Vacunas contra la gripe en adultos con cáncer e inmunodeprimidos
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised controlled study Location: Brazil | |
| Participants | Patients more than 7 days before allogeneic HSCT 78 patients, of which 19 children. 40 were vaccinated and 38 were unvaccinated Mean age not reported | |
| Interventions | Influenza vaccination versus no vaccination, assigned by randomisation | |
| Outcomes | 1. All‐cause mortality 2. Influenza‐related mortality 3. Vaccine immunogenicity reported as seroconversion rate or geometric mean titres 4. Laboratory‐confirmed influenza infections | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers with their respective randomisation group was generated by a website |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes sequentially numbered and sealed |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to follow up for the mortality outcome. |
| Selective reporting (reporting bias) | High risk | No primary endpoint defined in methods, no registry. Seroconversion reported at multiple time points |
| Other bias | Low risk | No baseline imbalances, sponsored by academic grant, no sample size calculation |
| Blinding of participants and personnel (performance bias) | Low risk | No blinding, but outcomes are objective |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding, but outcomes are objective |
| Methods | Retrospective, observational cohort study | |
| Participants | Stage 4 colorectal adenocarcinoma, with active chemotherapy treatment 1225 adults, 1577 person‐years: 626 person‐years vaccinated, 951 person‐years unvaccinated Mean age 74 year in both groups | |
| Interventions | Yearly influenza vaccination, examined through medical bills | |
| Outcomes |
| |
| Notes | Results given per person‐years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random |
| Allocation concealment (selection bias) | High risk | Non‐random |
| Incomplete outcome data (attrition bias) | High risk | Mortality data were given only for 697 of 1054 patients. |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | High risk | No control for cancer stage and functional capacity, no sample size calculation, funding not mentioned. |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded |
| Methods | Retrospective case‐control study (confirmed influenza versus no influenza) | |
| Participants | HSCT recipients (CML, acute leukaemia, severe aplastic anaemia, NHL, MM, other) 43 participants eligible to receive influenza vaccination: 19 vaccinated 24 unvaccinated Mean age not reported | |
| Interventions | Influenza vaccination, obtained from review of participants' charts | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random |
| Allocation concealment (selection bias) | High risk | Non‐random |
| Incomplete outcome data (attrition bias) | High risk | Mortality ‐ no record |
| Selective reporting (reporting bias) | High risk | The only outcome reported was documented influenza |
| Other bias | High risk | No control for cancer stage and functional capacity, funding not mentioned, no sample size calculation |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded |
| Methods | Randomised trial, open‐label Location: Italy | |
| Participants | MM, with active chemotherapy treatment 50 adults: 25 vaccinated 25 unvaccinated Mean age not reported | |
| Interventions | Influenza vaccination versus no vaccination, assigned by randomisation | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to follow up |
| Selective reporting (reporting bias) | Unclear risk | Mortality reported only due to influenza pneumonia |
| Other bias | Unclear risk | No reported baseline imbalances, funding not mentioned, no sample size calculation |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used |
| Blinding of outcome assessment (detection bias) | High risk | None |
| Methods | Randomised controlled trial Location: Canada, Toronto | |
| Participants | At least 12 weeks post HSCT 73 adults: 35 received adjuvanted vaccine and 38 received non‐adjuvanted vaccine Median age 54.5 years in adjuvanted vaccine group versus 52.5 years in non‐adjuvanted vaccine group | |
| Interventions | Adjuvanted influenza vaccination versus non‐adjuvanted influenza vaccination, assigned by randomisation | |
