Prostaciclinas en aerosol para el síndrome de dificultad respiratoria aguda (SDRA)
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | 2‐group cross‐over RCT, 1 centre. ITT: yes. Overall study quality: low risk of bias. Sample size calculation: not reported. Country: the Netherlands. | |
| Participants | 14 children included first after 24 hours of admission with ALI defined by the criteria of the American‐European Consensus Conference in 1994 (Bernard 1994). Inclusion criteria: acute onset of respiratory failure; PaO2/FIO2 ratio ≤ 300 torr; no clinical signs of atrial hypertension (suspected clinically); bilateral infiltrates on chest radiographs, children intubated with endotracheal tubes with an internal diameter > 3.5 mm. ALI classified as either primary (intrapulmonary) or secondary (extrapulmonary) lung injury. Exclusion criteria: congenital heart disease, decreased cardiac shortening fraction < 30%, mitral regurgitation, enlarged left atrium suspected to have raised left atrial pressure and cardiogenic pulmonary oedema, thrombocytopenia (< 50,000/L), bleeding diathesis, activated partial thromboplastin time > 43 seconds, intracranial haemorrhage, acute renal failure, chronic lung disease or poor prognosis with the probability of death, or withdrawal of therapy within the following 24 hours. | |
| Interventions | Intervention group: 8 children, first treated with aerosolized prostacyclin (epoprostenol sodium), stepwise increase of doses (10, 20, 30, 40 and 50 ng/kg/minute) followed by normal saline (designated as placebo). Each dose administered over 20‐minute period, followed by 5‐minute period between each dose increment. To achieve washout, there was 30‐minute period between prostacyclin and placebo nebulization. Control group: 6 children, initially treated with 5 doses of normal saline followed by aerosolized prostacyclin. Ventilation strategy and weaning standardized. No cross‐over of treatment failures. Standard critical care therapy to both groups. | |
| Outcomes | Primary outcomes: improved oxygenation. Secondary outcomes: mortality, adverse effects, oxygenation index, FiO2, improved ventilation and respiratory variables, primary versus secondary lung injury, changes in haemodynamics, bleeding. | |
| Notes | Aerosolized prostacyclin over < 24 hours did not reduce overall mortality at 28 days (RR 1.50, 95% CI 0.17 to 12.94, 14 participants) compared with aerosolized saline (total of 3 deaths). Letter sent to authors in December 2009. Authors replied in December 2009. The authors were unable to provide additional information except data for the analysis of mortality based on origin of the lesion (primary versus secondary lung injury) without finding statistical significance. Length of longest follow‐up: 28 days. Authors conclusion: "Aerosolized prostacyclin improves oxygenation in children with acute lung injury. Future trials should investigate whether this treatment will positively affect outcome." Funding: not for profit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by numbered envelopes, following a cross‐over randomization procedure. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) | Low risk | Investigators and carers blinded to assignment of participants. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals specified. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess based on available information. |
| Other bias | Low risk | Appeared free of such biases. |
| Methods | Single‐centre, RCT. Country: Pakistan. | |
| Participants | 67 adults aged ≥ 18 years with ARDS. | |
| Interventions | Intervention group: PGE1 (alprostadil) 20 μg in 5 mL normal saline in a nebulizer continuously over 30 minutes. Control group: 5 mL normal saline in a nebulizer continuously over saline over 30 minutes. Used concealed syringes. | |
| Outcomes | Primary endpoint: proportion of participants achieving 25% improvement in diastolic dysfunction, left ventricular end diastolic pressure, pulmonary artery systolic pressures and PaO2/FiO2 ratio from baseline as measured by repeat transthoracic echo and arterial blood gas analysis 30 minutes after treatment. No secondary outcomes. | |
| Notes | Participant enrolment from May 2006 to February 2008. Contacted study author twice, 20 June 2016 and 24 March 2017 and received relevant response. Study took place in an adult, multidisciplinary, "open‐policy," 11 bed ICU in a tertiary care hospital of Karachi, Pakistan. The authors stated that measurement of pulmonary artery pressure was carried out with the application of echocardiography instead of pulmonary artery catheterization with the inherent risk of inaccuracies and bias in regards to measurements and inter‐observer variability. However, we were advised to approach the authors due to some questions in regards to the accuracy of the reported values of the pulmonary artery pressures in the publication (> 80 mmHg in both the intervention and control group). It became apparent that the authors had mistakenly reported on the systemic vascular mean systolic pressure and not the mean pulmonary artery systolic pressure. Furthermore, the authors provided additional information on lack of mortality data during the trial follow‐up. The trial was funded by the Pakistan Medical Research Council. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Parallel‐group study with balanced randomizations from a computer‐generated randomization list. |
| Allocation concealment (selection bias) | Low risk | Independent pharmacists dispensed either the intervention or the control from pharmacy in a syringe form concealed with aluminium foil. |
| Blinding (performance bias and detection bias) | Low risk | All investigators, staff and participants were masked to outcome measurements and allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | All randomized participants seemed to be accounted for in the tables. However, the authors were unable to report data on mean artery pulmonary pressure and had provided data on systemic artery pressure instead in the manuscript. |
| Selective reporting (reporting bias) | Low risk | Trial registration available on ClinicalTrials.gov: NCT00314548. |
| Other bias | Low risk | Appeared free of other bias. |
For explanation of acronyms and abbreviations used in this table, see Appendix 1.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Randomized, multicentre, double‐blind, placebo‐controlled, phase II clinical trial of intravenous liposomal PGE1 versus placebo for people with ARDS. No inhalational therapy of prostacyclin. | |
| Multicentre, double‐blind, placebo‐controlled, phase III clinical trial; 350 people with ARDS randomized to receive either liposomal PGE1 or placebo. No inhalational therapy of prostacyclin. | |
| Retrospective, non‐interventional cohort study. 94 participants included, with 47 participants receiving prostacyclin and 47 receiving placebo. Reason for exclusion: retrospective study. | |
| Randomized, placebo‐controlled trial of intravenous prostacyclin in acute respiratory failure in people with COPD. No inhalational therapy of prostacyclin. | |
| Case report. No randomization. | |
| Randomized, double‐blind, cross‐over study; 16 people with COPD randomized to either a single dose of iloprost 10 mg (low dose), iloprost 20 mg (high dose) or placebo. All participants excluded because of chronic lung disease. | |
| Randomized double‐blind, multicentre study of intravenous PGE1 in people with the ARDS versus placebo. No inhalational therapy of prostacyclin. There are multiple publications in different journals based on this trial. | |
| Prospective, non‐randomized interventional study examining the effect of inhaled prostacyclin in 15 consecutive, mechanically ventilated people with ARDS and severe hypoxaemia. | |
| 16 participants in an observational study. Reason for exclusion: retrospective study. | |
| Randomized, interventional clinical study comparing INO and aerosolized prostacyclin on haemodynamics and gas exchange in people with septic shock and pulmonary hypertension. Excluded since majority of participants did not have ARDS or ALI. | |
| Randomized, placebo‐controlled, double‐blind trial of intravenous PGE1 in surgical participants with ARDS. No inhalational therapy of prostacyclin. | |
| 28 adults with end‐stage cirrhosis (18 men and 10 women) underwent modified piggyback liver transplantations. Reason for exclusion: observational study on elective patients. | |
| 15 people with ALI treated with PGE1 inhalation in addition to standard intensive care. No randomization. | |
| Trial terminated prior to enrolment. Accessed 26 April 2016. | |
| Case report. No randomization. | |
| 10 people with ARDS received in random order: nitric oxide inhalation, aerosolized PGE1, infusion of PGE1 or no intervention. No control group and thus not an RCT. | |
| Randomized double‐blind placebo‐controlled study on the activity of intravenous PGE1 in people with ARDS. No inhalational therapy with prostacyclin. | |
| Prospective, non‐randomized interventional study examining the effect of nebulized iloprost in 20 people admitted to medical and surgical ICUs. No control group. | |
| Case report. PGE1 infusion. No randomization. | |
| RCT enrolling only neonates and therefore excluded. All 7 participants INO prior to enrolment. 4 participants received pulmonary vasodilators (milrinone, sildenafil), 1 participant, randomized to high‐dose inhaled PGE1 received low‐dose inhaled PGE1 for the first 24 hours, thereafter high‐dose inhaled PGE1. 5/7 participants received surfactant. 5/7 received neuromuscular blockade and 4/7 received steroids before randomization. 6 participants received extracorporeal membrane oxygenation. | |
| Retrospective, single‐centre analysis of mechanically ventilated adults receiving INO or PGI2 for improvement in oxygenation; 105 mechanically ventilated people evaluated. Retrospective analysis and thus not an RCT. | |
| Same cohort as Torbic 2013. | |
| Case report. Comparison of INO and inhaled prostacyclin. No randomization. | |
| Unblinded, non‐randomized interventional, prospective clinical study of inhaled aerosolized prostacyclin in people with ARDS. | |
| Double‐blind, placebo‐controlled trial evaluating the efficacy of early infusion of PGE1 for reducing the incidence of severe respiratory failure and mortality. No inhalational prostacyclin therapy. | |
| Multicentre, randomized, double‐blind, placebo‐controlled clinical study evaluating the safety of intravenous liposomal PGE1 (TLC C‐53) in people with ARDS. No inhalational therapy of prostacyclin. | |
| Trial examining the effects of aerosolized PGI2 on gas exchange and haemodynamics in mechanically ventilated people with severe community‐acquired pneumonia. Both groups received active treatment of inhalational prostacyclin. No control group. | |
| 16 people with ARDS selected to receive initially either INO and then inhaled PGI2, or vice versa for very short period of time. No control group. | |
| Case report of 8 participants receiving both inhaled prostacyclin and INO at various concentration. Not an RCT. |
For explanation of acronyms and abbreviations used in this table, see Appendix 1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
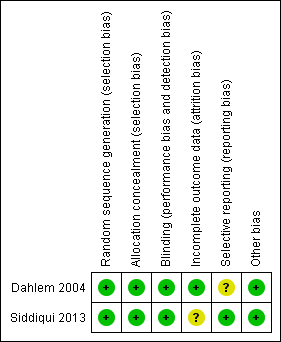
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
| Aerosolized prostacyclin compared to placebo for acute respiratory distress syndrome (ARDS) | ||||||
| Patient or population: people with ARDS Setting: intensive care unit in the Netherlands and Pakistan Intervention: aerosolized prostacyclin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with aerosolized prostacyclin | |||||
| Mortality | Study population | RR 1.50 | 14 | ⊕⊝⊝⊝ | Only 1 small paediatric trial with cross‐over design provided mortality data (Dahlem 2004). Thus, no meta‐analysis carried out. | |
| 167 per 1000 | 250 per 1000 | |||||
| PaO2/FiO2 ratio5 | ‐ | MD 25.35 lower | ‐ | 67 | ⊕⊝⊝⊝ | Only 1 trial provided data (Siddiqui 2013). Thus, no meta‐analysis was carried out. |
| Improvement in mean pulmonary arterial pressure | ‐ | ‐ | ‐ | ‐ | ‐ | No data is available for meta‐analysis (Characteristics of included studies, Siddiqui 2013) |
| Adverse events7 | ‐ | ‐ | ‐ | 81 (2 studies) | ⊕⊝⊝⊝ | Only descriptive assessment of safety with no available data to carry out meaningful analyses (Dahlem 2004; Siddiqui 2013). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FiO2: fraction of inspired oxygen; MD: mean difference; PaO2: partial pressure of oxygen in arterial blood; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Mortality at 28 to 30 days. 2Required information size for paediatric population depending on the level of heterogeneity adjustment was between 2897 (I2 = 0) and 3862 (I2 = 25%). 4This outcome was downgraded from high to low quality of evidence due to limitations in design (small sample size, few events, cross‐over design) suggesting high likelihood of bias, indirectness of evidence and high probability of publication bias. (Dahlem 2004). 5Despite the fact that biochemical markers of clinical outcomes are often not included in SoF tables, we have chosen to include this outcomes since it is widely used in clinical practice to guide treatment. 6The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size, few events and wide 95% CI suggesting high likelihood of bias and indirectness of evidence. (Siddiqui 2013). 7Adverse events such as bleeding or organ dysfunction 8The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size and few events and since only descriptive assessment of safety and adverse events were provided in the included trials with no data being available for meta‐analyses. | ||||||


