تاثیر تناوب تعویض پانسمان وسایل دسترسی به ورید مرکزی بر عفونتهای وابسته به کاتتر
چکیده
پیشینه
افرادی که در بخش مراقبتهای ویژه بستری میشوند و آنهایی که نیازمند مراقبت سلامت مزمن هستند، غالبا به دسترسی عروقی طولانی‐مدت نیاز دارند. وسایل دسترسی به ورید مرکزی (central venous access devices; CVADs) به منظور تجویز داروهای داخل‐وریدی و نمونهگیری از خون استفاده میشوند. CVADها دارای یک پوشش بوده و با یک چسب یا نوار چسب محفوظ شدهاند تا از عفونی شدن آنها جلوگیری شود و بیتحرک بمانند. پانسمان به هنگام آلوده شدن با خون یا هنگام برداشتن آن از روی پوست تعویض میشود. برداشتن و جایگذاری مکرر پانسمان میتواند به پوست آسیب برساند. پوست یک لایه مهم است که بدن را از عفونت مصون نگاه میدارد. تعویض کمتر پانسمان میتواند آسیب به پوست را کاهش دهد، اما هنوز مشخص نیست که تا چه اندازه روی بروز عفونت وابسته به کاتتر تاثیر دارد.
اهداف
بررسی تاثیر دفعات تعویض پانسمان CVAD بر بروز عفونتهای وابسته به کاتتر و پیامدهای دیگر شامل درد و آسیب پوستی.
روشهای جستوجو
در جون 2015 منابع زیر را جستوجو کردیم: پایگاه ثبت تخصصی گروه زخم در کاکرین (Cochrane Wounds Specialised Register)؛ پایگاه مرکزی ثبت کارآزماییهای بالینی کاکرین (CENTRAL) (کتابخانه کاکرین (The Cochrane Library))؛ ؛Ovid MEDLINE؛ Ovid MEDLINE (استنادات نمایه نشده و در حال انجام دیگر)؛ Ovid EMBASE؛ و EBSCO CINAHL. همچنین، پایگاههای ثبت کارآزماییهای بالینی را برای یافتن کارآزماییهای ثبت شده جستوجو کردیم. هیچ گونه محدودیتی از نظر زبان، تاریخ یا وضعیت نشر یا محیط انجام مطالعه اعمال نکردیم.
معیارهای انتخاب
تمامی کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) که تاثیر تناوب تعویض پانسمان CVAD را بر بروز عفونتهای وابسته به کاتتر در بیماران تمامی بخشهای مراقبت سلامت ارزیابی کرده بودند.
گردآوری و تجزیهوتحلیل دادهها
از متدولوژی استاندارد مرور کاکرین استفاده کردیم. دو نویسنده مرور بهطور مستقل از هم مطالعات را برای ورود ارزیابی کردند و به بررسی خطر سوگیری (bias) و استخراج دادهها پرداختند. در صورت نیاز، متاآنالیز (meta‐analysis) انجام دادیم یا در زمانی که ناهمگونی در دادهها وجود داشت، دادهها را سنتز کردیم.
نتایج اصلی
پنج کارآزمایی (با 2277 شرکتکننده) انتخاب کردیم که دفعات مختلف تعویض پانسمان CVAD را با هم مقایسه کرده بودند. تمامی مطالعات در اروپا انجام و بین 1995 تا 2009 منتشر شده بودند. شرکتکنندگان از بخشهای مراقبتهای ویژه و بخشهای مراقبت سرطانی از یک بیمارستان اطفال و چهار بیمارستان بزرگسالان انتخاب شده بودند. در مطالعات از انواع مختلف پانسمانهای شفاف استفاده شده بود و فواصل طولانی تعویض پانسمان (5 تا 15 روز، گروه مداخله) را با فواصل کوتاهتر تعویض پانسمان (2 تا 5 روز، گروه کنترل) با هم مقایسه کرده بودند. شرکتکنندگان مطالعه هر یک تا زمان خروج CVAD یا تا زمان ترخیص از ICU یا بیمارستان پیگیری شدند.
عفونت خونی وابسته به کاتتر (catheter‐related bloodstream infection; CRBI) تایید شده
یک کارآزمایی 995 بیمار را که کاتتر ورید مرکزی دریافت کردند، به صورت تصادفی به دو گروه با فواصل تعویض پانسمان کوتاه یا بلند تقسیم کرده و وقوع CRBSI را اندازهگیری کردند. مشخص نبود که تفاوتی از نظر CRBSI بین فواصل تعویض پانسمان کوتاه یا طولانی وجود داشت یا خیر (RR: 1.42؛ 95% فاصله اطمینان (CI): 0.40 تا 4.98؛ شواهد با کیفیت پائین).
عفونت خونی وابسته به کاتتر مشکوک
دو کارآزمایی 151 نفر را از نظر فواصل تعویض پانسمان به دو گروه فواصل کوتاه یا طولانی تقسیم کرده و به اندازهگیری CRBSI مشکوک پرداختند. مشخص نبود بین افرادی که فواصل تعویض پانسمانشان طولانی یا کوتاه بود، تفاوتی از نظر خطر CRBSI مشکوک وجود داشت یا خیر (RR: 0.70؛ 95% CI؛ 0.23 تا 2.10؛ شواهد با کیفیت پائین).
مورتالیتی به هر علتی
سه کارآزمایی 896 شرکتکننده را به صورت تصادفی به دو گروه با فواصل تعویض طولانی یا کوتاه تقسیم و مورتالیتی به هر علتی را اندازهگیری کردند. مشخص نبود که از نظر خطر مرگومیر به هر علتی، تفاوتی بین این دو گروه وجود دارد یا خیر (RR: 1.06؛ 95% CI؛ 0.90 تا 1.25؛ شواهد با کیفیت پائین).
عفونت محل کاتتر
دو کارآزمایی 371 نفر را از نظر فواصل تعویض پانسمان به دو گروه کوتاه یا طولانی تقسیم کرده و ابتلا به عفونت محل کاتتر را اندازهگیری کردند. مشخص نبود که بین افرادی که فواصل تعویض پانسمانشان بلند یا کوتاه بود، تفاوتی از نظر عفونت محل کاتتر وجود داشت یا خیر (RR: 1.07؛ 95% CI؛ 0.71 تا 1.63؛ شواهد با کیفیت پائین).
آسیب پوستی
یک کارآزمایی کوچک (112 کودک) و سه کارآزمایی (1475 بزرگسال) میزان آسیب پوستی را اندازهگیری کرده بودند. شواهدی با کیفیت بسیار پائین در مورد تاثیر فواصل زمانی طولانی‐مدت بین تعویض پانسمان بر آسیب پوستی در مقایسه با فواصل کوتاهتر وجود داشت (کودکان: RR برای امتیازدهی ≥ 2 در مقیاس آسیب پوستی 0.33؛ 95% CI؛ 0.16 تا 0.68؛ دادههای مربوط به بزرگسالان تجمیع نشده بود).
درد
دو مطالعه با 193 شرکتکننده به اندازهگیری درد پرداخته بودند. مشخص نبود که تفاوتی بین فواصل طولانیتر تعویض پانسمان CVAD با فواصل کوتاهتر، از نظر درد هنگام برداشتن پانسمان وجود دارد یا خیر (RR: 0.80؛ 95% CI؛ 0.46 تا 1.38) (شواهد با کیفیت پائین).
نتیجهگیریهای نویسندگان
بهترین شواهدی که در حال حاضر در دسترس هستند، غیر‐قابل نتیجهگیریاند، چرا که مشخص نیست فواصل طولانیتر تعویض پانسمان CVAD از نظر افزایش یا کاهش عفونت ناشی از کاتتر، مورتالیتی یا درد چه تفاوتی نسبت به فواصل کوتاهتر دارند.
