of how allocation was concealed. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition in each group: 15.6% |
| Methods | Setting: Exercise and Health Psychology Laboratory, Canada Recruitment: from local businesses, hospitals, academic institutions and organisations and through advertisements placed in newspapers, radio stations and city buses in London, Ontario. Motivated to quit | |
| Participants | 409 participants, 100% female, average age: exercise plus smoking maintenance: 41.96 (± 12.70); exercise plus contact control 43.47 (± 14.02); smoking maintenance plus contact control: 43.45 (± 12.22); contact control: 40.36 (± 11.92) Average cigs/day: exercise plus smoking maintenance: 17.04 (± 6.79); exercise plus contact control 16.71 (± 6.96); smoking maintenance plus contact control: 16.88 (± 5.16); contact control: 16.41 (± 6.78) Therapists: trained facilitator | |
| Interventions | Pharmacotherapy: transdermal NRT after 4 weeks of exercising (10 week programme: 21 mg once daily for weeks 4 to 9, followed by 14 mg once daily for weeks 10 to 11 and 7 mg once daily during weeks 12 to 13) Exercise maintenance + smoking cessation maintenance ‐ 14‐week exercise‐aided smoking cessation programme (33 x 45‐minute sessions) ‐ Weeks 8 to 14: five 25‐minute weekly cognitive behavioural therapy group sessions, for long‐term exercise adherence ‐ Received a set of Brandon’s “Forever Free” booklets after first 14 weeks ‐ After week 14: seven 15‐minute telephone counselling sessions biweekly for the first months + monthly for the next two months + bimonthly for the last 8 months to maintain exercise behaviour ‐ Total contact: 64 sessions, 1985 minutes Exercise maintenance + contact control ‐ 14‐week exercise‐aided smoking cessation programme (33 x 45‐minute sessions) ‐ Weeks 8 to 14: five 25‐minute weekly cognitive behavioural therapy group sessions, for long‐term exercise adherence ‐ After week 14: seven 15‐minute telephone counselling sessions biweekly for the first months + monthly for the next two months + bimonthly for the last 8 months ‐ to maintain exercise behaviour ‐ Total contact: 64 sessions, 1985 minutes Smoking cessation maintenance + contact control ‐ 14‐week exercise programme (33 x 45‐minute sessions) and 10 weeks NRT (starting from week 4) ‐ Weeks 8 to 14: received messages reinforcing women’s health issues ‐ Received a set of Brandon’s 'Forever Free' booklets after first 14 weeks ‐ After week 14: seven 15‐minute telephone counselling sessions biweekly for the first months + monthly for the next two months + bimonthly for the last 8 months ‐ messages reinforcing the Forever Free booklets and/or women’s health issues (e.g. vitamin D intake, oral hygiene, sleep disorders) ‐ Total contact: 59 sessions, 1860 minutes Contact control ‐ 14‐week exercise programme (33 x 45‐minute sessions) and 10 weeks NRT (starting from week 4) ‐ Weeks 8 to 14: received messages reinforcing women’s health issues ‐ After week 14: seven 15‐minute telephone counselling sessions biweekly for the first months + monthly for the next two months + bimonthly for the last 8 months ‐ messages reinforcing the Forever Free booklets and/or women’s health issues (e.g. vitamin D intake, oral hygiene, sleep disorders) ‐ Total contact: 59 sessions, 1860 minutes | |
| Outcomes | Abstinence: continuous abstinence at 14, 26, and 56 weeks Validation: CO < 6 ppm considered abstinent | |
| Source of Funding/CoI | Funding: Canadian Cancer Society No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | The project manager for trial used numbered containers to implement the random allocation sequence, and the sequence was concealed until interventions were assigned. However, the method of concealment was not specified. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 54.8% of participants lost to follow‐up, but attrition similar between groups |
| Methods | Setting: community, Canada | |
| Participants | 396 smokers interested in quitting within 30 days, smoking ≥ 15 cpd; 48% F, av age 38, av cpd 23 to 24 | |
| Interventions | Pharmacotherapy: NRT; patch (15 mg x 8 wks, 10 mg x 2 weeks, 5 mg x 2 weeks) free 1. Physician advice (3 x 15‐min, 2 weeks before, 4 weeks, 12 weeks after quit date) | |
| Outcomes | Abstinence at 12 m (PP) | |
| Source of Funding/CoI | National Cancer Institute of Canada with funds from the Canadian Cancer Society Nicotine replacement therapy was provided at no cost by McNeil Consumer Products. "The University of Ottawa Heart Institute Research Corporation has a contract with Johnson & Johnson–Merck Consumer Pharmaceuticals to manage the 'Stop Smoking Now!' telephone counselling service offered to users of Nicotrol NRT. The authors received a grant from Johnson & Johnson–Merck to conduct a pilot study before the clinical trial; no payment was received from the company for the clinical trial or its analysis and write‐up." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using table of random numbers, stratified by sex and nicotine dependence |
| Allocation concealment (selection bias) | Unclear risk | Concealment unclear but physician blind to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 84% intervention, 86% control, followed up at 12 m |
| Methods | Setting: residential substance abuse treatment programme, USA Recruitment: research therapist assessed patients for eligibility | |
| Participants | 165 alcoholic smokers (≥ 10 cpd for 6 m), 60% M, av age 34, av cpd 21 Therapists: research therapists | |
| Interventions | Pharmacotherapy: NRT; patch preferred, mostly used for 2‐3 months 1. Brief advice ˜15 mins, assessed smoking rate and interest in quitting, ± 2 x 5 to 15‐min boosters at 7 & 30 days 2. Motivational interviewing, 45 min, ± 2 x 5 to 15‐min boosters at 7 & 30 days | |
| Outcomes | Abstinence at 12 months (7‐day PP) Validation: CO < 10 ppm | |
| Source of Funding/CoI | National Institute of Alcohol Abuse and Alcoholism, United States Department of Veterans Affairs. No declarations of interest | |
| Notes | Booster and no‐booster conditions combined in analyses. Only 51% used NRT during the first month, 34% during the subsequent 2 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Assignment in sealed envelope opened just before the first treatment session |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Overall 32% lost to follow‐up; MI 35%, (28/80 including 1 death), BA 29% (25/85 including 3 deaths) |
| Methods | Setting: smoking cessation clinic, Greece | |
| Participants | 205 smokers | |
| Interventions | Pharmacotherapy: bupropion 300 mg/day for 19 weeks 1. Control: 15 mins physician counselling 2. Nonspecific group therapy (NSGT), 1‐hour weekly for 1 month, then every 3 weeks until 19 weeks 3. Cognitive behavioral group therapy (CBGT), same schedule 4. CBGT without bupropion ‐ not used in review | |
| Outcomes | Abstinence at 12 months after end of treatment (continuous) Validation: CO ≤ 10 ppm | |
| Source of Funding/CoI | No source of funding reported. Authors declared no conflicts of interest. | |
| Notes | 2 & 3 vs 1 in primary analysis, same intensity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not stated, 3:1:1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 90% followed up at 12 months |
| Methods | Setting: USA, primary care clinics Recruitment: from primary care clinics, participants willing to quit | |
| Participants | 544 participants, 59% female, average age: 46.2 ± 12.8, average cigs/day: 18.6 ± 8.8 Therapists: bachelor's level study staff supervised a licensed clinical psychologist | |
| Interventions | Pharmacotherapy: 8 weeks OR 26 weeks of nicotine patch plus nicotine gum (factorial design) Intervention: 4 sessions of face‐to‐face counselling plus 8 sessions of telephone counselling Control: 4 sessions of face‐to‐face counselling | |
| Outcomes | Abstinence: 7‐day point prevalence abstinence at 6 and 12 months Validation: none | |
| Source of Funding/CoI | National Cancer Institute, Wisconsin Partnership Program, and Department of Veterans Affairs. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Staff could not view the allocation sequence. The database did not reveal participants' treatment condition to staff until participants' eligibility was confirmed. Participants did not know treatment allocation until they provided consent. |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "The percentage of participants missing abstinence outcome data was 20.4% at Week 26 and 30.0% at Week 52, with no differences observed in missingness across the two levels (on vs. off) of any of the factors." No further details provided |
| Methods | Setting: outpatient treatment research clinic, Department of Psychiatry and Behavioral Sciences Substance Abuse Research Center, USA Recruitment: by local radio, television and print adverts. Motivated to quit | |
| Participants | 154 participants (78 in 2 groups receiving pharmacotherapy), 100% female, average age: 47.8 ± 9.3, average cigs/day: 21.4 ± 9.1 Therapists: a therapist and co‐therapist pair; four female, master's level therapists were trained on each therapy manual and supervised weekly by a doctoral‐level clinical psychologist | |
| Interventions | Pharmacotherapy: 6 weeks of sustained‐release bupropion (300 mg/day; 150 mg/day for 3 days, followed by 150 mg twice daily) Intervention: 7 x 60‐minute sessions of cognitive behavioural therapy (CBT) Control: 7 x 60‐minute sessions of standard therapy (ST) | |
| Outcomes | Abstinence: 7‐day point prevalence at 3, 6, 9, and 12 months Validation: CO (abstinent if ≤ 10 ppm) and salivary cotinine (abstinent if ≤ 15 ng/mL) | |
| Source of Funding/CoI | Funded by National Institute on Drug Abuse. GlaxoSmithKline provided the bupropion. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators and research staff were blind to the randomization codes, which were kept by a faculty member independent of the research and treatment team." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was high, but similar between study arms. Control: 56.8%; intervention: 53.7% |
| Methods | Setting: hospital for military veterans, USA | |
| Participants | 223 smokers, ≥ 20 cigs in week before admission, contemplation or action stage of change, able to use NRT, av age 55, av cpd 23 | |
| Interventions | Pharmacotherapy: NRT; patches (tailored dose) in hospital and for 8 weeks post‐discharge 1. Intervention: nurse or health educator counselling; 30 to 60 mins initial session. 5 calls at 1, 3 weeks, 1 month, 2 months, 3 months, < 30 min/call & S‐H materials | |
| Outcomes | Abstinence: 7‐day PP at 12 months | |
| Source of Funding/CoI | California Tobacco‐Related Disease Research Program. No declarations of interest | |
| Notes | Relative effect similar if spousal corroboration allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer algorithm |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up (3 intervention, 4 control) included as smokers. Deaths (5 intervention, 9 control) excluded from denominator |
| Methods | Setting: clinic, USA | |
| Participants | 677 smokers (> 10/day) attempted to quit for 1 week; 57% F, av age 42; av cpd 25 | |
| Interventions | Pharmacotherapy: NRT, patches for 8 wks. All participants had attended 3 brief (5 to 10‐min) individual counselling sessions pre‐quit, quit day and 8 days post‐TQD & NCI booklet 'Clearing The Air'. | |
| Outcomes | Abstinence at 12 months (7‐day PP) | |
| Source of Funding/CoI | National Institute on Drug Abuse. Lederle Laboratories supplied the nicotine patches. No declarations of interest | |
| Notes | Marginal to include as the counselling was intended for relapse prevention. 1 vs 3 in primary analysis. Including 2 did not alter findings; 17.6% quit in 1, 18.8% in 2. No evidence found for hypothesised differences in relative efficacy for smokers at high or low risk of relapse. High‐risk smokers expected to do better with motivational intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 1 wk after TQD, stratified by ± any smoking post‐TQD. Method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow‐up not reported, all missing included as smokers |
