Anticoagulación para pacientes con cáncer y catéteres venosos centrales
Resumen
Antecedentes
La colocación de un catéter venoso central (CVC) aumenta el riesgo de trombosis en los pacientes con cáncer. A menudo la trombosis requiere la extracción del CVC, lo que provoca retrasos en el tratamiento y morbilidad y mortalidad relacionadas con la trombosis. Ésta es una actualización de la revisión Cochrane publicada en 2014.
Objetivos
Evaluar la eficacia y la seguridad de la anticoagulación para la tromboprofilaxis en los pacientes con cáncer con un CVC.
Métodos de búsqueda
En mayo de 2018 se realizó una búsqueda exhaustiva de la bibliografía que incluyó una búsqueda electrónica importante en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL), MEDLINE (Ovid) y Embase (Ovid); una búsqueda manual en las actas de congresos; la verificación de las referencias de los estudios incluidos; la búsqueda de los estudios en curso; y el uso de la función de "citas relacionadas" en PubMed. Esta actualización de la revisión sistemática se basó en los resultados de una búsqueda bibliográfica realizada el 14 de mayo de 2018.
Criterios de selección
Ensayos controlados aleatorizados (ECA) que evaluaron los efectos beneficiosos y perjudiciales de la heparina no fraccionada (HNF), la heparina de bajo peso molecular (HBPM), los antagonistas de la vitamina K (AVK) o el fondaparinux, o que compararon los efectos de dos de estos anticoagulantes en pacientes con cáncer y un CVC.
Obtención y análisis de los datos
Se extrajeron los datos y se evaluó el riesgo de sesgo mediante un formulario estandarizado. Los resultados incluyeron mortalidad por todas las causas, tromboembolismo venoso (TEV) sintomático relacionado con el catéter, embolia pulmonar (EP), hemorragia grave, hemorragia leve, infección relacionada con el catéter, trombocitopenia y calidad de vida relacionada con la salud (CVRS). La certeza de la evidencia para cada resultado se evaluó mediante los criterios GRADE (Balshem 2011).
Resultados principales
Trece ECA (23 documentos) cumplieron los criterios de inclusión. Estos ensayos reclutaron 3420 participantes. Siete ECA compararon HBPM con ninguna HBPM (seis en adultos y uno en niños), seis ECA compararon AVK con ningún AVK (cinco en adultos y uno en niños) y tres ECA compararon HBPM con AVK en pacientes adultos.
HBPM versus ninguna HBPM
Seis ECA (1537 participantes) compararon HBPM con ninguna HBPM en pacientes adultos. Los metanálisis mostraron que la HBPM probablemente disminuyó la incidencia de TEV sintomático relacionado con el catéter hasta los tres meses de seguimiento en comparación con ninguna HBPM (riesgo relativo [RR] 0,43; intervalo de confianza [IC] del 95%: 0,22 a 0,81; diferencia de riesgos [DR] 38 menos por 1000; IC del 95%: 13 menos a 52 menos; evidencia de certeza moderada). Sin embargo, el análisis no confirmó ni excluyó un efecto beneficioso o perjudicial de la HBPM sobre la mortalidad a los tres meses de seguimiento (RR 0,82; IC del 95%: 0,53 a 1,26; DR 14 menos por 1000; IC del 95%: 36 menos a 20 más; evidencia de certeza baja), hemorragia grave (RR 1,49; IC del 95%: 0,06 a 36,28; DR 0 más por 1000; IC del 95%: 1 menos a 35 más; evidencia de certeza muy baja), hemorragia leve (RR 1,35; IC del 95%: 0,62 a 2,92; DR 14 más por 1000; IC del 95%: 16 menos a 79 más; evidencia de certeza baja), y trombocitopenia (RR 1,03; IC del 95%: 0,80 a 1,33; DR 5 más por 1000; IC del 95%: 35 menos a 58 más; evidencia de certeza baja).
AVK versus ningún AVK
Cinco ECA (1599 participantes) compararon dosis bajas de AVK con ningún AVK en pacientes adultos. Los metanálisis no confirmaron ni excluyeron un efecto beneficioso o perjudicial de dosis bajas de AVK en comparación con ningún AVK sobre la mortalidad (RR 0,99; IC del 95%: 0,64 a 1,55; DR 1 menos por 1000; IC del 95%: 34 menos a 52 más; evidencia de certeza baja), TEV sintomático relacionado con el catéter (RR 0,61; IC del 95%: 0,23 a 1,64; DR 31 menos por 1000; IC del 95%: 62 menos a 51 más; evidencia de certeza baja), hemorragia grave (RR 7,14; IC del 95%: 0,88 a 57,78; DR 12 más por 1000; IC del 95%: 0 menos a 110 más; evidencia de certeza baja), hemorragia leve (RR 0,69; IC del 95%: 0,38 a 1,26; DR 15 menos por 1000; IC del 95%: 30 menos a 13 más; evidencia de certeza baja), retiro prematuro del catéter (RR 0,82; IC del 95%: 0,30 a 2,24; DR 29 menos por 1000; IC del 95%: 114 menos a 202 más; evidencia de certeza baja), e infección relacionada con el catéter (RR 1,17; IC del 95%: 0,74 a 1,85; DR 71 más por 1000; IC del 95%: 109 menos a 356; evidencia de certeza baja).
HBPM versus AVK
Tres ECA (641 participantes) compararon HBPM con AVK en pacientes adultos. La evidencia disponible no confirmó ni excluyó un efecto beneficioso o perjudicial de la HBPM en relación con el AVK sobre la mortalidad (RR 0,94; IC del 95%: 0,56 a 1,59; DR 6 menos por 1000; IC del 95%: 41 menos a 56 más; evidencia de certeza baja), la TEV sintomático relacionado con el catéter (RR 1,83; IC del 95%: 0,44 a 7,61; DR 15 más por 1000; IC del 95%: 10 menos a 122 más; evidencia de certeza muy baja), la EP (RR 1,70; IC del 95%: 0,74 a 3,92; DR 35 más por 1000; IC del 95%: 13 menos a 144 más; evidencia de certeza baja), hemorragia grave (RR 3,11; IC del 95%: 0,13 a 73,11; DR 2 más por 1000; IC del 95%: 1 menos a 72 más; evidencia de certeza muy baja) o hemorragia leve (RR 0,95, IC del 95%: 0,20 a 4,61; DR 1 menos por 1000; IC del 95%: 21 menos a 95 más; evidencia de certeza muy baja). Los metanálisis mostraron que la HBPM probablemente aumentaba el riesgo de trombocitopenia en comparación con el AVK a los tres meses de seguimiento (RR 1,69; IC del 95%: 1,20 a 2,39; DR 149 más por 1000; IC del 95%: 43 menos a 300 más; evidencia de certeza moderada).
Conclusiones de los autores
La evidencia no fue concluyente en cuanto al efecto de la HBPM en la mortalidad, el efecto del AVK en la mortalidad y el TEV relacionado con el catéter, y el efecto de la HBPM en comparación con el AVK en la mortalidad y el TEV relacionado con el catéter. Se encontró evidencia de certeza moderada de que la HBPM reduce el TEV relacionado con el catéter en comparación con ninguna HBPM. Los pacientes con cáncer con CVC en los que se considere la anticoagulación se debe equilibrar el posible efecto beneficioso de la reducción de las complicaciones tromboembólicas con los posibles efectos perjudiciales y la carga de los anticoagulantes.
PICOs
Resumen en términos sencillos
Anticoagulantes para prevenir los coágulos sanguíneos en pacientes con cáncer y catéteres venosos centrales
Antecedentes
Un catéter venoso central (CVC) es un tubo que se inserta en una vena grande para administrar líquidos o fármacos. La colocación de un CVC aumenta el riesgo de coágulos sanguíneos en los pacientes con cáncer. Esta revisión evaluó la efectividad y la seguridad de los agentes anticoagulantes en pacientes con cáncer y un CVC.
Características de los estudios
Se realizaron búsquedas en la literatura científica de estudios de anticoagulantes en pacientes con cáncer y un CVC. La evidencia está actualizada hasta el 14 de mayo de 2018.
Resultados clave
Se incluyeron 13 ensayos que reclutaron a 3420 pacientes con cáncer y un CVC. La mayoría de los ensayos incluyeron a pacientes con varios tipos y etapas del cáncer. Siete estudios compararon anticoagulantes inyectables con ningún anticoagulante, seis estudios compararon pastillas anticoagulantes con ningún anticoagulante y tres estudios compararon anticoagulantes inyectables con pastillas anticoagulantes. Al considerar a los pacientes con cáncer y un CVC, los anticoagulantes inyectables probablemente redujeron el riesgo de coágulos sanguíneos relacionados con el CVC en comparación con ninguna anticoagulación y probablemente aumentaron el riesgo de trombocitopenia (niveles bajos de plaquetas en la sangre, lo que causa hemorragias en los tejidos) en comparación con las pastillas anticoagulantes.
Certeza de la evidencia
Al comparar los anticoagulantes inyectables con ninguna anticoagulación, se consideró que la certeza de la evidencia fue moderada en el caso de los coágulos sanguíneos en el sitio del catéter, baja en el caso de la mortalidad, la infección en el sitio del catéter y las hemorragias leves, y muy baja en el caso de las hemorragias graves.
Al comparar las pastillas anticoagulantes con ninguna anticoagulación, se consideró que la certeza de la evidencia fue baja para la mortalidad por hemorragias graves y leves, la retirada prematura del catéter y la infección relacionada con el catéter fue baja, y muy baja para los coágulos sanguíneos en el lugar del catéter.
Al comparar los anticoagulantes inyectables con las pastillas anticoagulantes, se consideró que la certeza de la evidencia fue baja para la mortalidad y los coágulos sanguíneos en las extremidades y muy baja para los coágulos sanguíneos en el lugar del catéter y las hemorragias graves y leves.
Authors' conclusions
Summary of findings
| Low‐molecular‐weight heparin (LMWH) compared to no LMWH for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: no LMWH | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no LMWH | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 1236 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 77 per 1000 | 14 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊕⊕⊝ | RR 0.43 | Study population | |
| 67 per 1000 | 38 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 96 per 1000 | 5 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1018 | ⊕⊝⊝⊝ | RR 1.49 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 0 fewer per 1000 | ||||
| Minor bleeding (up to 3 months) | 544 | ⊕⊕⊝⊝ | RR 1.35 | Study population | |
| 41 per 1000 | 14 more per 1000 | ||||
| Catheter‐related infection (up to 3 months) | 474 | ⊕⊕⊝⊝ | RR 0.97 | Study population | |
| 92 per 1000 | 3 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months) | 1002 | ⊕⊕⊝⊝ | RR 1.03 | Study population | |
| 176 per 1000 | 5 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in three studies, incomplete outcome data not addressed in three studies, and unclear or no allocation concealment in four out of five studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (20 per 1000 absolute increase), including 79 events in total. cDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. dDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. eDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (40 per 1000 absolute increase), including 94 events in total. fDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in four studies; and unclear or no allocation concealment in three out of four studies. hDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (35 per 1000 absolute increase), including five events in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. iDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in two out of two studies. jDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (79 per 1000 absolute increase), including 26 events in total. kDowngraded by one level due to concern about risk of bias; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in one out of two studies. lDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase), including 36 events in total. mDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in three out of four studies. nDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase) including 163 events in total. | |||||
| Vitamin K antagonist (VKA) compared to no VKA for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: VKA Comparison: no VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no VKA | Risk difference with VKA | ||||
| All‐cause mortality (up to 3 months) | 701 | ⊕⊕⊝⊝ | RR 0.99 | Study population | |
| 95 per 1000 | 1 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1271 | ⊕⊕⊝⊝ | RR 0.61 | Study population | |
| 80 per 1000 | 31 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 384 | ⊕⊝⊝⊝ | RR 0.61 | Study population | |
| 73 per 1000 | 29 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 7.14 | Study population | |
| 2 per 1000 | 12 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 0.69 | Study population | |
| 48 per 1000 | 15 fewer per 1000 | ||||
| Catheter‐related infection | 88 | ⊕⊕⊝⊝ | RR 1.17 | Study population | |
| 419 per 1000 | 71 more per 1000 | ||||
| Premature CVC removal | 88 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 163 per 1000 | 29 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVC: central venous catheter; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and unclear or no allocation concealment in two out of four studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (34 per 1000 absolute reduction) and the possibility of important harm (52 per 1000 absolute increase), including 67 events in total. cThe trial WARP showed no overall survival advantage in participants taking warfarin compared with participants in the no‐warfarin group (hazard ratio 0.98, 95% CI 0.77 to 1.25; P = 0.26) (Young 2009). dDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and no clear information concerning allocation concealment in one out of four studies). eDowngraded by one level due to unexplained inconsistency (I2 = 70%). Imprecision was partially driven by the inconsistency between the studies and was taken into consideration when downgrading by two levels for serious risk of bias and serious inconsistency. fDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (63 per 1000 absolute reduction) and the possibility of important harm (57 per 1000 absolute increase), including 87 events in total. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. hDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (54 per 1000 absolute reduction) and the possibility of important harm (29 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. kDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for no effect (0 per 1000 absolute reduction) and the possibility of important harm (120 per 1000 absolute increase), including eight events in total. lDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear or no allocation concealment in two out of three studies. mDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (30 per 1000 absolute reduction) and the possibility of important harm (16 per 1000 absolute increase), including 42 events in total. nDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the included study). oDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (109 per 1000 absolute reduction) and the possibility of important harm (356 per 1000 absolute increase), including 40 events in total. pDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (114 per 1000 absolute reduction) and the possibility of important harm (202 per 1000 absolute increase), including 13 events in total. | |||||
| Low‐molecular‐weight heparin (LMWH) compared to vitamin K antagonist (VKA) for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with VKA | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 561 | ⊕⊕⊝⊝ | RR 0.94 | Study population | |
| 94 per 1000 | 6 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 327 | ⊕⊝⊝⊝ | RR 1.83 | Study population | |
| 19 per 1000 | 15 more per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 317 | ⊕⊝⊝⊝ | RR 1.61 | Study population | |
| 63 per 1000 | 39 more per 1000 | ||||
| Pulmonary embolism (up to 3 months) | 327 | ⊕⊕⊝⊝ | RR 1.70 | Study population | |
| 49 per 1000 | 35 more per 1000 | ||||
| Major bleeding (up to 3 months) | 289 | ⊕⊝⊝⊝ | RR 3.11 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 2 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 234 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 26 per 1000 | 1 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months)n | 327 | ⊕⊕⊕⊝ | RR 1.69 | Study population | |
| 216 per 1000 | 149 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear allocation concealment in two out of three studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (41 per 1000 absolute reduction) and the possibility of important harm (56 per 1000 absolute increase), including 51 events in total. cDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and unclear allocation concealment in one out of two studies. dDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (10 per 1000 absolute reduction) and the possibility of important harm (122 per 1000 absolute increase), including nine events in total. eDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and unclear allocation concealment in one out of two studies. fDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. gDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (156 per 1000 absolute increase), including 26 events in total. hDowngraded by one level due to concern both risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (13 per 1000 absolute reduction) and the possibility of important harm (144 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. kDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (51 per 1000 absolute increase), including one event in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. lDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the study and unclear allocation concealment). mDowngraded by two levels due to concern about imprecision (95% CI was consistent with the possibility for benefit (21 per 1000 absolute reduction) and the possibility of important harm (95 per 1000 absolute increase), including six events in total. nThe study by Lavau‐Denes and colleagues included all grades of thrombocytopenia (even mild cases) (Lavau‐Denes 2013). oDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. | |||||
Background
See the glossary for the definitions of technical terms (Table 1).
| Term | Definition |
| Adjuvant therapy | Assisting in the amelioration, or cure of disease |
| Anticoagulation | Process of hindering the clotting of blood especially by treatment with an anticoagulant. |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting) |
| Bacteremia | Presence of bacteria in the blood |
| Central venous line | Synthetic tube that is inserted into a central (large) vein to provide temporary intravenous access for the administration of fluid, medication, or nutrients. |
| Coagulation | Clotting |
| Deep venous (vein) thrombosis (DVT) | Condition marked by the formation of a thrombus within a deep vein (as of the leg or pelvis) that may be asymptomatic or be accompanied by symptoms (as swelling and pain) and that is potentially life threatening if dislodgment of the thrombus results in pulmonary embolism |
| Fibrin | White insoluble fibrous protein formed from fibrinogen by the action of thrombin especially in the clotting of blood |
| Fondaparinux | Anticoagulant medication |
| Hemostatic system | System that shortens the clotting time of blood and stops bleeding |
| Heparin | Enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. 2 forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low‐molecular‐weight heparin (LMWH) |
| Impedance plethysmography | Technique that measures the change in blood volume (venous blood volume as well as the pulsation of the arteries) for a specific body segment |
| Kappa statistics | Measure of degree of non‐random agreement between observers or measurements of a specific categorical variable or both |
| Metastasis | Spread of cancer cells from the initial or primary site of disease to another part of the body |
| Oncogene | Gene having the potential to cause a normal cell to become cancerous |
| Osteoporosis | Condition that affects especially older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness |
| Parenteral nutrition | Practice of feeding a person intravenously, circumventing the gastrointestinal tract |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or 1 of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock, and sometimes death. |
| Stroma | Supporting framework of an organ typically consisting of connective tissue |
| Thrombin | Proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions |
| Thrombosis | Formation or presence of a blood clot within a blood vessel |
| Vitamin K antagonists (VKA) | Anticoagulant medications that are used for anticoagulation. Warfarin is a vitamin K antagonist |
| Warfarin | Anticoagulant medication that is a vitamin K antagonist that is used for anticoagulation |
| Ximelagatran | Anticoagulant medication |
Description of the condition
People with cancer often require long‐term central venous access to receive chemotherapy, transfusions, parenteral nutrition, and antibiotics. However, central venous catheter (CVC) placement increases the risk of thrombosis in people with cancer (Agnelli 2005a). Even in the absence of a CVC, people with cancer have a four‐ to six‐fold increased risk of thrombosis compared with the general population (Blom 2005; Blom 2006; Sutherland 2003). Moreover, cancer treatments, including surgery, radiotherapy (Guy 2017), and systemic therapy, also increase the risk of thrombosis (Agnelli 2005a). As a result, the incidence of clinically overt CVC‐related deep venous thrombosis (DVT) in people with cancer ranges from 0.3% to 28.3% (Verso 2003). The incidence of clinically overt pulmonary embolism (PE) with CVC‐related DVT varies between 15% and 25% (Verso 2003).
Both thrombosis and infection often necessitate the removal of the CVC, resulting in treatment delays as well as the clinical consequences of thrombosis and infections themselves (Kuter 2004). More importantly, these complications can lead to significant morbidity and mortality (Kreuziger 2015). Indeed, people with cancer with venous thromboembolism (VTE), compared with people with cancer without VTE, have a significantly reduced survival with an estimated mortality ratio of 2.2 (Sorensen 2000). In addition, people with cancer treated for VTE have higher thrombosis recurrence rates and more hemorrhagic complications compared with people without cancer treated for VTE (Hutten 2000; Prandoni 2002). For example, among people with venous thrombosis on anticoagulation, bleeding was related to cancer status and cancer severity but could not be explained by subtherapeutic or supratherapeutic levels of anticoagulation (Prandoni 2002).
Description of the intervention
Parenteral anticoagulants such as unfractionated heparin (UFH), low‐molecular‐weight heparins (LMWH), and fondaparinux do not have intrinsic anticoagulant activity but potentiate the activity of antithrombin III in inhibiting activated coagulation factors. Heparin and its low‐molecular‐weight derivatives are not absorbed orally and must be administered parenterally by intravenous infusion or subcutaneous injections (Motlekar 2006).
Oral anticoagulants such as vitamin K antagonists (VKA) have been the mainstay of oral anticoagulant therapy since the 1950s. Well‐designed clinical trials have shown their effectiveness for the primary and secondary prevention of several venous and arterial thrombotic diseases (Ansell 2008).
How the intervention might work
Researchers have hypothesized that, because of the risk of thromboembolic events related to a CVC, anticoagulants may improve outcomes by reducing the incidence of these events. Moreover, researchers have hypothesized that anticoagulants may improve outcomes in people with cancer through an antitumor effect in addition to its antithrombotic effect (Smorenburg 2001; Thodiyil 2002). In addition, anticoagulation has the potential to reduce infections by preventing thrombus formation, a suspected risk factor for catheter‐related infection (Lordick 2003). However, anticoagulants increase the risk for bleeding. In fact, in people with venous thrombosis on anticoagulation the risk of bleeding was higher if people had cancer and correlated with the extent of cancer (Prandoni 2002). Heparins are also known to cause thrombocytopenia and heparin‐induced thrombocytopenia (HIT) syndrome (Girolami 2006).
Why it is important to do this review
Several studies have evaluated the efficacy of thromboprophylaxis with anticoagulants in people with cancer with CVC. One 2003 systematic review of thrombosis prophylaxis in people with CVC identified two randomized controlled trials (RCTs) conducted in people with cancer (Klerk 2003). Our systematic review conducted in 2014 identified 12 trials reporting follow‐up data on 2823 participants (Bern 1990; De Cicco 2009; Heaton 2002; Karthaus 2006; Lavau‐Denes 2013; Massicotte 2003; Mismetti 2003; Monreal 1996; Niers 2007; Ruud 2006; Verso 2008; Young 2009). The included trials provided evidence of reduction in catheter‐related thrombosis when comparing LMWH to no LMWH, of a decrease in asymptomatic catheter‐related thrombosis when comparing VKA to no VKA, and an increase in thrombocytopenia when comparing LMWH to VKA. Since then, we are aware of two new reports of a previously included study addressing this question (Verso 2008).
Objectives
To evaluate the efficacy and safety of anticoagulation for thromboprophylaxis in people with cancer and a CVC.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
People of any age with cancer and a CVC, with either solid or hematologic cancer, at any stage of their cancer, and irrespective of the type of cancer therapy received. Participants had to have no clinical evidence of VTE at enrolment.
Types of interventions
Intervention:
-
parenteral anticoagulants (including UFH, LMWH, and fondaparinux) irrespective of the dose;
-
VKA irrespective of the dose;
-
direct oral anticoagulant (DOACs) irrespective of the dose.
Control:
-
placebo or no intervention;
-
any of the anticoagulants listed under intervention.
Types of outcome measures
Primary outcomes
-
All‐cause mortality.
Secondary outcomes
-
Catheter‐related thrombosis. The diagnosis of CVC thrombosis could have resulted either from screening with the described screening methods or from clinical suspicion with subsequent confirmation by one of the described tests.
-
Non‐catheter‐related thrombosis. DVT events diagnosed using an objective diagnostic test such as: venography, 125I‐fibrinogen‐uptake test, impedance plethysmography, or compression ultrasound.
-
PE events diagnosed using an objective diagnostic test such as: pulmonary perfusion/ventilation scan, computed tomography, pulmonary angiography, or autopsy.
-
Major bleeding: authors' definitions of major bleeding.
-
Minor bleeding: authors' definitions of minor bleeding.
-
Catheter‐related infection: author's definition of catheter‐related infection.
-
Thrombocytopenia: authors' definitions of thrombocytopenia.
-
Health‐related quality of life (HRQoL): measured using a validated tool.
-
Premature CVC removal.
-
HIT.
-
Heparin‐induced thrombocytopenia with thrombosis (HITT).
Search methods for identification of studies
Electronic searches
The search was part of a comprehensive search for studies of anticoagulation in people with cancer. We did not use language restrictions. We conducted comprehensive searches on 14 May 2018, following the original electronic searches in January 2007, February 2010, February 2013, and February 2016 (last major search). We electronically searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid; starting 1966), and Embase (Ovid; starting 1980). For each database, the search strategies combined terms for anticoagulants, terms for cancer, and a search filter for RCTs. We used no language restrictions. We list the full search strategies for each of the electronic databases in Appendix 1; Appendix 2; and Appendix 3.
Searching other resources
We handsearched the conference proceedings of the American Society of Clinical Oncology (ASCO, starting with its first volume, 1982 up to May 2018) and of the American Society of Hematology (ASH, starting with its 2003 issue up to May 2018). We also searched ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform for ongoing studies. We reviewed the reference lists of papers included in this review and of other relevant systematic reviews. We used the 'related citation' feature in PubMed to identify additional articles and 'citation tracking' of included studies in Web of Science Core Collection. In addition, we contacted experts in the field for information about unpublished and ongoing trials.
Data collection and analysis
Selection of studies
Two review authors independently screened the title and abstract of identified article citations for potential eligibility. We retrieved the full text of articles judged potentially eligible by at least one review author. Two review authors then independently screened the full‐text article for eligibility using a standardized form with explicit inclusion and exclusion criteria (as detailed in the Criteria for considering studies for this review section) and resolved their disagreements by discussion or by consulting a third review author.
Data extraction and management
Two review authors independently extracted data from each included study and resolved any disagreements by discussion. We collected data related to the following.
Participants
-
Number of participants randomized to each study arm.
-
Number of participants followed up in each study arm.
-
Number of participants who discontinued the intervention in each arm.
-
Population characteristics (e.g. age, gender, co morbidities, co interventions).
-
Type of cancer.
-
Stage of cancer.
-
Time since cancer diagnosis.
-
CVC characteristics: type (e.g. port, external, tunneled versus non‐tunneled, coated versus not coated), site (e.g. subclavian, internal jugular, external jugular, femoral), placement side, type of medication/fluids infused, specialty of physician placing the CVC (e.g. surgeon, radiologist, physician‐in‐training), aseptic insertion technique, and antibiotic coverage during placement.
Interventions
-
Type of anticoagulant studied: LMWH, UFH, VKA, fondaparinux.
-
Type and dosage of LMWH (therapeutic versus prophylactic). We classified the dosage of LMWH as either prophylactic or therapeutic using a standardized chart (Table 2).
-
Dosage of UFH.
-
Intensity of VKA therapy (international normalized ratio (INR) target or dose).
-
Initiation of treatment relative to time of CVC insertion.
-
Duration of treatment.
-
Cointerventions including radiation therapy or systemic therapy (type and duration).
-
Control: placebo or no intervention.
| LMWH | Generic name | Prophylactic dose | Therapeutic dose |
| Lovenox | Enoxaparin | 40 mg once daily | 1 mg/kg twice daily |
| Fragmin | Dalteparin | 2500‐5000 U once daily | 200 U/kg once daily or |
| Innohep | Tinzaparin, logiparin | 4500 U once daily | 90 U/kg twice daily |
| Fraxiparine | Nadroparin | 35‐75 anti‐Xa IU/kg/day | 175 anti‐Xa IU/kg/day |
| Certoparin | Sandoparin | 3000 anti‐Xa IU once daily | – |
| Reviparin | Reviparin | 1750‐4200 anti‐Xa IU | 7000‐12,600 anti‐Xa IU |
IU: international units; U: units; Xa: factor Xa.
Outcomes
We attempted to extract both time‐to‐event data (for survival outcome) and categorical data (for all outcomes). However, none of the studies reported time‐to‐event data for participants with cancer.
For dichotomous data, we extracted data necessary to conduct complete‐case analysis as the primary analysis. We aimed to abstract data for all‐cause mortality at three and six months (time point defined a priori in the protocol).
We attempted to contact study authors for incompletely reported data. We decided a priori to consider abstracts in the main analysis only if authors supplied us with full reports of their methods and results; otherwise abstracts were included only in the sensitivity analysis.
Other
We extracted from each included trial any information on the following points.
-
Source of funding.
-
Ethical approval.
-
Conflict of interest.
Assessment of risk of bias in included studies
We assessed risk of bias at the study level using Cochrane's 'Risk of bias' tool (Higgins 2011). Two review authors independently assessed the methodologic quality of each included study and resolved their disagreements by discussion. 'Risk of bias' criteria included the following:
-
adequate sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
percentage of follow‐up, and whether incomplete outcome data were addressed;
-
whether the study was free of selective reporting;
-
whether the study was stopped early for benefit.
See Dealing with missing data section for information on assessing risk of bias associated with participants with missing data per outcome and across studies.
Measures of treatment effect
We collected and analyzed risk ratios (RRs) with 95% confidence intervals (CI) for dichotomous data. None of the outcomes of interest was meta‐analyzed as a continuous variable.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
Determining participants with missing data
It was unclear whether certain participant categories (e.g. those described as 'withdrew consent' or 'experienced adverse events') were followed up by the trial authors (versus had missing participant data) (Akl 2016). To deal with this issue, we made the following considerations:
-
'ineligible participants' and 'did not receive the first dose' participant categories, which were defined prior to the initiation of the study intervention, most likely had missing participant data;
-
'withdrew consent,' 'lost to follow‐up' (LTFU), and 'outcome not assessable' participant categories and any other category explicitly reported as not being followed up, which were defined after the initiation of the study intervention, most likely had missing participant data;
-
'dead,' 'experienced adverse events,' 'non‐compliant,' and 'discontinued prematurely' (and similarly described) participant categories, less likely to have had missing participant data.
Dealing with participants with missing data in the primary meta‐analysis
In the primary meta‐analysis, we used a complete‐case analysis approach, that is, we excluded participants considered to have missing data (Guyatt 2017).
For categorical data, we used the following calculations for each study arm:
-
denominator: (number of participants randomized) – (number of participants most likely with missing data, both pre‐ and postintervention initiation);
-
numerator: number of participants with observed events (i.e. participants who had at least one event for the outcome of interest during their available follow‐up time).
For continuous data, we planned to use for each study arm, the reported mean and standard deviation (SD) for participants followed up by the trial authors.
Assessing the risk of bias associated with participants with missing data
When the primary meta‐analysis of a specific outcome found a statistically significant effect, we conducted sensitivity meta‐analyses to assess the risk of bias associated with missing participant data. Those sensitivity meta‐analyses used a priori plausible assumptions about the outcomes of participants considered to have missing data. The assumptions we used in the sensitivity meta‐analyses were increasingly stringent to challenge the statistical significance of the results of the primary analysis progressively (Akl 2013; Ebrahim 2013).
For categorical data, and for RR showing a reduction in effect (RR less than 1), we used the following increasingly stringent but plausible assumptions (Akl 2013).
-
For the control arm, relative incidence (RI) among participants with missing data (LTFU) compared to participants with available data (followed up, FU) in the same arm (RILTFU/FU) = 1; for the intervention arm, RILTFU/FU = 1.5.
-
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 2.
-
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 3.
-
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 5.
For RR showing an increase in effect (RR greater than 1), we switched the above assumptions between the control and interventions arms (i.e. used RILTFU/FU = 1 for the intervention arm).
Specifically, we used the following calculations for each study arm:
-
denominator: (number of participants randomized) – (number of participants most likely with missing data, pre intervention initiation);
-
numerator: (number of participants with observed events) + (number of participants most likely with missing data postintervention initiation, with assumed events).
Assumed events were calculated by applying the a priori plausible assumptions to the participants considered most likely with missing data postintervention initiation.
For continuous data, we planned to use the four strategies suggested by Ebrahim and colleagues (Ebrahim 2013). The strategies imputed the means for participants with missing data based on the means of participants followed up in individual trials included in the systematic review. To impute SD, we used the median SD from the control arms of all included trials (Ebrahim 2013).
Assessment of heterogeneity
We assessed heterogeneity between trials by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (I2 test; Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, we attempted to investigate the possible reasons for this (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We assessed selective outcome reporting bias by trying to identify whether the study was included in a trial registry, whether a protocol was available, and whether the methods section provided a list of outcomes. We compared the list of outcomes from those sources to the outcomes reported in the published paper. We planned to create funnel plots for outcomes including 10 or more trials.
Data synthesis
For dichotomous data, we calculated the RR separately for each study (DerSimonian 1986; RevMan 2014). When analyzing data related to participants who were reported as not compliant, we attempted to adhere to the principles of intention‐to‐treat (ITT) analysis. We approached the issue of non‐compliance independently from that of missing data (Alshurafa 2012). We then pooled the results of the different studies using a random‐effects model. We assessed the certainty of evidence at the outcome level using the GRADE approach (Balshem 2011).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses based on characteristics of participants but did not conduct them as the data were not available.
Sensitivity analysis
We planned for sensitivity meta‐analyses to assess the risk of bias associated with missing participant data when the primary meta‐analysis of a specific outcome found a statistically significant effect.
Results
Description of studies
Results of the search
Figure 1 shows the study flow diagram. As of May 2018, the search strategy identified 7602 unique citations after removal of duplicates. The title and abstract screening identified 140 potentially eligible citations. The full‐text screening identified 13 eligible RCTs published as full reports (Bern 1990; Couban 2005; De Cicco 2009; Heaton 2002; Karthaus 2006; Lavau‐Denes 2013; Massicotte 2003; Mismetti 2003; Monreal 1996; Niers 2007; Ruud 2006; Verso 2008; Young 2009). In the previous version of this review, we excluded the study by Couban and colleagues due to different follow‐up times between the study arms (Couban 2005). For the current update, we update to include it in the systematic review but not in the meta‐analysis. The February 2016 search identified two new reports for each of two previously included trials (Couban 2005; Verso 2008).

Study flow diagram.
Agreement between review authors for study eligibility was excellent (kappa = 0.94).
Included studies
The 13 included RCTs enrolled 3420 participants. They examined either prophylactic‐dose heparin (UFH or LMWH) or low‐dose VKA (either fixed low dose or target INR less than 2). Two of the RCTs conducted a three‐way comparison (LMWH versus VKA versus no anticoagulation) (De Cicco 2009; Lavau‐Denes 2013). Most studies administered treatments for the specified fixed period or until CVC removal or thrombosis diagnosis. The studies varied in the thromboembolic outcomes they assessed (e.g. whether thrombosis was symptomatic or not, at the site of the catheter or not, CVC‐related or not) and the definitions of CVC‐related infection. The study by Couban and colleagues followed up participants for three months after catheter removal (which was different for the two study groups) and was not included in the meta‐analysis due to a differential follow‐up relative to randomization (63 days for the warfarin group and 84 days for the placebo group) (Couban 2005). This study was excluded from the previous version of this review (Akl 2014), but we decided to include it in the current version, only in the narrative section without including it in the meta‐analysis.
Bern 1990 recruited 121 adults with solid or hematologic cancer and CVC with a minimum life expectancy of three months. Participants were randomized to receive warfarin (1 mg/day for 90 days starting three days prior to catheter placement) or no intervention. Surgeons placed subclavian ports with no antibiotic prophylaxis and flushed with heparin. Assessed outcomes were CVC‐associated thrombosis and death. Participants were followed up for three months. The authors reported 96% follow‐up.
Couban 2005 recruited 255 participants with cancer and CVC. Participants were randomized to receive fixed‐dose warfarin 1 mg daily or placebo until the study endpoint or CVC removal. Assessed outcomes were CVC‐related thrombosis, bleeding, or death. Participants were followed up for three months after removal of CVC or until reaching a study endpoint. The study authors reported complete follow‐up.
De Cicco 2009 recruited 450 participants with cancer and CVC aged at least 18 years, and a minimum life expectancy of three months. Participants were randomized to receive acenocumarine (1 mg/day for three days before and eight days after CVC insertion) or dalteparin (two hours before and daily for eight days after CVC insertion) or no anticoagulation. Assessed outcomes were death, CVC‐related thrombosis, major bleeding, and minor bleeding. Participants were followed up for three months. The study authors reported 78% follow‐up.
Heaton 2002 recruited 88 hematologic participants with cancer and one or more CVCs. Participants were randomized to receive warfarin (1 mg/day for 90 days) or no intervention. Radiologists placed external, tunneled subclavian catheters with no antibiotic prophylaxis and flushed with heparin or saline. Seventy‐seven participants had one catheter, eight had two catheters, and three had three catheters. Assessed outcomes were CVC‐related thrombosis, CVC‐related infection, and premature removal of CVC. Participants were followed up for 90 days. The study authors report complete follow‐up.
Karthaus 2006 recruited 425 participants with solid or hematologic cancers and CVC aged at least 18 and a minimum life expectancy of 16 weeks. Participants were randomized to dalteparin (prophylactic dose for 16 weeks starting seven days prior to catheter placement) or placebo. Participants had mostly ports that were placed either proximal or distal to the axilla and flushed with normal saline or heparin. Assessed outcomes were catheter‐associated thrombosis and death. Participants were followed up for 16 weeks. The study authors reported 91% follow‐up.
Lavau‐Denes 2013 recruited 420 participants with solid invasive cancer, locally advanced or metastatic, and a minimum life expectancy of three months. Participants were randomized to LMWH (dalteparin, nadroparin, or enoxaparin) once daily, or warfarin (1 mg/day), or no anticoagulation. Participants had a subclavian CVC inserted for fewer than seven days. Assessed outcomes were catheter‐associated thrombosis and death. Participants were followed up for 90 days. The study authors reported 97% follow‐up.
Massicotte 2003 recruited 94 participants with cancer and a CVC, aged 0 to 18 years. Overall, the study recruited 186 participants. Participants were randomized to twice daily reviparin sodium or no treatment. Assessed outcomes were death, CVC‐related thrombosis, and bleeding. Participants were followed up for 30 days. The study authors reported complete follow‐up.
Mismetti 2003 recruited 59 participants with solid cancer and CVC aged at least 18 years, and a minimum life expectancy of three months. Participants were randomized to nadroparin (prophylactic dose for 90 days started two hours prior to CVC placement) or warfarin (1 mg/day for 90 days started three days prior to CVC placement) or no treatment. Assessed outcomes were upper extremity thrombosis, bleeding, and death. Participants were followed up for six months. The study authors reported 98% follow‐up.
Monreal 1996 recruited 32 participants with solid tumors and a minimum life expectancy of three months. Participants were randomized to dalteparin (prophylactic dose for 90 days starting two hours prior to catheter placement) or no intervention. Surgeons placed subclavian ports with no antibiotic prophylaxis and flushed with heparin (Monreal 1996). Assessed outcomes were catheter‐related DVT, death, and thrombocytopenia. Participants were followed up for 90 days. Study was stopped early for benefit. The study authors reported complete follow‐up.
Niers 2007 recruited 113 participants with hematologic malignancies aged at least 18 years. Participants were randomized to daily prophylactic‐dose nadroparin or placebo injections. The study medication was started two hours before insertion of the CVC, and was continued for three weeks or until the day of CVC removal, whichever came first. The CVC was inserted according to a standard protocol under sterile conditions (Niers 2007). Assessed outcomes were catheter‐related thrombosis, bleeding, and infection. Participants were followed up for 21 days. The authors reported 77% follow‐up.
Ruud 2006 recruited 73 children with solid or hematologic cancers and CVC with mean age of 6.8 years (Ruud 2006). Participants were randomized to receive warfarin starting from the day of CVC insertion (target INR 1.3 to 1.9) or no intervention. Participants had jugular external tunneled catheters or ports. Assessed outcomes were VTE, bleeding, infection, and removal of CVC. Participants were followed up for six months (Ruud 2006). The authors reported 84% follow‐up.
Verso 2008 recruited 385 participants with solid or hematologic cancers aged at least 18 years and a minimum life expectancy of three months (Verso 2008). Participants were randomized to enoxaparin (prophylactic dose for six weeks starting two hours prior to catheter placement) or placebo. Participants had either jugular or subclavian second‐generation catheters. Assessed outcomes were CVC‐related thrombosis, bleeding, and death. Participants were followed up for three months. The authors reported 81% follow‐up.
Young 2009 randomized 812 participants with solid or hematologic cancers aged at least 16 years who were receiving chemotherapy through CVC to no warfarin, fixed‐dose warfarin (1 mg/day), or dose‐adjusted warfarin to maintain INR between 1.5 and 2.0 (Young 2009). The study pooled data from fixed‐dose VKA and adjusted dose VKA arms. Assessed outcomes were catheter‐related thrombosis, bleeding, and death. Participants were followed up for a median of 45 months (range 26 to 88 months). Study authors reported 99% follow‐up.
Excluded studies
We excluded 117 studies from the review for the following reasons: not population of interest: people without a CVC (69 studies), people with VTE (19 studies); not design of interest: not an RCT (13 studies), review (four studies), editorial or letter to the editor (two studies), not intervention of interest: intervention used was not relevant (four studies), intervention was compared with urokinase (two studies), required data not obtainable from authors (three studies), concerns about accuracy and validity of study (one study) (see Characteristics of excluded studies table).
Risk of bias in included studies
The judgments for the risk of bias are summarized in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
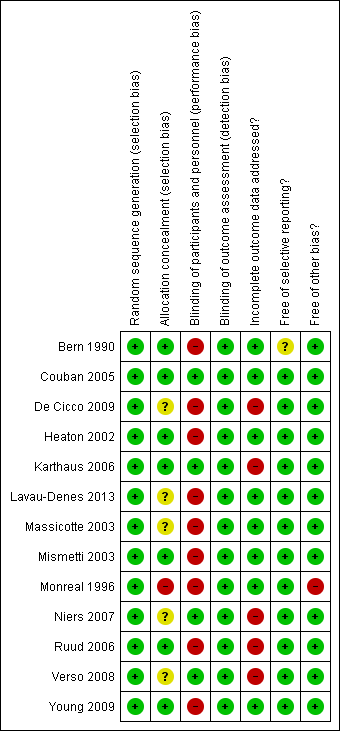
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
The method of sequence generation was clear for all studies (low risk of bias).
Allocation was adequately concealed in seven studies (low risk of bias; Bern 1990; Couban 2005; Heaton 2002; Karthaus 2006; Mismetti 2003; Ruud 2006; Young 2009), allocation concealment was not reported in five studies (unclear risk of bias; De Cicco 2009; Lavau‐Denes 2013; Massicotte 2003; Niers 2007; Verso 2008), and allocation was not concealed in one study (high risk of bias; Monreal 1996).
Blinding
Blinding of participants and personnel (performance bias)
We judged participants and personnel to be definitely blinded in four studies (low risk of bias; Couban 2005; Karthaus 2006; Niers 2007; Verso 2008), and definitely not blinded in nine studies (high risk of bias; Bern 1990; De Cicco 2009; Heaton 2002; Lavau‐Denes 2013; Massicotte 2003; Mismetti 2003; Monreal 1996; Ruud 2006; Young 2009).
Blinding of outcome assessment (detection bias)
We judged outcome assessors to be definitely blinded in 11 studies (low risk of bias; Bern 1990; Couban 2005; De Cicco 2009; Karthaus 2006; Massicotte 2003; Mismetti 2003; Monreal 1996; Niers 2007; Ruud 2006; Verso 2008; Young 2009), and probably not blinded in two studies (Heaton 2002; Lavau‐Denes 2013).
Incomplete outcome data
Four studies reported complete follow‐up (low risk of bias; Couban 2005; Heaton 2002; Massicotte 2003; Monreal 1996).
Bern 1990 reported 96% overall follow‐up, Lavau‐Denes 2013 reported 97% follow‐up, Mismetti 2003 reported 98% follow‐up, and Young 2009 reported 99% follow‐up (all low risk of bias).
De Cicco 2009 reported 78% overall follow‐up, Karthaus 2006 reported 91% follow‐up, Niers 2007 reported 77% follow‐up, Ruud 2006 reported 84% follow‐up, and Verso 2008 reported 81% follow‐up (all high risk of bias).
Selective reporting
Only two studies were registered and all outcomes listed in the registry were reported (Lavau‐Denes 2013; Young 2009). None of the other studies had a published protocol. All studies reported on the outcomes listed in their methods section except Bern 1990, who did not provide a list of outcomes in the methods section.
Other potential sources of bias
Two studies were stopped early: one for insufficient accrual (Massicotte 2003), and one for benefit (Monreal 1996). Nine studies used the ITT principle (Couban 2005; De Cicco 2009; Heaton 2002; Karthaus 2006; Lavau‐Denes 2013; Massicotte 2003; Mismetti 2003; Verso 2008; Young 2009), two studies were unclear about the use of the ITT principle (Bern 1990; Niers 2007), and two studies did not use the ITT principle (Monreal 1996; Ruud 2006).
The study by Young and colleagues pooled data from the fixed‐dose VKA and adjusted‐dose VKA arms (Young 2009).
Effects of interventions
See: Summary of findings for the main comparison Low‐molecular‐weight heparin (LMWH) compared to no LMWH for people with cancer and central venous catheters; Summary of findings 2 Vitamin K antagonist (VKA) compared to no VKA for people with cancer and central venous catheters; Summary of findings 3 Low‐molecular‐weight heparin (LMWH) compared to vitamin K antagonist (VKA) for people with cancer and central venous catheters
Low‐molecular‐weight heparin versus no low‐molecular‐weight heparin
We did not include the study by Massicotte and colleagues with the trials in the same meta‐analysis given it focused on children (Massicotte 2003), so we report its results here separately. The trial did not confirm or exclude a beneficial or detrimental effect of LMWH compared with no intervention on VTE (odds ratio (OR) 1.15, 95% CI 0.42 to 3.23) or infection (OR 0.59, 95% CI 0.32 to 1.10). The paper reported no statistically significant differences between groups in incidences of minor bleeding (48/90 with heparin versus 41/94 with no heparin; P = 0.24), major bleeding (0/90 with heparin versus 1/94 with no heparin; P = 1.0), and mortality (0/92 with heparin versus 2/94 with no heparin; P = 1.0).
We present below the meta‐analysis of the remaining five studies that were conducted in adults.
All‐cause mortality
Meta‐analysis of the five RCTs including 1236 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on mortality at three months compared to no LMWH (RR 0.82, 95% CI 0.53 to 1.26; RD 14 fewer per 1000, 95% CI 36 fewer to 20 more; Analysis 1.1) (De Cicco 2009; Karthaus 2006; Lavau‐Denes 2013; Monreal 1996; Verso 2008). There was no heterogeneity (I2 = 0%). We did not create funnel plots due to the low number of included trials for the outcome of mortality. The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table for the main comparison).
Symptomatic catheter‐related thrombosis
Meta‐analysis of five RCTs including 1089 participants showed that LMWH probably decreased the risk of symptomatic catheter‐related thrombosis compared to no LMWH (RR 0.43, 95% CI 0.22 to 0.81; RD 38 fewer per 1000, 95% CI 52 fewer to 13 fewer; moderate‐certainty evidence; Analysis 1.2) (Karthaus 2006; Lavau‐Denes 2013; Monreal 1996; Niers 2007; Verso 2008). There was low heterogeneity (I2 = 2%). Data from De Cicco 2009 was not included because the study authors did not specify the number of symptomatic versus non‐symptomatic events. Since the primary meta‐analysis found a statistically significant effect, and in order to assess the risk of bias associated with missing participant data, we conducted sensitivity meta‐analyses using the a priori plausible assumptions detailed in the Methods section. The effect estimate remained statistically significant for at least four stringent plausible assumptions. However, it lost significance with the most stringent plausible assumptions (see Appendix 7). The certainty of evidence was moderate due to serious risk of bias (summary of findings Table for the main comparison).
Meta‐analysis of five RCTs including 1089 participants did not show or exclude a beneficial or detrimental effect of LMWH on asymptomatic catheter‐related thrombosis (RR 0.95, 95% CI 0.62 to 1.46; RD 5 fewer per 1000, 95% CI 36 fewer to 44 more; very low‐certainty evidence) (Karthaus 2006; Lavau‐Denes 2013; Monreal 1996; Niers 2007; Verso 2008). There was low heterogeneity (I2 = 9%).
Pulmonary embolism
None of the studies reported PE.
Major bleeding
Meta‐analysis of four RCTs including 1018 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on major bleeding compared to no LMWH (RR 1.49, 95% CI 0.06 to 36.28; RD 0 fewer per 1000, 95% CI 1 fewer to 35 more; Analysis 1.4) (De Cicco 2009; Karthaus 2006; Niers 2007; Verso 2008). There was no heterogeneity (I2 = 0%). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table for the main comparison).
Minor bleeding
Meta‐analysis of two RCTs including 544 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on minor bleeding compared to no LMWH (RR 1.35, 95% CI 0.62 to 2.92; RD 14 more per 1000, 95% CI 16 fewer to 79 more; Analysis 1.5) (De Cicco 2009; Verso 2008). There was no heterogeneity (I2 = 0%). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table for the main comparison).
Catheter‐related infection
Meta‐analysis of two RCTs including 474 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on catheter‐related infection compared to no LMWH (RR 0.97, 95% CI 0.52 to 1.79; RD 3 fewer per 1000, 95% CI 44 fewer to 73 more; Analysis 1.6) (Karthaus 2006; Niers 2007). There was no heterogeneity (I2 = 0%). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table for the main comparison).
Thrombocytopenia
Meta‐analysis of four RCTs including 1002 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on thrombocytopenia compared to no LMWH (RR 1.03, 95% CI 0.80 to 1.33; RD 5 more per 1000, 95% CI 35 fewer to 58 more; Analysis 1.7) (Karthaus 2006; Lavau‐Denes 2013; Monreal 1996; Verso 2008). There was no heterogeneity (I2 = 0%). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table for the main comparison).
Health‐related quality of life
None of the included studies assessed HRQoL.
Premature central venous catheter removal
None of the included studies assessed premature CVC removal.
Heparin‐induced thrombocytopenia
None of the included studies assessed HIT.
Heparin‐induced thrombocytopenia with thrombosis
None of the included studies assessed HITT.
Low‐dose vitamin K antagonist versus no vitamin K antagonist
We did not include the study by Ruud and colleagues with the trials in the same meta‐analysis given its focus on children (Ruud 2006), so we report its results here separately. The study was stopped early because of a higher incidence of ultrasound‐detected VTE in the warfarin group (15/31 with warfarin versus 17/42 with no warfarin; P = 0.36). There was only one symptomatic VTE event in each group.
We present below the meta‐analysis of the remaining five studies that were conducted in adults.
All‐cause mortality
Meta‐analysis of the four RCTs including 701 participants did not confirm or exclude a beneficial or detrimental effect of low‐dose VKA on mortality at three months compared to no VKA (RR 0.99, 95% CI 0.64 to 1.55; RD 1 fewer per 1000, 95% CI 34 fewer to 52 more; Analysis 2.1) (Bern 1990; De Cicco 2009; Heaton 2002; Lavau‐Denes 2013). There was no heterogeneity (I2 = 0%). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table 2).
Symptomatic catheter‐related thrombosis
Meta‐analysis of the four RCTs including 1271 participants did not include or exclude a beneficial or detrimental effect of low‐dose VKA on symptomatic catheter‐related thrombosis compared to no VKA (RR 0.61, 95% CI 0.23 to 1.64; RD 31 fewer per 1000, 95% CI 62 fewer to 51 more; Analysis 2.2) (Bern 1990; Heaton 2002; Lavau‐Denes 2013; Young 2009). There was moderate heterogeneity (I2 = 70%). The certainty of evidence was low due to serious risk of bias and serious heterogeneity (summary of findings Table 2).
Meta‐analysis of two studies including 384 participants did not confirm or exclude a beneficial or detrimental effect of low‐dose VKA on asymptomatic catheter‐related thrombosis compared to no VKA (RR 0.61, 95% CI 0.27 to 1.40; RD 29 fewer per 1000, 95% CI 54 fewer to 29 more) (Bern 1990; Lavau‐Denes 2013). There was no heterogeneity (I2 = 0%). The certainty of evidence was very low due to serious risk of bias, serious imprecision, and serious indirectness (summary of findings Table 2).
Pulmonary embolism
None of the studies reported PE.
Major bleeding
One of the two studies assessing major bleeding reported no events (De Cicco 2009). The other study including 751 participants did not show or exclude a beneficial or detrimental effect of low‐dose VKA on major bleeding compared to no VKA (RR 7.14, 95% CI 0.88 to 57.78; RD 12 more per 1000, 95% CI 0 fewer to 110 more; Analysis 2.4) (Young 2009). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table 2).
Minor bleeding
Two studies assessing minor bleeding including 1026 participants did not confirm or exclude a beneficial or detrimental effect of low‐dose VKA on minor bleeding compared to no VKA (RR 0.69, 95% CI 0.38 to 1.26; RD 15 fewer per 1000, 95% CI 30 fewer to 13 more; Analysis 2.5) (De Cicco 2009; Young 2009). There was no heterogeneity (I2 = 0%). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table 2).
Catheter‐related infection
One study including 88 participants did not confirm or exclude a beneficial or detrimental effect of low‐dose VKA on catheter‐related infection compared to no VKA (RR 1.17, 95% CI 0.74 to 1.85; RD 71 more per 1000, 95% CI 109 fewer to 356 more) (Heaton 2002). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table 2).
Thrombocytopenia
None of the included studies assessed thrombocytopenia.
Health‐related quality of life
None of the included studies assessed HRQoL.
Premature central venous catheter removal
One study did not confirm or exclude a beneficial or detrimental effect of low‐dose VKA on premature CVC removal compared to no VKA (RR 0.82, 95% CI 0.30 to 2.24; RD 29 fewer per 1000, 95% CI 114 fewer to 202 more) (Heaton 2002). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table 2).
Heparin‐induced thrombocytopenia
None of the included studies assessed HIT.
Heparin‐induced thrombocytopenia with thrombosis
None of the included studies assessed HITT.
Low‐molecular‐weight heparin versus low‐dose vitamin K antagonist
All‐cause mortality
Meta‐analysis of three RCTs including 561 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on mortality compared to VKA (RR 0.94, 95% CI 0.56 to 1.59; RD 6 fewer per 1000, 95% CI 41 fewer to 56 more; Analysis 3.1) (De Cicco 2009; Lavau‐Denes 2013; Mismetti 2003). There was no heterogeneity (I2 = 0%). The certainty of evidence was low due to serious risk of bias and serious imprecision (summary of findings Table 3).
Symptomatic catheter‐related thrombosis
Meta‐analysis of two RCTs including 327 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on symptomatic catheter‐related thrombosis compared to VKA (RR 1.83, 95% CI 0.44 to 7.61; RD 15 more per 1000, 95% CI 10 fewer to 122 more; Analysis 3.2) (Lavau‐Denes 2013; Mismetti 2003). There was no heterogeneity (I2 = 0%). The certainty of evidence was very low due to serious risk of bias and very serious imprecision (summary of findings Table 3).
Meta‐analysis of two RCTs including 327 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on asymptomatic catheter‐related thrombosis compared to VKA (RR 1.61, 95% CI 0.75 to 3.46; RD 39 more per 1000, 95% CI 16 fewer to 156 more) (Bern 1990; Lavau‐Denes 2013). There was no heterogeneity (I2 = 0%). The certainty of evidence was very low due to serious risk of bias, serious imprecision, and serious indirectness (summary of findings Table 3).
Pulmonary embolism
Two studies including 327 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on the risk of PE compared to VKA (RR 1.70, 95% CI 0.74 to 3.92; RD 35 more per 1000, 95% CI 13 fewer to 144 more; Analysis 3.4) (Lavau‐Denes 2013; Mismetti 2003). The certainty of evidence was low due to serious risk of bias and serious imprecision.
Major bleeding
One of two studies assessing major bleeding reported no events (De Cicco 2009). The other study including 60 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on major bleeding compared to VKA (RR 3.11, 95% CI 0.13 to 73.11; RD 2 more per 1000, 95% CI 1 fewer to 72 more; Analysis 3.5) (Mismetti 2003). The certainty of evidence was very low due to serious risk of bias and very serious imprecision (summary of findings Table 3).
Minor bleeding
One study including 234 participants did not confirm or exclude a beneficial or detrimental effect of LMWH on minor bleeding compared to VKA (RR 0.95, 95% CI 0.20 to 4.61; RD 1 fewer per 1000, 95% CI 21 fewer to 95 more; Analysis 3.6) (De Cicco 2009). The certainty of evidence was very low due to serious risk of bias and very serious imprecision (summary of findings Table 3).
Catheter‐related infection
None of the included studies assessed catheter‐related infection.
Thrombocytopenia
Lavau‐Denes and colleagues included all grades of thrombocytopenia in their study (including mild cases). Meta‐analysis of two RCTs including 327 participants showed that LMWH increased thrombocytopenia compared to VKA at three months of follow‐up (RR 1.69, 95% CI 1.20 to 2.39; RD 149 more per 1000, 95% CI 43 fewer to 300 more; moderate‐certainty evidence; Analysis 3.7) (Lavau‐Denes 2013; Mismetti 2003). There was no heterogeneity (I2 = 0%). Since the primary meta‐analysis found a statistically significant effect, and in order to assess the risk of bias associated with missing participant data, we conducted sensitivity meta‐analyses using the a priori plausible assumptions detailed in the Methods section. The effect estimate remained statistically significant for the all plausible assumptions. The certainty of evidence was moderate due to serious risk of bias (summary of findings Table 3).
Health‐related quality of life
None of the included studies assessed HRQoL.
Premature central venous catheter removal
None of the included studies assessed premature CVC removal.
Heparin‐induced thrombocytopenia
None of the included studies assessed HIT.
Heparin‐induced thrombocytopenia with thrombosis
None of the included studies assessed HITT.
Discussion
Summary of main results
The evidence was not conclusive for the effect of LMWH compared to no LMWH on mortality, the effect of VKA compared to no VKA on mortality and catheter‐related thrombosis, and the effect of LMWH compared to VKA on mortality and catheter‐related thrombosis. We found moderate‐certainty evidence that LMWH reduced catheter‐related thrombosis compared to no LMWH. In considering anticoagulation, people with cancer with CVCs should balance the possible benefit of reduced thromboembolic complications with the possible harms and burden of anticoagulants.
We noted an interesting decrease in the baseline risk of symptomatic DVT by year of publication. This decline could reflect technologic advances in CVC materials and designs, better CVC management strategies (e.g. shorter insertion times), and advances in clinical care in general (e.g. early mobilization of participants). There was also a progressive decrease in the effect size of anticoagulation on thrombosis in people with cancer with CVC by year of publication, particularly in studies testing heparin therapy. A similar decrease in effect size of specific interventions has been shown in studies of mental health as more data have become available (Trikalinos 2004). The smaller size of earlier studies (Bern 1990: 121 participants; Monreal 1996: 32 participants) provides a possible explanation for this phenomenon in our review, as small trials have been shown to exaggerate intervention effects compared with larger trials (Kjaergard 2001). Another potentially contributing explanation is the early stopping of the study by Monreal and colleagues for benefit (Monreal 1996); Montori have shown that RCTs stopped early for benefit show implausibly large treatment effects (Montori 2005).
Overall completeness and applicability of evidence
The included studies recruited participants with a variety of cancer types and stages, which should increase the applicability of the results. The results apply best to LMWH given that only one study evaluated UFH. Unfortunately, we found no data to evaluate the impact of the intervention on HRQoL. This outcome is important given the potential burden of both oral and subcutaneous anticoagulants.
Quality of the evidence
When comparing LMWH to no LMWH, we judged the certainty of evidence to be moderate for symptomatic catheter‐related thrombosis due to serious risk of bias; low for mortality, minor bleeding, and catheter‐related infection due to serious risk of bias and serious imprecision; very low for asymptomatic catheter‐related thrombosis due to serious risk of bias, serious indirectness, and serious imprecision; and very low for major bleeding due to serious risk of bias and very serious imprecision.
When comparing VKA to no VKA, we judged the certainty of evidence to be low for mortality, major bleeding, minor bleeding, premature catheter removal, and catheter‐related infection due to serious risk of bias and serious imprecision, low for symptomatic catheter‐related thrombosis due to serious risk of bias and serious indirectness, very low for asymptomatic catheter‐related thrombosis due to serious risk of bias, serious indirectness, and serious imprecision; and
When comparing LMWH to VKA, we judged the certainty of evidence to be moderate for thrombocytopenia due to serious risk of bias; low for mortality and PE due to serious risk of bias and serious imprecision; very low for symptomatic catheter‐related thrombosis, major bleeding, and minor bleeding due to serious risk of bias and very serious imprecision; very low for asymptomatic catheter‐related thrombosis due to serious risk of bias, serious indirectness, and serious imprecision.
Potential biases in the review process
Our systematic approach to searching, study selection, and data extraction should have minimized the likelihood of missing relevant studies or relevant data.
Additional strengths of the review include the high agreement between review authors and the low likelihood of publication bias. One limitation of this review is that the 'no difference' findings may be related to the relatively small number of RCTs, small number of participants and events, and the absence of a true effect. Another limitation related to the small number of RCTs was our inability to conduct subgroup analyses exploring the impact on the treatment effect of the characteristics of participants (adult versus children, solid versus hematologic cancer, type of catheter), outcomes (symptomatic versus screening‐detected DVT, early versus late DVTs), and methodologic quality criteria (allocation concealment and blinding).
Agreements and disagreements with other studies or reviews
Chaukiyal and colleagues conducted a systematic review to assess primary thromboprophylaxis in people with cancer with CVCs, and included eight RCTs with 1428 participants (Chaukiyal 2008). While we included six of these trials. We excluded Couban and colleagues because of unequal follow‐up of the two study groups (Couban 2005). We excluded Abdelkefi and colleagues because the publishing journal published an "Expression of Concern," reportedly, concerns about the accuracy and validity of the trial findings were raised by readers, and when asked by the editors "to provide evidence that the study was conducted, the authors were unable to provide this evidence" (Abdelkefi 2004). Chaukiyal and colleagues found no statistically significant difference in catheter‐related thrombosis for the use of warfarin versus placebo; heparin versus placebo; or warfarin, UFH, or LMWH versus placebo. Moreover, the results did not confirm a detrimental effect of thromboprophylaxis on the risk of overall bleeding and thrombocytopenia.
A more recently published systematic review focused on children with cancer with CVC (Schoot 2013). The authors could not confirm or exclude any effect of preventive systemic treatments (including LMWH, antithrombin supplementation, low‐dose warfarin, and cryoprecipitate/fresh frozen plasma) compared with no intervention on VTE (symptomatic and asymptomatic), major bleeding, or minor bleeding.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 1 All‐cause mortality (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 3 Asymptomatic catheter‐related thrombosis (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 4 Major bleeding (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 5 Minor bleeding (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 6 Catheter‐related infection (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 7 Thrombocytopenia (up to 3 months).
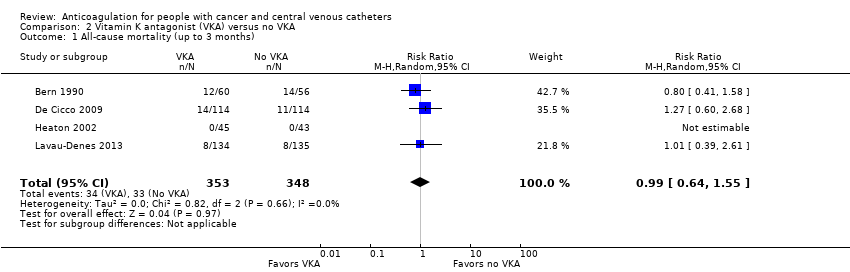
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 1 All‐cause mortality (up to 3 months).

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 3 Asymptomatic catheter‐related thrombosis.

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 4 Major bleeding (up to 3 months).
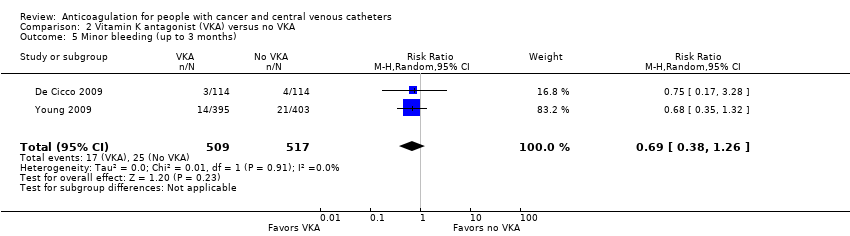
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 5 Minor bleeding (up to 3 months).
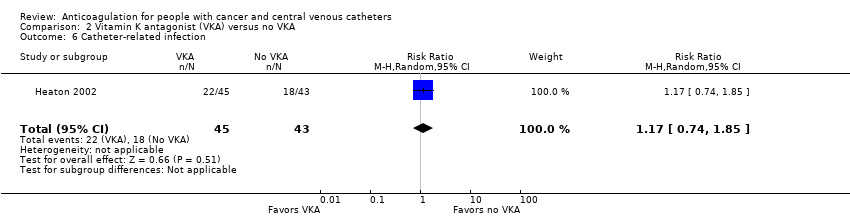
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 6 Catheter‐related infection.

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 7 Premature central venous catheter removal.

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 1 All‐cause mortality (up to 3 months).
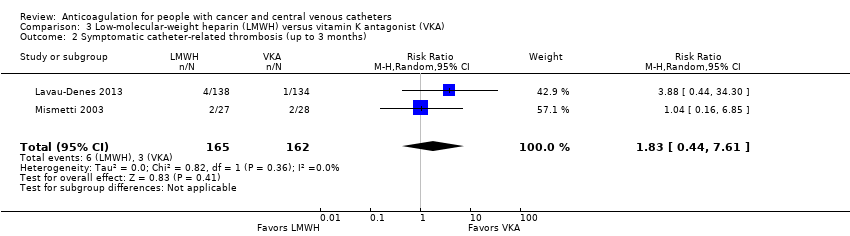
Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 3 Asymptomatic catheter‐related thrombosis.

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 4 Pulmonary embolism (up to 3 months).
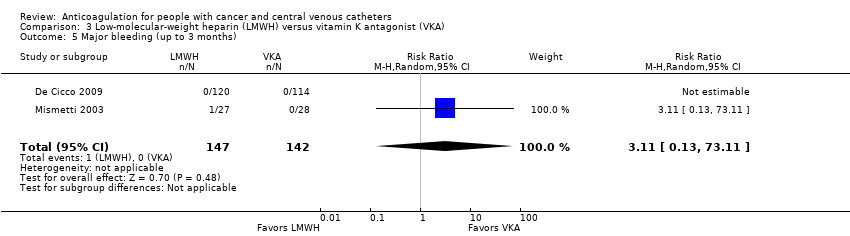
Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 5 Major bleeding (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 6 Minor bleeding (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 7 Thrombocytopenia (up to 3 months).
| Low‐molecular‐weight heparin (LMWH) compared to no LMWH for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: no LMWH | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no LMWH | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 1236 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 77 per 1000 | 14 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊕⊕⊝ | RR 0.43 | Study population | |
| 67 per 1000 | 38 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 96 per 1000 | 5 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1018 | ⊕⊝⊝⊝ | RR 1.49 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 0 fewer per 1000 | ||||
| Minor bleeding (up to 3 months) | 544 | ⊕⊕⊝⊝ | RR 1.35 | Study population | |
| 41 per 1000 | 14 more per 1000 | ||||
| Catheter‐related infection (up to 3 months) | 474 | ⊕⊕⊝⊝ | RR 0.97 | Study population | |
| 92 per 1000 | 3 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months) | 1002 | ⊕⊕⊝⊝ | RR 1.03 | Study population | |
| 176 per 1000 | 5 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in three studies, incomplete outcome data not addressed in three studies, and unclear or no allocation concealment in four out of five studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (20 per 1000 absolute increase), including 79 events in total. cDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. dDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. eDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (40 per 1000 absolute increase), including 94 events in total. fDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in four studies; and unclear or no allocation concealment in three out of four studies. hDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (35 per 1000 absolute increase), including five events in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. iDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in two out of two studies. jDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (79 per 1000 absolute increase), including 26 events in total. kDowngraded by one level due to concern about risk of bias; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in one out of two studies. lDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase), including 36 events in total. mDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in three out of four studies. nDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase) including 163 events in total. | |||||
| Vitamin K antagonist (VKA) compared to no VKA for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: VKA Comparison: no VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no VKA | Risk difference with VKA | ||||
| All‐cause mortality (up to 3 months) | 701 | ⊕⊕⊝⊝ | RR 0.99 | Study population | |
| 95 per 1000 | 1 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1271 | ⊕⊕⊝⊝ | RR 0.61 | Study population | |
| 80 per 1000 | 31 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 384 | ⊕⊝⊝⊝ | RR 0.61 | Study population | |
| 73 per 1000 | 29 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 7.14 | Study population | |
| 2 per 1000 | 12 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 0.69 | Study population | |
| 48 per 1000 | 15 fewer per 1000 | ||||
| Catheter‐related infection | 88 | ⊕⊕⊝⊝ | RR 1.17 | Study population | |
| 419 per 1000 | 71 more per 1000 | ||||
| Premature CVC removal | 88 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 163 per 1000 | 29 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVC: central venous catheter; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and unclear or no allocation concealment in two out of four studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (34 per 1000 absolute reduction) and the possibility of important harm (52 per 1000 absolute increase), including 67 events in total. cThe trial WARP showed no overall survival advantage in participants taking warfarin compared with participants in the no‐warfarin group (hazard ratio 0.98, 95% CI 0.77 to 1.25; P = 0.26) (Young 2009). dDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and no clear information concerning allocation concealment in one out of four studies). eDowngraded by one level due to unexplained inconsistency (I2 = 70%). Imprecision was partially driven by the inconsistency between the studies and was taken into consideration when downgrading by two levels for serious risk of bias and serious inconsistency. fDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (63 per 1000 absolute reduction) and the possibility of important harm (57 per 1000 absolute increase), including 87 events in total. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. hDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (54 per 1000 absolute reduction) and the possibility of important harm (29 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. kDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for no effect (0 per 1000 absolute reduction) and the possibility of important harm (120 per 1000 absolute increase), including eight events in total. lDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear or no allocation concealment in two out of three studies. mDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (30 per 1000 absolute reduction) and the possibility of important harm (16 per 1000 absolute increase), including 42 events in total. nDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the included study). oDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (109 per 1000 absolute reduction) and the possibility of important harm (356 per 1000 absolute increase), including 40 events in total. pDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (114 per 1000 absolute reduction) and the possibility of important harm (202 per 1000 absolute increase), including 13 events in total. | |||||
| Low‐molecular‐weight heparin (LMWH) compared to vitamin K antagonist (VKA) for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with VKA | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 561 | ⊕⊕⊝⊝ | RR 0.94 | Study population | |
| 94 per 1000 | 6 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 327 | ⊕⊝⊝⊝ | RR 1.83 | Study population | |
| 19 per 1000 | 15 more per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 317 | ⊕⊝⊝⊝ | RR 1.61 | Study population | |
| 63 per 1000 | 39 more per 1000 | ||||
| Pulmonary embolism (up to 3 months) | 327 | ⊕⊕⊝⊝ | RR 1.70 | Study population | |
| 49 per 1000 | 35 more per 1000 | ||||
| Major bleeding (up to 3 months) | 289 | ⊕⊝⊝⊝ | RR 3.11 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 2 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 234 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 26 per 1000 | 1 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months)n | 327 | ⊕⊕⊕⊝ | RR 1.69 | Study population | |
| 216 per 1000 | 149 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear allocation concealment in two out of three studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (41 per 1000 absolute reduction) and the possibility of important harm (56 per 1000 absolute increase), including 51 events in total. cDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and unclear allocation concealment in one out of two studies. dDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (10 per 1000 absolute reduction) and the possibility of important harm (122 per 1000 absolute increase), including nine events in total. eDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and unclear allocation concealment in one out of two studies. fDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. gDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (156 per 1000 absolute increase), including 26 events in total. hDowngraded by one level due to concern both risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (13 per 1000 absolute reduction) and the possibility of important harm (144 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. kDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (51 per 1000 absolute increase), including one event in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. lDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the study and unclear allocation concealment). mDowngraded by two levels due to concern about imprecision (95% CI was consistent with the possibility for benefit (21 per 1000 absolute reduction) and the possibility of important harm (95 per 1000 absolute increase), including six events in total. nThe study by Lavau‐Denes and colleagues included all grades of thrombocytopenia (even mild cases) (Lavau‐Denes 2013). oDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. | |||||
| Term | Definition |
| Adjuvant therapy | Assisting in the amelioration, or cure of disease |
| Anticoagulation | Process of hindering the clotting of blood especially by treatment with an anticoagulant. |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting) |
| Bacteremia | Presence of bacteria in the blood |
| Central venous line | Synthetic tube that is inserted into a central (large) vein to provide temporary intravenous access for the administration of fluid, medication, or nutrients. |
| Coagulation | Clotting |
| Deep venous (vein) thrombosis (DVT) | Condition marked by the formation of a thrombus within a deep vein (as of the leg or pelvis) that may be asymptomatic or be accompanied by symptoms (as swelling and pain) and that is potentially life threatening if dislodgment of the thrombus results in pulmonary embolism |
| Fibrin | White insoluble fibrous protein formed from fibrinogen by the action of thrombin especially in the clotting of blood |
| Fondaparinux | Anticoagulant medication |
| Hemostatic system | System that shortens the clotting time of blood and stops bleeding |
| Heparin | Enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. 2 forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low‐molecular‐weight heparin (LMWH) |
| Impedance plethysmography | Technique that measures the change in blood volume (venous blood volume as well as the pulsation of the arteries) for a specific body segment |
| Kappa statistics | Measure of degree of non‐random agreement between observers or measurements of a specific categorical variable or both |
| Metastasis | Spread of cancer cells from the initial or primary site of disease to another part of the body |
| Oncogene | Gene having the potential to cause a normal cell to become cancerous |
| Osteoporosis | Condition that affects especially older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness |
| Parenteral nutrition | Practice of feeding a person intravenously, circumventing the gastrointestinal tract |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or 1 of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock, and sometimes death. |
| Stroma | Supporting framework of an organ typically consisting of connective tissue |
| Thrombin | Proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions |
| Thrombosis | Formation or presence of a blood clot within a blood vessel |
| Vitamin K antagonists (VKA) | Anticoagulant medications that are used for anticoagulation. Warfarin is a vitamin K antagonist |
| Warfarin | Anticoagulant medication that is a vitamin K antagonist that is used for anticoagulation |
| Ximelagatran | Anticoagulant medication |
| LMWH | Generic name | Prophylactic dose | Therapeutic dose |
| Lovenox | Enoxaparin | 40 mg once daily | 1 mg/kg twice daily |
| Fragmin | Dalteparin | 2500‐5000 U once daily | 200 U/kg once daily or |
| Innohep | Tinzaparin, logiparin | 4500 U once daily | 90 U/kg twice daily |
| Fraxiparine | Nadroparin | 35‐75 anti‐Xa IU/kg/day | 175 anti‐Xa IU/kg/day |
| Certoparin | Sandoparin | 3000 anti‐Xa IU once daily | – |
| Reviparin | Reviparin | 1750‐4200 anti‐Xa IU | 7000‐12,600 anti‐Xa IU |
| IU: international units; U: units; Xa: factor Xa. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 5 | 1236 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.53, 1.26] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.81] |
| 3 Asymptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.62, 1.46] |
| 4 Major bleeding (up to 3 months) Show forest plot | 4 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.06, 36.28] |
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.92] |
| 6 Catheter‐related infection (up to 3 months) Show forest plot | 2 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.79] |
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 4 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.80, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 4 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.64, 1.55] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 4 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.64] |
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.27, 1.40] |
| 4 Major bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 7.14 [0.88, 57.78] |
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.26] |
| 6 Catheter‐related infection Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.74, 1.85] |
| 7 Premature central venous catheter removal Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.30, 2.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 3 | 561 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.56, 1.59] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.44, 7.61] |
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.75, 3.46] |
| 4 Pulmonary embolism (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.74, 3.92] |
| 5 Major bleeding (up to 3 months) Show forest plot | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 73.11] |
| 6 Minor bleeding (up to 3 months) Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.20, 4.61] |
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.20, 2.39] |

