Esteroides intranasales para la sinusitis aguda
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised: method of randomisation not mentioned | |
| Participants | N = 89; 42 male, 47 female | |
| Interventions | Tx group: budesonide 50 µg bid nasal spray to each nostril, N = 43 | |
| Outcomes | Difference in weekly symptom scores for cough and nasal discharge in the first, second and third week of the study in both groups, as difference between groups or change from baseline | |
| Notes | Marmara University Hospital Outpatient Clinic patients enrolled from November 1993 to October 1994 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | No separate data for groups, ITT not mentioned |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | ‐ |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | ‐ |
| Methods | Multicentre randomisation ‐ permuted blocks scheme stratified by site with a block size of 4 generated using SAS version 6.12 | |
| Participants | N = 95; 30 men, 65 women | |
| Interventions | Tx group: nasal spray fluticasone propionate 2 puffs (total dose 200 µg) once daily in each nostril; N = 47 | |
| Outcomes | Proportion of patients with clinical success (cured or much improved) at 10, 21, 56 days on telephone follow‐up | |
| Notes | Study conducted between October 1998 to April 2000 at 22 sites (12 primary care and 10 otolaryngology) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See methods |
| Allocation concealment (selection bias) | Low risk | See methods |
| Incomplete outcome data (attrition bias) | Low risk | See methods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | ‐ |
| Blinding of participants and personnel (performance bias) | Unclear risk | See methods |
| Blinding of outcome assessment (detection bias) | Unclear risk | ‐ |
| Methods | Multicentre randomisation | |
| Participants | N = 981; 338 men, 643 women | |
| Interventions | 4 groups | |
| Outcomes | Mean MSS | |
| Notes | Study conducted at 71 medical centres in 14 countries from January to September 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See methods |
| Allocation concealment (selection bias) | Unclear risk | See methods |
| Incomplete outcome data (attrition bias) | Low risk | See methods |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Unclear risk | ‐ |
| Blinding of participants and personnel (performance bias) | Unclear risk | See methods |
| Blinding of outcome assessment (detection bias) | Unclear risk | ‐ |
| Methods | Multicentre, randomised; method of randomisation not mentioned | |
| Participants | N = 967; 402 men, 565 women | |
| Interventions | 3 groups | |
| Outcomes | Improvement in total symptoms score | |
| Notes | Outpatients from 61 Tx centres in the US | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See methods |
| Allocation concealment (selection bias) | Unclear risk | See methods |
| Incomplete outcome data (attrition bias) | Unclear risk | See notes |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | ‐ |
| Blinding of participants and personnel (performance bias) | Low risk | See methods |
| Blinding of outcome assessment (detection bias) | Unclear risk | ‐ |
bid: twice daily
C: control
CT: computed tomography
FEV1: forced expiratory volume in one second
INCS: intranasal corticosteroid
ITT: intention‐to‐treat
MFNS: mometasone furoate
MSS: major symptom score
NSAID: non‐steroidal anti‐inflammatory drugs
PND: post‐nasal drip
Rx: radiological
tid: three times daily
TSS: total symptom score
Tx: treatment
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Study on quality of life. Outcome for a subset of patients from one of the included studies (Meltzer 2005) | |
| Allocation: randomised, parallel | |
| Abstract and full paper not available | |
| Allocation: randomised, parallel | |
| Missing data ‐ number randomised, numbers included in analyses, drop‐outs and reasons for drop‐out. The numbers reported do not add up to 100%. An email was sent to the author but there was no reply | |
| Allocation: randomised, parallel | |
| Missing data ‐ not mentioned acute/chronic sinusitis, diagnostic criteria not reported, drop‐outs not reported. Email was sent to the author but there was no reply | |
| Inclusion criteria for the review were not met | |
| Allocation: randomised, parallel |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.02, 1.18] |
| Analysis 1.1 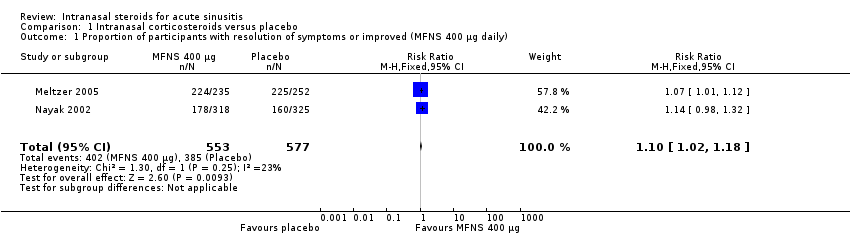 Comparison 1 Intranasal corticosteroids versus placebo, Outcome 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily). | ||||
| 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.11] |
| Analysis 1.2  Comparison 1 Intranasal corticosteroids versus placebo, Outcome 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily). | ||||
| 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| Analysis 1.3 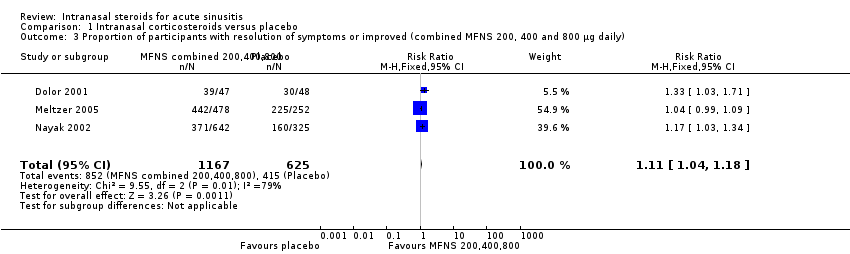 Comparison 1 Intranasal corticosteroids versus placebo, Outcome 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily). | ||||
| 4 Number of participants that dropped out from the study (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| Analysis 1.4  Comparison 1 Intranasal corticosteroids versus placebo, Outcome 4 Number of participants that dropped out from the study (MFNS 400 µg daily). | ||||
| 5 Number of participants that dropped out from the study (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.21] |
| Analysis 1.5  Comparison 1 Intranasal corticosteroids versus placebo, Outcome 5 Number of participants that dropped out from the study (MFNS 200 µg daily). | ||||
| 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.12] |
| Analysis 1.6  Comparison 1 Intranasal corticosteroids versus placebo, Outcome 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily). | ||||
| 7 Relapse (combined 200 and 400 µg daily) Show forest plot | 2 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.44, 1.15] |
| Analysis 1.7  Comparison 1 Intranasal corticosteroids versus placebo, Outcome 7 Relapse (combined 200 and 400 µg daily). | ||||
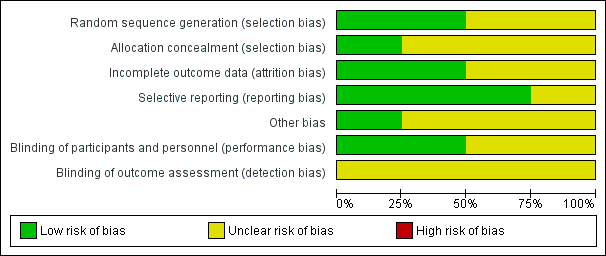
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
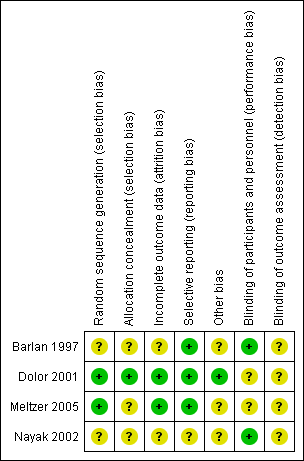
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 4 Number of participants that dropped out from the study (MFNS 400 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 5 Number of participants that dropped out from the study (MFNS 200 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 7 Relapse (combined 200 and 400 µg daily).
| Study | Intervention | Side effects | Comments |
| Fluticasone propionate 2 puffs ‐ total dose 200 µg or placebo nasal spray once daily in addition to 250 mg cefuroxime axetil orally twice daily and 2 puffs of xylometazoline hydrochloride twice daily | Headache, bloody nose, vaginal itching, yeast infection, nausea, stomach irritation, diarrhoea, increased congestion, hay fever, light‐headed, sore throat, thirsty, itching, rash, cough, fatigue, metallic taste, felt dried out, nasal tissue felt inflamed | No serious unexpected adverse events reported | |
| Amoxicillin‐clavulanate potassium 875 mg | Epistaxis was the most frequently reported adverse event | Treatment well‐tolerated, adverse events similar for all 3 arms of mild/moderate intensity: 12%, 15%, 15% in the MFNS 400, 800 µg and placebo arms | |
| Budesonide 50 µg or placebo nasal spray to each nostril bid in addition to amoxicillin clavulanate potassium 40 mg/kg/day tid | Rash after 1 week attributed to the antibiotic in 1 subject that was switched to cefaclor | No specific adverse events related to the INCS use were reported | |
| MFNS 200 µg once daily or twice daily nasal spray | Headache and epistaxis were most common reported | Most adverse events were mild or moderate with a similar incidence among treatment groups: 36.2%, | |
| bid: twice daily | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.02, 1.18] |
| 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.11] |
| 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| 4 Number of participants that dropped out from the study (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 5 Number of participants that dropped out from the study (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.21] |
| 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.12] |
| 7 Relapse (combined 200 and 400 µg daily) Show forest plot | 2 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.44, 1.15] |