| Methods | Setting: quitline, USA Recruitment: adult smokers willing to quit who called the Wisconsin Tobacco Quit Line | |
| Participants | 987 participants, 57.6% female, average age: 41.9 ± 13, average cigs/day: 20.7 ± 9.6 Therapists: trained cessation counsellors | |
| Interventions | Pharmacotherapy: 2 or 6 weeks of NRT (nicotine patch only vs patch plus nicotine gum) (factorial design) Intervention: 4 telephone counselling sessions including medication adherence counselling Control: 4 telephone counselling sessions | |
| Outcomes | Abstinence: 7‐day point prevalence at 2, 6, and 12 weeks, and at 6 months Validation: none | |
| Source of Funding/CoI | National Cancer Institute. Authors declared potential conflicts of interest. | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation computer‐randomised |
| Allocation concealment (selection bias) | Unclear risk | No detail reported |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐report only but similar amounts of contact between groups |
| Incomplete outcome data (attrition bias) | Low risk | Attrition low and similar between groups. Intervention: 24.9%, control: 21.6% |
| Methods | Setting: Menominee Tribal Clinic (primary care centre), USA Recruitment: all participants were receiving health care at the Menominee Tribal Clinic and were motivated to quit smoking. | |
| Participants | 103 participants, 62.1% female, average age: 39.8 (SD 13.1), average cigs/day: 14.4 (SD 7.9) Therapists: a study coordinator who was an enrolled member of the Menominee Tribe and trained as a counsellor | |
| Interventions | Pharmacotherapy: 12 weeks of varenicline Intervention: 5 x face‐to‐face culturally tailored counselling sessions, duration not reported Control: 5 x face‐to‐face standard counselling sessions, duration not reported | |
| Outcomes | Abstinence: 7‐day point prevalence abstinence at weeks 1, 3, and 7, and at 3 and 6 months Validation: CO < 10 ppm | |
| Source of Funding/CoI | Wisconsin Partnership Program, the Spirit of Eagles Community Network Program, the University of Wisconsin Carbone Cancer Center, and the University of Wisconsin Center for Tobacco Research and Intervention. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | High risk | Attrition rate high and differed between study arms: intervention: 47.2%; control: 66.0% |
| Methods | Setting: community, USA | |
| Participants | 214 female smokers, > 4 cpd, intending to quit in next 2 weeks; av age 33, av cpd 24 | |
| Interventions | Pharmacotherapy: NRT; free nicotine patch (dose based on smoking level) for up to 10 weeks, after 1 m contingent on abstinence 1. Access to Nicoderm support line | |
| Outcomes | Abstinence at 6 months (multiple PP; 7 days at 3 months & 6 months) | |
| Source of Funding/CoI | Vermont Department of Health (part). SmithKline Beecham provided nicotine patches. No declarations of interest | |
| Notes | Intervention participants received on average 7 calls of 9 mins. Classified in 4 to 8 subgroup analysis. 95% received at least 1 call. Participants could call Nicoderm support line, 21% of control vs 8% of intervention did so. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Approximately 73% followed up in each group |
| Methods | Setting: community, USA | |
| Participants | 330 female smokers > 4 cpd, intending to quit in next 2 weeks; av age 34, av cpd 24 | |
| Interventions | Pharmacotherapy: NRT; free nicotine patch (dose based on smoking level) for up to 10 weeks, 2nd & 3rd prescriptions dependent on reporting abstinence 1. No additional support | |
| Outcomes | Abstinence at 6 m (30 days at 3 months & 6 months) | |
| Source of Funding/CoI | Vermont Department of Health. SmithKline Beecham provided nicotine patches. No declarations of interest | |
| Notes | Similar to Solomon 2000 with more extended telephone contact | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and differential amounts of contact between groups |
| Incomplete outcome data (attrition bias) | Low risk | 87% response in both conditions at 6 m |
| Methods | Setting: immunology clinics, USA Recruitment: adult smokers who have been diagnosed with HIV and identified themselves as Latino/Hispanic. Not selected for motivation to quit | |
| Participants | 302 participants, 36% female, average age: 45, average cigs/day: not reported but stated 50% of the participants were heavy (> 10 cigarettes per day) smokers Therapists: 10 health educators who were at least Masters level professionals or had equivalent years of clinical research experience | |
| Interventions | Pharmacotherapy: 8 weeks of nicotine patches Intervention: self‐help and culturally sensitive print materials and videos, tailored behavioural counselling, two in‐person sessions, two additional in‐person sessions focused on tailored relapse prevention, one phone call on the quit date, two 10‐minute booster phone calls, option to bring a social support buddy to attend all sessions Control: self‐help print materials, two in‐person sessions, one phone call on the quit date | |
| Outcomes | Abstinence: 7‐day point prevalence at 3, 6 and 12 months Validation: exhaled carbon monoxide level < 10 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse, National Cancer Institute, National Institute of Allergy and Infectious Diseases, Lifespan/Tufts/Brown Center for AIDS Research, Clinical Core of the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health. Authors declared no conflicts of interest. | |
| Notes | New for 2019 update Not included in analysis 1.3 because durations of sessions were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Similar follow‐up rates (control 67%; intervention 60%) |
| Methods | Setting: 5 methadone maintenance treatment programme centres, USA | |
| Participants | 383 methadone‐maintained adult smokers. 53% M, av age 40, av cpd 27 | |
| Interventions | Pharmacotherapy: NRT; all participants willing to make quit attempt offered patches (8 to 12 weeks, dose and duration tailored to smoking rate) | |
| Outcomes | Abstinence at 6 months (PP) | |
| Source of Funding/CoI | National Cancer Institute. GlaxoSmithKline provided nicotine patches. No declarations of interest | |
| Notes | Included since most participants in both conditions did make quit attempts and received NRT; 81% intervention and 80% control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, methods not stated |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Approx 82% followed up in both groups at 6 months |
| Methods | Setting: USA Recruitment: via newspaper, radio, and television advertisements | |
| Participants | 524 participants, 47.5% female, average age: 44.27 ± 10.38, average cigs/day: 24.6 ± 10 Therapists: doctoral level therapist | |
| Interventions | Pharmacotherapy: 12 weeks of bupropion, initiated during the second week of treatment, 2 weeks prior to quit day Intervention: 12 x 120‐minute sessions of standard cessation group counselling with CBT for depression Control: 12 x 120‐minute sessions of standard cessation group counselling | |
| Outcomes | Abstinence: 7‐day point prevalence abstinence at 2, 6, and 12 months Validation: CO (abstinent if ≤ 10 ppm) and salivary cotinine (abstinent if ≤ 15 ng/mL) | |
| Source of Funding/CoI | Funding: National Institutes of Health Declarations of interest: one of the authors served on the Pfizer Speakers Bureau and a Pfizer Scientific Advisory Board. | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Unclear risk | Study reported "8 smokers did not provide any follow‐up data". However, this was only for the 12‐week follow‐up. |
| Methods | Setting: HMO, USA | |
| Participants | 1524 smokers ≥ 10 cpd; 57% F, av age 45, av cpd 23, 44% history of depression | |
| Interventions | Pharmacotherapy: randomised to bupropion 300 mg/day or 150 mg/day | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Source of Funding/CoI | National Cancer Institute. "The authors have no relevant financial interest in this article, and received no financial support or medication from GlaxoSmithKline". | |
| Notes | Prescription was mailed. No face‐to‐face contact during enrolment or prescription. Estimated as 31 to 90 minutes contact. No dose/behavioural treatment interaction at 12 m, bupropion arms collapsed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Open‐label randomized trial...The computer code for the procedure calculated probabilities of group assignment that were dynamically modified based on the number of members in each group so that final group sizes were equal. No restrictions such as stratification or blocking were used as part of the randomization process." |
| Allocation concealment (selection bias) | Low risk | Procedure built into study database |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and differential levels of support |
| Incomplete outcome data (attrition bias) | Low risk | 83% intervention, 88% control followed up at 12m |
| Methods | Setting: HMO (Group Health), Seattle, WA, USA Recruitment: Group Health members contacted by phone & mail from Free & Clear | |
| Participants | 1202 smokers (≥ 10 cpd); 67% F, av age 47, av cpd 22 | |
| Interventions | Pharmacotherapy: varenicline for 12 weeks (1 mg x 2/day, titrated 1st week). All received 5 to 10‐min orientation call, printed Quit Guides and access to a free support line for ad hoc calls. | |
| Outcomes | Abstinence at 6 m (PP) | |
| Source of Funding/CoI | National Cancer Institute. "Varenicline and nominal support for recruiting participants was provided by Pfızer, Inc. Neither entity [NCI or Pfizer] had any role in the study design; the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication." Authors declared potential conflicts of interest. | |
| Notes | 3 vs 1 in main analysis, 2 & 3 vs 1 had little effect on result. 60‐min contact on average for 3 64% were no longer taking varenicline at 3 months, but no between‐group differences in non‐compliance or reasons for stopping | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was randomly allocated using an automated algorithm built into the study database". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and differential levels of support |
| Incomplete outcome data (attrition bias) | Low risk | Participants lost to follow‐up counted as smokers in ITT analysis; equal losses between groups (103 web, 107 phone, 100 web + phone) |
| Methods | Setting: 7 chest clinics, Denmark | |
| Participants | 370 smokers of > 1 cpd with COPD (185 in relevant arms); 52% F, av age 61, av cpd 20 Therapists: 20 nurses with cessation experience, trained to support medication use and provide standardised counselling | |
| Interventions | Pharmacotherapy: NRT; sublingual. Factorial trial included placebo tablets; only active treatment groups used in this review. | |
| Outcomes | Sustained abstinence at 12 m (validated at all visits from wk 2, PP also reported) | |
| Source of Funding/CoI | "The Danish Medical Research Council provided the major grant for this study ($375,000). Pfizer Consumer Healthcare, Sweden, supplied the study drugs used in the trial and provided grant support ($25,000)." First author declared potential conflicts of interest. | |
| Notes | Also contributed to review of combined therapy review (Stead 2016), using placebo low‐support arm as control. Therapists were not full‐time specialist counsellors. Using PP outcome did not alter effect. Only contacts before 12 wks counted for classification of intensity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation list at each centre |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not described |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Unclear risk | 42/185 (23%) of active NRT participants not followed up at 12 m and counted as smokers. Not reported by support condition. Of those who were followed up at 12 m, 52% had withdrawn from study treatment. Authors stated: "One potential bias may have been the large early dropout of failures from the study. Consequently, these patients were not exposed to the possible effect of more intensive support." |
