Statins for aortic valve stenosis
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | Men and women between 18 and 82 years of age (mean of age = 58 years). Total participants = 269 (from 23 Canadian Centres) with asymptomatic mild aortic valve stenosis defined by maximum aortic valve velocity between 2.5 and 4.0 m/s. Participants were followed for a minimum of 3 years to a maximum of 5 years (median follow‐up = 3.5 years). Baseline Characteristics of Participants: Men = 60.5% (rosuvastatin group) and 63% (placebo group). Tricuspid Aortic Valve = 29.1% (rosuvastatin group) and 34.1% (placebo group). Bicuspid Aortic Valve = 53.7% (rosuvastatin group) and 45.2% (placebo group). Uncertain Aortic Valve Morphology = 17.2% (rosuvastatin group) and 20.7% (placebo group). Mean Pressure Gradient, mean (± SD) mmHg = 22.5 (± 7.6) (rosuvastatin group) and 23.1(± 7.6) (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 40.8 (± 11.1) (rosuvastatin group) and 41.6 (± 10.9) (placebo group). Aortic Valve Area, mean (± SD) cm² = 1.49 (± 0.71) (rosuvastatin group) and 1.56 (± 0.70) (placebo group). Peak AS Velocity, mean (± SD) m/s = 3.16 (± 0.42) (rosuvastatin group) and 3.19 (± 0.42) (placebo group). LDL value (± SD) = 3.18 mmol/L (± 0.63 SD) (rosuvastatin group) and 3.12 mmol/L (± 0.74 SD) (placebo group). Systolic blood pressure, mean (± SD) mmHg = 128.8 (± 15.67) (rosuvastatin group) and 128.4 (± 15.94) (placebo group). Diastolic blood pressure, mean (± SD) = 76.5 (± 10.04) (rosuvastatin group) and 75.9 (± 10.92) (placebo group). Most of the participants were white. | |
| Interventions | Rosuvastatin 40 mg daily (n = 134) or matching placebo (n = 135). Participants were randomised in 1:1 fashion in blocks of 4. | |
| Outcomes | Primary Outcome: Transvalvular aortic stenosis gradient and aortic valve area measured by Doppler echocardiography. Secondary Outcome: Aortic valve replacement and cardiovascular death. | |
| Notes | The trial was supported by the Canadian Institute of Health Research with additional support from Astra Zeneca Canada Inc, but Astra Zeneca Canada had no input into the study design and data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Rosuvastatin and placebo groups were randomised using a computer program at Astra Zeneca Canada Inc, which has no access to the rest of the data. |
| Allocation concealment (selection bias) | Low risk | A randomisation number was from the study database via a secure Internet line. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, site co‐ordinators, investigators and statisticians were all blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | The members of Committee who evaluated all outcomes and serious adverse events were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Participants who withdrew from the study were well‐described and all the randomised patients were evaluated for outcomes. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and is well‐described. |
| Other bias | High risk | The study received funding from the Canadian Institute of Health Research and Astra Zeneca Canada, but Astra Zeneca Canada had no input into the study design and a data analysis. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 155 participants were randomised (older than 18 years of age, mean of age = 68 years) with calcific aortic stenosis, an aortic jet velocity of at least 2.5 m per second and aortic valve calcification measured by Doppler Echocardiography. Participants were followed between 7 and 36 months (median of 25 months). Baseline Characteristics of Participants: Men = 68% (atorvastatin group) and 72% (placebo group). Tricuspid Aortic Valve = 96% (atorvastatin group) and 97% (placebo group). Bicuspid Aortic Valve = 4% (atorvastatin group) and 3% (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 47.8 (± 17.4) (atorvastatin group) and 49.5 (± 19.5) (placebo group). Aortic Valve Area, mean (± SD) cm² = 1.03 (± 0.40) (atorvastatin group) and 1.02 (± 0.41) (placebo group). Aortic Jet Velocity, mean (± SD) m/s = 3.39 (± 0.62) (atorvastatin group) and 3.45 (± 0.67) (placebo group). Most of the participants had hypertension and used aspirin. Current Smoker = 27% (atorvastatin group) and 28% (placebo group). Coronary heart disease = 23% (atorvastatin group) and 26% (placebo group). β‐blocker use = 27% (atorvastatin group) and 34% (placebo group). LDL value (± SD) = 137 mg/dL (± 34) (atorvastatin group) and 133 mg/dL (± 30) (placebo group). | |
| Interventions | Atorvastatin 80 mg daily (n = 77) or matching placebo (n = 78). | |