PICOs
خلاصه به زبان ساده
جهت کاهش عفونت وابسته به کاتتر، با چه فاصله زمانی باید پانسمان وسایل دسترسی به ورید مرکزی (central venous access devices; CVADs) تعویض شود؟
پیشینه
یک وسیله دسترسی به ورید مرکزی (CVAD، که تحت عنوان کاتتر ورید مرکزی نیز شناخته میشود) یک لوله توخالی است که در یک ورید بزرگ قرار داده شده و سر آن در نزدیکی قلب قرار میگیرد. CVAD این امکان را فراهم میکند که داروها، مایعات و فرآوردههای خونی به طور مستقیم وارد گردش خون شوند و نیز بتوان جهت آنالیز، نمونههای خونی گرفت. یکی از اثرات سوء CVAD عفونت گردش خون است که عفونت خونی وابسته به کاتتر یا CRBSI نامیده میشود و میتواند خطرناک و حتی تهدید کننده حیات باشد. برخی CVADها میتوانند تا هفتهها، ماهها یا سالها در محل باقی بمانند. بیشتر بیمارانی که در بخش مراقبتهای ویژه بستری میشوند، یک CVAD خواهند داشت، به بیمارانی که وریدهای ضعیف داشته یا به درمان طولانی‐مدت نیاز دارند نیز این روش پیشنهاد میشود. پانسمانها روی محل جایگزاری کاتتر، در جایی که کاتتر وارد وریدها شده، معمولا قفسه سینه، گردن یا بازو قرار داده میشوند تا از پوست اطراف محافظت به عمل آورند. پانسمان از ابتلا به عفونت پیشگیری کرده و حرکت CVAD را متوقف میکند. وقتی پانسمان کثیف یا جابجا شود، آن را تعویض میکنند. تعویض مکرر پانسمان میتواند به پوست اطراف آسیب برساند، لذا ممکن است در هنگام برداشتن پانسمان بیماران دچار درد یا آسیب پوستی شوند. همچنین تعویض مکرر پانسمان امری پُر‐هزینه است.
ما بر آن شدیم تا ببینیم تعویض پانسمان در فواصل طولانی یا کوتاه چه منفعت یا ضررهایی به همراه دارد. برخی بیمارستانها یا مراکز مراقبت سلامت توصیه میکنند هرچند روز پانسمان تعویض شود، در حالی که برخی دیگر به مدت طولانیتری پانسمان را در محل نگه میدارند.
سوال مطالعه مروری
شواهد در دسترس را در رابطه با تاثیر فاصله زمانی تعویض پانسمان CVADها و اینکه چه تاثیری بر خطر بروز CRBSI و دیگر عوارض دارند، مرور کردیم. پنج مطالعه را شناسایی کردیم که دادههای لازم را برای این مرور ارائه میکردند.
ویژگیهای مطالعه
پنج مطالعهای که در این مرور وارد شدند، بین سالهای 1995 تا 2009 منتشر شده و در مجموع شامل 2277 شرکتکننده میشدند. این مطالعات در چهار کشور انجام شده بودند (دو مطالعه در فرانسه، و در هر کدام از کشورهای ایتالیا، سودان و جمهوری چک یک مطالعه). یک مطالعه شامل کودکان و چهار کارآزمایی دیگر فقط شامل بزرگسالان میشد. چهار مطالعه در بیماران سرطانی و یکی در بیماران بستری در بخش مراقبتهای ویژه به انجام رسیده بودند.
فاصله زمانی بین تعویض پانسمان را به دو دسته کوتاه (2 تا 5 روز) در گروهی که تعویض پانسمان بیشتر انجام میشد و طولانی (5 تا 15 روز) در گروهی که کمتر پانسمان تعویض میشد، تقسیمبندی کردیم. تمامی مطالعات از پانسمان شفاف ساخته شده از مواد مصنوعی استفاده و در دو مطالعه، در زمانی که پوست دچار آسیب میشد از یک گاز (یک پانسمان پارچهای که به پوست نمیچسبد) که با نوار چسب محکم شده بود، استفاده کردند. در تمامی کارآزماییها پانسمان CVAD به طور روزانه کنترل میشد و شرکتکنندگان حداقل تا زمان برداشته شدن CVAD یا تا زمان ترخیص پیگیری شدند. در یک مطالعه، تدارک یکی از محصولات را تولید کننده آن بر عهده گرفته بود، اما این مساله تاثیری بر طراحی یا چگونگی آنالیز نتایج و گزارش آنها نداشت.
نتایج کلیدی
شواهد فعلی در مورد اینکه تکرر تعویض پانسمان CVADها بر خطر بروز CRBSI یا مرگومیر تاثیری میگذارد یا خیر، نامطمئن هستند. برخی موارد خاصی که به بیماران بستگی داشت، مثل درد هنگام برداشتن پانسمان یا آسیب پوستی که پانسمان میتواند ایجاد کند، از مشکلاتی بودند که به خود پانسمان کردن مربوط میشدند. هیچ گونه شواهدی پیدا نکردیم که نشان دهد درد، که به طور روزانه ارزیابی میشد، تحت تاثیر فراوانی تعویض پانسمان قرار داشته باشد.
کیفیت شواهد
کیفیت شواهد، در سطح بسیار پائین یا پائین بود. به دلیل کم بودن تعداد مطالعات و کوچک بودن آنها، طراحی ضعیف مطالعه و تفاوت در نتایج بین مطالعات، کیفیت آنها را پائین در نظر گرفتیم. هنوز لازم است مطالعاتی با طراحی بهتر انجام شود تا نشان دهند که فواصل طولانیتر یا کوتاهتر بین تعویض پانسمان در پیشگیری از عفونت وابسته به کاتتر، مورتالیتی، آسیب پوستی، درد هنگام برداشتن پانسمان، کیفیت زندگی و هزینه چه تاثیری دارند.
این خلاصه به زبان ساده تا 10 جون 2015 بهروز است.
Authors' conclusions
Summary of findings
| Patient or population: patients with a central venous access device | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Quality of the evidence | What happens | ||
| Without longer interval (5 ‐ 15 days) | With longer interval (5 ‐ 15 days) | Difference | ||||
| Catheter‐related blood stream infection (CRBSI) | RR 1.42 | Study population | ⊕⊕⊝⊝ | Longer intervals between dressing changes may have little or no effect on catheter‐related blood stream infection | ||
| 8 per 1000 | 12 per 1000 | 4 more per 1000 | ||||
| All‐cause mortality | RR 1.06 | Study population | ⊕⊕⊝⊝ | Longer intervals between dressing changes probably have little or no effect on death from any cause | ||
| 354 per 1000 | 375 per 1000 | 21 more per 1000 | ||||
| Skin damage Follow up: unclear | Not estimable | Skin damage was reported in four studies. Two provided data but their results were not combined due to inconsistency of size and direction of the effects. One study in children found less skin damage in the longer interval group (8/56) compared with the shorter interval group (24/56). Rates of skin damage in one study in adults were similar (7/39 in longer interval versus 6/42 in shorter interval).9 | ⊕⊝⊝⊝ | It is uncertain whether longer (compared with shorter) intervals between dressing changes reduce skin damage | ||
| Pain Follow up: unclear | RR 0.80 | Study population | ⊕⊕⊝⊝ | It is uncertain whether longer (compared with shorter) intervals between dressing changes affect pain on dressing removal | ||
| 347 per 1000 | 278 per 1000 | 69 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for risk of bias due to lack of blinding of participants and personnel and for a probable unit of analysis error (individual participants randomised but numbers of infections reported) 2 Downgraded for serious imprecision: result consistent with a reduction in CRBSI or an almost 5 fold increase 3 Downgraded for risk of bias due to lack of blinding of participants and personnel 4 Downgraded for imprecision: result consistent with a 10% reduction in mortality or a 25% increase 5 Downgraded twice for serious risk of bias: risk of performance bias due to lack of blinding of participants and personnel; different dressings were used in response to skin damage 6 Downgraded for inconsistency: experimental and control groups were different between studies and frequency of dressing changes overlapped between longer and shorter groups 7 Downgraded for imprecision 8 Downgraded for risk of bias: blinding of outcome assessment not described 9 Data from two additional RCTs could not be extracted and used within the analysis. One study presented toxicity on a 5‐point scale and reported no differences between groups. We are unable to use the data from the fourth study due to the 2 x 2 factorial design. | ||||||
Background
See Appendix 1 for glossary of terms.
Description of the condition
Central venous access devices (CVADs), also commonly called central venous catheters, are inserted when a patient requires venous access over an extended period of time. These devices are commonly used in patients admitted to intensive care units, for patients with oncological and haematological malignancies and other chronic health problems. CVADs are used to administer intravenous drugs including chemotherapy and immunosuppression, fluids, blood products, total parenteral nutrition, and for blood sampling.
The external portion of the CVAD can be partially tunnelled under the skin or non‐tunnelled. Non‐tunnelled catheters are those where the insertion site is directly above the entry into the vein (CNSA 2007); they are for short‐term use and can be sited using the jugular, subclavian or femoral veins (Hayden 2005). Peripherally inserted central catheters (PICCs) are also non‐tunnelled and are inserted into the central circulation usually from a peripheral vein in the upper arm ‐ they can remain in place for months (Gabriel 2005; Hayden 2005; RNAO 2005). Tunnelled CVADs are surgically implanted with a section of the catheter positioned in a subcutaneous tunnel between the entry site, which heals over, to the vein and the skin exit site (CNSA 2007), and are typically placed into the superior vena cava.
CVADs are covered with a dressing and secured with a separate securement device or skin adhesive, such as tape or transparent adhesive film, to prevent infection and movement (Hunt 1997; Wilson 2006 and Elkabir 2001; Rippon 2007, respectively). Newer products are available that combine both the dressing and securement function. The repeated application and removal of adhesives or adhesive tapes and dressings from the same site can cause damage to the skin by skin stripping, that is the removal of the superficial stratum corneum, which can cause development of inflammatory skin reactions, oedema and soreness (Cutting 2008).The entry and exit sites of CVADs are inspected visually daily for signs of infection, and this may require removal of the dressing. Dressings are replaced if they become loose, soiled or wet. Frequent dressing changes can impact upon the skin integrity surrounding the CVAD entry and exit sites. If skin integrity is compromised, rates of catheter‐related infection (CRI) including CRBSI may be affected.
Description of the intervention
The intervention of interest in this review is the frequency of dressing changes. Adhesives or adhesive tapes are designed to bond to the skin under a variety of conditions, such as flexure, changing temperatures, in the presence of perspiration and external moisture, but should also be easy to peel off in order to ensure minimal discomfort and trauma (Karwoski 2004). Choice of dressings and frequency of changes depends upon clinical practice protocols, and patient and clinician preferences (CNSA 2007; Gillies 2003; O'Grady 2011). The general consensus is for gauze dressings to be changed every 48 hours (CNSA 2007; Hadaway 2003; O'Grady 2011; Rosenthal 2003; RNAO 2005), and transparent semi‐permeable dressings every seven days, or earlier if the integrity of the dressings is compromised or there is blood underneath the dressing (Camp‐Sorrell 2004; CNSA 2007; Loveday 2014; Hadaway 2003; INS 2011; IVNNZ 2012; O'Grady 2011; Rosenthal 2003; RNAO 2005).
How the intervention might work
CVADs are commonly used in patients admitted to intensive care and those diagnosed with chronic diseases and cancer. These patients are often immunocompromised and healing processes are diminished due to their disease or treatment (Cutting 2008; Lotti 1998). The skin provides protection as a barrier to infection (Tortura 2000), so maintaining skin integrity is particularly important for these patients. Chemotherapy and radiation regimens can cause adverse skin changes (DeSpain 1992; Glean 2001; Hopewell 1990). Other patients at particular risk of skin damage are older adults, babies and young children who, by nature of their age, have fragile skin (Hollingworth 2009), and patients with disease‐related factors associated with dermatological changes (Cutting 2008). Constantly removing adhesives or adhesive tapes to change dressings may further aggravate already damaged skin (Hollingworth 2009). Thus, reducing the frequency of dressing changes may reduce skin damage, pain, costs, incidence of skin colonization and the potential for CRIs. A theoretical risk exists that transparent dressings increase surface humidity, which may lead to increased microbial colonisation at the catheter site and so increase the risk of CRI (Wille 1993). Therefore, prolonging the interval between dressing changes may increase infection due to increased skin colonisation underneath the dressing.
Why it is important to do this review
There is a lack of clear evidence concerning the optimal frequency of dressing changes for CVADs. Clinical guidelines have influenced a general consensus around the timing of dressing changes for CVADs, but the guidelines themselves are based on limited evidence. For example, recommendations in the Centers for Disease Control and Prevention (CDC) guidelines suggest only tunnelled CVADs with well‐healed sites might not require dressings (O'Grady 2011), but there is no recommendation about frequency of changes before sites are healed. Patients diagnosed with cancer are particularly vulnerable to skin damage because of the treatment they receive (Cutting 2008; Lotti 1998). Extending the time between dressing changes may reduce the damage and also reduce the associated costs. However, it remains unclear whether prolonging the time between changes results in other complications, such as an increased risk of bloodstream infection. We will examine the existing research to determine how frequently dressings that are used to protect CVADs should be changed. We are primarily interested in the incidence of CRI, but will also consider outcomes such as pain and skin damage.
Objectives
To assess the effect of the frequency of CVAD dressing changes on the incidence of CRIs and other outcomes including pain and skin damage.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) evaluating the effect of the frequency of CVAD dressing changes on the incidence of CRIs. We excluded studies comparing different dressing products and studies where the frequency of dressing change was not the only systematic difference between treatment arms as we required an explicit focus upon the frequency of changing the same type of dressing. Cluster‐randomised controlled trials, quasi‐randomised trials and cross‐over trials were not included in order to minimise potential bias in accordance with Reeves 2011.
Types of participants
Participants of any age requiring a CVAD in any healthcare or community setting.
Types of interventions
Trials comparing any frequency of changing the same type of dressings for the securement of a CVAD.
Types of outcome measures
Primary outcomes
-
Incidence of confirmed catheter‐related bloodstream infection (CRBSI) defined as bacteraemia or fungaemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (i.e. fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter. One of the following should be present for a positive diagnosis: a positive semi‐quantitative (> 15 colony forming units (CFU)/catheter segment) or quantitative (> 10³ CFU/catheter segment) culture from a catheter segment in which the same organism (species and antibiogram) is isolated from the catheter segment and peripheral blood (CDC 2002).
-
Incidence of suspected CRBSI, as described by the trial investigator.
-
All‐cause mortality.
Secondary outcomes
-
Incidence of catheter entry and exit site infection, as described by the trial investigator.
-
Skin damage, using an assessment tool (such as the Eastern Cooperative Oncology Group Common Toxicity Criteria for Skin (ECOG 2007; see Appendix 2).
-
Pain, using any validated measure or scale described by the trial investigator.
-
Quality of life, using any validated measure or scale described by the trial investigator.
-
Cost.
Search methods for identification of studies
Electronic searches
In June 2015 we searched the following electronic databases to identify reports of relevant randomised clinical trials:
-
The Cochrane Wounds Specialised Register (searched 11 June 2015);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 6);
-
Ovid MEDLINE (1946 to 10 June 2015);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 10 June 2015);
-
Ovid EMBASE (1974 to 10 June 2015);
-
EBSCO CINAHL (1982 to 11 June 2015).
The search strategies used can be found in Appendix 3. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no restrictions with respect to language, date of publication or study setting.
In July 2014 we searched the following clinical trials registers:
-
Australian and New Zealand Clinical Trials Register (www.anzctr.org.au)
-
ClinicalTrials.gov (www.clinicaltrials.gov/)
-
Current Controlled Trials (www.controlled‐trials.com/)
-
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/)
-
European Union Clinical Trials Register (www.clinicaltrialsregister.eu/)
Searching other resources
We searched reference lists of all retrieved and relevant publications identified by these strategies for further studies not identified by the methods outlined above.
Data collection and analysis
Selection of studies
Two review authors (NG and JW) acting independently located potentially eligible studies by screening titles and abstracts from the search. We obtained full copies of potentially eligible studies and acting independently, decided on inclusion based on the predefined inclusion and exclusion criteria. We have listed the excluded studies with reasons for their exclusion (Characteristics of excluded studies). Disagreements were resolved by discussion among the review authors.
Data extraction and management
We extracted data from eligible studies using a data extraction sheet. This summary contained baseline characteristics of study and control group participants and included the number of participants, age, gender, disease, treatment, reason for insertion of CVAD, method of insertion, profession of inserter (doctor, radiographer or nurse), anatomical location of insertion, type of CVAD, number of lumens on the CVAD, dwell time of the CVAD, dressing protocol, deviation from planned dressing day and reason, number of dressing changes during dwell time of the CVAD, known allergies to dressings, skin complexion and known history of or current positive blood cultures. We extracted the criteria for patient inclusion and exclusion, a description of the intervention and the number of patients randomised to each intervention. We recorded the healthcare settings in which the interventions were performed. In addition, we extracted the duration of follow‐up and numbers lost to follow‐up as well as outcomes.
When more than one publication arose from a study, we extracted data from all relevant publications, but did not duplicate data in analyses. Two review authors (NG and JW) extracted all data independently. Disagreements were resolved by discussion. If this had not resulted in consensus, the third review author's opinion would have been decisive (RC).
Assessment of risk of bias in included studies
Two review authors (NG and JW) independently assessed each eligible study using the Cochrane tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (for example, extreme baseline imbalance; see Appendix 4 for details of criteria on which the judgements were based). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study. Disagreements were discussed in a consensus meeting.
The assessment of risk of bias is presented using a 'Risk of bias' summary figure, which includes all the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the confidence the reader may give to the results of the particular studies.
Measures of treatment effect
Event rates for dichotomous outcomes are presented as risk ratios (RR) with 95% confidence intervals (CI). There were no continuous outcomes or time to event outcomes.
Unit of analysis issues
The unit of analysis was individual patients with a CVAD in situ. All five studies included in the review randomised the patients and not their CVAD, but three studies presented some results per CVAD or per dressing and we contacted the authors to obtain the results per patient (Engervall 1995; Rasero 2000; Timsit 2009). Timsit 2009 was the only paper to present CRBSI, our primary outcome. A decision was made to present the data per catheter rather than per patient for this one outcome in the absence of any other data. Cross‐over and cluster‐randomised trials were not included.
Dealing with missing data
If data were missing from the published trial reports, we made attempts to contact the study authors to complete the information necessary for the analysis and 'Risk of bias' assessment. We did not impute data if the missing data were not obtained after several attempts to contact the author. If no further information was provided we used an available case analysis. We addressed the potential impact of missing data on the findings of the review in the Discussion.
Assessment of heterogeneity
We included trials in a meta‐analysis if the study population and the interventions studied were sufficiently similar. We assessed statistical heterogeneity using the I² statistic (Higgins 2003), which examines the percentage of total variance across studies due to heterogeneity rather than chance. Values of I² under 25% indicate a low level of heterogeneity and justify use of a fixed‐effect model for meta‐analysis. Values of I² between 25% and 75% are considered moderate and a random‐effects model can be used. Values of I² higher than 75% indicate high levels of heterogeneity and pooling should not be undertaken.
Assessment of reporting biases
We reported each outcome separately. We were not able to use funnel plots to assess reporting biases, as an insufficient number of studies was included.
Data synthesis
If the studies were sufficiently similar we pooled them using a fixed‐effect model for values of I² under 25%. In the event of moderate heterogeneity we employed a random‐effects model. If the studies were statistically heterogenous (I² ≥ 75%) we produced a qualitative summary (O'Rourke 1989).
'Summary of findings' tables
We have presented the main results of the review in 'Summary of findings' (SoF) tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The SoF tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence with regard to the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). The following outcomes, which we believe to be the most important both clinically and to the consumer, are presented in the SoF tables:
-
CRBSI;
-
all‐cause mortality;
-
skin damage;
-
pain.
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analyses. We planned to investigate heterogeneity using sensitivity analysis (see below).
Sensitivity analysis
Too few studies were included in the meta‐analyses to conduct a sensitivity analysis. We were not able to explore the effect of concealment of allocation (adequate versus not reported, unclear or not undertaken).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
In this review comparisons were grouped by longer duration between dressing changes versus shorter duration between dressing changes with the shorter duration treated as the control group as this is considered standard practice by the trial authors.
Results of the search
The electronic search identified 471 titles. Of these, 453 were excluded by an examination of the titles and abstracts: two were duplicates; 136 were excluded because they did not contain information about CVADs; and 315 compared different dressings or were on other topics. The remaining 18 full texts were retrieved and reviewed. Of these, 10 did not meet the inclusion criteria and were excluded (see Characteristics of excluded studies). Five published RCTs met the inclusion criteria (Benhamou 2002; Engervall 1995; Rasero 2000; Timsit 2009; Vokurka 2009), and three were supplementary references to included papers (see Criteria for considering studies for this review and Characteristics of included studies and Figure 1).
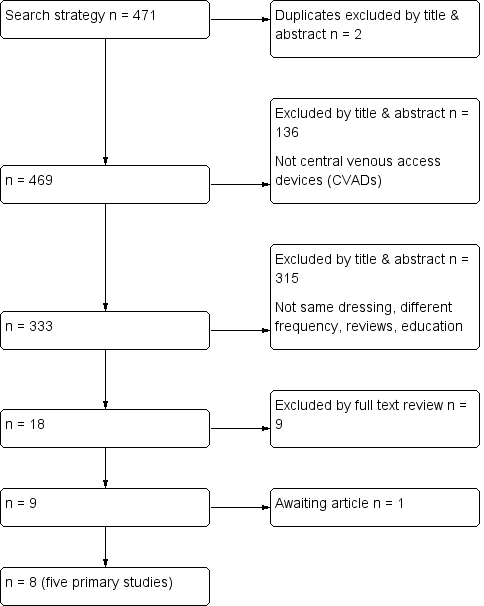
Flow diagram of included and excluded studies
A search of the clinical trials registers did not identify any additional trials. Only one study had been pre‐registered (Timsit 2009).
The reference lists of all retrieved and relevant publications were searched. One study was considered to be relevant but was not available through library resources (Fessard 1994). Attempts to contact the author and locate the journal are continuing.
Included studies
Types of patients
The five trials involved a total of 2277 participants, with the totals in individual trials ranging from 32 to 1653. One study involved children (Benhamou 2002), and the remaining four included adults only (Engervall 1995; Rasero 2000; Timsit 2009; Vokurka 2009). Two studies were set in a bone marrow transplant unit (Benhamou 2002; Rasero 2000), two studies consisted of patients undergoing treatment for a haematological malignancy (Engervall 1995; Vokurka 2009), and one study recruited patients receiving treatment in intensive care (Timsit 2009). Four countries were represented (two studies from France and one each from Italy, Sweden, and the Czech Republic). All studies were conducted in acute in‐patient settings. Patients were excluded from the studies if their skin was already damaged (Benhamou 2002; Rasero 2000; Vokurka 2009); were having treatment that would make them more susceptible to skin damage, such as the chemotherapeutic drug busulphan‐thiotepa (Benhamou 2002), or radiation to the chest (Vokurka 2009); or if they had allergies to polyurethane dressings (Rasero 2000; Timsit 2009; Vokurka 2009), chlorhexidine (Timsit 2009), or disinfectant (Vokurka 2009).
Types of interventions
Time frames for dressing changes varied between 2 and15 days. One study planned to compare 15‐day and 4‐day dressing changes for tunnelled catheters (Benhamou 2002). CVAD dressings were monitored on a daily basis in all trials and patients were followed up until the CVAD was removed or until discharge as a minimum. However, in this study there were a large number of protocol violations, that is, dressings were changed on days other than the day indicated in the protocol. In the 15‐day group, only 67 (17%) of the 365 dressing were changed on day 15 and, in the 4‐day group, 516 (76%) of the 678 dressings were changed on the correct day. This meant that dressing changes in the 15‐day group were actually changed, on average, every eight days and, in the 4‐day group, every four days. Reasons for the protocol violations included soiled and dislodged dressings and problems with the catheter that required the dressing to be removed. Two studies compared once versus twice‐weekly dressing changes for tunnelled CVADs (Engervall 1995; Timsit 2009), two studies compared once versus twice‐weekly dressing changes for non‐tunnelled CVADs (Timsit 2009; Vokurka 2009), and one study compared 5‐day versus 10‐day dressing changes for tunnelled CVADs and 2‐day versus 5‐day dressing changes for non‐tunnelled CVADs (Rasero 2000). Again however, reflecting the reality of pragmatic research in clinical settings, many of the dressings were not changed according to the group schedule. In the tunnelled CVAD 10‐day group 9.6% were not changed on the correct day, while in the tunnelled CVAD 5‐day group the proportion was 8.0%; in addition 6.8% of non‐tunnelled CVCs in the 5‐day group were not changed as scheduled and in the non‐tunnelled CVC 2‐day group the rate was 12.5%.
The dressings were applied under controlled conditions in all groups. Three studies stated that nurses were responsible for the dressing changes (Benhamou 2002; Engervall 1995; Rasero 2000). Four studies used Tegaderm (3M, St Paul, USA) dressings (Benhamou 2002; Engervall 1995; Rasero 2000; Timsit 2009), and one study used Bioclusive (Johnson and Johnson, New Jersey, USA; Vokurka 2009). One study used chlorhexidine gluconate‐impregnated sponges (Biopatch, Ethicon, New Jersey, USA) around the entry or exit site of the CVAD under the dressings (Timsit 2009).
Three studies used the same dressings throughout the period of observation. Two studies used different dressings that depended upon skin damage. One study used a Tegaderm (3M) covering a sterile gauze for grade 0 to 1 skin damage (48/56; 85% in the 15‐day group and 32/56; 57% in the 4‐day group), sterile gauze with Mefix for grade 2 to 3 skin damage (7/56; 13% in the 15‐day group and 23/56; 41% in the 4‐day group) and sterile gauze with tape for grade 4 skin damage (1/56; 2% in both the 15‐ and 4‐day groups; Benhamou 2002). The other study used a Tegaderm (3M) for undamaged skin or an exit site with mild erythema, but if the exit site had extensive erythema or other signs of local infection then the dressings were changed daily using a gauze dressing moistened with 10% ethanol with aluminium acetotartrate 10% until the erythema had disappeared, at which point the patient was returned to the allocated group (Engervall 1995). Patients in the once‐weekly group had more extra dressings due to erythema compared to the twice‐weekly group (3%; 0 to 91% once‐weekly group; 0%; 0 to 17% twice‐weekly group; P value 0.08 expressed as extra dressings days per CVAD days).
Skin decontamination varied between the groups. Two studies used the same antiseptic solution to clean the skin before the insertion of the CVAD and at dressing changes; one study used an alcohol‐based povidone‐iodine solution (Timsit 2009); and one used povidone‐iodine solution; whether the antiseptic was alcohol‐based was unclear (Vokurka 2009). One study used a 0.5% alcohol based chlorhexidine solution during insertion and at dressing changes, but changed to aqueous‐based povidone‐iodine if the skin became damaged (Benhamou 2002). One study did not describe skin decontamination that occurred prior to CVAD insertion but used 70% ethanol at dressing changes (Engervall 1995). One study did not mention which antimicrobial solution was used for skin decontamination (Rasero 2000).
Types of outcomes
Only one trial used a standard definition for confirmed CRBSI (Timsit 2009), two trials reported blood culture results (Benhamou 2002; Vokurka 2009), and one study reported blood culture and CVAD‐tip culture results separately (Engervall 1995). Blood cultures were performed on clinical suspicion of infection or determined by a temperature threshold stipulated by each study author. Three studies provided information about suspected CRBSI but these studies used different definitions (Benhamou 2002; Engervall 1995; Timsit 2009): Benhamou 2002 did not provide a definition; Engervall 1995 defined suspected CRBSI as not responding to antibiotics; and Timsit 2009 had an investigator blinded to the study group review the patient's case including the medical chart in order to perform an independent blinded review. In all five studies, catheter‐site infection was defined by skin colonisation and additionally in three studies by local signs of catheter‐site infection such as the presence of inflammation, erythema, tenderness, swelling or discharge (Benhamou 2002; Engervall 1995; Timsit 2009). Two studies measured pain: Benhamou 2002 used categories of none, moderate or severe; and Vokurka 2009 used a visual analogue score ranging from 0 to 10 (0: no pain, 5: moderate pain, 10: severe pain). No study measured quality of life. Two studies measured cost (Rasero 2000; Timsit 2009).
In the Timsit 2009 trial, a 2 x 2 factorial design was used, in which participants were randomised to a 3‐ or 7‐day dressing change and to a dressing alone or with chlorhexidine gluconate‐impregnated sponge (CHGIS). They also combined arterial and central catheters in their analysis. The authors were contacted and provided information based on central catheters only and reported separately for the CHGIS and non‐CHGIS groups. For our analysis we have included only the non‐CHGIS group, to maintain consistency with other trials.
Excluded studies
The Table of Characteristics of excluded studies specifies our reasons for excluding 10 studies. One was a systematic review (Zitella 2003); three studies compared different dressings (Davidson 1986; Hagerstrom 1994; Lucas 1996); one was a study protocol (Bystricka 2004); one was a letter to the editor commenting on a study of dressings (Dickerson 1989); in three studies the frequency of dressing change was not the only systematic difference between treatment groups (Powell 1985; Samsoondar 1985; Young 1988); and one was a cluster RCT (Ishizuka 2011).
Risk of bias in included studies
See the 'Risk of bias' tables in the Characteristics of included studies section and Figure 2; Figure 3; Table 1 and Table 2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
| CRBSI | Not applicable | Not applicable | Not applicable | High risk | Not applicable |
| Suspected CRBSI | High risk | High risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | High risk | High risk | High risk | High risk | High risk |
| Skin damage | High risk | Not applicable | High risk | High risk | High risk |
| Pain | HIgh risk | Not applicable | Not applicable | Not applicable | High risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | High risk | Not applicable | Not applicable |
| CRBSI | Not applicable | Not applicable | Not applicable | Low risk | Not applicable |
| Suspected CRBSI | Unclear risk | Unclear risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Skin damage | Unclear risk | Not applicable | Unclear risk | Unclear risk | Unclear risk |
| Pain | Unclear risk | Not applicable | Not applicable | Not applicable | Unclear risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | Unclear risk | Not applicable | Not applicable |
Allocation
Random sequence generation
Three studies used computer‐generated lists to generate the allocation sequence (Benhamou 2002; Timsit 2009; Vokurka 2009). One study used manually mixed envelopes (Engervall 1995). One study did not describe how the random sequence was generated (Rasero 2000).
Allocation concealment
Vokurka 2009 used computer software to conceal the allocation of trial patients into individual groups. We contacted the trialists of Engervall 1995 who stated that they had used randomisation envelopes. The other three studies did not describe how the allocation was concealed (Benhamou 2002; Rasero 2000; Timsit 2009).
Blinding
Blinding of participants and personnel
None of the studies was able to blind participants or staff involved in direct care from identifying the allocated intervention due to the nature of the intervention.
Blinding of outcome assessment
Timsit 2009 mentioned blinding of outcome assessment, stating that staff involved in analysing catheter cultures and reviewing CRBSI were blinded to the study groups. It was unclear in the remaining four trials whether outcome assessors were blinded (Benhamou 2002; Engervall 1995; Rasero 2000; Vokurka 2009).
Incomplete outcome data
A flow chart was provided by Timsit 2009 that included the numbers of patients screened, excluded, randomised to each group and withdrawals and reasons for exclusions from the per‐protocol analysis. Four studies accounted for all randomised participants and their withdrawal from each group (Benhamou 2002; Engervall 1995; Rasero 2000; Vokurka 2009). Two studies reported sample size calculations and used an intention‐to‐treat analysis (Benhamou 2002; Timsit 2009). Two studies presented results per patient (Benhamou 2002; Vokurka 2009). Two studies presented results per catheter and per patient (Engervall 1995; Timsit 2009). One study presented results per dressing and per patient (Rasero 2000). Overall, reported attrition rates were low and well balanced. There was a proportionally higher attrition rate in one arm of the Timsit 2009 trial, but losses were marginal and unlikely to have had an impact on outcomes.
Four studies monitored the CVAD sites closely and dressings were changed if they were loose or soiled (Benhamou 2002; Rasero 2000; Timsit 2009; Vokurka 2009). This meant that approximately one‐third of the participants had additional dressing changes that constituted protocol violations. These violations were reported in the results.
Selective reporting
A study protocol was available for one study (Timsit 2009). All other authors provided results for outcomes mentioned in their published methods section (Benhamou 2002; Engervall 1995; Rasero 2000; Vokurka 2009).
Other potential sources of bias
Benhamou 2002 and Engervall 1995 varied their dressing protocol according to the grade of skin damage. The interim analysis in the Engervall 1995 study showed no statistical significance for the rates of the primary outcome between groups, so the study was stopped and the secondary outcomes analysed. Rasero 2000 did not present baseline data. In one study, the manufacturer provided one of the products but they had no influence in the design or how the results were analysed and reported (Timsit 2009).
Effects of interventions
Primary outcomes
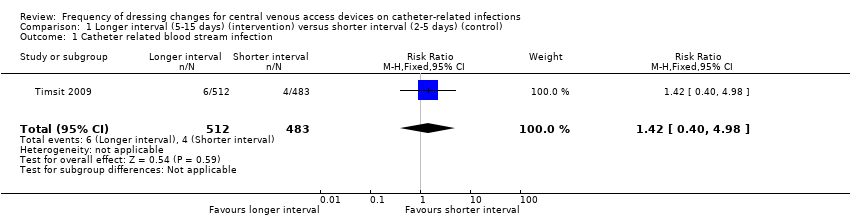
Confirmed catheter‐related bloodstream infection (995 central venous catheters)
Only one study (Timsit 2009), that had uncertain risk of bias for allocation concealment, reported confirmed CRBSI as per our protocol. There was no clear evidence of a difference between groups for this outcome (RR 1.42; 95% CI 0.40 to 4.98; Analysis 1.1). LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision): (summary of findings Table for the main comparison).
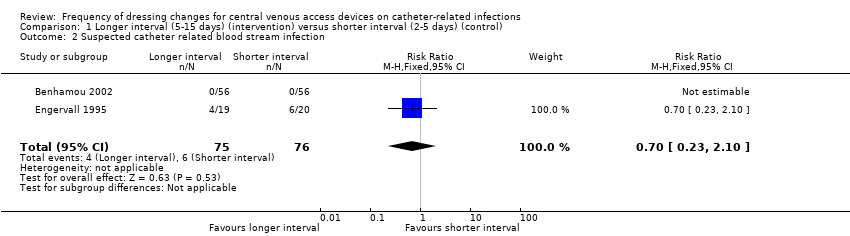
Suspected catheter‐related bloodstream infection (151 participants)
We were able to extract data from two studies that reported suspected CRBSI (Benhamou 2002; Engervall 1995). Benhamou 2002 stated that no CVADs were removed due to suspicion of CRBSI. In the Engervall 1995 trial 6/20 (30%) of CVADs were removed in the once‐weekly group and 4/19 (21%) in the twice‐weekly group due to suspected CRBSI (RR 0.70; 95% CI 0.23 to 2.10; Analysis 1.2). Both studies were at uncertain risk of bias for allocation concealment, blinding of outcome assessment and selective reporting. There was no clear evidence of a difference between the groups for this outcome. LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision).
All‐cause mortality (896 participants)
Three studies at uncertain risk of bias, included information about all‐cause mortality (Benhamou 2002; Engervall 1995; Timsit 2009). It was possible to combine the data from all these studies; the studies were homogenous so the fixed‐effect model was used for data synthesis (I² = 0%). There was no clear difference in all‐cause mortality between longer (5‐15 days) and shorter (2‐5 days) time intervals between dressing changes (RR 1.06; 95% CI 0.90 to 1.25; Analysis 1.3). LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision): (summary of findings Table for the main comparison).
Secondary outcomes
Catheter‐site infection (371 participants)
All five studies reported catheter‐site infection but in a variety of different ways. Benhamou 2002 and Rasero 2000 reported the proportions of participants developing a catheter‐site infection. Engervall 1995 reported the rate of exit site infections per 100 CVAD days; Vokurka 2009 reported positive skin swabs and Timsit 2009 reported rates of skin colonisation, Data from the two studies (Benhamou 2002; Rasero 2000) that reported risk of catheter‐site infection in a similar way were pooled using a fixed effect model (I2 = 0%). There was no clear evidence of a difference in the risk of catheter‐site infection rate between longer (5‐15 days) and shorter (2‐5 days) time intervals between dressing changes (RR 1.07; 95% CI 0.71 to 1.63; Analysis 1.4). LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision).
Engervall 1995 reported 1.6 exit site infections per 100 CVAD days (median, range 0 to 13.3) in the longer interval (less frequent) group compared with 0 per 100 CVAD days (median, range 0 to 9.1) in the short interval (more frequent) group. Vokurka 2009 reported 13 positive skin swabs across both treatment groups but did not report by group. We contacted the trialists of Timsit 2009 but they were unable to provide per patient data. The Timsit 2009 study reported catheter‐site infection rates per catheter rather than by patient in their published paper.
Consequently it remains unclear whether longer or shorter intervals between dressing changes for CVADs influences the risk of catheter‐site infection.
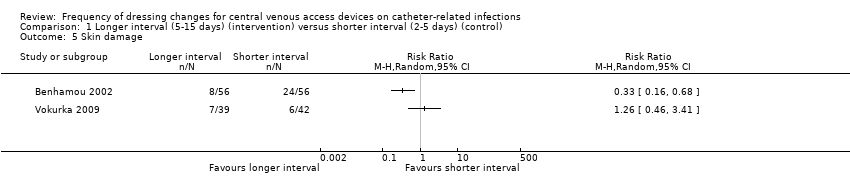
Skin damage (1587 participants)
Skin damage was reported in four studies (Benhamou 2002; Rasero 2000; Timsit 2009; Vokurka 2009). Data from two trials were included in the forest plot (Benhamou 2002; Vokurka 2009). Results were highly heterogenous (I² = 78%), probably due to different scales being used to assess skin damage and dissimilar time frames for assessment, so we did not pool the data. One of these trials (Benhamou 2002) included only children and showed that fewer participants in the longer interval group (8/56) scored grade ≥ 2 on the skin damage scale compared with 24/56 in the shorter interval group (RR 0.33; 95% CI 0.16 to 0.68; P value 0.012; Analysis 1.5). There was no clear evidence of a difference in rates of skin damage between long and short intervals in adult patients (Vokurka 2009) (RR 1.26; 95% CI 0.46 to 3.41; Analysis 1.5. VERY LOW QUALITY EVIDENCE (downgraded for risk of bias, imprecision and heterogeneity).
Two trials could not be included in the skin damage forest plot. In the Rasero 2000 trial toxicity was graded on a 5‐point scale, but there were no reported differences between groups. We are unable to use the data from the Timsit 2009 trial due to the 2 x 2 factorial design.
Pain
Pain was assessed on a daily basis in two studies (Benhamou 2002; Vokurka 2009). The maximum intensity of pain reported was analysed. When data from the two studies were combined there was no clear evidence of a difference in pain however this comparison is underpowered (RR 0.80; 95% CI 0.46 to 1.38; Analysis 1.6; Benhamou 2002; Vokurka 2009). This was rated as LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision): (summary of findings Table for the main comparison). The pain classification systems used are detailed in the Characteristics of included studies. The pain data were dichotomised on the basis of a judgement that any pain experienced and reported by the patient was clinically significant.
Quality of life
None of the studies reported quality of life.
Cost
Rasero 2000 reported the costs of nursing time and dressings and stated that less frequent dressing changes would reduce costs by 400% in the tunnelled CVAD group and by 50% in the non‐tunnelled CVAD group when compared to the standard practice of changing dressings every second day. The monetary figures presented in the text and the table were different. Several attempts have been made to contact the authors for clarification but without success.
Discussion
Summary of main results
This systematic review included five RCTs (2277 participants) at unclear or high risk of bias. We assessed the effects of prolonging the frequency of dressing changes for CVADs on the incidence of confirmed CRBSI, suspected CRBSI, all‐cause mortality, CVAD entry and exit site infection, skin damage, pain, quality of life and cost. All studies used transparent polyurethane dressings, which are often favoured over gauze dressings because they allow the catheter site to be monitored visually for signs of infection without removal of the dressing. The longer intervals of dressing changes ranged from 5 to 15 days and the shorter intervals from 2 to 5 days. It was not possible to obtain data that would facilitate analysis at the level of the patient rather than the catheter from two of the trial authors. Rasero 2000 presented data for every dressing change and Timsit 2009 reported data per catheter. One of the authors who was contacted for additional information no longer had the data in an accessible form due to technological advances (Engervall 1995). Most published literature in this field was ineligible for this review as it compared the effect of different dressings on CRIs rather than different frequencies of dressing change within the context of a constant dressing type.
From the available data, we can draw no conclusions about the incidence of confirmed or suspected CRBSI associated with different intervals of dressing frequency. We used the CDC definition of confirmed CRBSI (CDC 2002), which requires the CVAD to be removed so that the tip can be quantitatively or semi‐quantitatively cultured. Clinically this definition is impractical as it requires the removal of the CVAD. Mermel 2009 offers a more practical definition of two blood samples drawn (one from a catheter hub and the other from a peripheral vein) that, when cultured, meet CRBSI criteria for quantitative blood cultures or differential time to positivity which would enable CVADs to remain in place until the results of the blood cultures become available.
Similarily, neither benefits or harms of the intervention could be demonstrated for all‐cause mortality, CVAD entry and exit site infection, pain, quality of life and cost. Most comparisons are underpowered and therefore clinically important effects cannot be excluded.
As highlighted in the Included studies section, each study used a variety of antimicrobial solutions for skin decontamination. The recent guidelines recommend using a > 0.5% chlorhexidine skin preparation with 70% alcohol for skin decontamination or a 1% to 2% tincture of iodine or povidone‐iodine for sensitive skin (Loveday 2014; INS 2011; IVNNZ 2012; O'Grady 2011). At the time of these studies this preparation was not available. When patients' skin damage worsened in Benhamou 2002, Tegaderm (3M) was no longer used and it is unclear how frequently the Mefix tape or sterile gauze and tape dressing were changed. Skin damage grade ≥ 2 occurred more frequently in the 4‐day group, which may have an effect upon the rates of skin infection.
It was not possible to provide an overall estimate of the effect of changing dressings less frequently on skin damage. Data from two small studies of limited quality reported contradictory results; one trial favoured shorter times between dressing changes (two dressing changes per week; Vokurka 2009), and the other favoured longer times (up to 15 days; Benhamou 2002). In addition, the Benhamou 2002 study was powered to detect a 30% improvement in the rate of grade ≥ 2 skin damage in the 15‐day group, but only 17% of dressings in this group remained intact for this length of time. In the Benhamou 2002 study, on average, the longer interval dressings were in place for 8 days with no adverse events occurring in either trial. Consequently, this raises the possibility of replacing dressings only when clinically indicated, especially in the paediatric and neonatal population where skin is fragile. Patients receiving radiotherapy, or those with existing sensitivities, may also benefit from extending the time between dressing changes.
Overall completeness and applicability of evidence
The primary and secondary outcomes of clinical interest included confirmed and suspected CRBSI, all‐cause mortality, catheter‐site infection, skin damage, pain, quality of life and cost, but these were poorly reported and many results could not be extracted for this review. These outcomes should be included in any future clinical trials involving frequency of dressing changes.
The five studies included in this review were undertaken in acute care settings in Europe. CVADs are usually placed in patients requiring intensive care, treatment for malignancies and other patients requiring long‐term treatment. Four of the studies recruited participants with haematological malignancies or those undergoing a bone marrow transplant. This population is immunocompromised due to their underlying disease or treatment, hence these results may not be easily applied to patients with chronic health problems or those being cared for in other settings.
Dressings and products for decontamination continue to evolve, with new products constantly being developed and marketed. So, although all of the studies in this review used transparent dressings, older studies may have used products that are no longer available. Other reviews have been published comparing different dressings.
The final limitation to the completeness and generalisability of results is that all of the studies compared changing the frequency of transparent polyurethane dressings only. Consequently, studies comparing the frequency of changing other types of dressings, such as gauze and tape, may provide different results. Only one trial used chlorhexidine impregnated sponges, which are now commonly used, as part of the dressing regimen, but we could not extract these data.
Quality of the evidence
Limitations in study design and implementation
Risk of bias was assessed according to six components: sequence generation; allocation concealment; blinding; selective outcome reporting, incomplete follow‐up and other potential biases. The risk of bias was difficult to assess due to poor reporting in most of the studies (Figure 2; Figure 3). Only three studies provided sufficient information to assess how the randomisation sequence was generated; and two study authors we contacted described the method used for allocation concealment. It would not be possible for the participants and personnel to be blinded to the frequency of dressing changes, but only one study blinded outcome assessments.
Two of the studies comprised 81% (2163/2675) of the total participants (Benhamou 2002; Timsit 2009). These two studies calculated the required sample size, used random‐number generation to allocate the sequence and used an intention‐to‐treat analysis. However, neither was rated as being at low risk of bias for allocation concealment.
Protocol deviations were common in the treatment and control arms. Dressings were changed early as they were soiled or not intact. This issue reflects the reality of pragmatic research in clinical settings and the importance of visual inspections of the dressing to improve care and maintenance of the CVAD.
Indirectness of evidence
This review was limited by a lack of uniformity in the experimental and control groups. The frequency of the dressing changes overlapped at the outer limits of the longer (5 to 15 days ) and shorter (2 to 5 days) intervals between dressings. Confirmed CRBSI was reported in only one trial (Timsit 2009). These limitations restrict confident decision making regarding the effect of frequency of dressing changes on CRIs.
Unexplained heterogeneity or inconsistency of results
All‐cause mortality and catheter‐site infection were the only outcomes that could be pooled using fixed‐effect model for meta‐analysis. Pain was pooled using a random‐effects model for meta‐analysis. It was not possible to pool the skin damage results due to heterogeneity. Heterogeneity was generally due to differences in populations and different scales and definitions that were used for the various outcomes.
Imprecision of results
There was serious imprecision in all the results, even when meta‐analysis was undertaken, with wide confidence intervals due to the small sample sizes. Consequently, results reflect a lack of evidence of a difference rather than evidence of no difference (between CVAD dressing change intervals). Further research is therefore very likely to have an important impact on the confidence of the estimates of effect for all of the measured outcomes.
Publication bias
Lack of information about most of the important clinical outcomes could suggest selective outcome reporting, but we were unable to confirm this as only one study was registered with a trials registry.
Potential biases in the review process
The authors are confident that all studies meeting the inclusion criteria were selected. The reference lists were handsearched and only one additional title was found (Fessard 1994). The full paper was requested from the author and from the journal, but to date our requests remain unanswered. Clearly described procedures were followed to prevent potential biases in the review process. The methods used are transparent and reproducible. One of the authors (CR) has given lectures for 3M and received an unrestricted research Grant from Centurion. No products from these companies were included in this review.
Agreements and disagreements with other studies or reviews
Zitella 2003 reviewed the literature concerning CVAD care for patients undergoing bone marrow transplantation: only the Engervall 1995 and Rasero 2000 studies were included in both that review and ours. The Benhamou 2002 study was published in the month that the Zitella 2003 review was accepted for publication and the other studies in our review were published after 2003. With regard to frequency of dressing changes, Zitella 2003 concluded firstly that the Engervall 1995 study showed more positive catheter tip cultures in the once‐weekly group, but the study was limited by a small sample size, and secondly that the Rasero 2000 study showed no significant difference in skin colonisation between the four groups. Overall, the authors concluded that colonisation is a imperfect measure for CRBSI.
One of the excluded studies allocated participants to routine (every 72 hours) and non‐routine (until removal of the CVAD) intervals between dressing changes based upon the ward they were admitted to (cluster randomisation; Ishizuka 2011). There was a significant inter‐group difference in the duration of catheter dwell time (routine group 9.1 ± 0.5 days and non‐routine group 11.9 ± 0.7 days). There was a no significance in CRBSI between groups (13/241 in the routine group and 10/266 in the non‐routine group). Kaplan‐Meier analysis and the log rank test revealed a significant difference in the period from insertion to the development of CRBSI between the groups favouring the routine interval between dressing changes (P value 0.026).
The Timsit 2009 RCT was subjected to a secondary analysis in a later publication,Timsit 2012, which reported data on 1419 patients (3275 combined arterial catheters and CVADs) who had their dressings replaced on the allocated third or seventh day versus those with dressings replaced before any of the scheduled days. They found that early dressing disruption (replacement) occurred for 67% of scheduled dressings and was significantly associated with increased skin colonisation, CRBSI and major CRI (CRBSI or suspected CRBSI). For subclavian CVADs alone (n = 547), it was reported that both percentage of dressings disrupted (P value 0.0043), and disruption of the final dressing (P value 0.0004) were significantly associated with greater levels of skin colonisation at CVAD removal. Those authors concluded that disruption of dressings was common and an important risk factor for infection.
The wound and skin adhesive literature recognises that multiple factors influence the degree of adhesion of the same product to different people's skin (Rippon 2007). It is also acknowledged that trauma caused by repeated removal and application of adhesives or adhesive tapes causes an erythematous reaction that affects the barrier function of the skin (Cutting 2008; Hollingworth 2009). Compromised barrier function becomes important when bacterial overgrowth has been associated with occlusive dressings (Dykes 2007), such as the polyurethane dressings commonly used to secure CVADs. However, whether polyurethane dressings are more likely than other adhesive products to cause skin stripping remains unclear (Cutting 2008; Dykes 2001). It is also unclear whether there is an association between skin stripping and an increased incidence of infection. However, damaged skin provides a potential entry point for infection, so it makes sense to prevent the skin damage occurring. The notion of preventing skin damage to avoid CRI is supported by an infection control practice guideline, which recommends not shaving insertion sites, to avoid micro‐abrasions that may encourage bacterial colonisation (Wilson 2006).
The counter argument to the skin stripping theory is that organisms originating from patients’ own skin are likely to be the ones that cause many CRIs (Casey 2010; Elliott 1998; Gillies 2003; Maki 1997; Mermel 2000); these may be capable of migrating from the skin surface along the outside of the catheter to cause infection irrespective of skin damage (Wilson 2006). If skin around the catheter site is disinfected regularly then colonisation and CRBSI should be reduced. However, catheter‐site infections cannot be relied upon to identify or predict CRBSIs (Safdar 2002), and can exist independently of a systemic infection (Walshe 2002). Moreover, efforts to maintain skin integrity may assist in reducing CRBSIs. While this may be true, the proportion of positive skin cultures around the exit site has been found to be higher in the presence of erythema when compared with healthy skin (Engervall 1995).

Flow diagram of included and excluded studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 1 Catheter related blood stream infection.
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 2 Suspected catheter related blood stream infection.

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 3 All‐cause mortality.

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 4 Catheter‐site infection.
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 5 Skin damage.
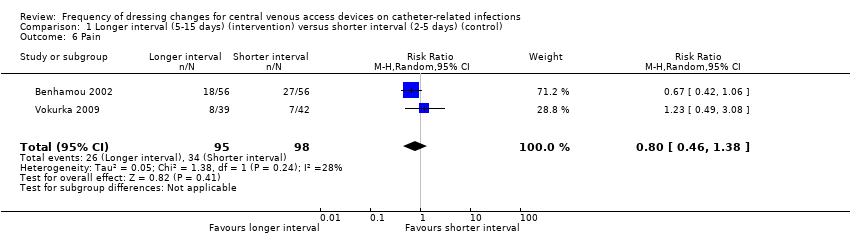
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 6 Pain.
| Patient or population: patients with a central venous access device | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Quality of the evidence | What happens | ||
| Without longer interval (5 ‐ 15 days) | With longer interval (5 ‐ 15 days) | Difference | ||||
| Catheter‐related blood stream infection (CRBSI) | RR 1.42 | Study population | ⊕⊕⊝⊝ | Longer intervals between dressing changes may have little or no effect on catheter‐related blood stream infection | ||
| 8 per 1000 | 12 per 1000 | 4 more per 1000 | ||||
| All‐cause mortality | RR 1.06 | Study population | ⊕⊕⊝⊝ | Longer intervals between dressing changes probably have little or no effect on death from any cause | ||
| 354 per 1000 | 375 per 1000 | 21 more per 1000 | ||||
| Skin damage Follow up: unclear | Not estimable | Skin damage was reported in four studies. Two provided data but their results were not combined due to inconsistency of size and direction of the effects. One study in children found less skin damage in the longer interval group (8/56) compared with the shorter interval group (24/56). Rates of skin damage in one study in adults were similar (7/39 in longer interval versus 6/42 in shorter interval).9 | ⊕⊝⊝⊝ | It is uncertain whether longer (compared with shorter) intervals between dressing changes reduce skin damage | ||
| Pain Follow up: unclear | RR 0.80 | Study population | ⊕⊕⊝⊝ | It is uncertain whether longer (compared with shorter) intervals between dressing changes affect pain on dressing removal | ||
| 347 per 1000 | 278 per 1000 | 69 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for risk of bias due to lack of blinding of participants and personnel and for a probable unit of analysis error (individual participants randomised but numbers of infections reported) 2 Downgraded for serious imprecision: result consistent with a reduction in CRBSI or an almost 5 fold increase 3 Downgraded for risk of bias due to lack of blinding of participants and personnel 4 Downgraded for imprecision: result consistent with a 10% reduction in mortality or a 25% increase 5 Downgraded twice for serious risk of bias: risk of performance bias due to lack of blinding of participants and personnel; different dressings were used in response to skin damage 6 Downgraded for inconsistency: experimental and control groups were different between studies and frequency of dressing changes overlapped between longer and shorter groups 7 Downgraded for imprecision 8 Downgraded for risk of bias: blinding of outcome assessment not described 9 Data from two additional RCTs could not be extracted and used within the analysis. One study presented toxicity on a 5‐point scale and reported no differences between groups. We are unable to use the data from the fourth study due to the 2 x 2 factorial design. | ||||||
| CRBSI | Not applicable | Not applicable | Not applicable | High risk | Not applicable |
| Suspected CRBSI | High risk | High risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | High risk | High risk | High risk | High risk | High risk |
| Skin damage | High risk | Not applicable | High risk | High risk | High risk |
| Pain | HIgh risk | Not applicable | Not applicable | Not applicable | High risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | High risk | Not applicable | Not applicable |
| CRBSI | Not applicable | Not applicable | Not applicable | Low risk | Not applicable |
| Suspected CRBSI | Unclear risk | Unclear risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Skin damage | Unclear risk | Not applicable | Unclear risk | Unclear risk | Unclear risk |
| Pain | Unclear risk | Not applicable | Not applicable | Not applicable | Unclear risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | Unclear risk | Not applicable | Not applicable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter related blood stream infection Show forest plot | 1 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.40, 4.98] |
| 2 Suspected catheter related blood stream infection Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.23, 2.10] |
| 3 All‐cause mortality Show forest plot | 3 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| 4 Catheter‐site infection Show forest plot | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.63] |
| 5 Skin damage Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Pain Show forest plot | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.38] |