| Methods | Setting: Netherlands, primary care Recruitment: by practice assistants, GPs, and practice nurses and via a leaflet displayed in the waiting room | |
| Participants | 311 participants, 52.9% female, average age: 48 ± 13.2, average cigs/day: 19 ± 8.1 Therapists: practice nurse or general practitioner | |
| Interventions | Pharmacotherapy: 12‐week course of varenicline Intervention: intensive counselling with practice nurse. 3 face‐to‐face plus 7 telephone sessions Control: brief advice with GP | |
| Outcomes | Abstinence: prolonged abstinence (maximum of five cigarettes after a grace period of 9 weeks) at weeks 9 and 26, and at 12 months Validation: CO < 10 ppm | |
| Source of Funding/CoI | Eindhoven Corporation of Primary Health Care Centres, Pfizer, and Research School CPHRI. Authors declared potential conflicts of interest. | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation randomised by computer |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer disclosed the allocation once during a phone call by a member of the research team with the assistants of the health‐care centre, who then contacted the patient to schedule an appointment with the GP or PN." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Dropout rates similar. Intervention: 18.6%, control: 25.8% |
| Methods | Setting: quitline, USA Recruitment: by mail to rural veteran daily cigarette smokers aged ≥ 18 years. Selected for motivation to quit | |
| Participants | 63 participants Therapists: doctoral‐level social worker with expertise in substance abuse and a masters‐level counsellor | |
| Interventions | Pharmacotherapy: free of charge 12‐week supply of pharmacotherapy mailed to the participants. Medication options included several forms of nicotine replacement therapy (patch, gum, lozenge), bupropion and varenicline. Combinatoin therapy was also available, as appropriate. 1. Quitline referral 2. Tailored tobacco intervention: 6 weekly sessions over phone each lasting 20 to 30 minutes | |
| Outcomes | 7‐day point prevalence abstinence at 12 weeks and 6 months after quit‐date Validation: none | |
| Source of Funding/CoI | Department of Veterans Affairs Office of Rural Health. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details provided |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report only and differential levels of support |
| Incomplete outcome data (attrition bias) | Low risk | Tailored tobacco intervention 25.8%; quitline referral 12.5% |
| Methods | Setting: USA Recruitment: from the Houston metropolitan area via local print media. Motivated to quit | |
| Participants | 412 participants, 54.9% female, average age: 48.7 ± 11.9, average cigs/day: 19.9 ± 10.1 Therapists: two master's level therapists | |
| Interventions | Pharmacotherapy: 6 weeks of NRT patches Mindfulness‐based addiction treatment (MBAT): 8 x 120‐minute in‐person group counselling sessions Cognitive behavioural treatment (CBT): 8 x 120‐minute in‐person group counselling sessions Control: 4 x 5‐ to 10‐minute individual counselling sessions | |
| Outcomes | Abstinence: 7‐day point prevalence abstinence at 4 weeks and 6 months Validation: CO (abstinent if < 6 ppm) and salivary cotinine (abstinent if < 20 ng/mL) | |
| Source of Funding/CoI | National Institute on Drug Abuse, Centers for Disease Control and Prevention, National Cancer Institute, National Center for Complementary and Integrative Health, and the Oklahoma Tobacco Settlement Endowment Trust. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Dropout rates similar between groups. Control: 35.9%; MBAT: 33.1%; CBT: 34.8% |
| Methods | Setting: USA, community‐based primary health care clinic Recruitment: word of mouth and flyers | |
| Participants | 400 participants, 58.7% female, average age: 45 ± 10.5, average cigs/day: ≥ 3 Therapists: individual sessions by a nurse practitioner or a physician. Group sessions by a social worker and a nurse practitioner | |
| Interventions | Pharmacotherapy: NRT (unclear about duration or type) Group counselling: could attend up to 12 sessions but frequency and scheduling determined by clinician according to the standard of care at the healthcare facility. Individual counselling: could attend up to 12 sessions but frequency and scheduling determined by clinician according to the standard of care at the healthcare facility | |
| Outcomes | Abstinence: planned follow‐up at 1, 2, 3, 4, 5, 6, and 9 months Validation: carbon monoxide | |
| Source of Funding/CoI | National Institute on Minority Health and Health Disparities, National Institute on Drug Abuse, and Pfizer Inc. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | High risk | Quote: "In addition, because of the very low follow‐up rates that could be achieved with this population, in spite of intensive efforts, the data was censored at the end of the 12th week, i.e., at the end of the intervention." |
| Methods | Setting: Mayo Clinic Hospitals, USA Recruitment: recruited from Mayo Clinic Hospitals | |
| Participants | 600 participants Sex: control: 49% female; intervention: 48% female, average age: control: 46.0 (± 14.7); intervention: 46.7 (± 14.9), average cigs/day: control: 14.2 (± 9.6); intervention: 14.6 (± 9.0) Therapists: study personnel | |
| Interventions | Pharmacotherapy: NRT while hospitalised and a free 2‐week supply of NRT at discharge, with instructions to purchase over‐the‐counter patches if desired Intervention: brief quitline facilitation session designed to overcome cognitive barriers to quitline utilisation. Also given a written brochure and a wallet‐sized 'quit‐card'. If amenable, directly referred to a quitline provider (1 x 5‐minute session) Control: brief advice (1 x 5‐minute session) | |
| Outcomes | Abstinence: 7‐day point prevalence abstinence at 7 days, 1 month, and 6 months Validation: urine continine < 2 ng/mL | |
| Source of Funding/CoI | ClearWay Minnesota. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized using dynamic randomization allocation based on the Mayo Clinic Study Data Management System, a proprietary web application for data entry and management." |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Similar attrition rates. Intervention: 30.3%; control: 26.0% |
| Methods | Setting: USA, university‐based research clinic Recruitment: through advertisements on public transportation, community‐based organisations, street outreach, and word‐of‐mouth. Inclusion criteria included motivation to quit | |
| Participants | 342 participants, intervention: 39% female; control: 48% female; average age: intervention: 49.48 (± 9.44); control: 49.52 (± 8.73); average cigs/day: intervention:18.20 (± 11.53); control: 17.88 (± 10.03) Therapists: doctoral and masters or bachelors level co‐therapy pairs and supervision by the principal investigator or a co‐investigator | |
| Interventions | Pharmacotherapy: 8 weeks of nicotine patches, including 4 weeks at 21 mg, 2 weeks at 14 mg, and 2 weeks at 7 mg (doses adjusted for smoking history) Intervention: NRT plus culturally‐specific CBT (9 x 90‐ to 120‐minute sessions) Control: NRT plus standard CBT (9 x 90‐ to 120‐minute sessions) | |
| Outcomes | Abstinence: 7‐day point prevalence abstinence at 3 and 6 months Validation: saliva cotinine < 7 ng/mL, exhaled CO < 8 ppm | |
| Source of Funding/CoI | National Cancer Institute of the National Institutes of Health. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Similar attrition rates: culturally‐specific CBT: 15.5%; standard CBT: 19.5% |
| Methods | Setting: USA, community Recruitment: recruited from Ohio Appalachian counties. Inclusion criteria included willingness to participate in study protocol. | |
| Participants | 707 participants Female: Community Health Worker Face‐to‐Face (CHWF2F): 65.7%; Community Health Worker Quitline (CHWQL): 69.8% Age: • CHWF2F: 18 to 24: 4.5%; 25 to 54: 62.9%; ≥ 55: 32.6% • CHWQL: 18 to 24: 5.4%; 25 to 54: 65.8%; ≥ 55: 28.8% Average cigs/day: CHWF2F: 22.3 (SD 11.7); CHWQL: 20.9 (SD 9.2) Therapists: • CHWF2F: community health worker and a registered nurse employed in the county public health department clinic • CHWQL: community health worker and quitline services provided by trained counsellors from National Jewish Health | |
| Interventions | Pharmacotherapy: • CHWF2F: a new 21 mg nicotine patch at the start of each visit, beginning on quit‐day and lasting for 8 weeks • CHWQL: up to two mailings of a 4‐week supply of free 21 mg nicotine patches. To receive the second 4‐week supply of free NRT, each participant was required to have completed at least two proactive counselling calls 1. CHWF2F: 7 face‐to‐face 30‐minute sessions with a community health worker 2. CHWQL: 1 face‐to‐face 30‐minute session with a community health worker, followed by up to five proactive telephone counselling sessions, and unlimited reactive calls from the participant, with a quitline | |
| Outcomes | Abstinence: prolonged abstinence at 3, 6, and 12 months, after a 2‐week post‐quit date grace period Validation: saliva cotinine level < 15 ng/mL, expired air CO level < 8 ppm | |
| Source of Funding/CoI | National Institutes of Health. No declarations of interest | |
| Notes | New for 2019 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Similar attrition in study groups. CHWF2F: 14.4%; CHWQL: 14.7% |
| Methods | Setting: cardiovascular outpatient department, Netherlands | |
| Participants | 385 smokers (8 deaths excluded from outcomes). 37% F, av age 59, av cpd 21 | |
| Interventions | Pharmacotherapy: NRT; patch (8 wks, dose based on smoking rate) for smokers making a quit attempt. In both groups, participants planning to quit received 8 wks nicotine patch with instruction from nurse | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Source of Funding/CoI | Netherlands Heart Foundation. Novartis Consumer Health provided nicotine patches 'for prime cost'. | |
| Notes | Participants were referred to nurse practitioner for counselling; not part of usual care. Unclear how many participants used NRT; in a subgroup who responded to a questionnaire (Wiggers Int J Behav Med 2006), 16% did not start patch therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerized balanced randomization programme taking prognostic factors (e.g. clinic attendance, age and gender) into account." |
| Allocation concealment (selection bias) | Low risk | "While patients completed their baseline questionnaire (and signed a written informed consent) nurses randomly assigned ...". Judged low risk as participant data had to be entered |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 89% intervention and 85% control followed up at 12 m. 8 deaths excluded from final denominators |
| Methods | Setting: mental health outpatient clinics, USA Recruitment: patients with schizophrenia or schizoaffective disorder, willing to use NRT | |
| Participants | 100 smokers (> 10 cpd) using an atypical antipsychotic; 16% F, av age ˜46, av cpd 23 Therapists: trained mental health clinicians provided both conditions. | |
| Interventions | Pharmacotherapy: nicotine patch (21 mg for 16 wks incl tapering) 1. Treatment of Addiction to Nicotine in Schizophrenia (TANS); 24 x 45‐min individual counselling sessions over 26 wks 2. Medical Management (MM); 9 x 20‐min over 26 wks | |
| Outcomes | Continuous abstinence at 12 m Validation: CO < 10 ppm | |
| Source of Funding/CoI | National Institute on Drug Abuse. Authors also reported support from Pfizer but unclear how it related to this study; "The authors are also supported in part by grants from the National Institute of Mental Health (JMW); National Institute on Drug Abuse (to MLS); Pfizer, Inc.; and the New Jersey Department of Health and Senior Services, Office of the State Epidemiologist, through funds from New Jersey Comprehensive Tobacco Control Program (JMW, MLS)." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "adaptive urn randomization procedure that accounts for motivation, gender, ethnicity, and heavy versus light smoking status" |
| Allocation concealment (selection bias) | Low risk | Judged that process for randomisation prevented prior knowledge of condition |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 75% followed up at 12 m, authors reported "not different between groups" |
| Methods | Setting: research unit for Asian health, NYC, USA | |
| Participants | Chinese smokers (any smoking in previous wk); 12% F, av age 44, av cpd NS, 25% smoked < 10 cpd, 49% had never attempted to quit | |
| Interventions | Pharmacotherapy: NRT. Patch for 8 wks (could start at any time in 6 m period) 1. Culturally‐tailored counselling in Chinese, 4 x 60‐min & S‐H 2. Health education in Chinese: 4 x 60‐min, including general health, nutrition, exercise & tobacco | |
| Outcomes | Abstinence at 6 m (PP) Validation: CO < 6 ppm | |
| Source of Funding/CoI | National Cancer Institute Community Network Program. Authors declared no conflicts of interest. | |
| Notes | Conditions had same contact time, but control did not focus on smoking. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not stated |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 10% intervention and 14% control lost to follow‐up at 6 m and counted as smokers in ITT analysis |
| Methods | Setting: general practice smoking cessation clinic, Turkey Recruitment: smokers motivated to quit within 6 months | |
| Participants | 350 smokers 50% M, av age 36.22, cpd not reported Therapists: smoking cessation clinic specialists | |
| Interventions | Pharmacotherapy: NRT (gum or patch), bupropion, or varenicline for 3 m or as long as necessary 1. Control; 8 visits & 1 call; baseline, day 8, 20, 23, 30, 45, 60, 120, 210, ˜150 mins 2. Same as control plus CBT‐oriented anger management and stress control programme, 5 x 90‐min sessions, in 1st month, ˜730 mins total | |
| Outcomes | Abstinence at 180 days, continuous abstinence (from quit‐day) Validation: CO ≤ 10 ppm | |
| Source of Funding/CoI | No funding. Authors declared no conflicts of interest. | |
| Notes | Pharmacotherapy was only used if the participant wanted to. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternated allocation, based on order that they were added to the participant list |
| Allocation concealment (selection bias) | High risk | Not specified whether this randomisation order was known to those enrolling |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | 6.3% lost to follow‐up |
ACS: American Cancer Society
ACT: acceptance and commitment therapy
av.: average
BCT‐S: behavioural couples treatment
CBGT: cogntive behavioural group therapy
CBT: cognitive behavioural therapy
CHWF2F: community health worker face‐to‐face
CHWQL: community health worker quitline
cigs: cigarettes
C‐MIS: "Minimal intervention strategy for cardiology patients"
CO: carbon monoxide
cpd: cigarettes per day
CQ: Committed Quitters programme
F: female
FAP: functional analytic psychotherapy
FC: face‐to‐face counselling
F&C: Free & Clear
HE: health education
HMO: health maintenance organisation
hr: hour
incl: included
ITT: intention‐to‐treat
LGBT: lesbian, gay, bisexual, transgender
M: male
m: month
MA: meta‐analysis
MBAT: mindfulness‐based addiction treatment
MDD: major depressive disorder
MI: motivational interviewing
mins: minutes
NCI: National Cancer Institute
NP: nurse practitioners
NRT: nicotine replacement therapy
NS: not specified
NSGT: non‐specific group therapy
PI: principal investigator
PP: point prevalence abstinence
ppm: parts per million
RP: relapse prevention
SC: smoking cessation
SD: standard deviation
S‐H: self help
TANS: treatment of addiction to nicotine in schizophrenia
TC: telephone counselling
TQD: target quit date
UC: usual care
VUMC: Vanderbild University Medical Center
vs: versus
wk(s): week(s)
yr: year
ZAP: Zyban advantage programme
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Compared delivery of quitline counselling: counsellor‐ versus participant‐initiated | |
| Tested motivational interviewing to promote smoking cessation. Low use of NRT; only 59% of participants requested nicotine patches. Included in Lindson‐Hawley 2015 Cochrane review of motivational interviewing | |
| Experimental intervention was tailored for at‐risk subgroups, and included recommendation to use combination NRT. Standard treatment control recommended single type of NRT. | |
| Only participants interested in quitting (17% at baseline) were offered NRT. Main intervention was motivational interviewing. | |
| Pharmacotherapy was only offered to the participants in the intervention arm. | |
| Pharmacotherapy was only offered to participants interested in quitting; 24% reported use. | |
| Only 3 months follow‐up | |
| Intervention delivered by computer, no personal support. | |
| Factorial trial of bupropion/placebo and mood management CBT or standard cessation CBT. Both behavioural interventions were intensive, and experimental treatment was specifically devised for people with depression. | |
| Only 3 months follow‐up (42 participants) | |
| Only 3 months follow‐up. Compared 2 versus 4 counselling callbacks for smokers calling a quitline who received up to 6 weeks of free NRT. | |
| Only 12 weeks follow‐up | |
| Pharmacotherapy was only offered to participants interested in quitting; 24% used. | |
| Pharmacotherapy differed by arm: control arm advised to use pharmacotherapy but had to pay for it; intervention arm provided with bupropion free of charge. | |
| Both pharmacotherapy and behavioural components varied by trial arm. | |
| Main study results have not been published. | |
| Only 5 weeks follow‐up. Compared 2 intensities of pharmacist‐led behavioural support for participants using NRT. | |
| Only 12 weeks follow‐up | |
| Control group participants may or may not have received NRT. | |
| Only 12 weeks follow‐up | |
| Compared web‐based versus print formats of smoking cessation intervention. | |
| Only 12 weeks follow‐up | |
| Only 3 months follow‐up | |
| Both behavioural interventions were of similar intensity, differing only in scheduling of sessions. | |
| Both behavioural interventions were of similar intensity. | |
| Factorial trial crossing extended behavioural support (CBT) with medical management only, and nortriptyline or placebo, for 1 year, as adjuncts to nicotine patch and 5 group counselling sessions. Placebo arms could have been compared, but no other trials confounded behavioural support with placebo, and the support common to all conditions was also much more intensive than in other trials. | |
| Similar design to Hall 2004: factorial trial crossing extended behavioural support (CBT) with medical management only, and bupropion or placebo, as adjunct to nicotine patch and 5 group support sessions over 11 weeks. As with Hall 2004, placebo arms could have been compared, but no other trials confounded behavioural support with placebo, and the support common to all conditions was also much more intensive than in other trials. | |
| Study population pregnant smokers, not eligible | |
| Only 3 months follow‐up. Test of motivational interviewing as adjunct to nicotine patch therapy for HIV+ smokers | |
| Intervention was access to an internet site; no person‐to‐person behavioural support | |
| Intervention and control did not differ on use of pharmacotherapy or intensity of behavioural support. Test of timing in relation to alcohol dependence treatment | |
| Test of reimbursement for pharmacotherapy and counselling | |
| Pilot study of a culturally‐tailored intervention for Koreans, with only 30 participants | |
| Main intervention was exercise, not eligible for this review. Recruitment to the standard care control was halted early. | |
| Compared proactive and reactive telephone counselling. Both conditions could get same intensity of counselling. | |
| Tested a specific behavioural intervention: feedback of biomedical information. | |
| Behavioural interventions were matched for intensity; specifically tested a weight‐related intervention. | |
| Only offer of nicotine gum | |
| Only 10 weeks follow‐up | |
| Only 3 months follow‐up | |
| Only 3 months follow‐up. Small study of pharmacist advice as adjunct to NRT | |
| Only 3 months follow‐up | |
| Only 4 months follow‐up. Intervention was offer of group support and free NRT. | |
| No difference in intensity of behavioural support | |
| Only 3 months follow‐up. Study of an intervention to increase adherence to varenicline | |
| All participants received same intensity of motivational interviewing, group sessions and offer of NRT. Tested different targets for motivational interviewing. | |
| Pharmacotherapy not offered in same way to both arms | |
| Pharmacotherapy not offered in same way to both arms | |
| Not all participants were offered pharmacotherapy. | |
| Intervention participants did not automatically receive additional behavioural support; intervention consisted of automated telephone calls to identify participants at risk of relapse. Only this subgroup then received further counselling. | |
| Only 3 months follow‐up, behavioural interventions similar in intensity as adjuncts to nicotine patch | |
| Participants were smokeless tobacco users not smokers | |
| Only 12 weeks follow‐up from start of treatment. Study of computer‐tailored materials as adjunct to nicotine gum | |
| Only 12 weeks follow‐up from start of treatment. Study of computer‐tailored materials as adjunct to nicotine patch | |
| Short follow‐up (preoperative period) | |
| Only 12 weeks follow‐up from start of treatment. No personal behavioural support, study of web‐based tailored materials as adjunct to nicotine patch | |
| Intervention was automated telephone counselling messages, no personal contact. | |
| Compared intervention from 2 different types of pharmacist, not between different intensities of support. | |
| Compared 2 group‐based behavioural interventions similar in intensity as adjuncts to nicotine patch, see Stead 2017. | |
| Use of nicotine gum was substantially different between the relevant arms of the trial, and the intervention condition was also a test of the impact of training. | |
| Only 3 month follow‐up. Test of multifaceted intervention including offer of NRT at preoperative clinics | |
| Only 12 weeks follow‐up from start of treatment | |
| Trial of methods of delivery of care rather than of intensity of support |
CBT: cognitive behavioural therapy
NRT: nicotine replacement therapy
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Improving radiotherapy outcomes in head and neck cancer patients: a preliminary comparison of smoking cessation intervention ‘Varenicline plus support’ with ‘treatment as usual’ |
| Methods | Study design: randomised controlled trial Setting: New South Wales, Australia Recruitment: potential participants identified in the month preceding the new patient clinic using treatment planning software |
| Participants | Target: 40 |
| Interventions | Pharmacotherapy: varenicline for 3 months course initially then an offer of an additional 3 months course depending on the successful completion of the first course 1. Treatment as usual: standard New South Wales Health Tobacco assessment and smoking cessation advice 2. Multicomponent smoking cessation programme including 10 behaviour change sessions with a psychologist |
| Outcomes | Abstinence at 6 months post‐radiotherapy Validation: not specified |
| Starting date | August 2014 |
| Contact information | Benjamin Britton, University of Newcastle |
| Notes | Stopped due to a higher than anticipated number of ineligible patients and time‐limited funding Only the intervention group was offered varenicline. |
| Trial name or title | A cluster‐randomised pilot trial of a tailored worksite smoking cessation intervention targeting Hispanic/Latino construction workers: intervention development and research design |