| Outcomes | Primary Outcome: Progression of aortic valve stenosis by changes in aortic jet velocity on doppler echocardiography and progression of valvular calcification by computer tomography. Secondary Outcome: Aortic valve replacement, death from any cause, death from cardiovascular causes, hospitalisation for severe aortic valve, hospitalisation for any cause and hospitalisation for cardiovascular causes. | |
| Notes | The study drug was provided by Pfizer, which has no access to the rest of the data.The authors received support from Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Atorvastatin and placebo groups were randomised using a minimisation technique with a dedicated, locked computer program (Edinburgh University). |
| Allocation concealment (selection bias) | Low risk | Numbered containers were used. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was no information to say that the investigators were blinded to evaluate the data.The paper just mentioned what each author did in the study. |
| Incomplete outcome data (attrition bias) | Low risk | The number of missing values was well‐balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and well‐described. |
| Other bias | High risk | The study drug was provided by Pfizer, which had no access to the rest of the data.The authors received support from Pfizer. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | Men and women between the ages of 45 and 85 years (mean of age = 67.9) who had asymptomatic, mild to moderate aortic valve stenosis, as assessed on echocardiography, with peak aortic jet velocity of 2.5 to 4 m/s. 1873 participants were randomised. The minimum follow‐up was 4 years (median = 52.2 months). Baseline Characteristics of Participants: Men = 61.5% (simvastatin plus ezetimibe group) and 61.2 % (placebo group). Bicuspid Aortic Valve = 5% (simvastatin plus ezetimibe group) and 6.3% (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 39.3 (± 13.9) (simvastatin plus ezetimibe group) and 39.6 (± 8.7) (simvastatin plus ezetimibe group). Mean Pressure Gradient, mean (± SD) mmHg = 22.7 (± 8.8) (simvastatin plus ezetimibe group) and 23 (± 13.8) (simvastatin plus ezetimibe group). Aortic Valve Area, mean (± SD) cm² = 1.29 (± 0.48) (simvastatin plus ezetimibe group) and 1.27 (± 0.46) (placebo group). Peak Aortic Jet Velocity, mean (± SD) m/s = 3.09 (± 0.55) (simvastatin plus ezetimibe arm) and 3.10 (± 0.54) (placebo arm). Most of the participants were white and had hypertension. Former smoking = 37% (simvastatin plus ezetimibe group) and 35 %(placebo group). Neoplasm = 11% (simvastatin plus ezetimibe group ) and 8.4% (placebo group). β‐blocker and Aspirin use: 28% (simvastatin plus ezetimibe group) and 25% (placebo group). Calcium antagonist use = 17% (simvastatin plus ezetimibe group) and 16% (placebo group). Diuretic (including spironolactone) = 22% (simvastatin plus ezetimibe group) and 28% (placebo group). The mean of left ventricular ejection fraction was 66% for both groups. LDL value, (± SD) = 139 mg/dL (± 35) (simvastatin plus ezetimibe group) and 140 mg/dL (±36) (placebo group). | |
| Interventions | Simvastatin (40 mg) plus ezetimibe (10 mg) (n = 944) or matching placebo (n = 929). | |
| Outcomes | Primary outcome: death from cardiovascular causes, aortic valve replacement, congestive heart failure, non‐fatal myocardial infarction, hospitalisation for unstable angina, coronary artery bypass grafting, percutaneous coronary intervention, or non‐haemorrhagic stroke. Secondary outcome: All primary outcomes above and progression of aortic stenosis as seen on echocardiography and safety of the study drugs. | |
| Notes | 173 study sites in seven European countries. The authors received support from Merck. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not mention how the random sequence generation was carried out. |
| Allocation concealment (selection bias) | Unclear risk | Does not mention how the allocation concealment was carried out. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | The Committee members who evaluated all outcomes were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The number of withdrawals were reported. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and well‐described. |
| Other bias | High risk | The study was provided by Merck, but the scientific responsibility remained with the independent steering committee. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | Women and men between 18 and 45 years of age (mean of age 33 years). Total participants = 242; 63 randomised with congenital aortic valve stenosis and peak aortic valve velocity of 2.5 m/s. The median follow‐up was 2.4 years. Baseline Characteristics of Participants: Men: 70% (rosuvastatin group) and 73% (placebo group). Bicuspid Valve: 93% (rosuvastatin group) and 88% (placebo group). Tricuspid Valve: 7 % (rosuvastatin group) and 12% (placebo group). Mean Pressure Gradient, mean (± SD) mmHg = 27 (± 10) (rosuvastatin group) and 32 (± 17) (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 48 (± 18) (rosuvastatin group) and 56 (± 28) (placebo group). Aortic Valve Area, mean (± SD) cm² = 1.3 (± 0.4) (rosuvastatin group) and 1.3 (± 0.5) (placebo group). Peak AS Velocity, mean (± SD) m/s = 3.4 (± 0.7) (rosuvastatin group) and 3.6 (± 0.9) (placebo group). Most of the participants had a previous intervention (surgical valvulotomy or balloon valvuloplasty) and none or a grade 1 of aortic regurgitation. Systolic blood pressure, mean (± SD) mmHg = 129 (± 16) (rosuvastatin group) and 131 (±16) (placebo group). Diastolic blood pressure, mean (± SD) mmHg = 76 (± 10) (rosuvastatin group) and 78 (± 10) (placebo group). Aortic valve calcium = 40% (rosuvastatin group) and 36% (placebo group). Left ventricular hypertrophy = 20% (rosuvastatin group) and 33%(placebo group). LDL value, (± SD) = 106 mg/dL (± 31) (rosuvastatin group) and 104 mg/dL (± 35) (placebo group). | |
| Interventions | Rosuvastatin 10 mg (n = 30) or matching placebo (n = 33). | |
| Outcomes | Primary outcome: progression of peak aortic valve velocity. Secondary outcome: temporal changes in the left ventricular mass, ascending aortic diameter and N‐terminal pro‐brain natriuretic peptide. | |
| Notes | Study Site: 6 tertiary referral centres for congenital heart disease in The Netherlands and Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Rosuvastatin and placebo groups were randomised using a computer program at the Erasmus Medical University Center pharmacology department, which had no access to the rest of data. |
| Allocation concealment (selection bias) | Low risk | A randomisation number was sent by pharmacology department to the site co‐ordinator and the study medication to the participants. The participants were unaware of the treatment assignment. |
| Blinding of participants and personnel (performance bias) | Low risk | Patients, physicians and investigators were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | The investigators that evaluated all outcomes were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Patients who withdrew from the study were well‐described all the randomised patients were evaluated. |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but all data were properly reported. |
| Other bias | Low risk | There was no other bias. The study did not received support from any organisations. |
LDL: low‐density lipoprotein
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Sub study of ASTRONOMER trial. | |
| Study with methodological flaws and high risk of bias. | |
| Study with methodological flaws and high risk of bias. |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Interest of statin in surgical aortic stenosis: from myocardial preconditioning to ventricular reverse remodeling |
| Methods | Randomised controlled trial. Allocation: Randomised. Endpoint Classification: Efficacy study. Intervention Model: Parallel assignment. Masking: Single‐blind (participant). Primary Purpose: Treatment. Phase: 3. |
| Participants | Inclusion Criteria: 1. Age > or = 70 years and < 80 years. 2. Severe aortic valve stenosis. 3. Indication for aortic valve replacement by bioprosthesis. 4. Ejection fraction > or = 50%. 5. Without treatment with statin and no renal failure. 6. Informed consent signed. Exclusion Criteria: 1. Ischemic heart disease. 2. Concomitant surgery to aortic valve replacement. 3. Emergency surgery and known intolerance for statin. 4. Pregnant woman. |
| Interventions | Atorvastatin 80 mg per day. |
| Outcomes | Primary outcomes: Phase I: To study changes on inflammatory markers after aortic valve replacement. Phase II: To study changes in left ventricular mass at the end of the study (12 months). [ Time Frame: 1 year ] [ Designated as safety issue: No ]. Secondary outcomes: Phase I: To study changes on mitochondrial function, reactive oxygen species, and perioperative systolic and diastolic functions. Phase II: To study changes on clinical status, systolic and diastolic functions during the one year follow‐up. [ Time Frame: 1 year ] [Designated as safety issue: No ]. |
| Starting date | December 2008. |
| Contact information | Michel KINDO, MD. University Hospital, Strasbourg. France. E‐mail: michel.kindo@chru‐strasbourg.fr. |
| Notes | ClinicalTrials.gov Identifier: NCT00811330. This study is currently recruiting participants. First received: December 17, 2008. Last updated: June 18, 2015. Last verified: June 2015. |
