Osmotic therapies added to antibiotics for acute bacterial meningitis
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 exp Meningitis/
2 meningit*.tw.
3 1 or 2
4 Osmosis/
5 Osmotic Pressure/
6 exp Diuretics, Osmotic/
7 (osmos* or osmot* or osmol*).tw.
8 exp Sugar Alcohols/
9 glycer*.tw,nm.
10 1,2,3‐propanetrio*.tw,nm.
11 mannitol*.tw,nm.
12 sorbit*.tw,nm.
13 Sodium Lactate/
14 (sodium adj2 lactat*).tw,nm.
15 Saline Solution, Hypertonic/
16 (hypertonic adj2 saline*).tw,nm.
17 or/4‐16
18 3 and 17
Appendix 2. Embase (Elsevier) search strategy
#20 #16 AND #19
#19 #17 OR #18
#18 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((doubl* OR singl*) NEAR/2 (blind* OR mask*)):ab,ti
#17 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp
#16 #3 AND #15
#15 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
#14 (hypertonic NEAR/2 saline):ab,ti
#13 'sodium chloride'/de
#12 (lactat* NEAR/2 sodium):ab,ti
#11 'lactate sodium'/de
#10 sorbit*:ab,ti
#9 mannitol*:ab,ti
#8 '1,2,3‐propanetriol':ab,ti OR propanetrio*:ab,ti
#7 glycer*:ab,ti
#6 'sugar alcohol'/exp
#5 osmotic*:ab,ti
#4 'osmotic diuretic agent'/exp
#3 #1 OR #2
#2 meningit*:ab,ti
#1 'meningitis'/exp
Appendix 3. CINAHL (Ebsco) search strategy
S12 S3 and S11
S11 S4 or S5 or S6 or S7 or S8 or S9 or S10
S10 TI hypertonic N2 saline or AB hypertonic N2 saline
S9 (MH "Saline Solution, Hypertonic")
S8 TI sodium N2 lactat* or AB sodium N2 lactat*
S7 TI ( glycerol* or 1,2,3‐propanetriol or propanetriol* or mannitol* or sorbit* ) or AB ( glycerol* or 1,2,3‐propanetriol or propanetriol* or mannitol* or sorbit* )
S6 AB sugar alcohol* or TI sugar alcohol*
S5 (MH "Sugar Alcohols+")
S4 TI osmotic* or AB osmotic*
S3 S1 or S2
S2 TI meningit* or AB meningit*
S1 (MH "Meningitis+")
Appendix 4. LILACS (BIREME) search strategy
> Search > (MH:Meningitis OR Meningite OR MH:C10.228.228.507$ OR C10.228.566$ OR MH:C01 252.200$ OR C10.228.228.180.500$ OR meningit$) AND (MH:"Diuretics, Osmotic" OR "Diuréticos Osmóticos" OR "Diuréticos Osmóticos" OR "Osmotic Diuretics" OR MH:D27.505.696.560.500.453$ OR osmot$ OR osmos$ OR osmol$ OR MH:"Sugar Alcohols" OR "Alcoholes del Azúcar" OR "Álcoois de Açúcar" OR MH:D02.033.800$ OR MH:D09.853$ OR "sugar alcohols" OR glycer$ OR "1,2,3‐propanetriol" OR mannitol$ OR sorbit$ OR MH:"Sodium Lactate" OR "Lactato de Sodio" OR "Lactato de Sódio" OR MH:D02.241.511.459.500$ OR "sodium lactate" OR MH:"Saline Solution, Hypertonic" OR "Solución Salina Hipertónica" OR "Solução Salina Hipertônica" OR "Hypertonic Saline Solution" OR "Sodium Chloride Solution, Hypertonic" OR "Hypertonic Solution, Saline" OR "Solución Hipertónica de Cloruro de Sodio" OR "Solução Hipertônica de Cloreto de Sódio" OR "hypertonic saline") > clinical_trials
Appendix 5. ClinicalTrials.gov search strategy
Search terms: (meningitis OR meningitides) AND (osmosis or osmoses or osmotic or osmolarity or glycerol OR glycerine OR glycerin OR or 1,2,3‐propanetriol or propanetriol or mannitol or sorbitol or sodium lactate or (hypertonic AND saline))
Appendix 6. WHO ICTRP search strategy
Search terms: meningit* AND osmos* or meningit* AND osmot* OR meningit* AND osmol* or meningit* AND glycer* OR meningit* AND 1,2,3‐propanetrio* or meningit* AND mannitol* or meningit* AND sorbit* or meningit* AND sodium lactate or meningit* AND hypertonic AND saline*

Study screening flow diagram
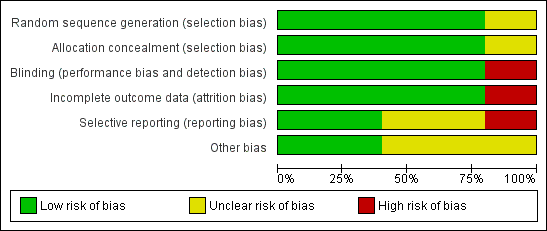
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
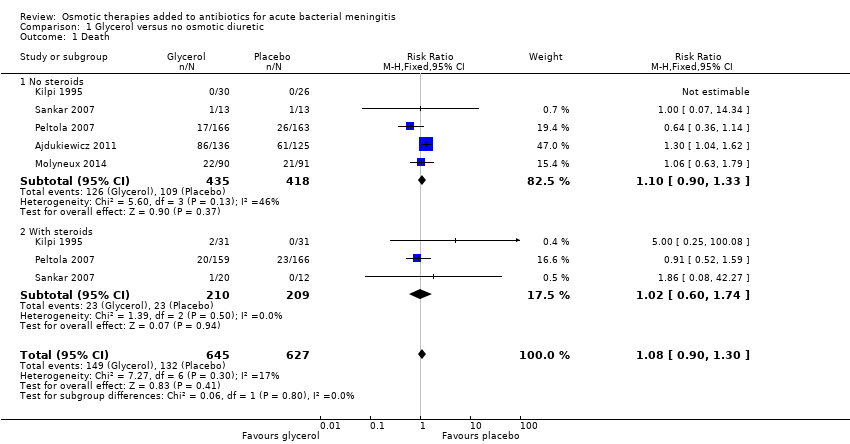
Comparison 1 Glycerol versus no osmotic diuretic, Outcome 1 Death.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 2 Neurological disability.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 3 Seizures.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 4 Hearing loss.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 5 Adverse effects: nausea, vomiting, diarrhoea.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 6 Adverse effects: gastrointestinal bleeding.
| Glycerol for acute bacterial meningitis | ||||||
| Patient or population: children and adults with acute bacterial meningitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Glycerol | |||||
| Death | 19 per 100 | 21 per 100 | RR 1.08 | 1272 | ⊕⊕⊕⊝ | Downgraded for imprecision. Glycerol probably has little or no effect on death |
| Neurological disability | 9 per 100 | 6 per 100 (5 to 9) | RR 0.73 (0.53 to 1.00) | 1270 (5 studies) | ⊕⊕⊝⊝ Low1,3,4,5 | Downgraded for imprecision and inconsistency. Glycerol may reduce disability |
| Seizures | 32 per 100 | 35 per 100 | RR 1.08 | 1090 | ⊕⊕⊝⊝ | Downgraded for inconsistency and imprecision. Glycerol may have little or no effect on seizures |
| Hearing loss | 16 per 100 | 10 per 100 | RR 0.64 | 922 | ⊕⊕⊕⊝ | Downgraded for imprecision. Glycerol probably reduces hearing loss |
| Adverse effects: nausea, vomiting, diarrhoea | 47 per 100 | 51 per 100 (38 to 69) | RR 1.09 (0.81 to 1.47) | 851 | ⊕⊝⊝⊝ | Downgraded for serious inconsistency and imprecision. The effect of glycerol on adverse events: nausea, vomiting and diarrhoea is uncertain |
| Adverse effects: gastrointestinal bleeding | 3 per 100 | 3 per 100 (13 to 8) | RR 0.93 (0.39 to 2.19) | 607 | ⊕⊕⊕⊝ | Downgraded for imprecision. Glycerol probably has little or no effect on adverse events: gastrointestinal bleeding |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval (CI)) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: allocation concealment was adequate in four trials and unclear (not reported) in one trial. 3Not downgraded for indirectness. The five trials were conducted in Finland, Malawi, India and South America. Four were in children and one in adults. All included patients with suspected meningitis and cerebrospinal fluid (CSF) changes suggestive of bacterial infection. 5Downgraded by one level for inconsistency: in the Finnish trial the risk of neurological sequelae was reduced with glycerol (RR 0.50, 95% CI 0.32 to 0.78, N = 329), but this was not found in the other studies and the meta‐analysis did not detect a difference (I² = 59%). 6Downgraded by one level for inconsistency: in the trial with adults the risk of seizures was higher with glycerol (RR 1.62, 95% CI 1.18 to 2.23, N = 250), but this was not found in the other studies and the meta‐analysis did not detect a difference (I² = 62%). 8Another two trials reported on this outcome but the results could not be added to the meta‐analysis; one reported more cases of vomiting with glycerol and the other that the incidence of vomiting was "similar" in the treatment groups. 9Downgraded by two levels for inconsistency: in the South American and Finnish trials the risk of adverse effects was increased with glycerol, but this was not found in the Malawi and India trials, and the meta‐analysis did not detect a difference (I² = 79%). | ||||||
| Drug | Class | Mechanism of action | Dose range and route | Studied/used in |
| Glycerol | Sugar alcohol | Probably osmosis plus possible vascular and metabolic benefit | IV 5% to 10% solution or 50 g Oral 1.5 g/kg | Meningitis (Peltola 2007), stroke (Righetti 2004) |
| Mannitol | Sugar alcohol | Osmotic diuretic | IV 20% solution 1 mL/kg to 10 mL/kg or 1 g/kg | Brain trauma (Wakai 2013), cerebral malaria (Namutangula 2007), stroke (Bereczki 2007)
|
| Sorbitol | Sugar alcohol | Osmotic diuretic (weak) | Oral, IV | Experimental brain perfusion, stroke |
| Hypertonic saline | Hypertonic solutions | Osmosis | IV | Brain trauma (Choi 2005), stroke (Schwarz 2002) |
| Sodium lactate | Hydroxy acids | Osmosis (weak) | IV | Brain trauma (Ichai 2009) |
| IV: intravenous | ||||
| Name of study | Population | Intervention and dose | Control used | Treatment duration | Study arms |
| Children in Finland | Oral glycerol 4.5 g/kg max 180 g/24 h in 3 divided doses Dexamethasone (dex) 1.5 mg/kg max 60 mg/day | No oral placebo IV saline | 3 days | 4 arms: IV dexamethasone + glycerol, oral glycerol, IV dexamethasone, neither treatment | |
| Children in India | Oral glycerol 1.5 g/kg 3 x daily Dexamethasone 0.15 mg/kg 3 x daily | Oral carboxymethylcellulose 2% IV saline | Not detailed | 4 arms: placebo oral and IV, IV dexamethasone + oral glycerol, IV placebo + oral glycerol, IV dexamethasone + oral placebo | |
| Children in South America | Oral glycerol 1.5 g/kg 3 x daily Dexamethasone 0.15 mg/kg 3 x daily | Oral carboxymethylcellulose 2% IV saline | 2 days | 4 arms: oral and IV placebo, IV dexamethasone + oral glycerol, IV placebo + oral glycerol, IV dexamethasone + oral placebo | |
| Adults in Malawi, Southern Africa | Oral glycerol 75 mg 4 x daily diluted in water or 50% dextrose solution | Oral 50% dextrose solution | 4 days | Oral glycerol versus oral 50% dextrose | |
| Children in Malawi, Southern Africa | Oral glycerol 25 mL/dose (maximum dose) = 100 mL/24 hours. Acetaminophen 35 mg/kg 6‐hourly | Oral carboxymethylcellulose 2% | 2 days | 3 arms: oral glycerol and oral acetaminophen, oral placebo and glycerol, oral acetaminophen and oral placebo | |
| IV: intravenous | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 5 | 1272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 1.1 No steroids | 5 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.90, 1.33] |
| 1.2 With steroids | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.60, 1.74] |
| 2 Neurological disability Show forest plot | 5 | 1270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.00] |
| 2.1 No steroids | 5 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.01] |
| 2.2 With steroids | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.38, 1.77] |
| 3 Seizures Show forest plot | 4 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 3.1 No steroids | 4 | 755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.44] |
| 3.2 With steroids | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| 4 Hearing loss Show forest plot | 5 | 922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.93] |
| 4.1 No steroids | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.41, 0.99] |
| 4.2 With steroids | 3 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.35] |
| 5 Adverse effects: nausea, vomiting, diarrhoea Show forest plot | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.47] |
| 5.1 No steroids | 2 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.81, 1.83] |
| 5.2 With steroids | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.13] |
| 6 Adverse effects: gastrointestinal bleeding Show forest plot | 3 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.19] |
| 6.1 No steroids | 3 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.06, 2.60] |
| 6.2 With steroids | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.44, 3.04] |