| Methods | Study design: cluster‐randomised pilot trial Setting: South Florida, USA Recruitment: identification of potential participants through research partnership with local construction companies |
| Participants | Target: 126 Hispanic/Latino smokers (63 per arm) |
| Interventions | Pharmacotherapy: 8 weeks of free NRT (6 weeks supply provided by the study team and 2 weeks by the quitline) 1. Enhanced care: single face‐to‐face behavioural group counselling session delivered at the food truck + two brief follow‐up counselling phone calls + usual care 2. Usual care: fax referral to the Florida quitline (quitline to provide four brief counselling sessions by phone) + informative handout about the quitline |
| Outcomes | Abstinence at 6 months Validation: saliva cotinine < 15 ng/mL |
| Starting date | April 2017 |
| Contact information | David Lee, University of Miami |
| Notes |
| Trial name or title | Culturally‐tailored smoking cessation for American Indians |
| Methods | Study design: RCT (cluster randomisation) Setting: American Indian and Alaskan Native smokers in 2 sites (Kansas and Oklahoma) Participants will form temporal clusters in recruiting order, and then pairs of clusters will be assigned to the groups using randomised permuted blocks based on computer‐generated random numbers. |
| Participants | 58 groups totaling 448 participants |
| Interventions | Pharmacotherapy: choice of free pharmacotherapy, including Chantix®, Zyban®, Nicotine Replacement Therapy (NRT, patches, gum, or lozenges), or a combination of the latter 2 1. Non‐native tailored intervention using American Cancer Society guide to educate about the risks of smoking + assisting with planning for cessation (included pharmacotherapy) 2. “All Nations Breath of Life” (ANBL) programme (culturally‐tailored) = group support sessions, telephone motivational interviewing, culturally‐tailored educational curriculum, pharmacotherapy, and participants' incentives |
| Outcomes | Abstinence: continuous abstinence |
| Starting date | September 2010 |
| Contact information | Won Choi, University of Kansas |
| Notes | Both usual care and intervention received intensive behaviour counselling; however the types of counselling were different. The study aimed to assess culturally‐tailored smoking cessation interventions among American Indian populations. ‐ study completed in January 2015 |
| Trial name or title | Nicotine patches and quitline counselling to help hospitalised smokers stay quit: study protocol for a randomised controlled trial |
| Methods | Setting: hospitalised patients recruited from 2 healthcare systems in San Diego county Recruitment: motivation: respiratory therapists/research recruiters at bedside; interested in quitting, selected if they were motivated |
| Participants | 1640 participants |
| Interventions | Pharmacotherapy: 6 to 10 cpd = 6 weeks 14 mg + 2 weeks 7 mg; ≥ 11 cpd = 4 weeks 21 mg, 2 weeks 14 mg & 2 weeks 7 mg nicotine patches 1. Usual care ‐ brief bedside intervention (< 10 minutes), educational materials & state quitline number provided 2. Just nicotine patches (8 weeks, step‐down programme) 3. Proactive telephone counselling provided by the state quitline after discharge 4. Both patch + telephone counselling |
| Outcomes | Abstinence at 6 months ‐ 30‐day PP Validation: cotinine‐validated smoking status |
| Starting date | Date of registration: February 1, 2011; date of first participant: August 3, 2011 |
| Contact information | Shu‐Hong Zhu, University of California San Diego |
| Notes | Analysis will use 4 vs 2 |
| Trial name or title | Duration of behavioural counselling treatment needed to optimise smoking abstinence |
| Methods | RCT |
| Participants | 450 |
| Interventions | Pharmacotherapy: 1. 3 months of counselling 2. 6 months of counselling 3. 12 months of counselling |
| Outcomes | Abstinence: 1 year Validation: not specified |
| Starting date | February 2008 |
| Contact information | |
| Notes | There are no study results yet. |
| Trial name or title | A pilot study of a smoking cessation intervention for women living with HIV: study protocol |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: convenience sampling. To be recruited offline and online across the nation |
| Participants | 50 women diagnosed with HIV and residing in a community |
| Interventions | Pharmacotherapy: eight weeks of nicotine patches Eight weekly individualised counselling sessions of 30‐minute cognitive behavioural therapy via: 1. telephone video call 2. telephone voice call |
| Outcomes | Abstinence at 6 months Validation: salivary cotinine < 10ng/mL |
| Starting date | Protocol published in February 2017 |
| Contact information | Sun S Kim, University of Massachusetts Boston |
| Notes |
| Trial name or title | Telephone counselling and the distribution of nicotine patches to smokers |
| Methods | Study design: randomised controlled trial (factorial) Setting: University of California, California Smokers’ Helpline, USA Recruitment: recruitment of eligible participants through the Helpline |
| Participants | 4200 participants |
| Interventions | Pharmacotherapy: eight weeks of nicotine patch 1. Telephone counselling: pre‐quit session + five proactive follow‐up calls 2. Self‐help materials: reading materials mailed to the participants |
| Outcomes | Abstinence at 6 months Validation: unspecified in the trials registry |
| Starting date | February 2009 |
| Contact information | Shu‐Hong Zhu, University of California |
| Notes |
| Trial name or title | Treatment of smoking among individuals with PTSD: a phase II, randomised study of varenicline and cognitive behavioural therapy |
| Methods | RCT |
| Participants | 166 |
| Interventions | Pharmacotherapy: varenicline 1 mg tablets, orally, twice daily x 12 weeks 1. Control = 5‐min weekly counselling x 12 weeks, focused on medication adherence and smoking cessation 2. Control = 75 to 90‐min weekly psychotherapy sessions x 12 weeks, focused on gradually confronting distressing trauma‐related memories and reminders |
| Outcomes | Abstinence: 7‐day PP at 6 months |
| Starting date | January 2009 |
| Contact information | Edna B Foa, University of Pennsylvania |
| Notes |
| Trial name or title | Reducing tobacco‐related health disparities |
| Methods | Study design: randomised controlled trial Setting: not known Recruitment: not known |
| Participants | 639 participants |
| Interventions | Pharmacotherapy: 300 pieces of nicotine gum issued at baseline visit 1. Standard treatment: mailed packet with standard self‐help materials delivered four times + referral to quitline 2. MAPS‐6 (standard treatment + six phone counselling sessions over a two‐year period) 3. MAPS‐12 (standard treatment + 12 phone counselling sessions over a two‐year period) 4. Standard treatment + NRT 5. MAPS‐6 + NRT 6. MAPS‐12 + NRT |
| Outcomes | Abstinence at 24 months Validation: carbon monoxide < 10 ppm, saliva cotinine < 20 ng/mL |
| Starting date | January 2011 |
| Contact information | Larkin Strong, M.D. Anderson Cancer Center |
| Notes |
| Trial name or title | Smoking cessation in rural hospitals |
| Methods | Study design: randomised controlled trial Setting: Recruitment: |
| Participants | 606 participants (303 in each arm) |
| Interventions | Pharmacotherapy: not specified in the trials registry 1. In‐hospital smoking cessation counselling by phone + four outpatient counselling sessions by phone 2. Counselling as above but with coordination of pharmacotherapy with their insurance coverage and their health care provider |
| Outcomes | Abstinence at 12 months Validation: not specified in the trials registry |
| Starting date | March 2010 |
| Contact information | Edward Ellerbeck, University of Kansas Medical Center |
| Notes |
| Trial name or title | Smoking cessation treatment for head & neck cancer patients: acceptance and commitment therapy |
| Methods | RCT |
| Participants | 108 |
| Interventions | Pharmacotherapy: varenicline 2 mg daily for 12 weeks 1. Acceptance and Commitment Therapy (ACT): 6 x 60‐min counselling sessions delivered over a 5‐week period 2. Motivational and Behavioral Counselling (MBC): 6 x 60‐min counselling sessions delivered over a 5‐week period |
| Outcomes | Abstinence: 14 and 26 weeks Validation: cotinine verification |
| Starting date | March 2010 |
| Contact information | Jan Blalock M. D. Anderson Cancer Center |
| Notes |
| Trial name or title | Maintaining nonsmoking |
| Methods | Setting: USA |
| Participants | 271 |
| Interventions | Pharmacotherapy: varenicline: 12 weeks, 1 mg bid 1. Participants have monthly meetings with medical staff 2. Participants receive monthly counselling with content based on a health education model 3. Participants receive monthly counselling with content based on a relapse prevention model plus access to ongoing medication treatment with varenicline 4. Participants receive monthly counselling with content based on a relapse prevention model |
| Outcomes | Abstinence at 12, 24, 52, 64, 104 months |
| Starting date | May 2010 |
| Contact information | University of California. No PI listed |
| Notes |
| Trial name or title | Developing genetic education for smoking cessation |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: not given in the trials registry |
| Participants | 103 participants |
| Interventions | Pharmacotherapy: 6 weeks of transdermal nicotine replacement therapy 1. Two educational sessions about genetics and smoking 2. Two educational sessions about nutrition |
| Outcomes | Abstinence at 6 months Validation: not given in the trials registry |
| Starting date | April 2012 |
| Contact information | Julia F Houfek, University of Nebraska |
| Notes |
| Trial name or title | Integrated smoking cessation treatment for low‐income community corrections |
| Methods | RCT |
| Participants | 689 |
| Interventions | Pharmacotherapy: bupropion 1. Brief physician advice to quit plus bupropion 2. 4 sessions of intensive counselling plus bupropion |
| Outcomes | Abstinence: at 3, 6, 9, 12 months Validation: verified by expired carbon monoxide |
| Starting date | October 2009 |
| Contact information | Karen L Cropsey, University of Alabama at Birmingham |
| Notes |
| Trial name or title | Providing free Nicotine patches to quitline smokers |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: smokers aged 18 years or older recruited from the quitline |
| Participants | 3710 participants |
| Interventions | Pharmacotherapy: nicotine patches 1. self‐help materials only 2. self‐help materials + a voucher for 2 weeks’ worth of nicotine patches 3. self‐help materials + 2 weeks’ worth of nicotine patches 4. up to 5 sessions of telephone counselling 5. up to 5 sessions of telephone counselling + a voucher for 2 weeks’ worth of nicotine patches 6. up to 5 sessions of telephone counselling + 2 weeks’ worth of nicotine patches |
| Outcomes | 6 months prolonged abstinence Validation: none specified in the trials registry |
| Starting date | April 2013 |
| Contact information | Shu‐Hong Zhu, University of California |
| Notes |
| Trial name or title | The Canadian HIV Quit Smoking Trial: tackling the comorbidities of depression and cardiovascular disease in HIV+ smokers |
| Methods | RCT |
| Participants | 256 |
| Interventions | Pharmacotherapy: NRT = 7 mg to 42 mg depending on cpd; varenicline = 0.5 mg/daily for 3 days, 0.5 mg twice daily for 4 days and 1 mg twice daily for the remainder of the treatment period 1. NRT only 2. NRT + HIV‐tailored smoking cessation counselling 3. Varenicline only 4. Varenicline + HIV‐tailored smoking cessation counselling |
| Outcomes | Abstinence: 7‐day PP at week 48 Validation: expired carbon monoxide levels measured using a piCO+ Smokerlyzer; CO < 10 ppm |
| Starting date | January 2014 |
| Contact information | Louise Balfour, Ottawa Research Hospital |
| Notes |
| Trial name or title | CPT and smoking cessation |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: US veteran smokers with post‐traumatic stress disorder, aged between 18 and 65 years. Selected for motivation to quit smoking |
| Participants | 69 participants |
| Interventions | Pharmacotherapy: bupropion, nicotine patches and a rescue method (e.g. nicotine gum, lozenge, inhaler) 1. 12 sessions of combined cognitive processing therapy and integrated care for smoking cessation, involvement in smokefreeVET.gov’s text messaging programme for smoking cessation 2. 12 sessions of integrated care for smoking cessation, involvement in smokefreeVET.gov’s text messaging programme for smoking cessation |
| Outcomes | Abstinence at 6 months Validation: exhaled carbon monoxide < 4ppm |
| Starting date | December 2013 |
| Contact information | Eric A Dedert, Durham VA Medical Center |
| Notes |
| Trial name or title | Behavioural smoking cessation for people living with HIV/AIDS |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: smokers with HIV or AIDS diagnosis and aged 18 years or older |
| Participants | 400 participants |
| Interventions | Pharmacotherapy: a prescription for bupropion for all groups 1. Brief counselling 2. Brief counselling + brief high‐magnitude prize contingency management 3. Continued counselling + monitored support to quit smoking 4. Monitored support to quit smoking + prize contingency management for abstinence 5. Pharmacotherapy only 6. Continued monitoring + low intensity prize contingency management |
| Outcomes | Abstinence at 6 and 12 months Validation: urinary cotinine, carbon monoxide |
| Starting date | August 2013 |
| Contact information | David Ledgerwood, Wayne State University |
| Notes |
| Trial name or title | Smoking cessation strategies in community cancer programmes for lung and head and neck cancer patients |
| Methods | Setting: USA |
| Participants | 180 |
| Interventions | 1. High‐intensity counselling + long‐acting NRT + PRN NRT |
| Outcomes | Abstinence: 7‐day PP at 8 weeks Validation: CO |
| Starting date | July 2014 |
| Contact information | Joseph Valentino, University of Kentucky |
| Notes |
| Trial name or title | A quit smoking study using smartphones |
| Methods | RCT |
| Participants | 30 |
| Interventions | Pharmacotherapy: nicotine patch 1.Nicotine patch plus behavioural cessation counselling without access to Mobile Games 2. Nicotine patch plus behavioural cessation counselling with access to Mobile Games |
| Outcomes | Abstinence: change between baseline mean cigarettes smoked per day and mean cigarettes smoked per day during the first 4 weeks of the quit attempt |
| Starting date | October 2014 |
| Contact information | Tanya R. Schlam, University of Wisconsin Center for Tobacco Research and Intervention |
| Notes |
| Trial name or title | Behavioural activation and varenicline for smoking cessation in depressed smokers |
| Methods | Study design: randomised controlled trial Setting: Chicago, USA Recruitment: smokers with major depressive disorder |
| Participants | 576 participants |
| Interventions | Pharmacotherapy: varenicline or placebo for 12 weeks 1. Standard behavioural cessation treatment (45 minutes x 8 sessions) 2. Behavioural activation integrated with standard behavioural cessation treatment (45 minutes x 8 sessions) |
| Outcomes | Abstinence at 27 weeks Validation: expired carbon monoxide ≤ 8 ppm |
| Starting date | June 2015 |
| Contact information | Brian L Hitsman, Northwestern University |
| Notes |
| Trial name or title | Optimising smoking cessation for people with HIV/AIDS who smoke |
| Methods | Study design: randomised controlled trial (factorial) Setting: University of Maryland Medical Center, USA Recruitment: not specified in the trials registry |
| Participants | 300 participants |
| Interventions | Pharmacotherapy: varenicline 1. Standard care: low intensity, brief counselling 2. Positively Smoke Free (details unspecified in the trials registry) |
| Outcomes | Abstinence at 24 weeks Validation: not specified in the trials registry |
| Starting date | July 2016 |
| Contact information | Seth Himelhoch, University of Maryland |
| Notes |
| Trial name or title | Hospital to home, smoker support trial |
| Methods | Study design: randomised controlled trial Setting: hospital and home Recruitment: smokers leaving hospital |
| Participants | 404 participants |
| Interventions | Pharmacotherapy: nicotine replacement products 1. Usual care: behavioural support before leaving hospital, referral to NHS Stop Smoking Services after discharge 2. Home visit as soon as practicable after discharge and typically within 48 hours to deliver a multicomponent intervention; tailored support package including telephone support, carbon dioxide measurements, home air quality measurements, signposting to support groups, self‐help materials |
| Outcomes | Abstinence at 4 weeks and 12 weeks post‐discharge according to the information on the trials registry Validation: exhaled carbone monoxide < 6 ppm |
| Starting date | June 2016 |
| Contact information | John Britton, University of Nottingham |
| Notes |
| Trial name or title | Smoking cessation intervention for women with HIV/AIDS |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: smokers with diagnosis of HIV infection and aged between 18 and 75 years |
| Participants | 50 participants |
| Interventions | Pharmacotherapy: nicotine replacement therapy (habitrol patch) 1. cognitive behavioural therapy via video‐conferencing 2. cognitive behavioural therapy via telephone |
| Outcomes | Abstinence at 6 months Validation: saliva cotinine |
| Starting date | June 2016 |
| Contact information | Sun S Kim, University of Massachusetts |
| Notes |
| Trial name or title | Strategies to promote cessation in smokers who are not ready to quit (PACE) |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: smokers aged 18 years or older |
| Participants | 828 participants |
| Interventions | Pharmacotherapy: nicotine gum 1. brief advice + typical smoking cessation resources 2. motivational interviewing 3. rate reduction 4. motivational interviewing + rate reduction |
| Outcomes | Abstinence at 12 months Validation: not specified in the trials registry |
| Starting date | September 2016 |
| Contact information | Robert Klesges, University of Virginia |
| Notes |
| Trial name or title | Pilot trial of a smoking cessation intervention informed by construal level theory |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: not specified in the trials registry |
| Participants | 23 participants |
| Interventions | Pharmacotherapy: eight weeks of transdermal nicotine patch 1. Standard informational treatment: in‐person session, text messaging 2. Spotlight on smoke‐free living 1.5 hour intervention session combined with daily text messaging for up to 1 week pre‐quit and 4 weeks post‐quit. |
| Outcomes | Abstinence at 13 weeks Validation: not specified in the trials registry |
| Starting date | December 2016 |
| Contact information | Richard Yi, University of Florida |
| Notes |
| Trial name or title | Smoking cessation interventions for people living with HIV in Nairobi, Kenya |
| Methods | Study design: randomised controlled trial (factorial) Setting: Nairobi, Kenya Recruitment: smokers living with HIV and receiving care in a methadone maintenance programme in Nairobi, Kenya |
| Participants | 300 participants |
| Interventions | Pharmacotherapy: bupropion 1. Standard care: brief advice to quit provided in a standardised format 2. Positively smoke free: eight sessions of tailored behavioural treatment for smoking cessation |
| Outcomes | Abstinence at 36 months Validation: carbon monoxide level < 7ppm |
| Starting date | January 2019 |
| Contact information | Seth Himelhoch, University of Maryland |
| Notes |
| Trial name or title | Improving quitline support study: optimising remotely delivered smoking cessation services for low‐income smokers |
| Methods | Study design: four‐factor, fully‐crossed randomised controlled trial Setting: USA Recruitment: smokers aged 18 years or older selected for motivation to quit |
| Participants | 1600 participants |
| Interventions | Pharmacotherapy: 2 weeks of nicotine patches and lozenges 16 conditions of four factors: phone call, SmokefreeTXT, financial incentive, nicotine replacement (patches +/‐ lozenges) |
| Outcomes | Abstinence at 6 months Validation: saliva cotinine < 4ng/mL |
| Starting date | July 2018 |
| Contact information | Danielle E McCarthy, University of Wisconsin |
| Notes |
| Trial name or title | Post‐discharge smoking cessation strategies: helping HAND 4 |
| Methods | Study design: randomised controlled trial Setting: three hospitals in USA Recruitment: not specified in the trials registry |
| Participants | 1350 participants |
| Interventions | Pharmacotherapy: eight weeks of nicotine replacement therapy 1. Electronic referral to State tobacco quitline 2. Personalised tobacco care management: seven proactive contacts over three months delivered by automated interactive voice response phone calls, text messaging and/or email + offer of a return call from the hospital‐based tobacco coach who offer counselling, medication advice and coordination of care with the patient’s outpatient health care team |
| Outcomes | Abstinence at 6 months after hospital discharge Validation: not specified in the trials registry |
| Starting date | August 2018 |
| Contact information | Nancy Rigotti, Massachusetts General Hospital |
| Notes |
| Trial name or title | Integrating smoking cessation and alcohol use treatment in homeless populations |
| Methods | Study design: randomised controlled trial Setting: homeless shelters Recruitment: homeless smokers aged 18 years or older |
| Participants | 645 participants |
| Interventions | Pharmacotherapy: 12 weeks of nicotine patch plus nicotine gum or lozenge 1. Integrated intensive smoking plus alcohol intervention using cognitive behavioural therapy 2. Intensive smoking intervention using cognitive behavioural therapy 3. Usual care: brief smoking cessation and brief alcohol counselling |
| Outcomes | Abstinence at 52 weeks Validation: cotinine‐verified 7‐day smoking abstinence |
| Starting date | January 2015 |
| Contact information | Olamide Ojo‐Fati, Universtiy of Minnesota |
| Notes |
| Trial name or title | Efficacy of smoking cessation therapy alone or integrated with prolonged exposure therapy for smokers with PTSD |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: smokers with post‐traumatic stress disorder aged between 18 and 64 years selected for motivation to quit |
| Participants | 80 participants |
| Interventions | Pharmacotherapy: nicotine patch 1. Standard smoking cessation treatment: once‐weekly 45‐minute sessions of cognitive behavioural therapy over a 12‐week period 2. Integrated PTSD and smoking treatment: once‐weekly 90‐minute sessions over a 12‐week period. Incorporated standard treatment with therapy for reducing PTSD symptoms and anxiety sensitivity and enhancing tolerance for nicotine withdrawal sensations. |
| Outcomes | Abstinence at 24 weeks Validation: saliva cotinine < 10ng/mL for stated abstinence of 2 weeks or more, carbon monoxide analysis of breath samples < 8ppm for stated abstinence of 24 hours to 2 weeks |
| Starting date | October 2013 |
| Contact information | Mark B Powers, University of Texas |
| Notes |
| Trial name or title | Interactive voice response telephone technology for the treatment of smoking in patients with heart disease (IVR) |
| Methods | Setting: smokers recently hospitalised with CHD, Canada Health Care Recruitment: study co‐ordinator recruited within 24 hours of admission |
| Participants | N randomised: 100 (but 99 used in calculations). Dropouts: 15 + 1 death Sex: 67.4% M, Age: 54, av cpd 16‐25 Therapists: nurse specialist |
| Interventions | Pharmacotherapy: NRT in hospital before quit date 1. Access to NRT during hospitalisation, brief bedside counselling by nurse, self‐help guide 2. Interactive Voice Response system posted questions "concerning current smoking status, confidence in staying smoke‐free, use of pharmacotherapy, and self‐help materials" |
| Outcomes | Abstinence at 12 m, 7‐day PP Validation: none |
| Starting date | July 2006 |
| Contact information | Robert Reid, University of Ottawa Heart Institute |
| Notes |
| Trial name or title | Planning a change easily: a randomised controlled trial for smokers who are not ready to quit |
| Methods | Study design: randomised controlled trial Setting: not specified in the protocol Recruitment: smokers recruited via flyers, business cards, medical referrals, Facebook, Pandora Radio, and ‘refer‐a‐friend’ programme |
| Participants | 840 participants |
| Interventions | Pharmacotherapy: 4 mg nicotine gum for rate reduction group and motivational interviewing + rate reduction group. 1. Brief advice 2. Motivational interviewing 3. Rate reduction 4. Motivational interviewing + rate reduction |
| Outcomes | Abstinence at 12 months Validation: saliva cotinine |
| Starting date | Not specified in the protocol |
| Contact information | Francisco I Salgado Garcia, Universtiy of Tennesse |
| Notes |
| Trial name or title | Community‐based physical activity as adjunctive smoking cessation treatment: rationale, design, and baseline data for the Lifestyle Enhancement Program (LEAP) randomised controlled trial |
| Methods | Study design: randomised controlled trial Setting: community, USA Recruitment: smokers who are sedentary or minimally active during leisure time, and aged between 18 and 65 years |
| Participants | 392 participants |
| Interventions | Pharmacotherapy: 6 weeks of transdermal nicotine 1. Behavioural counselling + physical activity intervention 2. Behavoural counselling + wellness intervention |
| Outcomes | Abstinence at 12 months Validation: expired carbon monoxide < 10ppm |
| Starting date | January 2003 |
| Contact information | Kenneth D Ward, University of Memphis |
| Notes |
| Trial name or title | Enhancing cancer outreach for low‐income adults with innovative smoking cessation. Project ACTION (Adult smoking Cessation Treatment through Innovative Outreach to Neighborhoods) |
| Methods | Cluster RCT Setting: community, USA |
| Participants | 756 |
| Interventions | 1. Standard care: brief coach advice to quit smoking, nicotine replacement therapy (NRT), and self‐help written materials 2. Enhanced care: As 1. plus a single motivational interviewing counselling session and a cell phone‐delivered text/graphical messaging component 3. Intensive care: As 2. plus a series of 11 cell phone‐delivered proactive counselling sessions and a cell phone‐delivered text/graphical messaging component |
| Outcomes | Abstinence at 12 months |
| Starting date | June 2010 |
| Contact information | Alex Prokhorov, University of Texas MD Anderson Cancer Center |
| Notes |
| Trial name or title | Reducing racial/ethnic tobacco cessation disparities via cognitive behavioural therapy: design of a dual‐site randomised controlled trial |
| Methods | Study design: randomised controlled trial Setting: USA Recruitment: African American/black, Hispanic, or white non‐Hispanic smokers aged 18 years or older |
| Participants | 354 participants |
| Interventions | Pharmacotherapy: up to 8 weeks of transdermal nicotine patch 1. Group cognitive behavioural therapy 2. General health education |
| Outcomes | Abstinence at 12 months Validation: not specified in the trials registry |
| Starting date | August 2015 |
| Contact information | Monica Webb Hooper, Case Western Reserve University School of Medicine |
| Notes |
ACT: Acceptance and commitment therapy
bid: bis in die (twice a day)
CHD: cornary heart disease
cpd: cigarettes per day
CPT: cognitive processing therapy
IVR: interactive voice response
NRT: nicotine replacement therapy
PI: prinicipal investigator
PRN: pro re nata (when necessary)
PTSD: post‐traumatic stress disorder
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Subgroups by type of pharmacotherapy Show forest plot | 65 | 23331 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| Analysis 1.1  Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 1 Subgroups by type of pharmacotherapy. | ||||
| 1.1 NRT | 49 | 16541 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.04, 1.21] |
| 1.2 Bupropion | 5 | 2298 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.46] |
| 1.3 Nortriptyline | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.60, 1.63] |
| 1.4 Varenicline | 2 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.27] |
| 1.5 NRT & bupropion | 3 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.00, 1.54] |
| 1.6 Choice of pharmacotherapy | 5 | 2490 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.00, 1.68] |
| 2 Subgroups by contrast in number of contacts between intervention & control Show forest plot | 63 | 21997 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| Analysis 1.2  Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 2 Subgroups by contrast in number of contacts between intervention & control. | ||||
| 2.1 4 to 8 or > 8 contacts versus no contact | 8 | 4018 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.02, 1.43] |
| 2.2 More than 8 contacts versus 1 to 3 contacts | 4 | 1063 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.70, 1.57] |
| 2.3 4 to 8 contacts versus 1 to 3 contacts | 18 | 9579 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.01, 1.19] |
| 2.4 More than 8 contacts versus 4 to 8 contacts | 12 | 1737 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.98, 1.33] |
| 2.5 Intervention & control in same contact category | 21 | 5600 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.16, 1.50] |
| 3 Subgroups by duration of contact in control condition (not prespecified) Show forest plot | 62 | 21695 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| Analysis 1.3 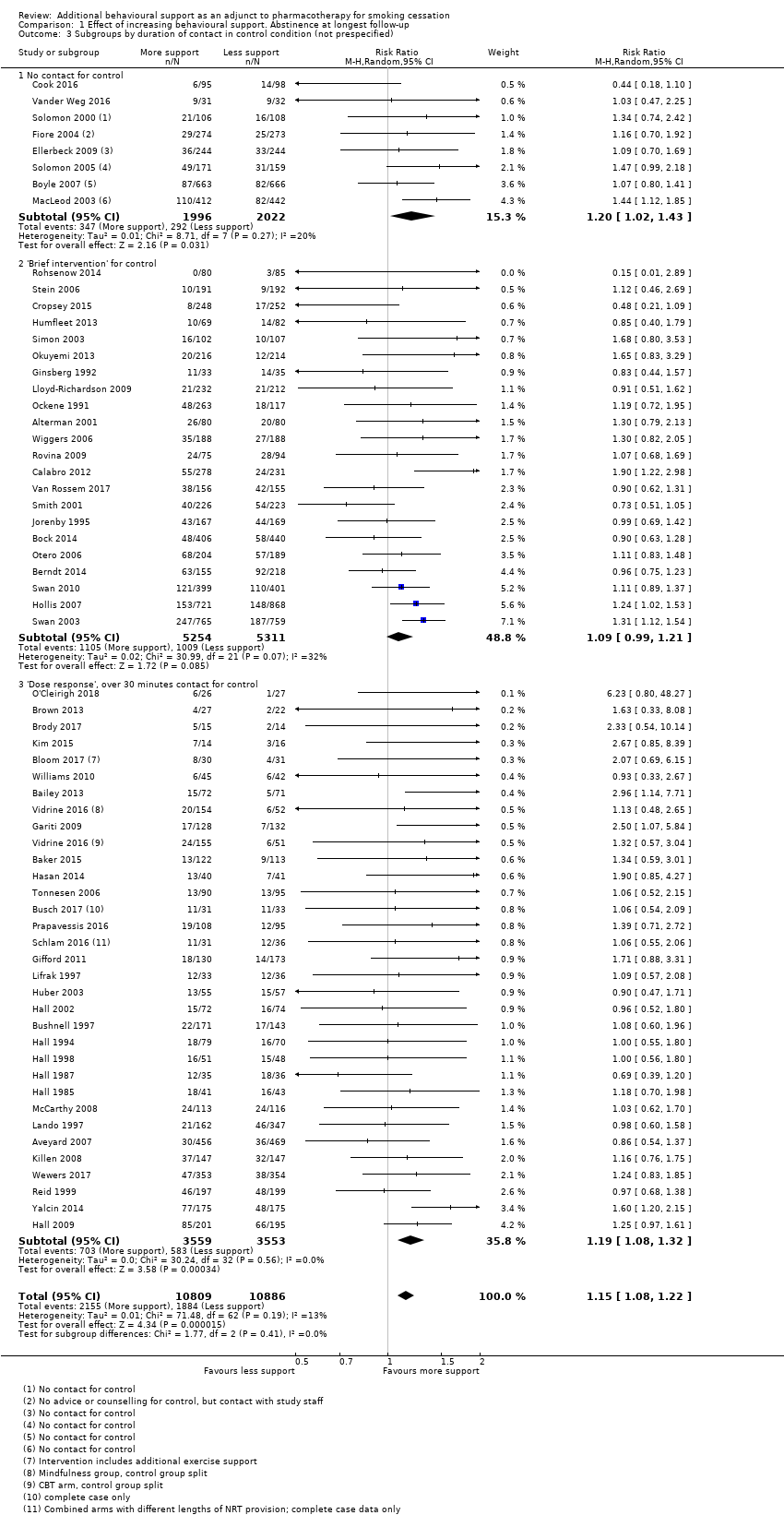 Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 3 Subgroups by duration of contact in control condition (not prespecified). | ||||
| 3.1 No contact for control | 8 | 4018 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.02, 1.43] |
| 3.2 'Brief intervention' for control | 22 | 10565 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.99, 1.21] |
| 3.3 'Dose response', over 30 minutes contact for control | 32 | 7112 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.08, 1.32] |
| 4 Subgroup by modality of intervention contact (not prespecified) Show forest plot | 65 | 23331 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| Analysis 1.4 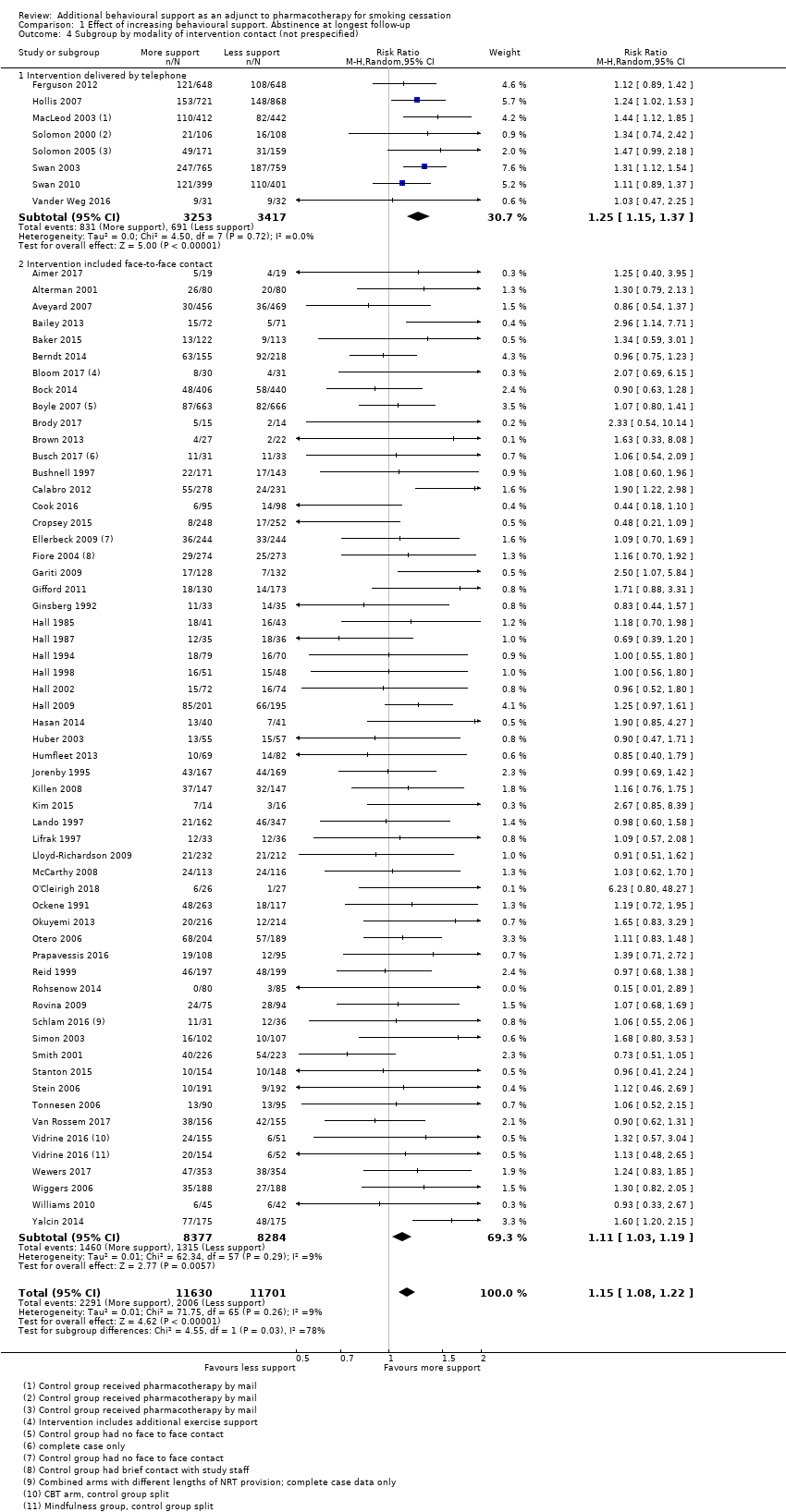 Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 4 Subgroup by modality of intervention contact (not prespecified). | ||||
| 4.1 Intervention delivered by telephone | 8 | 6670 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.37] |
| 4.2 Intervention included face‐to‐face contact | 57 | 16661 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.03, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sensitivity analysis including intermediate intensity conditions. Adjunct behavioural support versus pharmacotherapy alone Show forest plot | 65 | 27425 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.07, 1.20] |
| Analysis 2.1 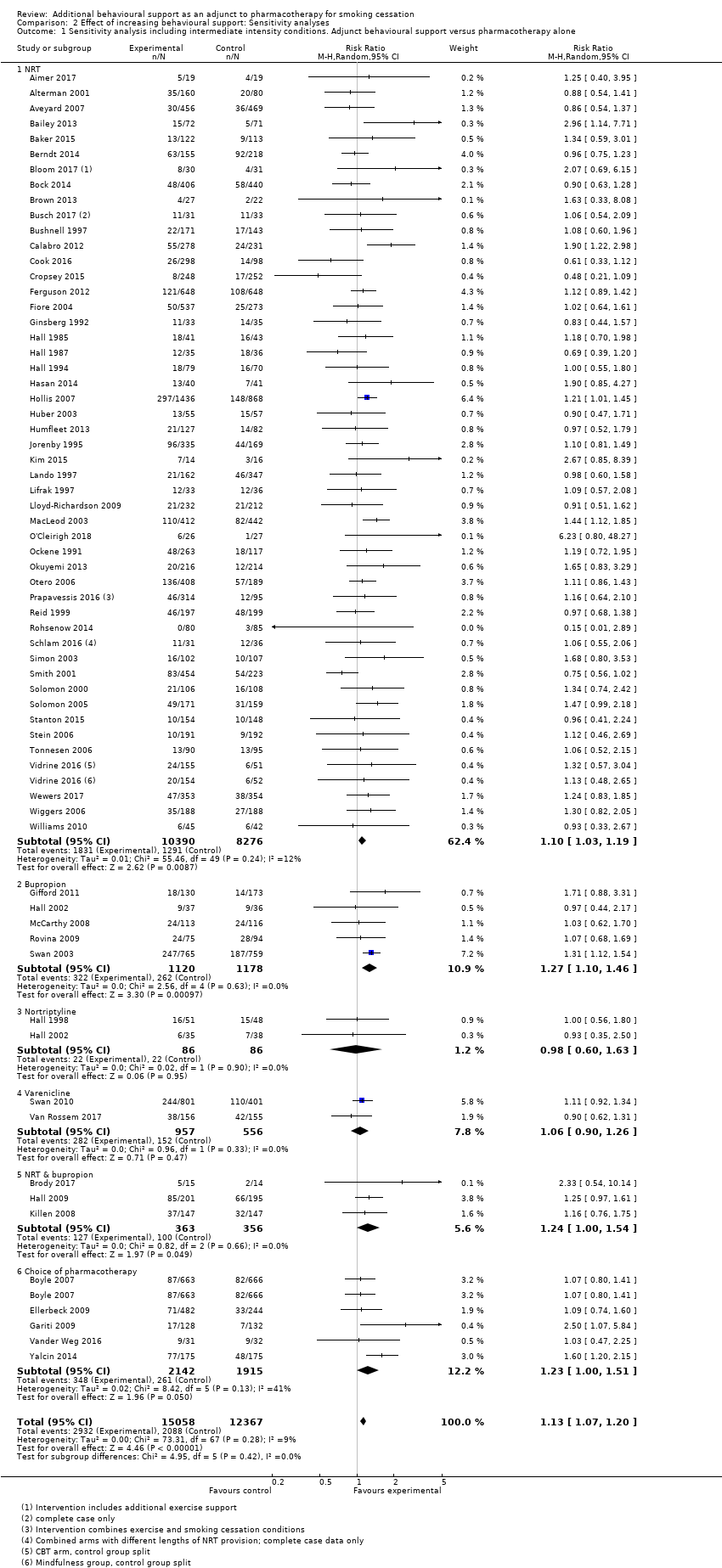 Comparison 2 Effect of increasing behavioural support: Sensitivity analyses, Outcome 1 Sensitivity analysis including intermediate intensity conditions. Adjunct behavioural support versus pharmacotherapy alone. | ||||
| 1.1 NRT | 49 | 18666 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.03, 1.19] |
| 1.2 Bupropion | 5 | 2298 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.46] |
| 1.3 Nortriptyline | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.60, 1.63] |
| 1.4 Varenicline | 2 | 1513 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.90, 1.26] |
| 1.5 NRT & bupropion | 3 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.00, 1.54] |
| 1.6 Choice of pharmacotherapy | 5 | 4057 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.00, 1.51] |
| 2 By outcome definition Show forest plot | 65 | 23389 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| Analysis 2.2 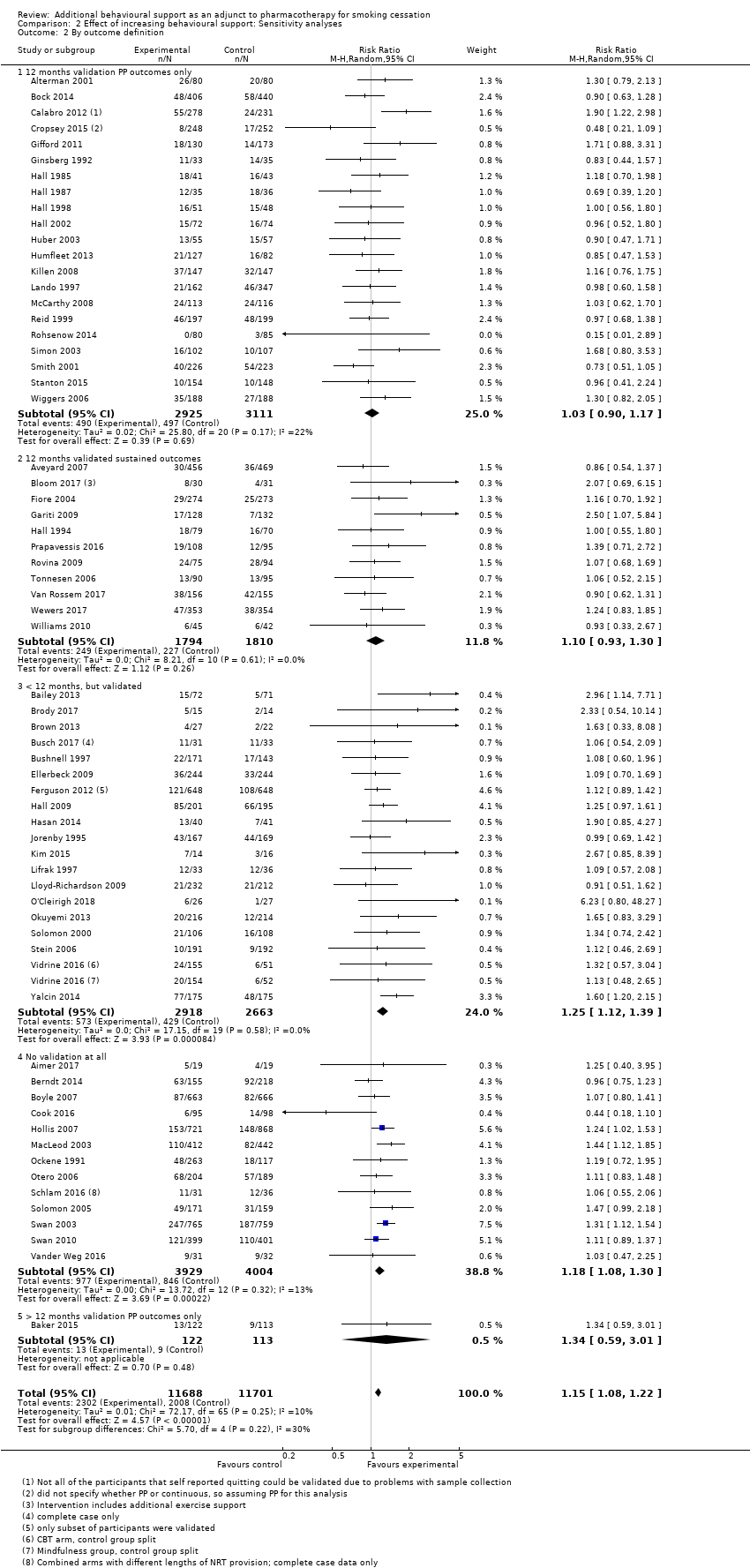 Comparison 2 Effect of increasing behavioural support: Sensitivity analyses, Outcome 2 By outcome definition. | ||||
| 2.1 12 months validation PP outcomes only | 21 | 6036 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.17] |
| 2.2 12 months validated sustained outcomes | 11 | 3604 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.30] |
| 2.3 < 12 months, but validated | 19 | 5581 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.12, 1.39] |
| 2.4 No validation at all | 13 | 7933 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.08, 1.30] |
| 2.5 > 12 months validation PP outcomes only | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.59, 3.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence at longest follow‐up Show forest plot | 15 | 4138 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.25] |
| Analysis 3.1  Comparison 3 Studies matched for contact time. Abstinence at longest follow‐up point, Outcome 1 Abstinence at longest follow‐up. | ||||
| 1.1 Family support versus usual care telephone counselling | 1 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.45] |
| 1.2 Face‐to‐face, tests attentional training v placebo training | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.48, 2.50] |
| 1.3 ACT versus CBT telephone counselling | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.74, 2.46] |
| 1.4 Positive psychotherapy versus usual care (face‐to‐face) | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 8.78 [0.49, 157.62] |
| 1.5 Couples treatment versus individual treatment (face‐to‐face) | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.37, 1.43] |
| 1.6 Behavioural activation versus standard treatment (face‐to‐face) | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [0.24, 94.85] |
| 1.7 Culturally tailored versus standard (face‐to‐face) | 4 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.68, 1.92] |
| 1.8 Exercise counselling versus health education (face‐to‐face) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.23, 1.89] |
| 1.9 Adherence counselling versus standard care (telephone) | 1 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.15] |
| 1.10 MIndfulness versus CBT (face‐to‐face) | 1 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.45] |
| 1.11 Quitline facilitation session versus brief advice (telephone) | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.62, 4.00] |
| 1.12 Motivational interviewing versus health education | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.94] |
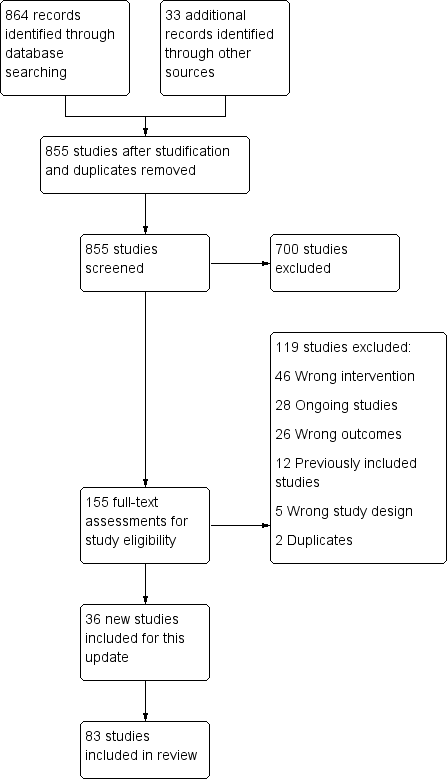
Study flow diagram for 2019 update

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Effect of increasing behavioural support. Abstinence at longest follow‐up.
Subgroups by type of pharmacotherapy
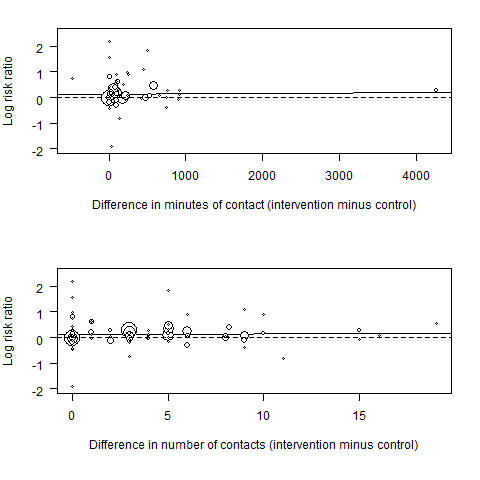
Meta‐regression results (the fitted meta‐regression trend is shown as the solid line)

Funnel plot of comparison: 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, outcome: 1.1 Subgroups by type of pharmacotherapy.

Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 1 Subgroups by type of pharmacotherapy.

Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 2 Subgroups by contrast in number of contacts between intervention & control.

Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 3 Subgroups by duration of contact in control condition (not prespecified).

Comparison 1 Effect of increasing behavioural support. Abstinence at longest follow‐up, Outcome 4 Subgroup by modality of intervention contact (not prespecified).

Comparison 2 Effect of increasing behavioural support: Sensitivity analyses, Outcome 1 Sensitivity analysis including intermediate intensity conditions. Adjunct behavioural support versus pharmacotherapy alone.

Comparison 2 Effect of increasing behavioural support: Sensitivity analyses, Outcome 2 By outcome definition.

Comparison 3 Studies matched for contact time. Abstinence at longest follow‐up point, Outcome 1 Abstinence at longest follow‐up.
| Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation | ||||||
| Patient or population: People using smoking cessation pharmacotherapy | ||||||
| Outcomes | Illustrative absolute effects* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed successful quitters without intervention | Estimated quitters with intervention | |||||
| Pharmacotherapy (with variable level of behavioural support) | Additional behavioural support (in addition to pharmacotherapy) | |||||
| Smoking cessation at longest follow‐up | Study population1 | RR 1.15 | 23,331 | ⊕⊕⊕⊕ | Effect very stable over time: updates of this analysis (15 new studies added 2015; 18 new studies added 2019) have had minimal impact on the effect estimate. Little evidence of differences in effect based on amount of support or type of pharmacotherapy provided. | |
| 171 per 1000 | 197 per 1000 | |||||
| The estimated rate of quitting with behavioural intervention (and its 95% confidence interval) is based on the assumed quit rate in the control group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on the control group crude average 2Sensitivity analysis removing studies at high risk of bias yielded results consistent with those from the overall analysis. A funnel plot was inconclusive but suggested there may have been slightly more small studies with large effect sizes than with small effect sizes. However, asymmetry was not clear and we did not downgrade on this basis; given the large number of included studies and the degree of homogeneity between them, it is unlikely that smaller unpublished studies showing no effect, if they existed, would significantly alter our results. | ||||||
| Intervention | Control | ||||||
| Study ID | Pharmacotherapy | Modality (included face‐to‐face/ telephone only) | Number of contacts | Total duration (minutes) | Number of contacts | Total duration (minutes) | Comments |
| NRT | Face‐to‐face | 6 | 120 | 6 | 120 | ||
| NRT | Face‐to‐face | 4 | Unclear | Unclear | Unclear | ||
| NRT | Face‐to‐face | 16 | 4290 | 1 | 30 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 7 | 140 | 4 | 80 | ||
| NRT | Face‐to‐face | 19 | 950 | 10 | 500 | ||
| NRT | Face‐to‐face | 17 | 1050 | 17 | 290 | ||
| NRT | Telephone | 5 | 100 | 5 | 100 | ||
| NRT | Face‐to‐face | 7 | 112 | 7 | 112 | ||
| NRT | Face‐to‐face | 7 | 285 | 7 | 105 | ||
| NRT | Face‐to‐face | 20 | 400 | 20 | 880 | Exercise sessions/time excluded | |
| NRT | Face‐to‐face | 3 | Unclear | 1 | Unclear | ||
| Choice | Face‐to‐face | 9 | Unclear | 0 | 0 | ||
| NRT | Telephone | 5 | 90 | 5 | 90 | ||
| NRT & Bupropion | Face‐to‐face | 22 | 970 | 12 | 720 | ||
| NRT | Face‐to‐face | Unclear | Unclear | Unclear | Unclear | ||
| NRT | Face‐to‐face | 6 | 220 | 6 | 87.5 | ||
| NRT | Face‐to‐face | 8 | 480 | 4 | 240 | ||
| NRT | Face‐to‐face | 2 | 120 | 1 | 5 | Intervention also had "access to 5 web‐based booster sessions" | |
| NRT | Face‐to‐face | 11 | 130 | 0 | 0 | Multifactorial ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 4 | 100 | 1 | Unclear | ||
| Choice | Face‐to‐face | 6 | Unclear | 0 | 0 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Telephone | 6 | Unclear | Unclear | Unclear | ||
| NRT | Face‐to‐face | 5 | Unclear | 0 | 0 | Multiple arms ‐ highest vs lowest intensity | |
| Choice | Face‐to‐face | 10 | 125 | 4 | 30 | ||
| Bupropion | Face‐to‐face | 20 | Unclear | 1 | 60 | ||
| NRT | Face‐to‐face | 5 | Unclear | 2 | Unclear | ||
| NRT | Face‐to‐face | 14 | 1050 | 4 | Unclear | ||
| NRT | Face‐to‐face | 14 | 1050 | 5 | 300 | ||
| NRT | Face‐to‐face | 10 | 1200 | 5 | 450 | ||
| Nortriptyline | Face‐to‐face | 10 | 1200 | 5 | 450 | ||
| Bupropion/Nortriptyline | Face‐to‐face | 5 | 450 | 4 | 30 | ||
| NRT & Bupropion | Face‐to‐face | 11 | 330 | 5 | Unclear | Multifactorial study design | |
| NRT | Face‐to‐face | 7 | 195 | 6 | 105 | ||
| NRT | Telephone | 4 | 100 | 1 | 15 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 5 | 450 | 5 | 225 | ||
| NRT | Face‐to‐face | 6 | 300 | 1 | 'Brief' | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 8 | 480 | 0 | 0 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 6 | 210 | 6 | 210 | ||
| NRT & Bupropion | Face‐to‐face | 10 | 300 | 10 | 200 | ||
| NRT | Face‐to‐face | 8 | 320 | 8 | 80 | ||
| NRT | Face‐to‐face | 7 | 420 | 7 | 420 | ||
| NRT | Face‐to‐face | 4 | 48 | 0 | 0 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 20 | 736.5 | 4 | 82.5 | ||
| NRT | Face‐to‐face | 5 | Unclear | 2 | Unclear | ||
| NRT | Telephone | 5 | 60 | 0 | 0 | ||
| NRT | Face‐to‐face | 8 | 480 | 8 | 480 | ||
| NRT | Face‐to‐face | 6 | 540 | 6 | 540 | ||
| Bupropion | Face‐to‐face | 13 | Unclear | 13 | Unclear | Control received 80 minutes less contact than intervention | |
| NRT & Varenicline | Face‐to‐face | 9 | Unclear | 5 | Unclear | ||
| NRT | Face‐to‐face | 5 | 45 | 2 | 15 | ||
| NRT | Face‐to‐face | 10 | 600 | 5 | 100 | ||
| NRT | Face‐to‐face | 6 | 105 | 1 | 12.5 | ||
| NRT | Face‐to‐face | 4 | 240 | 1 | 20 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 36 | 1080 | 36 | 1080 | Intervention group: "exercise counselling delivered while the participant was engaged in exercise" ‐ have left this time in as also counselling | |
| NRT | Face‐to‐face | 64 | 1985 | 59 | 1860 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 6 | Unclear | 3 | 45 | ||
| NRT | Face‐to‐face | 3 | 65 | 3 | 35 | ||
| Bupropion | Face‐to‐face | 9 | 540 | 1 | 15 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Face‐to‐face | 12 | 320 | 4 | 200 | Multifactorial study design | |
| Bupropion | Face‐to‐face | 7 | 420 | 7 | 420 | ||
| NRT | Face‐to‐face | 6 | 195 | 1 | 10 | ||
| NRT | Face‐to‐face | 6 | 90 | 0 | 0 | Multiple arms ‐ highest vs lowest intensity | |
| NRT | Telephone | 4 | 67 | 4 | 60 | Exact duration of contact not recorded, but averages given, intervention: 67.0 (± 25.8), control: 60.1 (± 23.9) | |
| Varenicline | Face‐to‐face | 5 | Unclear | 5 | Unclear | Comparing culturally‐tailored with standard counselling ‐ duration of sessions not stated | |
| NRT | Telephone | See note | See note | 0 | 0 | Control = "access to quitline"; intervention = "up to 12 calls" ‐ averaged 7 calls at 9 minutes each | |
| NRT | Telephone | 8.2 | 80 | 0 | 0 | Intervention numbers based on average number/duration of calls | |
| NRT | Face‐to‐face | 7 | Unclear | 3 | Unclear | ||
| NRT | Face‐to‐face | 3 | 65 | 2 | 5 | Control offered "up to 2 visits", intervention only offered 3rd visit if ready to quit | |
| Bupropion | Face‐to‐face | 12 | 1440 | 12 | 1440 | ||
| Bupropion | Telephone | 4 | Unclear | 1 | 7.5 | Multiple arms ‐ highest vs lowest intensity | |
| Varenicline | Telephone | 5 | 67 | 0 | 0 | ||
| NRT | Face‐to‐face | 12 | 270 | 10 | 150 | ||
| Varenicline | Face‐to‐face | 10 | 120 | 1 | 20 | Duration of sessions not stipulated, but maximum amounts recorded in paper. Intervention: 120, control: 20 | |
| Choice | Telephone | 6 | 150 | 0 | 0 | Intervention sessions listed as 20 to 30 minutes ‐ control was referral to a quitline, but there were no mandated sessions, so contact listed as 0 | |
| Vidrine 2016 (CBT) | NRT | Face‐to‐face | 8 | 960 | 4 | 40 | Vidrine study intervention 2 (control split) |
| Vidrine 2016 (MBAT) | NRT | Face‐to‐face | 8 | 960 | 4 | 40 | Vidrine study intervention 1 (control split) |
| NRT | Face‐to‐face | 12 | Unclear | 12 | Unclear | Sessions' duration not reported | |
| NRT | Face‐to‐face | 1 | 5 | 1 | 5 | ||
| NRT | Face‐to‐face | 9 | 945 | 9 | 945 | Exact duration not listed, but approximate range given | |
| NRT | Face‐to‐face | 7 | 210 | 6 | 180 | Compared 2 interventions, less intensive counted as control | |
| NRT | Face‐to‐face | 3 | Unclear | 1 | Unclear | ||
| NRT | Face‐to‐face | 24 | 1080 | 9 | 180 | ||
| NRT | Face‐to‐face | 4 | 240 | 4 | 240 | ||
| Choice | Face‐to‐face | 14 | 730 | 9 | 150 | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Subgroups by type of pharmacotherapy Show forest plot | 65 | 23331 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| 1.1 NRT | 49 | 16541 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.04, 1.21] |
| 1.2 Bupropion | 5 | 2298 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.46] |
| 1.3 Nortriptyline | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.60, 1.63] |
| 1.4 Varenicline | 2 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.27] |
| 1.5 NRT & bupropion | 3 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.00, 1.54] |
| 1.6 Choice of pharmacotherapy | 5 | 2490 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.00, 1.68] |
| 2 Subgroups by contrast in number of contacts between intervention & control Show forest plot | 63 | 21997 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| 2.1 4 to 8 or > 8 contacts versus no contact | 8 | 4018 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.02, 1.43] |
| 2.2 More than 8 contacts versus 1 to 3 contacts | 4 | 1063 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.70, 1.57] |
| 2.3 4 to 8 contacts versus 1 to 3 contacts | 18 | 9579 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.01, 1.19] |
| 2.4 More than 8 contacts versus 4 to 8 contacts | 12 | 1737 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.98, 1.33] |
| 2.5 Intervention & control in same contact category | 21 | 5600 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.16, 1.50] |
| 3 Subgroups by duration of contact in control condition (not prespecified) Show forest plot | 62 | 21695 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| 3.1 No contact for control | 8 | 4018 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.02, 1.43] |
| 3.2 'Brief intervention' for control | 22 | 10565 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.99, 1.21] |
| 3.3 'Dose response', over 30 minutes contact for control | 32 | 7112 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.08, 1.32] |
| 4 Subgroup by modality of intervention contact (not prespecified) Show forest plot | 65 | 23331 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| 4.1 Intervention delivered by telephone | 8 | 6670 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.37] |
| 4.2 Intervention included face‐to‐face contact | 57 | 16661 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.03, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sensitivity analysis including intermediate intensity conditions. Adjunct behavioural support versus pharmacotherapy alone Show forest plot | 65 | 27425 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.07, 1.20] |
| 1.1 NRT | 49 | 18666 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.03, 1.19] |
| 1.2 Bupropion | 5 | 2298 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.46] |
| 1.3 Nortriptyline | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.60, 1.63] |
| 1.4 Varenicline | 2 | 1513 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.90, 1.26] |
| 1.5 NRT & bupropion | 3 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.00, 1.54] |
| 1.6 Choice of pharmacotherapy | 5 | 4057 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.00, 1.51] |
| 2 By outcome definition Show forest plot | 65 | 23389 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.22] |
| 2.1 12 months validation PP outcomes only | 21 | 6036 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.17] |
| 2.2 12 months validated sustained outcomes | 11 | 3604 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.30] |
| 2.3 < 12 months, but validated | 19 | 5581 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.12, 1.39] |
| 2.4 No validation at all | 13 | 7933 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.08, 1.30] |
| 2.5 > 12 months validation PP outcomes only | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.59, 3.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence at longest follow‐up Show forest plot | 15 | 4138 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.25] |
| 1.1 Family support versus usual care telephone counselling | 1 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.45] |
| 1.2 Face‐to‐face, tests attentional training v placebo training | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.48, 2.50] |
| 1.3 ACT versus CBT telephone counselling | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.74, 2.46] |
| 1.4 Positive psychotherapy versus usual care (face‐to‐face) | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 8.78 [0.49, 157.62] |
| 1.5 Couples treatment versus individual treatment (face‐to‐face) | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.37, 1.43] |
| 1.6 Behavioural activation versus standard treatment (face‐to‐face) | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [0.24, 94.85] |
| 1.7 Culturally tailored versus standard (face‐to‐face) | 4 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.68, 1.92] |
| 1.8 Exercise counselling versus health education (face‐to‐face) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.23, 1.89] |
| 1.9 Adherence counselling versus standard care (telephone) | 1 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.15] |
| 1.10 MIndfulness versus CBT (face‐to‐face) | 1 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.45] |
| 1.11 Quitline facilitation session versus brief advice (telephone) | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.62, 4.00] |
| 1.12 Motivational interviewing versus health education | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.94] |