| Trial name or title | Statin therapy in asymptomatic aortic stenosis |
| Methods | Randomised controlled trial. Allocation: Randomised. Endpoint Classification: Efficacy study. Intervention Model: Single‐group assignment. Masking: Double‐blind (participant, investigator, outcomes assessor). Primary Purpose: Treatment. Phase: 2. |
| Participants | Inclusion Criteria: 1. Age 21 years to 80 years. 2. Gender: both. 3. Mild to moderate aortic stenosis. 4. No symptoms caused by aortic stenosis. 5. Written informed consent to participate in the study. 6. Aortic valve leaflet thickening with reduced systolic opening. 7. Reduced aortic valve area > 0.8 cm2 and < 1.5 cm2. 8. Maximum aortic jet velocity at rest > 2.5 m/s. Exclusion Criteria: 1. Symptoms caused by aortic stenosis. 2. Aortic valve area < 0.7 cm2. 3. Severe aortic regurgitation. 4. Reduced left ventricular ejection fraction (< 50%). 5. Any valve disease with indication for surgery. 6. Coronary artery disease. 7. Therapy refractory arterial hypertension. 8. Comorbid noncardiac diseases or other reasons which make a regular follow‐up impossible. 9. Other indication for treatment with statins. 10. Women of childbearing potential not using the contraception method(s) specified in this study (specify), as well as women who are breastfeeding. 11. Known sensitivity to study drug(s) or class of study drug(s). 12. Patients with severe medical condition(s) that in the view of the investigator prohibits participation in the study (specify as required). 13. Use of any other investigational agent in the last 30 days. |
| Interventions | 40 mg fluvastatin daily. |
| Outcomes | Primary outcomes: 1. Progression of calcified aortic stenosis measured by: [ Time Frame: 24 months ] [ Designated as safety issue: No ]. 2. Transthoracic echocardiography (peak max/ mean, velocity max and aortic valve area) [ Time Frame: 24 months ] [ Designated as safety issue: No ]. 3. Catheterisation (peak to peak gradient, left ventricular function and compliance) [ Time Frame: 24 months ] [ Designated as safety issue: No ]. Secondary outcomes: Number of cardiovascular events [ Time Frame: 24 months ] [ Designated as safety issue: No ]. |
| Starting date | January 2003. |
| Contact information | Claudia Walther, MD. University of Leipzig. Germany. Telephone: xx49‐341‐8651428. E‐mail: [email protected]‐leipzig.de. |
| Notes | ClinicalTrials.gov Identifier: NCT00176410. The recruitment status of this study is unknown because the information has not been verified recently. First received: September 13, 2005. Last updated: January 13, 2010. Last verified: September 2006. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean pressure gradient Show forest plot | 2 | 1935 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.88, 0.80] |
| Analysis 1.1  Comparison 1 Statin versus Placebo, Outcome 1 Mean pressure gradient. | ||||
| 2 Valve area Show forest plot | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.28, 0.14] |
| Analysis 1.2  Comparison 1 Statin versus Placebo, Outcome 2 Valve area. | ||||
| 3 Aortic jet velocity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Statin versus Placebo, Outcome 3 Aortic jet velocity. | ||||
| 4 Freedom from valve replacement Show forest plot | 4 | 2360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| Analysis 1.4  Comparison 1 Statin versus Placebo, Outcome 4 Freedom from valve replacement. | ||||
| 5 Death from cardiovascular cause Show forest plot | 3 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.15] |
| Analysis 1.5  Comparison 1 Statin versus Placebo, Outcome 5 Death from cardiovascular cause. | ||||
| 6 Hospitalisation for any reason Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Statin versus Placebo, Outcome 6 Hospitalisation for any reason. | ||||
| 7 Severe adverse events Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Statin versus Placebo, Outcome 7 Severe adverse events. | ||||
| 7.1 Muscle pain | 3 | 2204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.09] |
| 7.2 Hepatic enzymes elevation | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.24, 5.67] |
| 7.3 Hepatitis | 2 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.25] |
| 7.4 Gastrointestinal condition | 3 | 2296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.25] |
| 7.5 Creatine kinase | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.33] |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
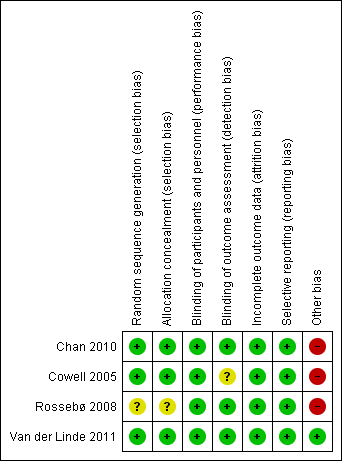
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Statin versus Placebo, Outcome 1 Mean pressure gradient.

Comparison 1 Statin versus Placebo, Outcome 2 Valve area.

Comparison 1 Statin versus Placebo, Outcome 3 Aortic jet velocity.

Comparison 1 Statin versus Placebo, Outcome 4 Freedom from valve replacement.

Comparison 1 Statin versus Placebo, Outcome 5 Death from cardiovascular cause.

Comparison 1 Statin versus Placebo, Outcome 6 Hospitalisation for any reason.

Comparison 1 Statin versus Placebo, Outcome 7 Severe adverse events.
| Statin versus Placebo for aortic valve stenosis | |||||
| Patient or population: patients with aortic valve stenosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Statin | ||||
| Mean pressure gradient (mmHg) Better indicated by lower scores. Follow‐up: median 2.4 to 4.5 years | The mean mean pressure gradient in the control groups was | The mean mean pressure gradient in the intervention groups was | MD ‐0.54 (‐1.88 to 0.80) | 1935 | ⊕⊕⊝⊝ |
| Valve area (cm2) Follow‐up: median 2.4‐ to 3.5 years | The mean valve area in the control groups was | The mean valve area in the intervention groups was | MD ‐0.07 (‐0.28 to 0.14) | 127 | ⊕⊕⊝⊝ |
| Aortic jet velocity (m/s) | The mean aortic jet velocity in the control groups was | The mean aortic jet velocity in the intervention groups was | MD ‐0.06 (‐0.26 to 0.14) | 155 | ⊕⊕⊝⊝ |
| Freedom from valve replacement | Study population | RR 0.93 | 2360 | ⊕⊕⊕⊝ | |
| 281 per 1000 | 261 per 1000 | ||||
| Moderate population | |||||
| 222 per 1000 | 206 per 1000 | ||||
| Death from cardiovascular cause | Study population | RR 0.80 | 2297 | ⊕⊕⊝⊝ | |
| 56 per 1000 | 45 per 1000 | ||||
| Moderate population | |||||
| 39 per 1000 | 31 per 1000 | ||||
| Hospitalisation for any reason | 154 per 1000 | 129 per 1000 | RR 0.84 | 155 | ⊕⊝⊝⊝ |
| Adverse events ‐ Muscle pain | Study population | RR 0.91 | 2204 | ⊕⊕⊕⊝ | |
| 168 per 1000 | 153 per 1000 | ||||
| Moderate population | |||||
| 30 per 1000 | 27 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded by one due to randomisation and allocation being unclear for Rossebø 2008. | |||||
| Mild Aortic stenosis | Moderate Aortic stenosis | Severe Aortic stenosis | |
| Valve area | 1.5 cm² | 1.0 to 1.5 cm² | < 1.0 cm² |
| Mean pressure gradient | < 20 mmHg | 20 to 39 mmHg | > 40 mmHg |
| Aortic jet velocity | < 2.0 to 2.9 m per second | 3.0 to 3.9 m per second | > 4.0 m per second |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean pressure gradient Show forest plot | 2 | 1935 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.88, 0.80] |
| 2 Valve area Show forest plot | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.28, 0.14] |
| 3 Aortic jet velocity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Freedom from valve replacement Show forest plot | 4 | 2360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| 5 Death from cardiovascular cause Show forest plot | 3 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.15] |
| 6 Hospitalisation for any reason Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Severe adverse events Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Muscle pain | 3 | 2204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.09] |
| 7.2 Hepatic enzymes elevation | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.24, 5.67] |
| 7.3 Hepatitis | 2 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.25] |
| 7.4 Gastrointestinal condition | 3 | 2296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.25] |
| 7.5 Creatine kinase | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.33] |