| Outcomes | 1. Vaccine immunogenicity reported as seroconversion rate, seroprotection rate or geometric mean titres 2. Laboratory‐confirmed influenza infections 3. Mortality related to influenza infections 4. All‐cause mortality 5. Hospitalisations attributed to influenza infections 6. Any hospitalisation 7. Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule in blocks of four |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to follow up for the mortality outcome. |
| Selective reporting (reporting bias) | Low risk | No significant differences in the secondary outcomes added to the manuscript after registration |
| Other bias | Low risk | No baseline imbalances, funding not mentioned, sample size achieved |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were not blinded, but low risk because of objective outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors of adverse events and lab workers were blinded |
| Methods | Prospective observational cohort study | |
| Participants | Solid malignancies, with active chemotherapy and haematological patients with active disease 806 adults: 387 vaccinated versus 419 unvaccinated Mean age 66 years in vaccinated group versus 60 years in unvaccinated | |
| Interventions | Patients were followed up through medical personal hard copy files and through electronic patients’ health records, including inpatient and outpatient records. Telephone or personal interviews were also conducted to collect data on clinical outcomes and assure vaccination status. | |
| Outcomes | 1. A composite of hospitalisations for fever or acute respiratory infection; and/or pneumonia necessitating antibiotic treatment; and/or chemotherapy interruptions related to an infectious condition. 2. All‐cause mortality 3. Influenza‐like illness 4. Laboratory‐confirmed influenza 5. Individual components of the primary outcome 6. Any hospitalisation and hospitalisation days 7. Antibiotic treatment necessity in hospitalisation 8. All delays in planned chemotherapy courses | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random |
| Allocation concealment (selection bias) | High risk | Non‐random |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes reported fully |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | Controlled for cancer stage and functional capacity, funded by an internal grant, sample size achieved |
| Blinding of participants and personnel (performance bias) | High risk | None blinded |
| Blinding of outcome assessment (detection bias) | High risk | None blinded |
HSCT: haematopoietic stem cell transplantation; CML: chronic myelogenous leukaemia; MM: multiple myeloma; NHL: non‐Hodgkin's lymphoma
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| All participants vaccinated | |
| All participants vaccinated | |
| All participants vaccinated | |
| All participants vaccinated | |
| All participants vaccinated | |
| Lymphoma participants compared to healthy participants | |
| Lymphoma participants compared to healthy participants | |
| All participants vaccinated | |
| Comparing different doses of influenza vaccine | |
| All participants vaccinated | |
| All participants vaccinated | |
| Healthy participants not vaccinated compared to breast cancer participants vaccinated | |
| Lymphoma participants compared to healthy participants | |
| All participants vaccinated Lymphoma participants compared to healthy participants | |
| All participants vaccinated Lymphoma participants compared to healthy participants | |
| All participants vaccinated | |
| Comparison to healthy participants | |
| Immunogenecity of vaccine compared to infection | |
| All participants vaccinated Comparing different doses of influenza vaccine | |
| Comparison to healthy participants | |
| Comparison to healthy participants | |
| All participants vaccinated | |
| Comparing different doses of influenza vaccine | |
| Comparison to healthy participants | |
| All participants vaccinated | |
| Comparing different doses of influenza vaccine | |
| Comparing different doses of influenza vaccine | |
| All participants vaccinated | |
| Vaccination rate in haematological participants | |
| Association between influenza vaccination and risk of developing Non‐Hodgkin lymphoma | |
| Comparing different doses of influenza vaccine | |
| Comparing different types of vaccines in different seasons | |
| Comparing different doses of influenza vaccine | |
| All participants vaccinated Lymphoma participants compared to healthy participants | |
| All participants vaccinated Comparison to healthy participants | |
| All participants vaccinated, healthy controls | |
| All participants vaccinated | |
| All participants vaccinated | |
| All participants vaccinated Serological outcomes only | |
| All participants vaccinated Lymphoma participants compared to healthy participants | |
| Myeloma participants vaccinated compared to healthy participants' serum | |
| Comparing different influenza vaccines in Non‐Hodgkin lymphoma patients | |
| All participants vaccinated Haematological participants compared to healthy participants | |
| All participants vaccinated | |
| All participants vaccinated | |
| No information about control group vaccination prior to study. Mortality is reported in a two‐year follow‐up, while we stated maximal follow‐up period until end of the influenza season following vaccination | |
| All participants vaccinated | |
| All participants vaccinated | |
| All participants vaccinated | |
| All participants vaccinated | |
| All participants vaccinated | |
| Comparing different doses of influenza vaccine | |
| All participants vaccinated | |
| All participants vaccinated Comparison to healthy participants | |
| All participants vaccinated |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Influenza vaccine versus none, Outcome 1 All‐cause mortality. | ||||
| 1.1 Non‐randomised, adjusted, events/person‐years | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Non‐randomised, adjusted, events/person | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Randomised, events/person | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Influenza‐like illness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Influenza vaccine versus none, Outcome 2 Influenza‐like illness. | ||||
| 2.1 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Non‐randomised, unadjusted, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Confirmed influenza Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 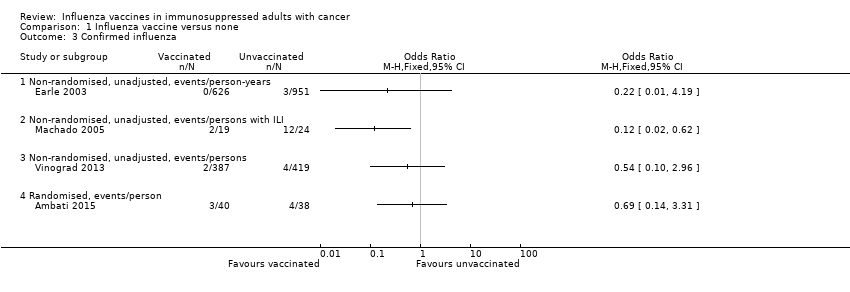 Comparison 1 Influenza vaccine versus none, Outcome 3 Confirmed influenza. | ||||
| 3.1 Non‐randomised, unadjusted, events/person‐years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Non‐randomised, unadjusted, events/persons with ILI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Non‐randomised, unadjusted, events/persons | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pneumonia Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 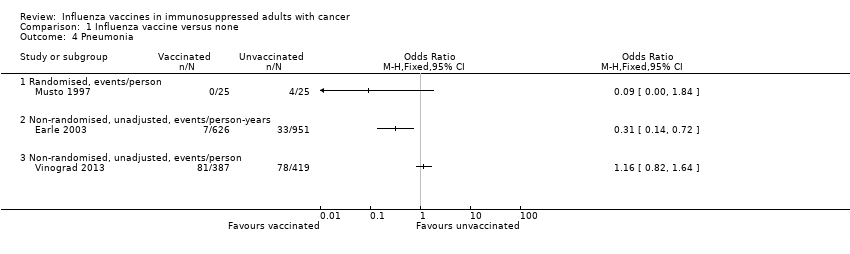 Comparison 1 Influenza vaccine versus none, Outcome 4 Pneumonia. | ||||
| 4.1 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Non‐randomised, unadjusted, events/person‐years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Non‐randomised, unadjusted, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Any hospitalisation Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Influenza vaccine versus none, Outcome 5 Any hospitalisation. | ||||
| 5.1 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Non‐randomised, unadjusted, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Influenza‐related mortality Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Influenza vaccine versus none, Outcome 6 Influenza‐related mortality. | ||||
| 6.1 Randomised, events/person | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Non‐randomised, unadjusted, events/person‐years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 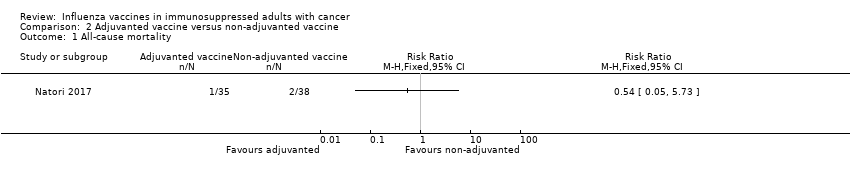 Comparison 2 Adjuvanted vaccine versus non‐adjuvanted vaccine, Outcome 1 All‐cause mortality. | ||||
| 2 Confirmed influenza Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Adjuvanted vaccine versus non‐adjuvanted vaccine, Outcome 2 Confirmed influenza. | ||||
| 3 Any hospitalisation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 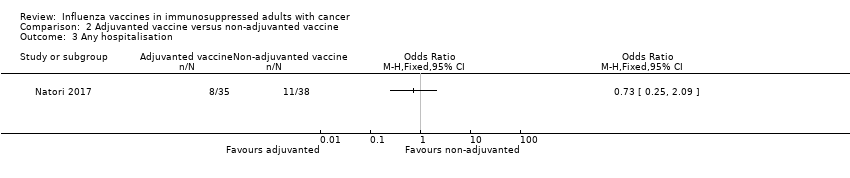 Comparison 2 Adjuvanted vaccine versus non‐adjuvanted vaccine, Outcome 3 Any hospitalisation. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Influenza vaccine versus none, Outcome 1 All‐cause mortality.

Comparison 1 Influenza vaccine versus none, Outcome 2 Influenza‐like illness.

Comparison 1 Influenza vaccine versus none, Outcome 3 Confirmed influenza.

Comparison 1 Influenza vaccine versus none, Outcome 4 Pneumonia.

Comparison 1 Influenza vaccine versus none, Outcome 5 Any hospitalisation.

Comparison 1 Influenza vaccine versus none, Outcome 6 Influenza‐related mortality.

Comparison 2 Adjuvanted vaccine versus non‐adjuvanted vaccine, Outcome 1 All‐cause mortality.

Comparison 2 Adjuvanted vaccine versus non‐adjuvanted vaccine, Outcome 2 Confirmed influenza.

Comparison 2 Adjuvanted vaccine versus non‐adjuvanted vaccine, Outcome 3 Any hospitalisation.
| Influenza vaccine compared to no vaccine for immunosuppressed adults with cancer | ||||||
| Patient or population: immunosuppressed adults with cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no vaccine | Risk with influenza vaccine | |||||
| All‐cause mortality, solid cancers | Study population | OR 0.88 | 1577 | ⊕⊝⊝⊝ | ||
| 417 per 1,000 | 387 per 1,000 | |||||
| All‐cause mortality, solid and haematological malignancies | Study population | OR 0.42 | 806 | ⊕⊝⊝⊝ | ||
| 456 per 1,000 | 260 per 1,000 | |||||
| All‐cause mortality, allogeneic BMT | Study population | OR 1.25 | 78 | ⊕⊕⊝⊝ | ||
| 211 per 1,000 | 250 per 1,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 observational study, low Newcastle Ottawa score 2 confidence interval up to 1 3 observational study 4 observational study, high Newcastle Ottawa score 5 reporting bias‐ no trial registry, vague description of outcomes in methods, small numbers 6 wide confidence interval crossing 1 | ||||||
| Adjuvanted influenza vaccine compared to non‐adjuvanted influenza vaccine in immunosuppressed adults with cancer | ||||||
| Patient or population: immunosuppressed adults with cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐adjuvanted influenza vaccine | Risk with adjuvanted influenza vaccine | |||||
| All‐cause mortality, allogeneic BMT, | Study population | RR 0.54 | 73 | ⊕⊕⊝⊝ | ||
| 53 per 1,000 | 28 per 1,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Small sample size, large confidence intervals | ||||||
| Ref. | Type of malignancy (influenza years) | No of cases | Influenza cases | Outcome |
| Allogeneic BMT/HSCT recipients (1997 to 1998) | 819 | 1.7% | Deaths 29% | |
| Autologous BMT/HSCT recipients (1997 to 1998) | 1154 | 0.2% | Deaths 0% | |
| Allogeneic BMT/HSCT recipients (1997 to 2000) | >819 |
| Deaths 23% | |
| Autologous BMT/HSCT recipients (1997 to 2000) | >1154 |
| Deaths 22% | |
| Allogeneic BMT/HSCT recipients (1996 to 2001) | 230 | 2.2% | Deaths 20% | |
| Autologous BMT/HSCT recipients (1996 to 2001) | 396 | 0% |
| |
| HSCT recipients (within 120 days after transplantation) (1989 to 2002) | 4797 | 1.3% | Deaths 10% Pneumonia 29% | |
| HSCT recipients (URTI symptoms present) (2001 to 2002) | 179 | 23% | Deaths 0% | |
| HSCT recipients AND haematologic malignancies (retrospective study of patients with laboratory‐confirmed viral respiratory infection) (2000 to 2002) | 343 | 33% | Deaths 4% Pneumonia 30% | |
| HSCT recipients | 230 | 29% |
| |
| Leukaemia | 61 | 33% |
| |
| Lymphoma | 37 | 51% |
| |
| Multiple myeloma | 15 | 40% |
| |
| CLL /acute leukaemia (hospitalised patients) (1993 to 1994) | 45 | 33% | Deaths 27% Pneumonia 80% | |
| CLL /acute leukaemia (1991 to 1992) | 37 | 11% | Deaths 25% Pneumonia 75% | |
| Solid cancers (H1N1 2009 pandemic) | 226 | 7% | 0% Deaths | |
| Haematologic malignancies (H1N1 2009 pandemic) | 167 (96 HSCT) | 17% (22%) | 0% Deaths | |
| HSCT recipients (prospective study of patients with laboratory‐confirmed H1N1 infection) (H1N1 2009 pandemic) | 286 | Deaths 6% Pneumonia 33% | ||
| Solid cancers (retrospective study of patients with laboratory‐confirmed H1N1 infection) (H1N1 2009 pandemic) | 115 | Deaths 9.5% Pneumonia 23% | ||
| BMT: bone marrow transplantation; CLL: chronic lymphocytic leukaemia; HSCT: haematopoietic stem cell transplantation; URTI: upper respiratory tract infection | ||||
| Selection | Comparability | Outcome | Total stars score | ||||||
| Representativeness of the exposed cohort | Selection of the non‐exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability * | Assessment of outcome | Was follow‐up long enough for outcomes to occur? | Adequacy of follow‐up of cohorts ** | ||
| c | a | a | a | No | d *** | a | a | 5 | |
| c | a | a | a | No | b | a | a | 6 | |
| b | a | a+b | a | a+b | b+c | a | a | 10 | |
| * The most important factor to control for was the cancer stage. The second most important factor was functional capacity ** A follow‐up rate of >=80% was considered adequate *** Procedure was described but considered inadequate (through billing accounts and other administrative databases) | |||||||||
| Outcome | Design | All‐cause mortality | Influenza‐like‐ illness | Influenza‐ related mortality | Confirmed influenza | Pneumonia | Any hospitalisation | Chemotherapy interruptions | GMT | |||||||
| Vaccination status | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
| Retrospective observational | Cox adjusted HR 0.88 (95% CI 0.78 to 1), 626 versus 951 py * | 0/626 py | 2/951 py | 0/626 py | 3/951 py | 7/626 py * | 33/951 py * | mean days 15.6, 95% CI 13.3 to 17.8 (N = 626 py) | mean days 16.4, 95% CI 14.3 to 18.4 (N = 951 py) | mean 5.06 days (N = 626 py) ** | mean 6.04 days (N = 951 py) ** | |||||
| Retrospective case‐control | 2/19 * | 12/24 * | ||||||||||||||
| Randomised, open‐label | 8/25 * | 18/25 * | 0/25 | 2/25 | 0/25 | 4/25 | 2/25 * | 12/25 * | ||||||||
| Prospective observational | MV adjusted OR 0.42 (95% CI 0.24 to 0.75) (387 versus 419p); MV adjusted OR in propensity‐matched cohort 0.42 (95% CI 0.24 to 0.76) (218p versus 218p) | 134/387 | 137/419 | 2/387 | 4/419 | 81/387 | 78/419 | 183/387 | 205/419 | 97/387 | 116/419 | |||||
| Randomised, open‐label | OR 1.25 (95%CI 0.43‐3.62) | 2/40 | 2/38 | 3/40 | 4/38 | 15*, 30*, 110 *** | 10*, 12.5*, 60 *** | |||||||||
| py= persons years * denoted statistically significant difference, P < 0.05 ** mean interval between chemotherapy bills *** data is for A/H1N1, A/H3N2 and B respectively CI: confidence interval;GMT: geometric mean titre. (Data are for 30 days post vaccination); HR: hazard ratio; OR: odds ratio; | ||||||||||||||||
| Outcome | Design | All‐cause mortality | Influenza‐related mortality | Confirmed influenza | Any hospitalisation | GMT | |||||
| Vaccination status | Adjuvanted | Non‐adjuvanted | Adjuvanted | Non‐adjuvanted | Adjuvanted | Non‐adjuvanted | Adjuvanted | Non‐adjuvanted | Adjuvanted | Non‐adjuvanted | |
| Randomised, open‐label | 1/35 | 2/38 | 0/35 | 0/38 | 5/35 | 3/38 | 8/35 | 11/38 | 319.6, 480.7, 298.9 * | 195.9, 359.3, 240.5 * | |
| * data is for A/H1N1, A/H3N2 and B respectively GMT: geometric mean titre. (Data are for 30 days post vaccination). | |||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Non‐randomised, adjusted, events/person‐years | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Non‐randomised, adjusted, events/person | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Randomised, events/person | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Influenza‐like illness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Non‐randomised, unadjusted, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Confirmed influenza Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Non‐randomised, unadjusted, events/person‐years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Non‐randomised, unadjusted, events/persons with ILI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Non‐randomised, unadjusted, events/persons | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pneumonia Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Non‐randomised, unadjusted, events/person‐years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Non‐randomised, unadjusted, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Any hospitalisation Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Randomised, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Non‐randomised, unadjusted, events/person | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Influenza‐related mortality Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Randomised, events/person | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Non‐randomised, unadjusted, events/person‐years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Confirmed influenza Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Any hospitalisation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |


