Esteroides intranasales para la sinusitis aguda
Appendices
Appendix 1. Previous search strategy
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 2, part of The Cochrane Library, www.thecochranelibrary.com (accessed 25 May 2011), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (September 2008 to May week 2, 2011) and Embase.com (October 2008 to May 2011). See Appendix 1 for details of previous searches.
Previously we searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2008, Issue 4) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to October 2008), EMBASE (1990 to October 2008) and bibliographies of included studies.
MEDLINE was searched using the following keywords and MeSH terms in conjunction with the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials (Lefebvre 2008). The same strategy was used to search CENTRAL and adapted to search EMBASE.
MEDLINE (OVID)
1 exp SINUSITIS/
2 sinusit*.tw.
3 rhinosinusit*.tw.
4 or/1‐3
5 exp STEROIDS/
6 steroid*.tw.
7 exp Adrenal Cortex Hormones/
8 adrenal cortex hormone*.tw.
9 exp Anti‐Inflammatory Agents/
10 anti‐inflammat*.tw.
11 corticosteroid*.tw.
12 or/5‐11
13 exp Administration, Intranasal/
14 exp Administration, Topical/
15 (nasal* or intranasal* or topical*).tw.
16 or/13‐15
17 12 and 16
18 4 and 17
Appendix 2. Embase.com search strategy
#24 #16 AND #23
#23 #22 NOT #21
#22 #17 OR #18
#21 #19 NOT #20
#20 'human'/de
#19 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de
#18 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR ((doubl* OR singl*) NEAR/1 blind*):ab,ti
#17 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp
#16 #4 AND #11 AND #15
#15 #12 OR #13 OR #14
#14 nasal*:ab,ti OR intranasal*:ab,ti OR topical*:ab,ti
#13 'topical drug administration'/de
#12 'intranasal drug administration'/de
#11 #5 OR #6 OR #7 OR #8 OR #9 OR #10
#10 'adrenal cortex hormone':ab,ti OR 'adrenal cortex hormones':ab,ti
#9 'anti‐inflammatory':ab,ti OR 'anti‐inflammatories':ab,ti OR antiinflammat*:ab,ti OR 'anti inflammatory':ab,ti OR 'anti inflammatories':ab,ti
#8 'antiinflammatory agent'/exp
#7 'corticosteroid'/exp
#6 steroid*:ab,ti
#5 'steroid'/exp
#4 #1 OR #2 OR #3
#3 rhinosinusit*:ab,ti OR nasosinusit*:ab,ti
#2 sinusit*:ab,ti
#1 'sinusitis'/exp
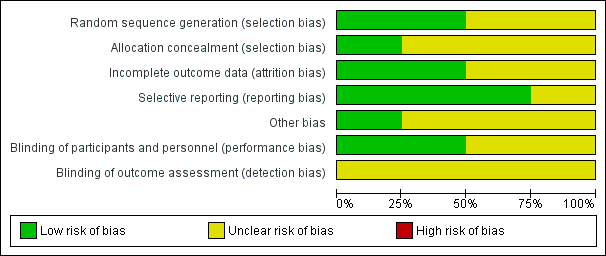
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
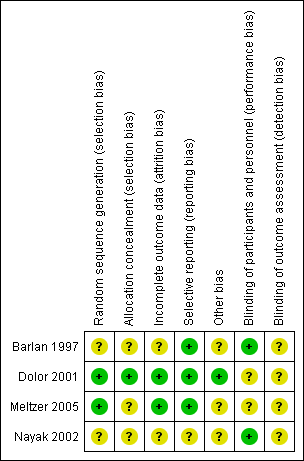
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
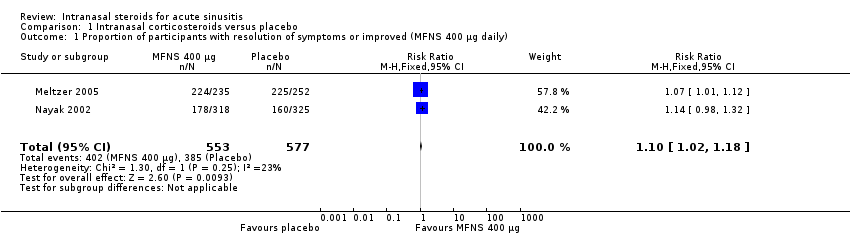
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily).
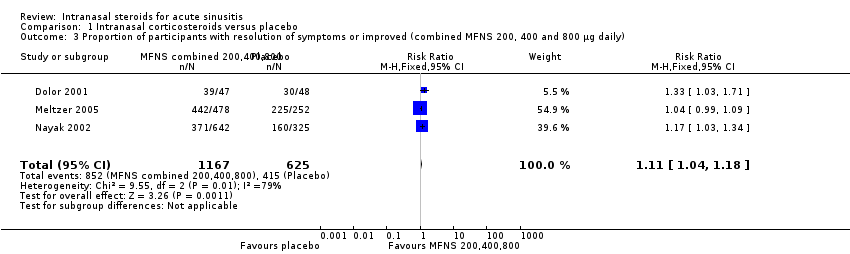
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 4 Number of participants that dropped out from the study (MFNS 400 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 5 Number of participants that dropped out from the study (MFNS 200 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 7 Relapse (combined 200 and 400 µg daily).
| Study | Intervention | Side effects | Comments |
| Fluticasone propionate 2 puffs ‐ total dose 200 µg or placebo nasal spray once daily in addition to 250 mg cefuroxime axetil orally twice daily and 2 puffs of xylometazoline hydrochloride twice daily | Headache, bloody nose, vaginal itching, yeast infection, nausea, stomach irritation, diarrhoea, increased congestion, hay fever, light‐headed, sore throat, thirsty, itching, rash, cough, fatigue, metallic taste, felt dried out, nasal tissue felt inflamed | No serious unexpected adverse events reported | |
| Amoxicillin‐clavulanate potassium 875 mg | Epistaxis was the most frequently reported adverse event | Treatment well‐tolerated, adverse events similar for all 3 arms of mild/moderate intensity: 12%, 15%, 15% in the MFNS 400, 800 µg and placebo arms | |
| Budesonide 50 µg or placebo nasal spray to each nostril bid in addition to amoxicillin clavulanate potassium 40 mg/kg/day tid | Rash after 1 week attributed to the antibiotic in 1 subject that was switched to cefaclor | No specific adverse events related to the INCS use were reported | |
| MFNS 200 µg once daily or twice daily nasal spray | Headache and epistaxis were most common reported | Most adverse events were mild or moderate with a similar incidence among treatment groups: 36.2%, | |
| bid: twice daily | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.02, 1.18] |
| 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.11] |
| 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| 4 Number of participants that dropped out from the study (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 5 Number of participants that dropped out from the study (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.21] |
| 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.12] |
| 7 Relapse (combined 200 and 400 µg daily) Show forest plot | 2 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.44, 1.15] |

