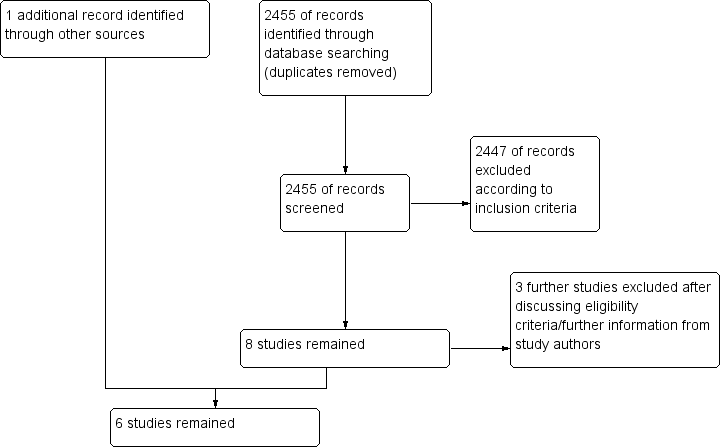
Hipotermia para la neuroprotección en adultos después de la reanimación cardiopulmonar
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomization: pre‐hospital | |
| Participants | Total number of participants 77, mean age 66 years, 33% female Out‐of‐hospital cardiac arrest of cardiac cause, ventricular fibrillation as first cardiac rhythm, comatose after resuscitation Participating sites: Australian university and community hospitals Multi‐centre: yes Language: English Allocation concealment: not applicable (odd and even days) Outcome assessor blind: yes Intention‐to‐treat analysis: yes Groups comparable: more females and more bystander CPR in hypothermia group Follow‐up > 80% of randomly assigned participants: yes | |
| Interventions | Therapeutic hypothermia vs standard pre‐hospital treatment protocols and intensive care treatment Means of cooling: ice packs placed around head, neck, torso and limbs Cooling rate: time from ROSC to target temperature: 2 hours Target temperature: 33°C Duration of cooling: 12 hours after target temperature was reached Rewarming: passive after 12 hours, active after 18 hours | |
| Outcomes | Survival with good neurological function to be sent home or to a rehabilitation facility at discharge In‐hospital death Haemodynamic, biochemical and haematological effects of hypothermia For IPD analysis, best ever reached CPC during hospital stay and CPC discharge provided | |
| Notes | Randomization: odd and even days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd and even days |
| Allocation concealment (selection bias) | High risk | Odd and even days |
| Blinding of participants and personnel (performance bias) | Low risk | Treating personnel not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Other bias | Low risk | No other major biases seen |
| Funding | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Good neurologic outcome | Low risk | Outcome assessment blinded |
| Blinding of outcome assessment (detection bias) Survivial | Low risk | Outcome assessment blinded |
| Methods | Randomization: in hospital | |
| Participants | Total number of participants 275, mean age 59 years, 24% female In‐hospital and out‐of‐hospital bystander‐witnessed cardiac arrest of presumed cardiac cause, ventricular fibrillation or non‐perfusing ventricular tachycardia as first cardiac rhythm, comatose after resuscitation Participating sites: European university and community hospitals Multi‐centre: yes Language: English Allocation concealment: opaque envelopes Outcome assessor blind: yes Intention‐to‐treat analysis: yes Groups comparable: significantly more diabetes and coronary heart disease and bystander CPR in control group Follow‐up > 80% of randomly assigned participants: yes | |
| Interventions | Therapeutic hypothermia vs standard intensive care treatment Means of cooling: cooling blanket that covered the whole body and released cooled air Cooling rate: time from ROSC to target temperature: median of 8 hours Target temperature: 32°C to 34°C Duration of cooling: median 24 hours Rewarming: passive over 8 hours | |
| Outcomes | Best CPC of 1, 2 vs CPC of 3, 4, 5 during 6 months Mortality at 6 months, rate of complication during first 7 days after cardiac arrest (bleeding of any severity) Pneumonia, sepsis, pancreatitis, renal failure, pulmonary oedema, seizures, arrhythmias and pressure sores For IPD analysis, best ever reached CPC during hospital stay and CPC discharge provided | |
| Notes | Randomization: computer‐generated random sequence Centre‐specific subsets of this study are also published as Tiainen 2003 (n = 70), Tiainen 2005 (n = 60) and Tiainen 2007 (n = 70) with more detailed investigations of neuropsychological and laboratory outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment assignments randomly generated by computer in blocks of 10, with stratification according to centre |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing treatment assignments provided by biostatistics centre |
| Blinding of participants and personnel (performance bias) | Low risk | Personnel involved in care of patients during first 48 hours after cardiac arrest could not be blinded to treatment assignments |
| Incomplete outcome data (attrition bias) | Low risk | Two participants lost to follow‐up, properly addressed |
| Other bias | Low risk | No other major biases seen |
| Funding | Low risk | Supported by grants of the Fourth RTD Framework Programme 1994–1998 of the European Union, the Austrian Ministry of Science and Transport and the Austrian Science Foundation |
| Blinding of outcome assessment (detection bias) Good neurologic outcome | Low risk | Personnel involved in care of patients during first 48 hours after cardiac arrest could not be blinded to treatment assignments. However, physicians responsible for assessing neurological outcome within first 6 months after the arrest unaware of treatment assignments |
| Blinding of outcome assessment (detection bias) Survivial | Low risk | Assessors of survival within first 6 months after arrest were unaware of treatment assignments |
| Methods | Randomization: in‐hospital | |
| Participants | Total number of participants 33, mean age 72 years, 39% female Out‐of‐hospital cardiac arrest of cardiac cause, pulseless electrical activity or asystole as first cardiac rhythm, comatose after resuscitation Participating site: Belgian university hospital Multi‐centre: no Language: English Outcome assessor blind: yes Intention‐to‐treat analysis: yes Groups comparable: yes, although groups were small, no significant differences Follow‐up > 80% of randomly assigned participants: yes | |
| Interventions | Therapeutic hypothermia vs standard post‐resuscitation care protocol Means of cooling: helmet device placed around head and neck and containing a solution of aqueous glycerol Cooling rate: starting point until target temperature not clearly stated Target temperature: 34°C Duration of cooling: start of cooling to start of rewarming, 4 hours Rewarming: passive over 8 hours | |
| Outcomes | Haemodynamic data, arterial pH, electrolytes, haematological data Complications such as pneumonia, sepsis, cardiac arrhythmia, coagulopathy Survival to hospital discharge and overall performance categories (OPCs) For IPD analysis, best ever reached CPC during hospital stay and CPC discharge provided | |
| Notes | Randomization: random number tables IPD included 33 participants; article reported on only 30, as follow‐up was not completed at the time of submission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated with random number tables |
| Allocation concealment (selection bias) | Low risk | After stabilization and insertion of catheters, participants prospectively blindly randomly assigned to 2 groups (treatment allocation concealed by use of opaque envelopes) |
| Blinding of participants and personnel (performance bias) | Low risk | Treating personnel not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Differences between published report and IPD properly reported |
| Other bias | Low risk | No other major biases seen |
| Funding | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Good neurologic outcome | Low risk | Outcome assessors blind to the intervention |
| Blinding of outcome assessment (detection bias) Survivial | Low risk | Outcome assessors blind to the intervention |
| Methods | Randomization: pre‐hospital | |
| Participants | Total number of participants 42, mean age 52 years in HF group, 56 years in HF+HT group, 19% female Out‐of‐hospital cardiac arrest of presumed cardiac cause, ventricular fibrillation or asystole as first cardiac rhythm, comatose after resuscitation Participating sites: French university and community hospital Multi‐centre: yes Language: English Allocation concealment: opaque envelopes Outcome assessor blind: not stated Intention‐to‐treat analysis: yes Groups comparable: yes Follow‐up > 80% of randomly assigned participants: yes | |
| Interventions | High‐flow haemofiltration vs high‐flow haemofiltration + therapeutic hypothermia vs standard supportive care Means of cooling: direct external cooling of blood Cooling rate: 4 hours after ICU admission, median temperature 31.7°C Target temperature: 32°C to 33°C Duration of cooling: 24 hours Rewarming: passive | |
| Outcomes | Survival at 6 months Rate of death by intractable shock in participants who had a favourable Glasgow Coma Scale (M5 or M6) or who required sedation Survival at CPC 1, 2 vs all else at 6 months | |
| Notes | Randomization pre‐hospital to save time for haemofiltration We did not pool data from this study with data from the other 3 studies, as treatment schemes with haemofiltration are not comparable with treatment schemes with non‐haemofiltration treatment (clinical heterogeneity) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 1/1/1 randomization sequence prepared for each centre |
| Allocation concealment (selection bias) | Low risk | Participants randomly allocated to treatment by use of sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding to assigned treatment not feasible |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Other bias | Low risk | No other major biases seen |
| Funding | Unclear risk | For this study, haemofiltration circuits, catheters and replacement fluid concentrates provided by GAMBRO AB, with estimated cost of €120 per participant treated by haemofiltration |
| Blinding of outcome assessment (detection bias) Good neurologic outcome | Unclear risk | Outcome assessor blind: not stated |
| Blinding of outcome assessment (detection bias) Survivial | Low risk | Outcome assessor blind: not stated, but for survival, risk of bias regarded as low |
| Methods | Randomization: unknown | |
| Participants | Total number of participants 54, mean age unknown, gender distribution unknown Out‐of‐hospital cardiac arrest of unknown cause with Glasgow Coma Scale score < 8 Participating site: Japanese university hospital Multi‐centre: unknown Language of abstract: English Allocation concealment: unknown Outcome assessor blind: not stated Intention‐to‐treat analysis: unknown Groups comparable: unknown Follow‐up > 80% of randomly assigned participants: yes | |
| Interventions | "Brain‐hypothermic treatment" vs "brain normothermic treatment" Means of cooling: water‐circulating blankets above and below participant with another ice mounted blanket over participant Cooling rate: unknown Target temperature: 32°C to 34°C Duration of cooling: 3 days Rewarming: unknown | |
| Outcomes | Glasgow outcome scale at 1 month (5‐point scale). Categories "moderate, mild, or no disabilities" defined as "good neurological outcome" | |
| Notes | Only abstract published Study now included in the pooled analysis, as we received information on the cooling method, whether cooling was applied locally or systemically | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available from abstract and correspondence |
| Allocation concealment (selection bias) | Unclear risk | No information available from abstract and correspondence |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information available from abstract and correspondence |
| Incomplete outcome data (attrition bias) | Unclear risk | No information available from abstract and correspondence |
| Other bias | Unclear risk | No information available from abstract and correspondence |
| Funding | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Good neurologic outcome | Unclear risk | No information available from abstract and correspondence |
| Methods | Randomization: | |
| Participants | Total number of participants 950, mean age 64 years, 19% female Age ≥ 18 years, out‐of‐hospital cardiac arrest of presumed cardiac cause, sustained return of spontaneous circulation comatose after resuscitation (GCS < 8) Participating sites: 36 intensive care units (ICUs) in Europe and Australia, university and community Multi‐centre: yes Language: English Allocation concealment: centrally Outcome assessor blind: yes Intention‐to‐treat analysis: modified intention‐to‐treat analysis Groups comparable: higher incidence of previous AMI and ischaemic heart disease in 33°C group Follow‐up > 80% of randomly assigned participants: yes | |
| Interventions | Group 1: target temperature 33°C Group 2: target temperature 36°C Means of temperature control: ice cold fluids, ice packs and intravascular or surface temperature‐management devices at the discretion of sites | |
| Outcomes | All‐cause mortality through the end of the trial CPC of 3 to 5 around 180 days Modified Rankin scale around 180 days Mortality at 180 days Individual neurological scores | |
| Notes | Control group differs (36°) from control group of other studies (no cooling) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization performed centrally with the use of a computer‐generated assignment sequence |
| Allocation concealment (selection bias) | Low risk | Randomization performed centrally |
| Blinding of participants and personnel (performance bias) | Low risk | Treating personnel not blinded |
| Incomplete outcome data (attrition bias) | Low risk | 11 out of 950 participants excluded after randomization (for various reasons) |
| Other bias | Unclear risk | Time from collapse to resuscitation 1 minute ‐ considerably shorter than in other trials Period between return of spontaneous circulation and start of therapy not defined, slow cooling rate Dose‐finding study with pragmatic study design ‐ risk of non‐effect in both groups and misleading interpretation of equivalence |
| Funding | Low risk | Funded by the Swedish Heart–Lung Foundation and others |
| Blinding of outcome assessment (detection bias) Good neurologic outcome | Low risk | Outcome assessment blinded |
| Blinding of outcome assessment (detection bias) Survivial | Low risk | Outcome assessment blinded |
AMI = acute myocardial infarction
CPC = cerebral performance category
CPR = cardiopulmonary resuscitation
GCS = Glasgow Coma Scale
HF = haemofiltration
HT = hypothermia
ICU = intensive care unit
IPD = individual patient data
M5 = localizes painful stimuli
M6 = obeys commands
OPC = overall performance category
ROSC = restoration of spontaneous circulation
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Substudy to Nielsen 2013; no outcome information on additional patients available | |
| Intervention and control groups did not meet inclusion criteria; control group of 34°C and lower | |
| Intervention and control groups did not meet inclusion criteria, relevant numbers of participants in control and intervention groups not continuously cooled |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All studies with subgroups Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 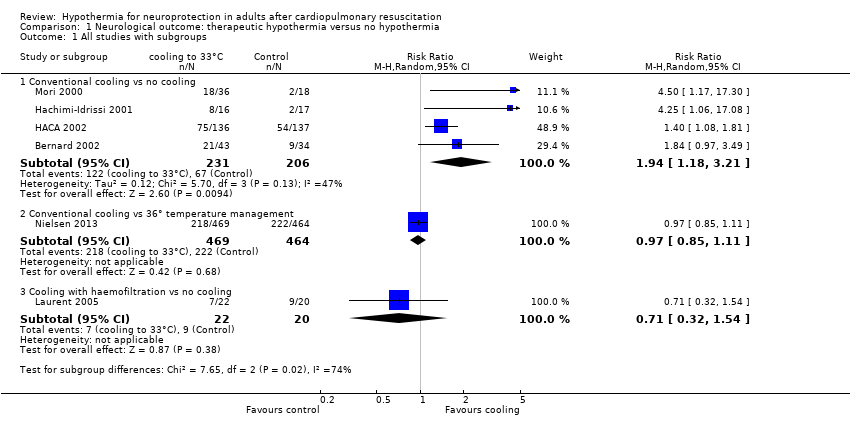 Comparison 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, Outcome 1 All studies with subgroups. | ||||
| 1.1 Conventional cooling vs no cooling | 4 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.18, 3.21] |
| 1.2 Conventional cooling vs 36° temperature management | 1 | 933 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.11] |
| 1.3 Cooling with haemofiltration vs no cooling | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.32, 1.54] |
| 2 Conventional cooling Show forest plot | 5 | 1370 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.02, 2.29] |
| Analysis 1.2 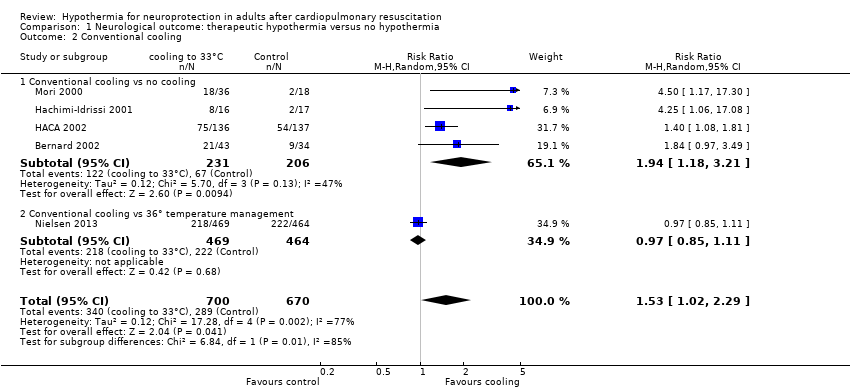 Comparison 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, Outcome 2 Conventional cooling. | ||||
| 2.1 Conventional cooling vs no cooling | 4 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.18, 3.21] |
| 2.2 Conventional cooling vs 36° temperature management | 1 | 933 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All studies with subgroups Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 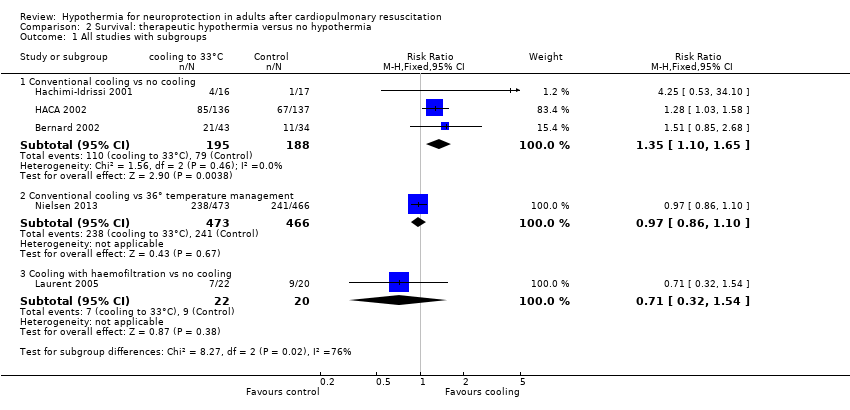 Comparison 2 Survival: therapeutic hypothermia versus no hypothermia, Outcome 1 All studies with subgroups. | ||||
| 1.1 Conventional cooling vs no cooling | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.65] |
| 1.2 Conventional cooling vs 36° temperature management | 1 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 1.3 Cooling with haemofiltration vs no cooling | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.32, 1.54] |
| 2 Conventional cooling Show forest plot | 4 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.96, 1.19] |
| Analysis 2.2 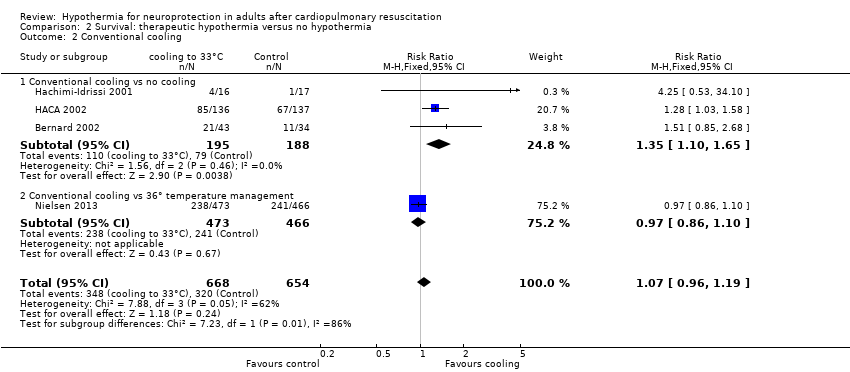 Comparison 2 Survival: therapeutic hypothermia versus no hypothermia, Outcome 2 Conventional cooling. | ||||
| 2.1 Conventional cooling vs no cooling | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.65] |
| 2.2 Conventional cooling vs 36° temperature management | 1 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
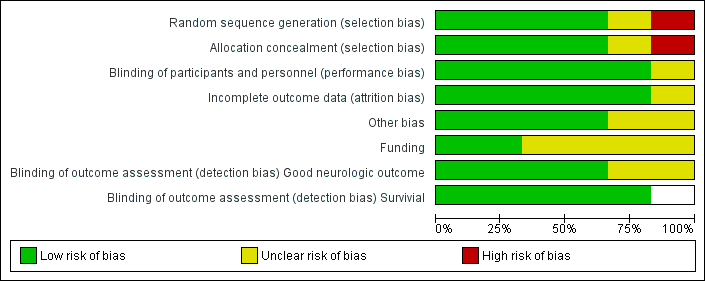
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
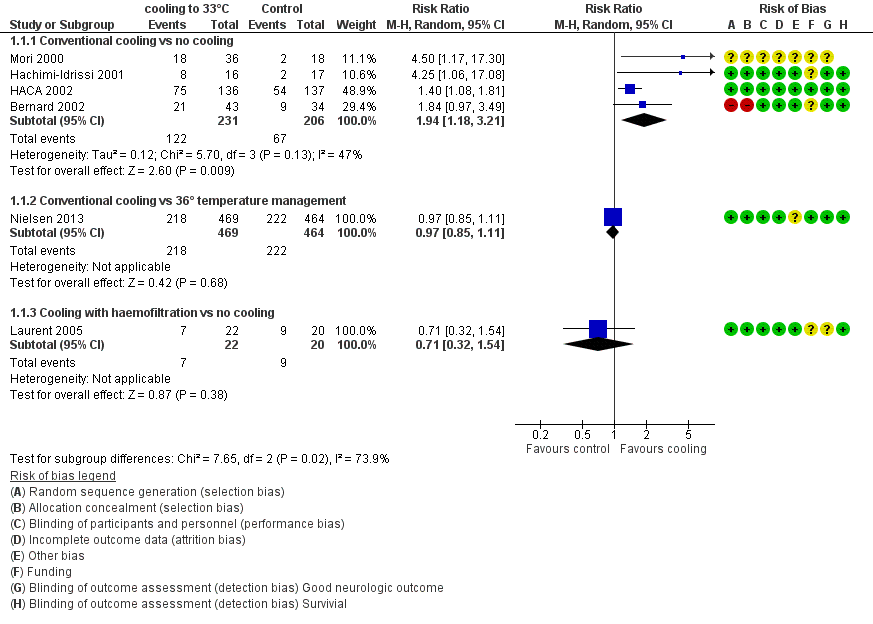
Forest plot of comparison: 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, outcome: 1.1 All studies with subgroups.
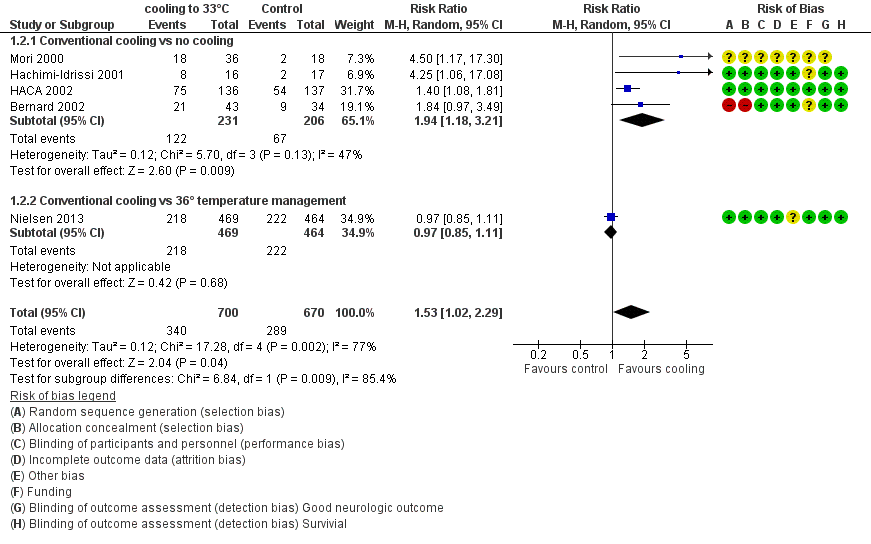
Forest plot of comparison: 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, outcome: 1.2 Conventional cooling.
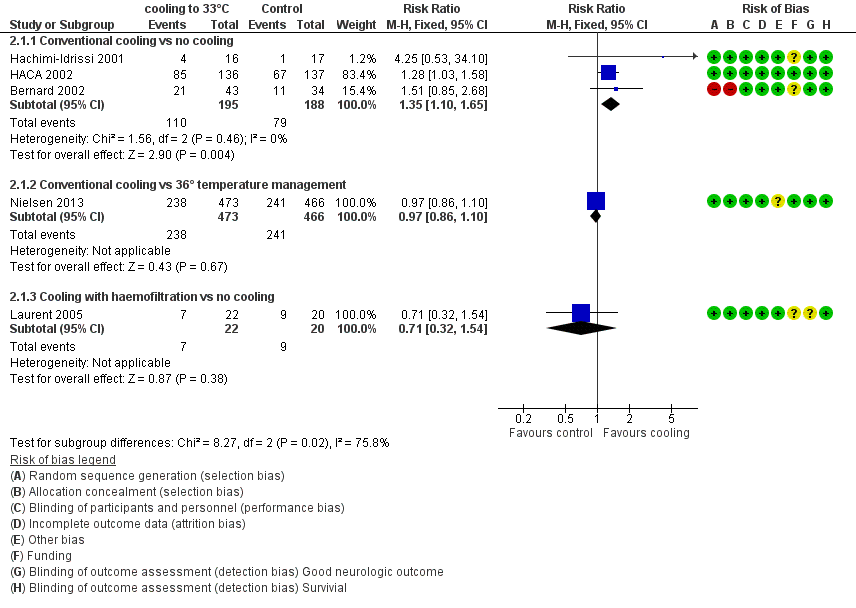
Forest plot of comparison: 3 Survival: therapeutic hypothermia versus no hypothermia, outcome: 3.1 All studies with subgroups.
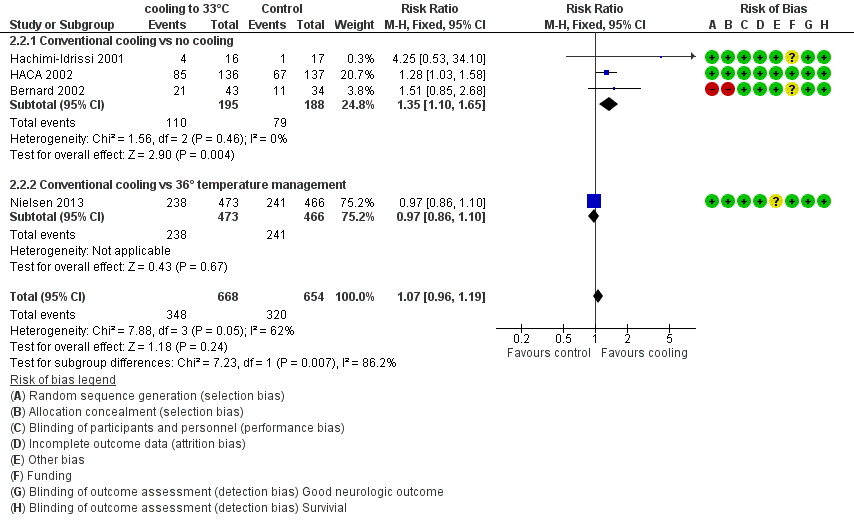
Forest plot of comparison: 3 Survival: therapeutic hypothermia versus no hypothermia, outcome: 3.2 Conventional cooling.

Comparison 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, Outcome 1 All studies with subgroups.

Comparison 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, Outcome 2 Conventional cooling.

Comparison 2 Survival: therapeutic hypothermia versus no hypothermia, Outcome 1 All studies with subgroups.

Comparison 2 Survival: therapeutic hypothermia versus no hypothermia, Outcome 2 Conventional cooling.
| Neurological outcome, survival and adverse events: conventional cooling compared with no cooling and 36°C for neuroprotection and survival in adults after cardiopulmonary resuscitation | ||||||
| Patient or population: adults after cardiopulmonary resuscitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No cooling | Cooling 32°C to 34°C | |||||
| Good neurological outcome | Study population | RR 1.94 (1.18 to 3.21) | 437 | ⊕⊕⊕⊝ | ||
| 325 per 1000 | 631 per 1000 | |||||
| Survival | Study population | RR 1.35 | 383 | ⊕⊕⊕⊝ | ||
| 420 per 1000 | 567 per 1000 | |||||
| Adverse events ‐ pneumonia | Study population | RR 1.15 | 1205 | ⊕⊕⊕⊝ | ||
| 423 per 1000 | 486 per 1000 | |||||
| Adverse events ‐ hypokalaemia | Study population | RR 1.38 | 975 | ⊕⊕⊝⊝ | ||
| 134 per 1000 | 185 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (with its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) GRADE Working Group grades of evidence | ||||||
| aOne quasi‐randomized trial (Bernard 2002) and one abstract (Mori 2000) but both not contributing to most data (see also Effects of interventions ‐ 'Sensitivity analysis') bTotal number of events < 300; imprecision therefore was rated as serious, and this resulted in downgrading of the overall quality of the evidence one level from high to moderate cOne quasi‐randomized trial not contributing to the majority of data (see also Effects of interventions ‐ 'Sensitivity analysis') dIndirectness was caused mostly by control group treatment (Nielsen 2013), which resulted in downgrading of the overall quality of the evidence one level from high to moderate eIndirectness was rated as very serious because of differences in intervention (haemofiltration, conventional cooling) and control group treatments (no cooling, 36°C), which resulted in downgrading of the overall quality of the evidence two levels from high to low fLaurent 2005 had some risk of bias but is not contributing to the majority of data | ||||||
| Outcome or subgroup | Studies | Participants | Risk ratio (M‐H, fixed, 95% CI) |
| Good neurological outcome by cardiac cause vs non‐cardiac cause | 3 | 383 | 1.54 (1.22 to 1.95) |
| Cardiac cause | 3 | 372 | 1.51 (1.19 to 1.91) |
| Non‐cardiac cause | 2 | 11 | 3.80 (0.55 to 26.29) |
| Good neurological outcome by location of cardiac arrest | 3 | 382 | 1.56 (1.23 to 1.98) |
| In‐hospital | 1 | 17 | 1.64 (0.47 to 5.73) |
| Out‐of‐hospital | 3 | 365 | 1.56 (1.23 to 1.99) |
| Good neurological outcome by witnessed cardiac arrest | 3 | 382 | 1.49 (1.18 to 1.88) |
| Witnessed cardiac arrest | 3 | 360 | 1.43 (1.13 to 1.81) |
| Non‐witnessed cardiac arrest | 3 | 22 | 5.31 (1.40 to 20.21) |
| Good neurological outcome by primary ECG rhythm | 3 | 382 | 1.51 (1.19 to 1.91) |
| VF/VT rhythm | 2 | 330 | 1.47 (1.15 to 1.88) |
| Non‐ VF/VT rhythm | 2 | 52 | 2.17 (0.68 to 6.93) |
| ECG = electrocardiogram VF/VT = ventricular fibrillation/ventricular tachycardia | |||
| Outcome or subgroup | Studies | Participants | Risk ratio (M‐H, Fixed, 95% CI) |
| Good neurological outcome for all studies with conventional cooling | 4 | 437 | 1.94 (1.18 to 3.21) |
| Studies with conventional cooling and adequate or unknown allocation concealment | 3 | 360 | 2.46 (0.96 to 6.28) |
| Studies with conventional cooling and adequate allocation concealment | 2 | 445 | 1.97 (0.71 to 5.45) |
| Studies with other cooling methods and adequate allocation concealment | 1 | 42 | 0.71 (0.32 to 1.54) |
| Outcome or subgroup | Studies | Participants | Risk ratio (M‐H, Fixed, 95% CI) |
| Bleeding of any severity | 2 | 1206 | 1.14 (0.96 to 1.35) |
| Need for platelet transfusion | 1 | 273 | 5.11 (0.25 to 105.47) |
| Significant haemorrhagic complications | 1 | 77 | Not estimable |
| Pneumonia | 2 | 1205 | 1.15 (1.02 to 1.30) |
| Pancreatitis | 1 | 273 | 0.51 (0.05 to 5.57) |
| Sepsis | 2 | 1206 | 1.14 (0.81 to 1.61) |
| Septic shock | 1 | 933 | 0.87 (0.50 to 1.52) |
| Renal failure or oliguria | 2 | 303 | 0.88 (0.48 to 1.61) |
| Haemodialysis | 3 | 1288 | 1.16 (0.80 to 1.67) |
| Seizures | 2 | 1202 | 1.18 (0.98 to 1.42) |
| Lethal or long‐lasting arrhythmia | 2 | 315 | 1.21 (0.88 to 1.67) |
| Any arrhythmia | 1 | 933 | 0.98 (0.93 to 1.04) |
| Pulmonary oedema | 1 | 269 | 1.76 (0.61 to 5.12) |
| Cardiac complications | 1 | No totals | |
| Hypokalaemia | 2 | 975 | 1.38 (1.03 to 1.84) |
| Hypophosphataemia | 2 | 975 | 1.10 (0.92 to 1.33) |
| Hypoglycaemia | 1 | 933 | 1.12 (0.64 to 1.97) |
| Hypomagnesaemia | 1 | 933 | 1.20 (0.88 to 1.65) |
| Pressure sores | 1 | 269 | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All studies with subgroups Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Conventional cooling vs no cooling | 4 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.18, 3.21] |
| 1.2 Conventional cooling vs 36° temperature management | 1 | 933 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.11] |
| 1.3 Cooling with haemofiltration vs no cooling | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.32, 1.54] |
| 2 Conventional cooling Show forest plot | 5 | 1370 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.02, 2.29] |
| 2.1 Conventional cooling vs no cooling | 4 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.18, 3.21] |
| 2.2 Conventional cooling vs 36° temperature management | 1 | 933 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All studies with subgroups Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Conventional cooling vs no cooling | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.65] |
| 1.2 Conventional cooling vs 36° temperature management | 1 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 1.3 Cooling with haemofiltration vs no cooling | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.32, 1.54] |
| 2 Conventional cooling Show forest plot | 4 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.96, 1.19] |
| 2.1 Conventional cooling vs no cooling | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.65] |
| 2.2 Conventional cooling vs 36° temperature management | 1 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |