Normobaric and hyperbaric oxygen therapy for the treatment and prevention of migraine and cluster headache
Appendices
Appendix 1. Search strategies used for this Cochrane review update
CENTRAL (the Cochrane Library)
#1 MeSH descriptor: [Headache] this term only
#2 MeSH descriptor: [Headache Disorders] explode all trees
#3 (headache* or migrain* or cephalgi* or cephalalgi* or cluster):ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Hyperbaric Oxygenation] this term only
#6 MeSH descriptor: [Oxygen Inhalation Therapy] this term only
#7 MeSH descriptor: [Oxygen] this term only and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU, Toxicity ‐ TO]
#8 MeSH descriptor: [Hyperoxia] this term only
#9 MeSH descriptor: [Atmosphere Exposure Chambers] this term only
#10 (hyperbar* or HBO*):ti,ab,kw (Word variations have been searched)
#11 (high pressure oxygen or 100% oxygen):ti,ab,kw (Word variations have been searched)
#12 ((monoplace or multiplace) near/5 chamber*):ti,ab,kw (Word variations have been searched)
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 #4 and #13 Publication Date from 2008 to 2015
MEDLINE (OVID)
1. Headache/
2. exp Headache Disorders/
3. (headache$ or migrain$ or cephalgi$ or cephalalgi$ or cluster).tw.
4. or/1‐3
5. Hyperbaric Oxygenation/
6. Oxygen Inhalation Therapy/
7. Oxygen/ae, tu, to
8. Hyperoxia/
9. Atmosphere Exposure Chambers/
10. (hyperbar$ or HBO$).tw.
11. (high pressure oxygen or 100% oxygen).tw.
12. ((monoplace or multiplace) adj5 chamber$).tw.
13. or/5‐12
14. randomized controlled trial.pt.
15. controlled clinical trial.pt.
16. randomized.ab.
17. placebo.ab.
18. drug therapy.fs.
19. randomly.ab.
20. trial.ab.
21. or/14‐20
22. exp animals/ not humans.sh.
23. 21 not 22
24. 4 and 13 and 23
25. (200805* or 200806* or 200807* or 200808* or 200809* or 200810* or 200811* or 200812* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015*).
26. 24 and 25
EMBASE (OVID)
1. Headache/
2. exp Headache/ and Facial Pain/
3. (headache$ or migrain$ or cephalgi$ or cephalalgi$ or cluster).tw.
4. or/1‐3
5. Hyperbaric Oxygenation/
6. Oxygen Therapy/
7. Oxygen/ae, tu, to
8. Hyperoxia/
9. Air Quality Control/
10. (hyperbar$ or HBO$).tw.
11. (high pressure oxygen or 100% oxygen).tw.
12. ((monoplace or multiplace) adj5 chamber$).tw.
13. or/5‐12
14. random$.tw.
15. factorial$.tw.
16. crossover$.tw.
17. cross over$.tw.
18. cross‐over$.tw.
19. placebo$.tw.
20. (doubl$ adj blind$).tw.
21. (singl$ adj blind$).tw.
22. assign$.tw.
23. allocat$.tw.
24. volunteer$.tw.
25. Crossover Procedure/
26. double‐blind procedure.tw.
27. Randomized Controlled Trial/
28. Single Blind Procedure/
29. or/14‐28
30. (animal/ or nonhuman/) not human/
31. 29 not 30
32. 4 and 13 and 31
33. (200805* or 200806* or 200807* or 200808* or 200809* or 200810* or 200811* or 200812* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015*).dd.
34. 32 and 33
CINAHL (EBSCO)
S24 S14 AND S23
S23 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S22 (allocat* random*)
S21 (MH "Quantitative Studies")
S20 (MH "Placebos")
S18 (random* allocat*)
S17 (MH "Random Assignment")
S16 (Randomi?ed control* trial*)
S15 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or
(doubl* mask* ) or (singl* mask* )
S14 S12 AND S13
S13 EM 20080501‐20140430
S12 S3 AND S11
S11 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10
S10 ((monoplace or multiplace) N5 (chamber*))
S9 (high pressure oxygen or 100% oxygen)
S8 (hyperbar* or HBO*)
S7 (MH "Hyperoxia")
S6 (MH "Oxygen/AE/TU")
S5 (MH "Oxygen Therapy")
S4 (MH "Hyperbaric Oxygenation")
S3 (S1 OR S2)
S2 (headache* OR migrain* OR cephalgi* OR cephalalgi* OR cluster)
S1 (MH "Headache+")
Appendix 2. Data extraction table for the relief of acute migraine attack
| Study ID | Participants and condition | Study design | Treatment | Treatment duration | Outcomes | Data extracted |
| 14 patients (23 to 67 years, 9 females) with a diagnosis of migraine documented by neurologist evaluation. | Acute therapy trial. RCT with partial cross‐over (patients with relief on first arm could not be crossed) with blinding of patients and investigators. Patients with no relief had the choice of undergoing the second arm of the study 30 minutes after completion of the first arm assigned. | Control: 10% oxygen breathing via Scott mask at 2 ATA. HBOT: 100% oxygen at 2 ATA on the same schedule. | 45 minutes | Proportion of patients with significant pain relief using a Blanchard pain inventory from 0 to 5. Significant relief defined as reduction on this scale of 2 or more points. | Fife 1992 gave the response of all patients from both treatment periods. We have included responders for both initial treatment and on crossover. Total of 8 of 11 individuals obtained pain relief from HBOT and 2 of 7 obtained similar relief from air. | |
| 19 patients with a diagnosis of migraine according to the AHC 1962. | Acute therapy trial. Cross‐over RCT with blinding of patients and investigators. Cross‐over was made 5 minutes after completing the first assigned treatment. | Control: Air breathing at 2.0 ATA. HBOT: 100% oxygen breathing on the same schedule. | 45 minutes | Complete or partial pain relief. | Hill 1992 reported no patients given air and all patients given oxygen obtained some pain relief. (10 had air first and 9 had HBOT first). We included the results given as prior to crossover because of limited reporting. This was a more conservation approach than imputing the response for all crossovers. | |
| 20 patients (14 female) with a diagnosis of migraine confirmed by a physician. | Acute therapy trial. RCT with randomisation not described. | Control: 100% oxygen at 1 ATA for 40 minutes. HBOT: 100% oxygen breathing using a hood at 2.0 ATA. | 40 minutes | Proportion with significant headache relief on a 6 category scale from 'none' to 'most severe ever'. | Myers 1995 reported 9 of 10 patients given HBOT obtained 'total' or 'near‐total' relief of headache versus 1 of the 10 patients given air. |
Abbreviations: ATA: atmospheres absolute; HBOT: hyperbaric oxygen therapy; RCT: randomised controlled trial.

'Risk of bias' graph: each 'Risk of bias' item presented as percentages across all included studies.
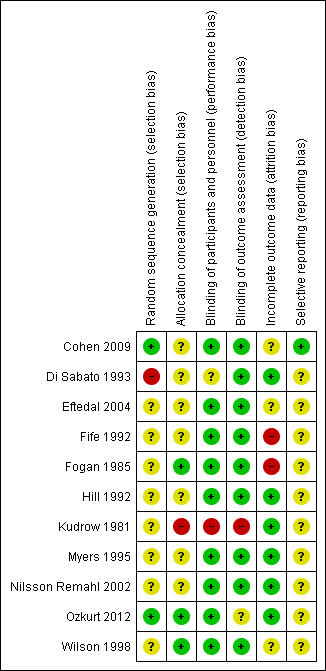
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
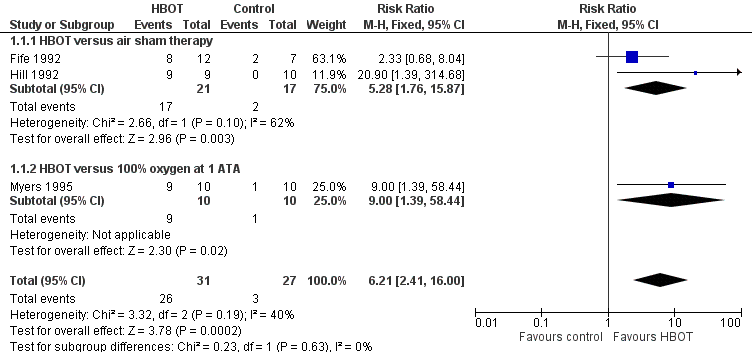
Forest plot of comparison: 1 HBOT versus control for acute migraine attack, outcome: 1.1 Substantial acute relief of headache.

Comparison 1 HBOT versus control for acute migraine attack, Outcome 1 Substantial acute relief of headache.
| Hyperbaric oxygen therapy for the relief of acute migraine | ||||||
| Patient or population: Acute migraine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with sham therapy | Risk with HBOT | |||||
| Chance of obtaining substantial headache relief (Relief). | Study population | RR 6.21 | 58 | Low¹ | We included 3 small RCTs but all showed large effect size with HBOT compared to either air or 100% oxygen sham. | |
| 111 per 1000 | 663 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹The evidence has been downgraded from moderate to low due to two of these trials being incompletely reported crossover trials reported only in abstract. [Two of the three included studies were planned as crossover trials. In those two, many patients obtained relief during the first treatment period and were not crossed to the second period. For Hill 1992, where all patients are not clearly accounted for we have included the results only from the first treatment period.] | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Substantial acute relief of headache Show forest plot | 3 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [2.41, 16.00] |
| 1.1 HBOT versus air sham therapy | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [1.76, 15.87] |
| 1.2 HBOT versus 100% oxygen at 1 ATA | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [1.39, 58.44] |


