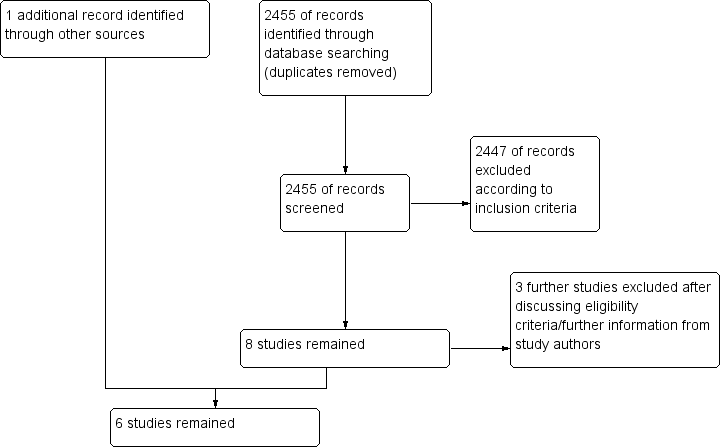
Hipotermia para la neuroprotección en adultos después de la reanimación cardiopulmonar
Appendices
Appendix 1. Search strategy: CENTRAL, The Cochrane Library
#1 MeSH descriptor Resuscitation explode all trees
#2 MeSH descriptor Cardiopulmonary Resuscitation explode all trees
#3 MeSH descriptor Resuscitation Orders explode all trees
#4 MeSH descriptor Heart Arrest explode all trees
#5 MeSH descriptor Heart Massage explode all trees
#6 ((cardio?pulmonary or order*) near2 resuscitation):ti,ab
#7 reanimation:ti,ab
#8 ((circulatory or circulation or cardiac) near arrest):ti,ab or heart standstill:ti,ab
#9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
#10 MeSH descriptor Cryotherapy explode all trees
#11 MeSH descriptor Hypothermia explode all trees
#12 MeSH descriptor Hypothermia, Induced explode all trees
#13 ((resuscitative or therapeutic or artificial or induced or extracorporeal) near hypothermia)
#14 artificial hibernation or body cooling or refrigeration anesthesia or body temperature:ti,ab or refrigeration:ti,ab
#15 (#10 OR #11 OR #12 OR #13 OR #14)
#16 (#9 AND #15)
Appendix 2. Search strategy: MEDLINE (Ovid SP)
1. Resuscitation/ or Cardiopulmonary Resuscitation/ or Resuscitation Orders/ or Heart Arrest/ or Heart Massage/ or advanced cardiac life support.mp. or ((cardio?pulmonary or order*) adj2 resuscitation).ti,ab. or reanimation.ti,ab. or ((circulatory or circulation or cardiac) adj3 arrest).ti,ab. or heart standstill.ti,ab.
2. Cryotherapy/ or Hypothermia/ or Circulatory Arrest, Deep Hypothermia Induced/ or Hypothermia, Induced/ or ((resuscitative or therapeutic or artificial or induced or extracorporeal) adj3 hypothermia).mp. or artificial hibernation.mp. or body cooling.mp. or chilling.mp. or refrigeration anesthesia.mp. or body temperature.ti,ab. or refrigeration.ti,ab.
3. 1 and 2
4. ((randomised controlled trial or controlled clinical trial).pt. or randomised.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
5. 3 and 4
Appendix 3. Search strategy: EMBASE (Ovid SP)
1. resuscitation/ or heart arrest/ or heart massage/ or advanced cardiac life support.mp. or ((cardio?pulmonary or order*) adj2 resuscitation).ti,ab. or reanimation.ti,ab. or ((circulatory or circulation or cardiac) adj3 arrest).ti,ab. or heart standstill.ti,ab.
2. cryotherapy/ or hypothermia/ or ((resuscitative or therapeutic or artificial or induced or extracorporeal) adj3 hypothermia).mp. or artificial hibernation.mp. or body cooling.mp. or chilling.mp. or refrigeration anesthesia.mp. or body temperature.ti,ab. or refrigeration.ti,ab.
3. 1 and 2
4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh.
5. 3 and 4
Appendix 4. Search strategy: CINAHL (EBSCO Host)
S1 ( (MH "Resuscitation") OR (MH "Resuscitation Orders") OR (MH "Resuscitation, Cardiopulmonary") OR (MH "Heart Arrest") OR (MH "Heart Massage") ) OR AB ( ((cardio?pulmonary or order*) and resuscitation) ) OR AB reanimation OR ( (circulatory or circulation or cardiac) and arrest ) OR heart standstill
S2 ( (MH "Cryotherapy") OR (MH "Hypothermia") OR (MH "Hypothermia, Induced") ) OR ( ((resuscitative or therapeutic or artificial or induced or extracorporeal) and hypothermia) ) OR artificial hibernation OR body cooling OR refrigeration anesthesia
S3 ( (MH "randomised Controlled Trials") OR (MH "Random Assignment") OR (MH "Prospective Studies") OR (MH "Multicenter Studies") OR (MH "Clinical Trials") OR (MH "Clinical Trial Registry") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Placebos") ) OR ( random* or controlled clinical trial or placebo )
S4 S1 and S2 and S3
Appendix 5. Search strategy: BIOSIS (Ovid SP)
1. advanced cardiac life support.mp. or ((cardio?pulmonary or order*) adj2 resuscitation).ti,ab. or reanimation.ti,ab. or ((circulatory or circulation or cardiac) adj3 arrest).ti,ab. or heart standstill.ti,ab.
2. (((resuscitative or therapeutic or artificial or induced or extracorporeal) adj3 hypothermia) or artificial hibernation or body cooling or chilling or refrigeration anesthesia).mp. or body temperature.ti,ab. or refrigeration.ti,ab.
3. 1 and 2
Appendix 6. Data extraction form
| Data extraction sheet Hypothermia for neuroprotection after cardiopulmonary resuscitation Update review | |
| Reviewer: | |
| Date: | |
| Decision: |
|
| Reasons for exclusion: | |
| Study characteristics | Publication type: |
| Language | |
| Setting | Multi‐centre:
|
| Participating sites:
| |
| Participants | Total number of patients: |
| Mean age: | |
| Percentage female: | |
| Cardiac arrest
| |
| Cause of cardiac arrest
| |
| Primary cardiac rhythm
| |
| Quality | Allocation concealment
|
| Outcome assessor blind
| |
| Intention‐to‐treat:
| |
| Groups comparable:
| |
| Follow‐up > 80% of randomly assigned participants:
| |
| Intervention | Type of intervention: |
| Controls: | |
| Time from restoration of spontaneous circulation to target temperature: | |
| Cooling rate: | |
| Duration of cooling: | |
| Rewarming: | |
| Outcomes | Types of outcome measures:
|
| Time point of assessment of outcome measures:
| |
| Funding | |
| Notes | |
Risk of bias assessment
| Allocation concealment | Short description: rating
|
| Inclusion/exclusion explicitly reported: | Short description: rating
|
| Intention‐to‐treat: | Short description: rating
|
| Appropriate participant description | Short description: rating
|
| Identical care | Short description: rating
|
| Outcomes appropriately defined: | Short description: rating
|
| Follow‐up > 80%: | Short description: rating
|
| Physician blinded: | Short description: rating
|
| Outcome assessor blinded (outcomes other than mortality): | Short description: rating
|
| Other risks of bias | Short description: |
Results primary:
| Type of outcome: | |||
| Cooling | No cooling | ||
| Events (n) | Total (N) | Events (n) | Total (N) |
Results secondary:
| Type of outcome: | |||
| Cooling | No cooling | ||
| Events (n) | Total (N) | Events (n) | Total (N) |
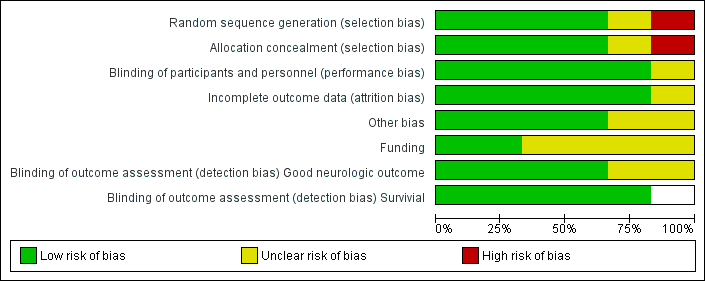
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
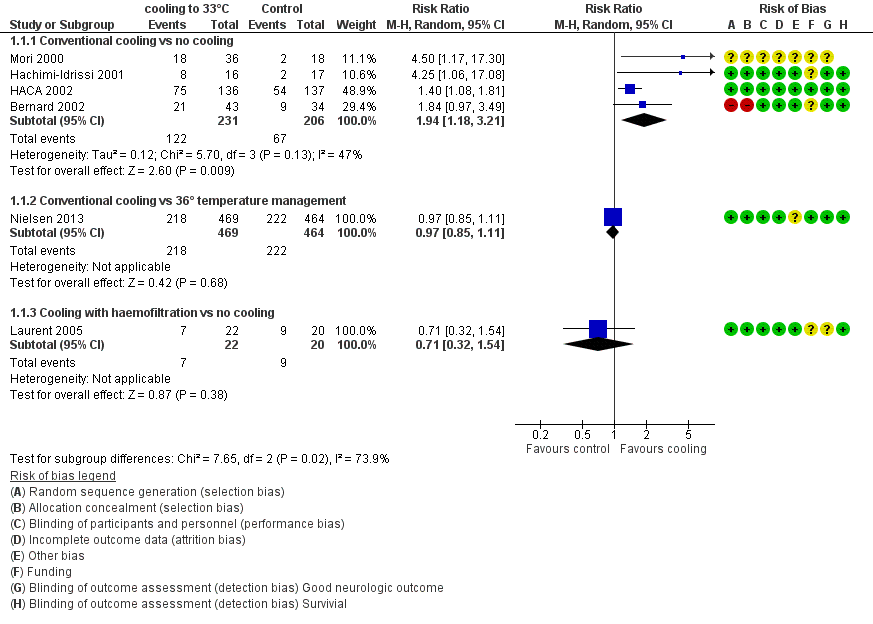
Forest plot of comparison: 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, outcome: 1.1 All studies with subgroups.
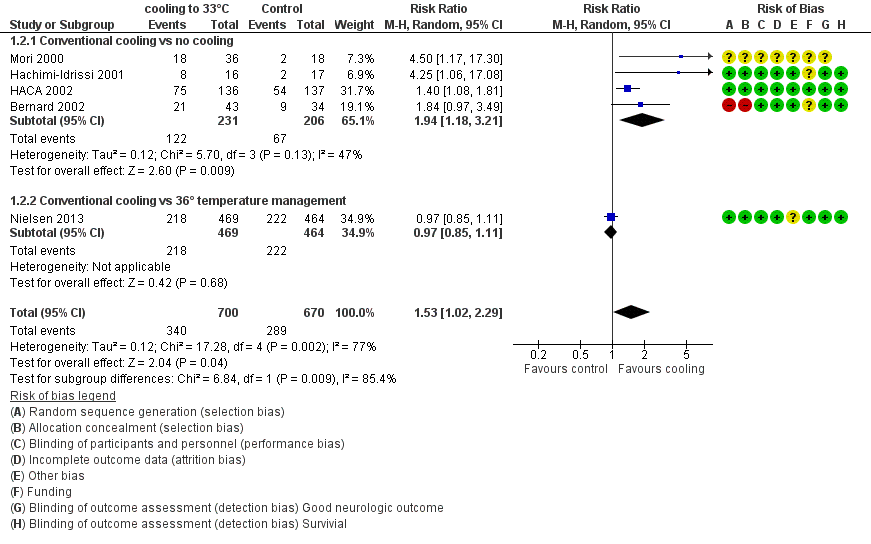
Forest plot of comparison: 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, outcome: 1.2 Conventional cooling.
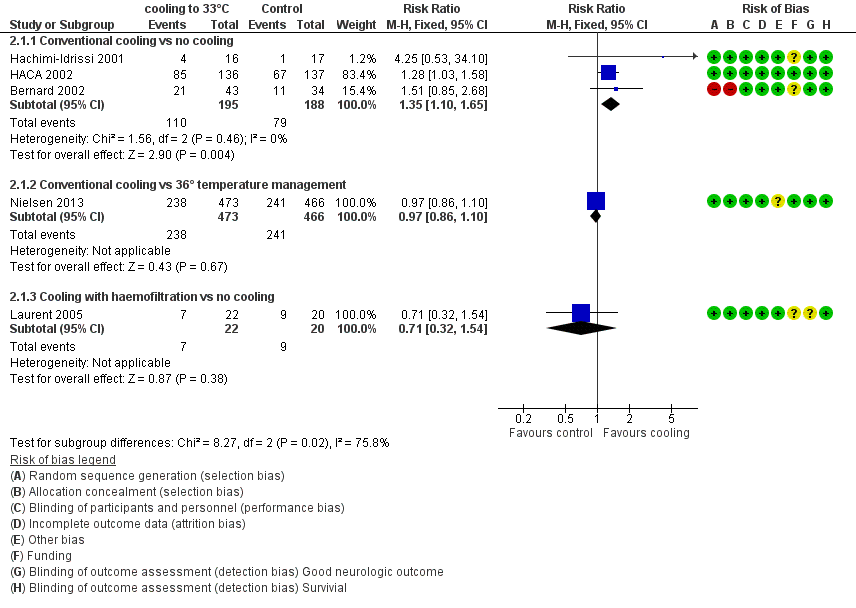
Forest plot of comparison: 3 Survival: therapeutic hypothermia versus no hypothermia, outcome: 3.1 All studies with subgroups.
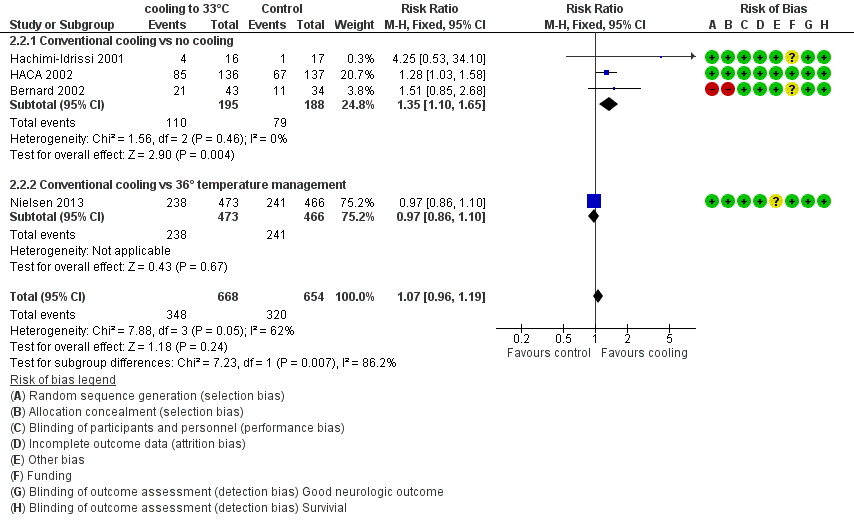
Forest plot of comparison: 3 Survival: therapeutic hypothermia versus no hypothermia, outcome: 3.2 Conventional cooling.
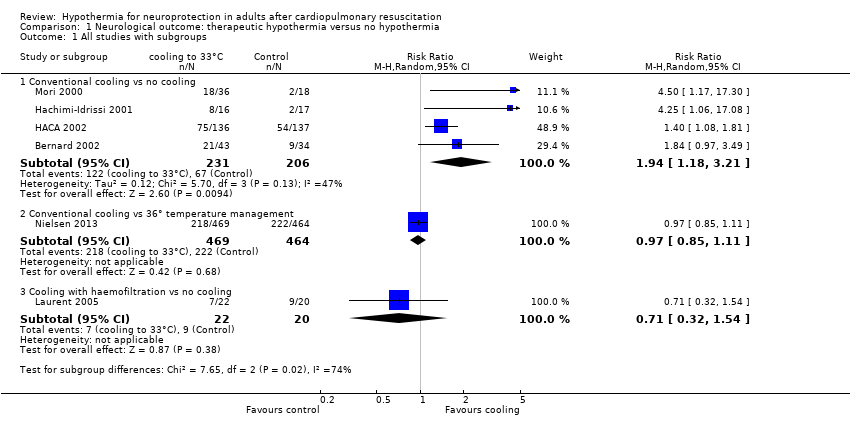
Comparison 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, Outcome 1 All studies with subgroups.
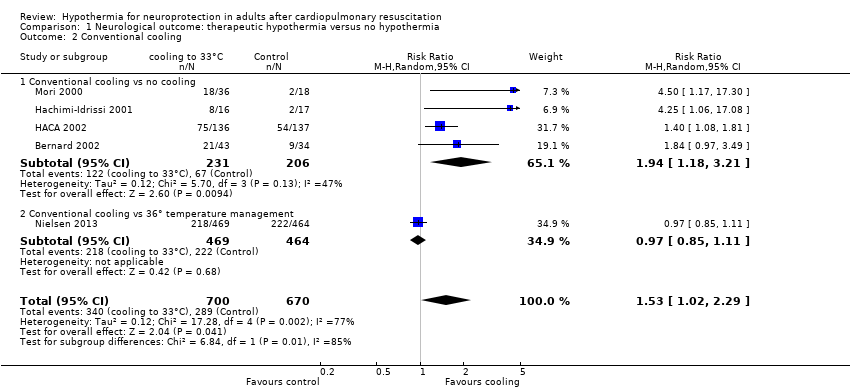
Comparison 1 Neurological outcome: therapeutic hypothermia versus no hypothermia, Outcome 2 Conventional cooling.
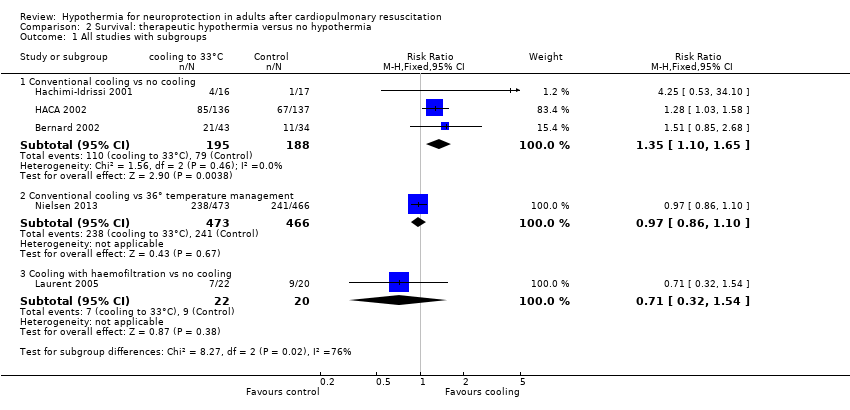
Comparison 2 Survival: therapeutic hypothermia versus no hypothermia, Outcome 1 All studies with subgroups.
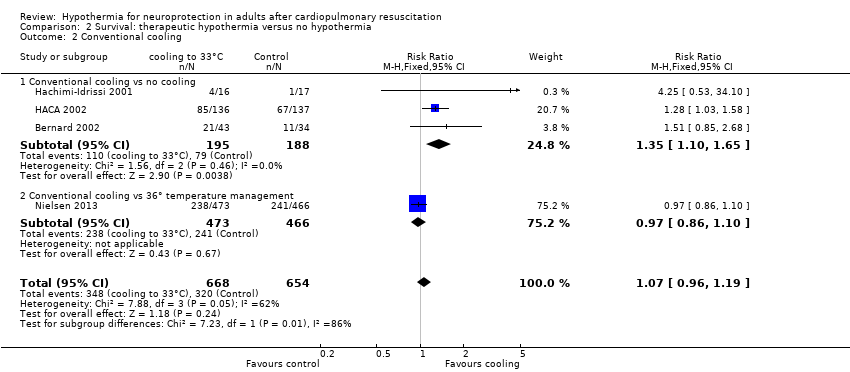
Comparison 2 Survival: therapeutic hypothermia versus no hypothermia, Outcome 2 Conventional cooling.
| Neurological outcome, survival and adverse events: conventional cooling compared with no cooling and 36°C for neuroprotection and survival in adults after cardiopulmonary resuscitation | ||||||
| Patient or population: adults after cardiopulmonary resuscitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No cooling | Cooling 32°C to 34°C | |||||
| Good neurological outcome | Study population | RR 1.94 (1.18 to 3.21) | 437 | ⊕⊕⊕⊝ | ||
| 325 per 1000 | 631 per 1000 | |||||
| Survival | Study population | RR 1.35 | 383 | ⊕⊕⊕⊝ | ||
| 420 per 1000 | 567 per 1000 | |||||
| Adverse events ‐ pneumonia | Study population | RR 1.15 | 1205 | ⊕⊕⊕⊝ | ||
| 423 per 1000 | 486 per 1000 | |||||
| Adverse events ‐ hypokalaemia | Study population | RR 1.38 | 975 | ⊕⊕⊝⊝ | ||
| 134 per 1000 | 185 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (with its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) GRADE Working Group grades of evidence | ||||||
| aOne quasi‐randomized trial (Bernard 2002) and one abstract (Mori 2000) but both not contributing to most data (see also Effects of interventions ‐ 'Sensitivity analysis') bTotal number of events < 300; imprecision therefore was rated as serious, and this resulted in downgrading of the overall quality of the evidence one level from high to moderate cOne quasi‐randomized trial not contributing to the majority of data (see also Effects of interventions ‐ 'Sensitivity analysis') dIndirectness was caused mostly by control group treatment (Nielsen 2013), which resulted in downgrading of the overall quality of the evidence one level from high to moderate eIndirectness was rated as very serious because of differences in intervention (haemofiltration, conventional cooling) and control group treatments (no cooling, 36°C), which resulted in downgrading of the overall quality of the evidence two levels from high to low fLaurent 2005 had some risk of bias but is not contributing to the majority of data | ||||||
| Outcome or subgroup | Studies | Participants | Risk ratio (M‐H, fixed, 95% CI) |
| Good neurological outcome by cardiac cause vs non‐cardiac cause | 3 | 383 | 1.54 (1.22 to 1.95) |
| Cardiac cause | 3 | 372 | 1.51 (1.19 to 1.91) |
| Non‐cardiac cause | 2 | 11 | 3.80 (0.55 to 26.29) |
| Good neurological outcome by location of cardiac arrest | 3 | 382 | 1.56 (1.23 to 1.98) |
| In‐hospital | 1 | 17 | 1.64 (0.47 to 5.73) |
| Out‐of‐hospital | 3 | 365 | 1.56 (1.23 to 1.99) |
| Good neurological outcome by witnessed cardiac arrest | 3 | 382 | 1.49 (1.18 to 1.88) |
| Witnessed cardiac arrest | 3 | 360 | 1.43 (1.13 to 1.81) |
| Non‐witnessed cardiac arrest | 3 | 22 | 5.31 (1.40 to 20.21) |
| Good neurological outcome by primary ECG rhythm | 3 | 382 | 1.51 (1.19 to 1.91) |
| VF/VT rhythm | 2 | 330 | 1.47 (1.15 to 1.88) |
| Non‐ VF/VT rhythm | 2 | 52 | 2.17 (0.68 to 6.93) |
| ECG = electrocardiogram VF/VT = ventricular fibrillation/ventricular tachycardia | |||
| Outcome or subgroup | Studies | Participants | Risk ratio (M‐H, Fixed, 95% CI) |
| Good neurological outcome for all studies with conventional cooling | 4 | 437 | 1.94 (1.18 to 3.21) |
| Studies with conventional cooling and adequate or unknown allocation concealment | 3 | 360 | 2.46 (0.96 to 6.28) |
| Studies with conventional cooling and adequate allocation concealment | 2 | 445 | 1.97 (0.71 to 5.45) |
| Studies with other cooling methods and adequate allocation concealment | 1 | 42 | 0.71 (0.32 to 1.54) |
| Outcome or subgroup | Studies | Participants | Risk ratio (M‐H, Fixed, 95% CI) |
| Bleeding of any severity | 2 | 1206 | 1.14 (0.96 to 1.35) |
| Need for platelet transfusion | 1 | 273 | 5.11 (0.25 to 105.47) |
| Significant haemorrhagic complications | 1 | 77 | Not estimable |
| Pneumonia | 2 | 1205 | 1.15 (1.02 to 1.30) |
| Pancreatitis | 1 | 273 | 0.51 (0.05 to 5.57) |
| Sepsis | 2 | 1206 | 1.14 (0.81 to 1.61) |
| Septic shock | 1 | 933 | 0.87 (0.50 to 1.52) |
| Renal failure or oliguria | 2 | 303 | 0.88 (0.48 to 1.61) |
| Haemodialysis | 3 | 1288 | 1.16 (0.80 to 1.67) |
| Seizures | 2 | 1202 | 1.18 (0.98 to 1.42) |
| Lethal or long‐lasting arrhythmia | 2 | 315 | 1.21 (0.88 to 1.67) |
| Any arrhythmia | 1 | 933 | 0.98 (0.93 to 1.04) |
| Pulmonary oedema | 1 | 269 | 1.76 (0.61 to 5.12) |
| Cardiac complications | 1 | No totals | |
| Hypokalaemia | 2 | 975 | 1.38 (1.03 to 1.84) |
| Hypophosphataemia | 2 | 975 | 1.10 (0.92 to 1.33) |
| Hypoglycaemia | 1 | 933 | 1.12 (0.64 to 1.97) |
| Hypomagnesaemia | 1 | 933 | 1.20 (0.88 to 1.65) |
| Pressure sores | 1 | 269 | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All studies with subgroups Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Conventional cooling vs no cooling | 4 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.18, 3.21] |
| 1.2 Conventional cooling vs 36° temperature management | 1 | 933 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.11] |
| 1.3 Cooling with haemofiltration vs no cooling | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.32, 1.54] |
| 2 Conventional cooling Show forest plot | 5 | 1370 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.02, 2.29] |
| 2.1 Conventional cooling vs no cooling | 4 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.18, 3.21] |
| 2.2 Conventional cooling vs 36° temperature management | 1 | 933 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All studies with subgroups Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Conventional cooling vs no cooling | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.65] |
| 1.2 Conventional cooling vs 36° temperature management | 1 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 1.3 Cooling with haemofiltration vs no cooling | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.32, 1.54] |
| 2 Conventional cooling Show forest plot | 4 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.96, 1.19] |
| 2.1 Conventional cooling vs no cooling | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.65] |
| 2.2 Conventional cooling vs 36° temperature management | 1 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |