Prostaciclinas en aerosol para el síndrome de dificultad respiratoria aguda (SDRA)
Appendices
Appendix 1. Abbreviations
| ALI = acute lung injury; ARDS = acute respiratory distress syndrome; CI = confidence interval; CINAHL = Cumulative Index to Nursing & Allied Health Literature; COPD = chronic obstructive lung disease; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; INO = inhaled nitric oxide; ITT = intention to treat analysis; LILACS = Latin American Caribbean Health Sciences Literature; MD = mean difference; MPAP = mean arterial pulmonary pressure; PaO2 = partial pressure of oxygen in arterial blood; PEEP = positive end expiratory pressure; PGE1 = prostaglandin E1; PGI2 = prostacyclin or epoprostenol or Flolan; PVR = pulmonary vascular resistance; RCT = randomized controlled trial; RR = risk ratio; TSA = trial sequential analysis. |
Appendix 2. Search strategies
| Database | Search strategy |
| Handsearch | Citation search of included studies and relevant reviews |
| CENTRAL,the Cochrane Library, 2017, Issue 4 | #1 MeSH descriptor Epoprostenol explode all trees #2 MeSH descriptor Prostaglandins explode all trees #3 prostaglandin*or Iloprost or Prostin or Flolan or Epoprostenol or Beraprost or Treprostinil or prostacyclin* #4 (#1 OR #2 OR #3) #5 MeSH descriptor Respiratory Distress Syndrome, Adult explode all trees #6 ARDS #7 respirator* or distress #8 distress and syndrome #9 (#5 OR #6 OR #7 OR #8) #10 (#9 AND #4) |
| Embase (OvidSP) | 1. exp prostacyclin/ or exp prostaglandin/ |
| ISI Web of Science | #1 TS = prostacyclin* or TS = prostaglandin* or TS = Iloprost or TS = Prostin or TS = Flolan or TS = Epoprostenol or TS = Beraprost or TS = Treprostinil Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI Timespan=All years #2 TS = ARDS or TS = (respirator* NEAR distress) or TS = (distress NEAR syndrome) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI Timespan=All years #3 (#1 AND #2) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI Timespan=All years |
| ISI BIOSIS Previews | #1 TS = prostacyclin* or TS = prostaglandin* or TS = Iloprost or TS = Prostin or TS = Flolan or TS = Epoprostenol or TS = Beraprost or TS = Treprostinil Indexes=BIOSIS Previews Timespan=All years #2 TS = ARDS or TS = (respirator* NEAR distress) or TS = (distress NEAR syndrome) Indexes=BIOSIS Previews Timespan=All years #3 (#1 AND #2) Indexes=BIOSIS Previews Timespan=All years |
| LILACS (via BIREME) | ("EPOPROSTENOL" or "EPOPROSTENOL/" or "PROSTAGLANDINS" or "prostaglandin$" or "Iloprost" or "Prostin" or "Flolan" or "Epoprostenol" or "Beraprost" or "Treprostinil" or "prostacyclin$") and ("RESPIRATORY DISTRESS SYNDROME, ACUTE/" or "RESPIRATORY DISTRESS SYNDROME, ADULT/" or "respirator$" or "distress") |
| MEDLINE (Ovid SP) | 1. exp Epoprostenol/ or exp Prostaglandins/ |
| CINAHL (EBSCOhost) | ((MM "Respiratory Distress Syndrome, Acute") or (MH "Respiratory Distress Syndrome+") or ARDS or respirator* or distress ) and ( prostaglandin*or Iloprost or Prostin or Flolan or Epoprostenol or Beraprost or Treprostinil or prostacyclin*) |
Appendix 3. Assessment of risk of bias in included studies
1. Random sequence generation
Assessment of randomization: sufficiency of the method in producing two comparable groups before intervention.
Grade: 'low risk': a truly random process (e.g. random computer number generator, coin tossing, throwing dice); 'high risk': any non‐random process (e.g. date of birth, date of admission by hospital or clinic record number or by availability of the intervention) or 'unclear risk': insufficient information.
2. Allocation concealment
Allocation method prevented investigators or participants from foreseeing assignment.
Grade: 'low risk': central allocation or sealed opaque envelopes; 'high risk': use of open allocation schedule or other unconcealed procedure or 'unclear risk': insufficient information.
3. Blinding
Assessment of appropriate blinding of the team of investigators and participants: person responsible for participant care, participants and outcome assessors.
Grade: 'low risk': blinding considered adequate if participants and personnel were kept unaware of intervention allocations after inclusion of participants into the study, and if the method of blinding involved a placebo indistinguishable from the intervention, as mortality is an objective outcome; 'high risk': not double‐blind, categorized as an open‐label study or without use of a placebo indistinguishable from the intervention or 'unclear risk': blinding not described.
4. Incomplete outcome data
Completeness of outcome data, including attrition and exclusions.
Grade: 'low risk': numbers and reasons for dropouts and withdrawals in the intervention groups described, or no dropouts or withdrawals specified; 'high risk': no description of dropouts and withdrawals provided; 'unclear risk': report gave the impression of no dropouts or withdrawals, but this was not specifically stated.
5. Selective reporting
Possibility of selective outcome reporting.
Grade: 'low risk': reported outcomes were prespecified in an available study protocol, or, if this was not available, published report included all expected outcomes; 'high risk': not all prespecified outcomes reported, reported using non‐prespecified subscales, reported incompletely or report failed to include a key outcome that would have been expected for such a study or 'unclear risk': insufficient information.
6. Funding bias
Assessment of any possible funding bias.
Grade: 'low risk': reported no funding, funding from universities or public institutions; 'high risk': funding from private investors, pharmaceutical companies or trial investigator employed by the pharmaceutical company or 'unclear risk': insufficient information.
7. Other bias
Assessment of any possible sources of bias not addressed in domains 1 to 6.
Grade: 'low risk': report appeared free of such biases; 'high risk': at least one important bias was present that was related to study design, early stopping because of some data‐dependent process, extreme baseline imbalance, academic bias, claimed fraudulence or other problems; or 'unclear risk': insufficient information, or evidence that an identified problem will introduce bias.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
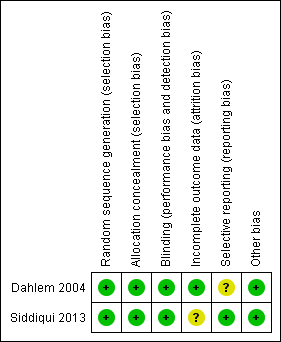
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
| Aerosolized prostacyclin compared to placebo for acute respiratory distress syndrome (ARDS) | ||||||
| Patient or population: people with ARDS Setting: intensive care unit in the Netherlands and Pakistan Intervention: aerosolized prostacyclin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with aerosolized prostacyclin | |||||
| Mortality | Study population | RR 1.50 | 14 | ⊕⊝⊝⊝ | Only 1 small paediatric trial with cross‐over design provided mortality data (Dahlem 2004). Thus, no meta‐analysis carried out. | |
| 167 per 1000 | 250 per 1000 | |||||
| PaO2/FiO2 ratio5 | ‐ | MD 25.35 lower | ‐ | 67 | ⊕⊝⊝⊝ | Only 1 trial provided data (Siddiqui 2013). Thus, no meta‐analysis was carried out. |
| Improvement in mean pulmonary arterial pressure | ‐ | ‐ | ‐ | ‐ | ‐ | No data is available for meta‐analysis (Characteristics of included studies, Siddiqui 2013) |
| Adverse events7 | ‐ | ‐ | ‐ | 81 (2 studies) | ⊕⊝⊝⊝ | Only descriptive assessment of safety with no available data to carry out meaningful analyses (Dahlem 2004; Siddiqui 2013). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FiO2: fraction of inspired oxygen; MD: mean difference; PaO2: partial pressure of oxygen in arterial blood; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Mortality at 28 to 30 days. 2Required information size for paediatric population depending on the level of heterogeneity adjustment was between 2897 (I2 = 0) and 3862 (I2 = 25%). 4This outcome was downgraded from high to low quality of evidence due to limitations in design (small sample size, few events, cross‐over design) suggesting high likelihood of bias, indirectness of evidence and high probability of publication bias. (Dahlem 2004). 5Despite the fact that biochemical markers of clinical outcomes are often not included in SoF tables, we have chosen to include this outcomes since it is widely used in clinical practice to guide treatment. 6The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size, few events and wide 95% CI suggesting high likelihood of bias and indirectness of evidence. (Siddiqui 2013). 7Adverse events such as bleeding or organ dysfunction 8The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size and few events and since only descriptive assessment of safety and adverse events were provided in the included trials with no data being available for meta‐analyses. | ||||||


