Dosis única oral de etoricoxib para el dolor posoperatorio agudo en pacientes adultos
Information
- DOI:
- https://doi.org/10.1002/14651858.CD004309.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 08 May 2014see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pain, Palliative and Supportive Care Group
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
RC and SD were involved with searching, data extraction, quality scoring, analysis, and writing for the original review. RAM was involved with analysis and writing. HJM acted as arbitrator and was involved with writing.
SD ran searches for the updates, and both SD and RAM carried out data extraction and updated the analyses and text.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
No sources of support supplied
Declarations of interest
SD and RAM have received research support from charities, government and industry at various times, but none related to this review. RAM has consulted for, and received lecture fees from, various pharmaceutical companies related to analgesics and other healthcare interventions in the last five years. None relate to this review.
Acknowledgements
The Oxford Pain Relief Trust supported both updates. The NHS Cochrane Collaboration Programme Grant Scheme supported the original review.
Jodie Barden and Jayne Rees developed the protocol and ran preliminary searches for the first version of this review, and Henry McQuay was an author.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 May 08 | Single dose oral etoricoxib for acute postoperative pain in adults | Review | Rachel Clarke, Sheena Derry, R Andrew Moore | |
| 2012 Apr 18 | Single dose oral etoricoxib for acute postoperative pain in adults | Review | Rachel Clarke, Sheena Derry, R Andrew Moore | |
| 2009 Apr 15 | Single dose oral etoricoxib for acute postoperative pain in adults | Review | Rachel Clarke, Sheena Derry, R Andrew Moore, Henry J McQuay | |
| 2003 Jul 21 | Single dose oral etoricoxib for postoperative pain | Protocol | Rachel Clarke, Sheena Derry, R Andrew Moore, Henry J McQuay | |
Differences between protocol and review
Since the protocol was published, 'Risk of bias' assessment and 'Summary of findings' tables have been introduced into Cochrane reviews. We have included them in this update.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Pain [*drug therapy];
- Administration, Oral;
- Cyclooxygenase 2 Inhibitors [*administration & dosage, adverse effects];
- Etoricoxib;
- Pain, Postoperative [*drug therapy];
- Pyridines [*administration & dosage, adverse effects];
- Randomized Controlled Trials as Topic;
- Sulfones [*administration & dosage, adverse effects];
- Toothache [drug therapy];
Medical Subject Headings Check Words
Adult; Humans;
PICOs

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
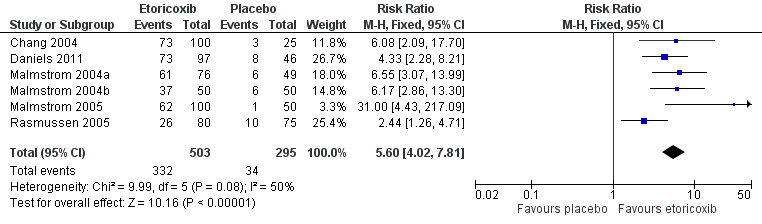
Forest plot of comparison: 1 Etoricoxib 120 mg versus placebo, outcome: 1.1 Participants with at least 50% pain relief over 6 hours

L'Abbé plot of etoricoxib 120 mg versus placebo for at least 50% pain relief. Size of circle is proportional to size of study (inset scale). Cream circles ‐ dental studies; pink circle ‐ orthopaedic study.

Forest plot of comparison: 1 Etoricoxib 120 mg vs placebo, outcome: 1.4 Participants using rescue medication within 24 hours

Forest plot of comparison: 2 Etoricoxib (all doses) versus placebo, outcome: 2.1 Participants with any adverse event

Comparison 1 Etoricoxib 120 mg vs placebo, Outcome 1 Participants with at least 50% pain relief over 6 hours.

Comparison 1 Etoricoxib 120 mg vs placebo, Outcome 2 Participants with at least 50% pain relief over 6 hours, dental.

Comparison 1 Etoricoxib 120 mg vs placebo, Outcome 3 Participants using rescue medication within 6 hours.

Comparison 1 Etoricoxib 120 mg vs placebo, Outcome 4 Participants using rescue medication within 24 hours.

Comparison 1 Etoricoxib 120 mg vs placebo, Outcome 5 Participants with any adverse event.

Comparison 2 Etoricoxib (all doses) versus placebo, Outcome 1 Participants with any adverse event.
| Etoricoxib compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: community or hospital Intervention: etoricoxib 120 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Relative effect and NNT or NNH (95% CI) | No of studies, events | Quality of the evidence | Comments | |
| comparator | intervention | |||||
| At least 50% of maximum pain relief over 4 to 6 hours | 120 in 1000 | 660 in 1000 | RR 5.6 (4.0 to 7.8) NNT 1.9 (1.7 to 2.1) | 6 studies 789 participants 366 events | High | Adequate numbers of studies, participants and events. Consistency across studies |
| Participants with at least 1 adverse event | 360 in 1000 | 330 in 1000 | RR 0.93 (0.74 to 1.2) NNH not calculated | 5 studies 643 participants 219 events | Moderate | Moderate numbers of studies, participants and events. Consistency across studies. Single dose studies may not reflect clinical practice |
| Participants with a serious adverse event | No serious adverse events | Low | Studies underpowered to detect rare events | |||
| Deaths | No deaths | Low | Studies underpowered to detect rare events | |||
| CI: Confidence interval; NNH: number needed to treat for harm; NNT: number needed to treat for benefit; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 6 hours Show forest plot | 6 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.60 [4.02, 7.81] |
| 2 Participants with at least 50% pain relief over 6 hours, dental Show forest plot | 5 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.68 [4.55, 9.82] |
| 3 Participants using rescue medication within 6 hours Show forest plot | 2 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.17, 0.34] |
| 4 Participants using rescue medication within 24 hours Show forest plot | 4 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.53, 0.67] |
| 5 Participants with any adverse event Show forest plot | 5 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.74, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 5 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.12] |


