Tratamiento con toxina botulínica tipo A para la distonía cervical
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised, double‐blind, parallel design Randomisation: carried out in blocks of four; method not described Setting: multicentre (22 centres in the USA, 1 in Canada) Duration: 10 weeks | |
| Participants | 170 participants enrolled (BtA group = 88; placebo group = 82) % Female: BtA: 70%; placebo: 80% Mean age, range: BtA: 55 years; placebo: 55 years Mean CD duration: BtA: 11.2 years; placebo: 9.1 years Mean CD severity, SD (CDSS): BtA: 9.2, 4.8; placebo: 9.3, 4.2 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | BtA: Botox (onabotulinumtoxinA); 25 ng of neurotoxin complex protein per 100 U, diluted with 1 mL sterile solution Placebo: 0.5 mg of human serum albumin and 0.9 mg of sodium chloride Study drug preparation: BtA provided in vials by Allergan Muscles injected: the doses and muscles injected were determined by the physician based on clinical assessment EMG guidance: no BtA dose per participant: maximum: 360 U; mean, range: 236 U, 91 U‐360 U | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | This was a 2‐period clinical trial consisting of a 10‐week open‐label period followed by a 10‐week double‐blind period, with up to 6 weeks between periods. Participants who successfully completed the open phase (i.e. responded to BtA and were compliant with the study protocol) were enrolled into the blinded phase. 214 participants were enrolled in Period I, of whom 170 continued into Period II. We only considered the results of the blinded phase in this review. Study discontinuations, reasons: BtA: n = 11 (13%), lack of efficacy: n = 8, unrelated reasons: n = 3 Placebo: n = 24 (29%), lack of efficacy: n = 19, unrelated reasons: n = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Comment: post‐randomisation exclusions were described as related to lack of efficacy or administrative reasons |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study |
| For‐profit bias | High risk | The study was supported by Allergan. |
| Enriched population – preferential enrolment of positive responders | High risk | Comment: to enrol in the study, participants had to have had at least 2 previous successful treatments with ≤ 360 U of Botox administered at 12‐ to 16‐week intervals. Also, all participants enrolled in phase II were compliant to treatment during phase I trial. |
| Enriched population – exclusion of poor responders | High risk | Quote: "Pure anterocollis and isolated head shift was exclusionary" |
| Methods | Randomised, double‐blind, parallel design Randomisation: block‐wise randomisation using a software‐generated code Setting: multicentre (37 centres in the USA) Duration: 8 weeks, follow‐up up to 20 weeks | |
| Participants | 233 participants enrolled (BtA 120 U group = 78; BtA 240 U group = 81; placebo group = 74) % Female: BtA 120 U: 51%; BtA 240 U: 54%; placebo: 49% Mean age, SD: BtA 120 U: 52.8 years, 11.5; BtA 240 U: 53.2 years, 12.2; placebo: 52.4 years, 10.8 Mean CD duration: BtA 120 U: 9.3 years, 8.4; BtA 240U: 9.7 years, 9.0; placebo: 10.8 years, 9.0 Mean CD severity, SD (TWSTRS total): BtA 120 U: 42.6, 9.7; BtA 240 U: 42.1, 9.3; placebo: 41.8, 7.9 Inclusion criteria:
Exclusion criteria:
Other medications for focal dystonia were required to be on a stable dose for at least 3 months | |
| Interventions | BtA: Xeomin (incobotulinumtoxinA); 120 U or 240 U, diluted in 4.8 mL Placebo: reconstitution of powder with 0.9% NaCl diluted in 4.8 mL Study drug preparation: vials and providers not mentioned Muscles injected: the number of injection sites per muscle and the volume injected into each muscle were determined at the discretion of the investigator EMG guidance: left at discretion of the investigator BtA dose per participant: 120 U or 240 U | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | Study discontinuations (at week 8), reasons: BtA 120 U: n = 3 (4%), adverse events: n = 1, consent withdrawal: n = 1, lost to F/U: n = 1 BtA 240 U: n = 5 (6%), adverse events: n = 2, consent withdrawal: n = 1, lost to F/U: n = 1, unrelated reasons: n = 1 Placebo: n = 6 (8%), lack of efficacy: n = 3, consent withdrawal: n = 1, lost to F/U: n = 1, unrelated reasons: n = 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using RANCODE version 3.6 (IDV, Gauting). Block‐wise randomization by previous treatment with botulinum toxin ensured a balanced treatment assignment for each center for pretreated and treatment‐naïve patients" |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects, investigators, medical staff, (…) data managers and monitors were blind to subjects' treatment group" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Subjects, investigators, medical staff, biostatisticians responsible for data analysis, data managers and monitors were blind to subjects' treatment group" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was identical to intervention, the fact that most of the participants had previously been treated with Bt could have led to a degree of bias |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Missing subject data was provided and their absence was regarded according to an ITT protocol" Comment: post‐randomisation exclusions were low and roughly distributed evenly between groups (BtA 120 U group = 3; BtA 240 U group = 5; Placebo group = 6). The reasons for exclusions were described. |
| Selective reporting (reporting bias) | Low risk | Comment: the outcomes mentioned in the study protocol matched the outcomes reported in the study. |
| For‐profit bias | High risk | Comment: study funded by Merz Pharmaceuticals GmbH, Frankfurt |
| Enriched population – preferential enrolment of positive responders | High risk | Quote: "A total of 233 subjects were randomized (...) Of these, 143 were previously treated with botulinum toxin" |
| Enriched population – exclusion of poor responders | High risk | Quote: "Subjects were excluded if they had (…) predominant anterocollis or retrocollis" |
| Methods | Randomised, double‐blind, parallel design Randomisation: stratified by CD classification; method not described Setting: single‐centre (USA) Duration: 12 weeks | |
| Participants | 55 participants enrolled (BtA group = 28; placebo group = 27) % Female: BtA: 61%; placebo: 67% Mean age: BtA: 46.8 years; placebo: 52.6 years Mean CD duration: BtA: 6.6 years; placebo: 9.8 years CD severity: BtA: 7% mild, 71% moderate, 21% severe; placebo: 11% mild, 48% moderate, 41% severe Inclusion criteria:
Exclusion criteria:
| |
| Interventions | BtA: Botox (onabotulinumtoxinA); diluted in saline solution to a concentration of 25 U per 1 mL Placebo: saline solution Study drug preparation: BtA provided in vials by Smith‐Kettlewell Eye Research Institute (USA) Muscles injected: the doses, muscles, and number of injected sites per muscle were determined by the physician based on clinical assessment and classification of CD EMG guidance: no BtA dose per participant: 150 U, rotational torticollis and torticollis plus retrocollis; 165 U, head tilt | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | Study discontinuations, reasons: BtA: n = 3 (11%), adverse events: n = 1, unrelated reasons: n = 2 Placebo: n = 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were divided into 3 cells (A, pure rotational torticollis; B, torticollis plus retrocollis; and C, head tilt with or without torticollis and retrocollis). In order to ensure reasonable balance of Botox and placebo injections in each cell, randomization was stratified by cell type, which was completed for blocks of 4 sequentially enrolled patients in each cell type" Comment: insufficient information about the method of randomisation to permit judgement of low or high risk |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The blinded physicians then injected them with Botox or saline, using syringes filled by the unblinded physicians according to the protocol" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Two blinded physicians gave the injections, determined the degree of head turning and disability, and videotaped the patients; but they did not examine the strength or size of the neck muscles, so that the presence of muscle atrophy would not identify patients receiving active injection. Videotapes of each patient visit were rated by the 2 blinded observers independently" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients previously treated with Botox were excluded from the trial" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: the study authors stated that some data were lost, accounting for up to 13% of total data, and it is unclear whether this had an impact on the overall results. |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | Low risk | Study drug was provided by Dr. A. Scott, from Smith‐Kettlewell Eye Research Institute (USA). |
| Enriched population – preferential enrolment of positive responders | Low risk | Comment: participants who had previously received Botox injections were excluded. |
| Enriched population – exclusion of poor responders | Low risk | Comment: Exclusion criteria did not include forms of dystonia known to have poorer response to treatment |
| Methods | Randomised, double‐blind, parallel design Randomisation: not described Setting: multicentre (Germany and Austria) Duration: 8 weeks | |
| Participants | 75 participants enrolled (BtA 250 U group = 19; BtA 500 U group = 18; BtA 1000 U group = 18; placebo group = 20). % Female: all groups: 48% Mean age, SD: all groups: 47 years, 11.5 Mean CD duration, SD: all groups: 7.4 years, 6.7 CD severity (Tsui modified scale): BtA 250 U: 14.3; BtA 500 U: 13.1; BtA 1000 U: 14.5; placebo: 14.4 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | BtA: Dysport (abobotulinumtoxinA); vials of 500 U, diluted with 1 mL sterile solution Placebo: 0.125 mg of human serum albumin and 2.5 mg of lactose, diluted with 1 mL sterile solution Study drug preparation: BtA provided in vials by Speywood Pharmaceuticals Muscles injected: a total of 2.5 mL of the study drug or placebo was injected in each participant (0.75 mL into 2 sites in the sternocleidomastoid muscle, and 1.75 mL into 2 sites in the splenius capitis muscle) EMG guidance: no BtA dose per participant: 250 U, 500 U, or 1000 U | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | Study discontinuations, reasons: BtA 250 U: n = 0 BtA 500 U: n = 2 (11%), lost to F/U: n = 2 BtA 1000 U: n = 0 Placebo: n = 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive treatment with placebo or total dose of 250, 500, or 1000 Dysport units of botulinum toxin type A in a double blind prospective study design" |
| Allocation concealment (selection bias) | Low risk | Comment: sequentially numbered drug containers of identical appearance |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were randomly assigned to receive treatment with placebo or total dose of 250, 500, or 1000 Dysport units of botulinum toxin type A in a double blind prospective study design" Quote: "All three vials were identical in appearance" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information to permit judgement of low risk or high risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information to permit judgement of low risk or high risk |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "One patient in the 500 unit group was lost to follow up and had to be excluded from result analysis. A further case of 500 unit group had missed one follow up visit and was excluded from efficacy analysis but included in analysis of adverse events" |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | High risk | Quote: "Toxin and placebo preparations was supplied by Speywood Pharmaceuticals Ltd" |
| Enriched population – preferential enrolment of positive responders | Low risk | Comment: all participants were previously untreated with botulinum toxin type A |
| Enriched population – exclusion of poor responders | High risk | Quote: "Seventy five patients (...) with rotational torticollis and hyperactivity clinically confined to one splenius capitis and the contralateral sternomastoid muscles" |
| Methods | Randomised, double‐blind, parallel design Randomisation: not adequately described Setting: multicentre (61 centres in 11 countries) Duration: 12 weeks | |
| Participants | 369 participants enrolled overall 213 participants enrolled with data contributing to the current review (BtA group = 159; placebo group = 54) % Female: BtA: 64%; placebo: 63% Mean age: BtA: 49 years; placebo: 50 years Mean CD duration: BtA: 7 years; placebo: 6 years Mean CD severity, SD (TWSTRS‐total): BtA: 46, 9; placebo: 47, 9 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | BtA: Dysport (abobotulinumtoxinA) Placebo: supplied in a 1‐mL prefilled syringe indistinguishable from the active products Study drug preparation: provided as a freeze‐dried powder containing 500 U of BtA haemagglutinin complex together with 125 µg of human albumin and 2.5 mg of lactose. The powder was reconstituted with 1.1 mL sodium chloride for injection using a glass syringe Muscles injected: administered into 2‐4 neck muscles (levator scapulae, trapezius, sternocleidomastoid, splenius capitis, scalenus (medius and anterior), semispinalis capitis, or longissimus capitis) in a single dosing session according to the physicians’ clinical judgment of the individual’s pattern of dystonic activity EMG guidance: left at discretion of the investigator BtA dose per patient: 500 U | |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "To maintain blinding, all study treatments were identical in appearance and smell. All injections during the double‐blind phase were prepared by dedicated and trained site personnel who were independent from investigators and had no contact with the investigators performing study assessment or the trial patients" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "To maintain blinding, all study treatments were identical in appearance and smell. All injections during the double‐blind phase were prepared by dedicated and trained site personnel who were independent from investigators and had no contact with the investigators performing study assessment or the trial patients" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Inclusion of a considerable proportion of non‐naive participants, meaning they may have been able to foresee group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| For‐profit bias | High risk | Quote: "This study was sponsored by Ipsen" |
| Enriched population – preferential enrolment of positive responders | High risk | Inclusion of a considerable proportion of non‐naive participants, meaning they may have been able to foresee group allocation |
| Enriched population – exclusion of poor responders | High risk | Exclusion of nonresponsive phenotypes |
| Methods | Randomised, double‐blind, parallel design Randomisation: Block‐wise randomisation using a software‐generated code, stratification by centre Setting: multicentre (16 centres in USA) Duration: 4 weeks, follow‐up up to 20 weeks | |
| Participants | 80 participants enrolled (BtA group = 37; placebo group = 43) % Female: BtA: 62%; placebo: 63% Mean age, SD: BtA: 53.4 years, 11.6; placebo: 53.6 years, 12.1 Mean CD duration, SD: BtA: 7.1 years, 7.1; placebo: 5.7 years, 5.2 Mean CD severity, SD (TWSTRS total): BtA: 45.1, 8.7; placebo: 46.2, 9.4 Inclusion criteria:
Exclusion criteria:
Medications such as muscle relaxants and benzodiazepines were required to be on a stable dose for ≥ 6 weeks | |
| Interventions | BtA: Dysport (abobotulinumtoxinA); 500 U Placebo: 0.125 mg of human serum albumin and 2.5 mg of lactose Study drug preparation: BtA provided in vials by Ipsen Ltd Muscles injected: the doses and number of injection sites per muscle were determined at the discretion of the investigator. EMG guidance: left at discretion of the investigator BtA dose per participant: 500 U | |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes | Participants who showed no benefit at week 4 were terminated from the study. Those who had evidence of response at week 4 continued in the study until additional injections were needed. Study discontinuations (at week 4), reasons: BtA: n = 15 (41%), reasons not described Placebo: n = 27 (63%), reasons not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was in blocks of four and was stratified by center and according to whether or not the patient had been treated previously with botulinum toxin" Quote: "All patients were randomly assigned to treatment using a randomization code generated before the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "Dysport was provided in a clear glass vial as a freeze dried white pellet (…). Placebo was provided in identical clear glass vials (…)" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Placebo was provided in identical clear glass vials (…). Study medication was supplied in individual patient boxes, containing one vial of either Dysport or placebo. Subjects, investigators, medical staff, (…) data managers and monitors were blind to subjects' treatment group" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Whenever possible, an investigator or research nurse other than the one performing the TWSTRS assessment who was blind to treatment condition performed the assessment for adverse events. All sites were asked to achieve as much consistency as possible with respect to assessors" Comment: insufficient information to permit judgement of low risk or high risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "At each post‐treatment visit, patients and investigators independently assessed the change from baseline" Comment: insufficient information to permit judgement of low risk or high risk. Although placebo was identical to intervention, the fact that most of the participants had previously been treated with Botox could have led to a degree of bias |
| Incomplete outcome data (attrition bias) | High risk | Comment: post‐randomisation exclusions at week 4 were high in both intervention arms, though this difference was asymmetrical, with more dropouts happening in the placebo arm |
| Selective reporting (reporting bias) | Low risk | Comment: the outcomes mentioned in the study protocol matched the outcomes reported in the study |
| For‐profit bias | High risk | Comment: study funded by Beauford Ipsen |
| Enriched population – preferential enrolment of positive responders | High risk | Comment: out of the 80 participants enrolled, 21 were de novo |
| Enriched population – exclusion of poor responders | High risk | Quote: "Patients with pure retrocollis were not permitted to participate" |
| Methods | Randomised, double‐blind, parallel design Randomisation: pre‐generated randomisation code Setting: multicentre (16 centres in USA, 4 in Russia) Duration: 12 weeks | |
| Participants | 116 participants enrolled (BtA group = 55; placebo group = 61) % Female: BtA: 67%; placebo: 62% Mean age, SD: BtA: 51.9, 13.4; placebo: 53.9, 12.5 Mean CD duration, SD: BtA: 12.0 years, 8.8; placebo: 11.8 years, 8.8 Mean CD severity, SD (TWSTRS total): BtA: 43.8, 8.0; placebo: 45.8, 8.8 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | BtA: Dysport (abobotulinumtoxinA) Placebo: not described Study drug preparation: BtA provided in vials by Ipsen Muscles injected: the doses and number of injection sites per muscle were determined at the discretion of the investigator EMG guidance: left at discretion of the investigator BtA dose per participant: 500 U | |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes | Study discontinuations, reasons: BtA: n = 10 (18%), lack of efficacy: n = 5, consent withdrawal: n = 2, lost to F/U: n = 1, unrelated reasons: n = 2 Placebo: n = 23 (38%), lack of efficacy: n = 23 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In the double‐blind treatment phase, patients were randomized using a pre‐generated randomization code to receive intramuscular injection of either 500 units Dysport or placebo (1:1)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Efficacy variables were assessed using intent‐to‐treat (ITT) analysis" Quote: "Safety assessments were based on the safety population, which included all patients who received at least one dose of study medication" Comment: the reasons for discontinuation were described |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | High risk | Quote: "This study was supported by the Ipsen Group. Editorial assistance for the preparation of this manuscript was provided by Ogilvy Healthworld Medical Education; funding was provided by Ipsen Limited, Slough, UK" |
| Enriched population – preferential enrolment of positive responders | High risk | Quote: "Patients were excluded if they had a (...) previous poor response to BoNT‐A or BoNT‐B treatments; current treatment with BoNT‐B due to lack of efficacy of BoNT‐A or the presence of neutralising antibodies to BoNT‐A" |
| Enriched population – exclusion of poor responders | High risk | Quote: "Patients were excluded if they had a diagnosis of pure anterocollis or retrocollis" |
| Methods | Randomised, double‐blind, parallel design Randomisation: method not described Setting: multicentre (Austria and Czech Republic) Duration: 4 weeks, follow‐up up to 16 weeks | |
| Participants | 68 participants enrolled (BtA group = 35; placebo group = 33) % Female: BtA: 46%; placebo: 56% Mean age, SD: BtA: 45.8 years, 13.2; placebo: 49.7 years, 9.6 Mean CD duration, SD: BtA: 6.5 years, 8.0; placebo: 4.8 years, 4.4 Mean CD severity, SD (Tsui scale): BtA: 11.1, 1.7; placebo: 11.5, 1.8 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | BtA: Dysport (abobotulinumtoxinA); 500 U, diluted with 1 mL 0.9% saline solution Placebo: 0.125 mg of human serum albumin and 2.5 mg of lactose, diluted with 1 mL 0.9% saline solution Study drug preparation: BtA and placebo provided in vials by Ipsen Muscles injected: based on clinical assessment 2 or 3 muscles were selected for injection: sternocleidomastoid (100 U‐200 U), splenius capitis (250 U‐350 U), trapezius (100 U‐200 U), and levator scapulae (100 U‐200 U). Each muscle was injected in 2 sites EMG guidance: no BtA dose per participant: 500 U | |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes | Participants were withdrawn from the study if they were considered non‐responders at week 4. Participants with an ongoing response at weeks 4 and 8 continued until re‐treatment was required. Study discontinuations (at week 4), reasons: BtA: n = 0 Placebo: n = 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either placebo or 500 units of Dysport" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Blinded study medication was supplied (...) as identical vials containing either Dysport (...) or placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information to permit judgement of low or high risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was identical to intervention, the fact that most of the participants had previously been treated with Bt could have led to a degree of bias. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "In order to remove the bias created by the withdrawal of the majority of placebo patients at week 4, a last observation carried forward technique was used for the week 8 analyses" |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | High risk | Quote: "Blinded study medication was supplied by Ipsen Ltd" |
| Enriched population – preferential enrolment of positive responders | High risk | Comment: out of the 68 participants enrolled, 47 had received BtA injections previously |
| Enriched population – exclusion of poor responders | High risk | Quote: "Patients with pure anterocollis were excluded" |
Bt: botulinum toxin
BtA: botulinum toxin type A
CD: cervical dystonia
CDSS: Cervical Dystonia Severity Scale
F/U: follow‐up
GAS: Global Assessment Scale
IGAE: Investigator Global Assessment of Efficacy
PEGR: Patient Evaluation of Global Response
TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Cross‐over design | |
| The primary outcome was not clinical; pharmacokinetic study | |
| Cross‐over design | |
| Cross‐over design | |
| This study has recruited part of the same population from Greene 1990; the primary outcome was not clinical | |
| Cross‐over design | |
| Cross‐over design | |
| Not randomised | |
| Cross‐over design | |
| Cross‐over design | |
| Cross‐over design | |
| Not randomised | |
| Cross‐over design | |
| The primary outcome was not clinical | |
| This study has recruited part of the same population from Gelb 1989 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cervical dystonia‐specific improvement Show forest plot | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| Analysis 1.1 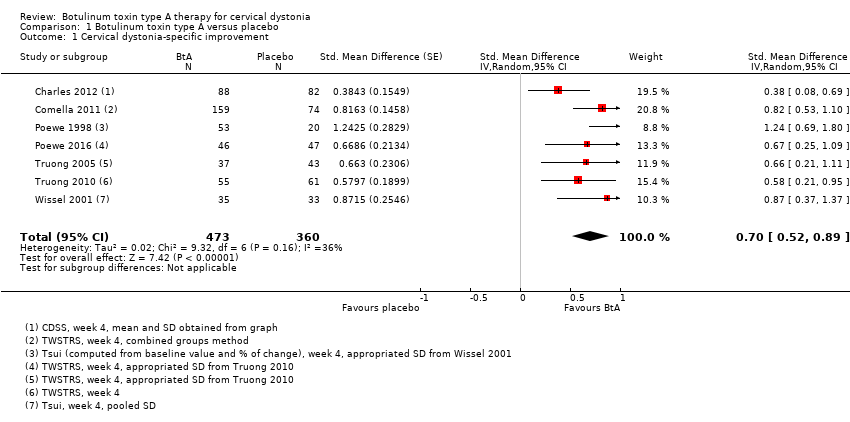 Comparison 1 Botulinum toxin type A versus placebo, Outcome 1 Cervical dystonia‐specific improvement. | ||||
| 2 Cervical dystonia‐specific improvement ‐ TWSTRS subgroup analysis Show forest plot | 4 | 522 | Mean Difference (IV, Random, 95% CI) | 8.06 [6.08, 10.05] |
| Analysis 1.2  Comparison 1 Botulinum toxin type A versus placebo, Outcome 2 Cervical dystonia‐specific improvement ‐ TWSTRS subgroup analysis. | ||||
| 3 Cervical dystonia‐specific severity ‐ as assessed with TWSTRS subscale Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 3.13 [2.15, 4.11] |
| Analysis 1.3 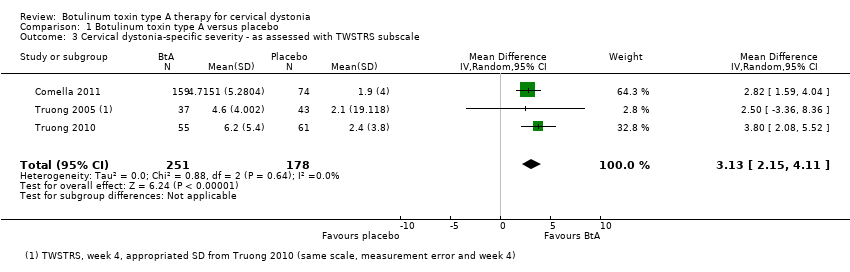 Comparison 1 Botulinum toxin type A versus placebo, Outcome 3 Cervical dystonia‐specific severity ‐ as assessed with TWSTRS subscale. | ||||
| 4 Cervical dystonia‐specific disability ‐ as assessed with TWSTRS subscale Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 2.52 [1.72, 3.31] |
| Analysis 1.4  Comparison 1 Botulinum toxin type A versus placebo, Outcome 4 Cervical dystonia‐specific disability ‐ as assessed with TWSTRS subscale. | ||||
| 5 Cervical dystonia‐specific improvement ‐ doses subgroup analysis Show forest plot | 6 | 777 | Std. Mean Difference (Random, 95% CI) | 0.84 [0.68, 1.00] |
| Analysis 1.5  Comparison 1 Botulinum toxin type A versus placebo, Outcome 5 Cervical dystonia‐specific improvement ‐ doses subgroup analysis. | ||||
| 5.1 Low dose | 1 | 39 | Std. Mean Difference (Random, 95% CI) | 1.24 [0.55, 1.94] |
| 5.2 Medium dose | 6 | 545 | Std. Mean Difference (Random, 95% CI) | 0.76 [0.59, 0.94] |
| 5.3 High dose | 2 | 193 | Std. Mean Difference (Random, 95% CI) | 1.08 [0.53, 1.63] |
| 6 Cervical dystonia‐specific improvement ‐ BtA formulation subgroup analysis Show forest plot | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| Analysis 1.6  Comparison 1 Botulinum toxin type A versus placebo, Outcome 6 Cervical dystonia‐specific improvement ‐ BtA formulation subgroup analysis. | ||||
| 6.1 Botox | 1 | 170 | Std. Mean Difference (Random, 95% CI) | 0.38 [0.08, 0.69] |
| 6.2 Dysport | 5 | 430 | Std. Mean Difference (Random, 95% CI) | 0.75 [0.54, 0.96] |
| 6.3 Xeomin | 1 | 233 | Std. Mean Difference (Random, 95% CI) | 0.82 [0.53, 1.10] |
| 7 Cervical dystonia‐specific improvement ‐ EMG‐guided versus non‐EMG‐guided subgroup analysis Show forest plot | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| Analysis 1.7  Comparison 1 Botulinum toxin type A versus placebo, Outcome 7 Cervical dystonia‐specific improvement ‐ EMG‐guided versus non‐EMG‐guided subgroup analysis. | ||||
| 7.1 EMG‐guided injection | 4 | 522 | Std. Mean Difference (Random, 95% CI) | 0.71 [0.52, 0.89] |
| 7.2 Non‐EMG‐guided injection | 3 | 311 | Std. Mean Difference (Random, 95% CI) | 0.79 [0.27, 1.31] |
| 8 Adverse events Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| Analysis 1.8  Comparison 1 Botulinum toxin type A versus placebo, Outcome 8 Adverse events. | ||||
| 9 Adverse events ‐ doses subgroup analysis Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Botulinum toxin type A versus placebo, Outcome 9 Adverse events ‐ doses subgroup analysis. | ||||
| 9.1 Low dose | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.56, 3.85] |
| 9.2 Medium dose | 6 | 664 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.06, 1.44] |
| 9.3 High dose | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.72, 5.02] |
| 10 Adverse events ‐ BtA formulation subgroup analysis Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| Analysis 1.10  Comparison 1 Botulinum toxin type A versus placebo, Outcome 10 Adverse events ‐ BtA formulation subgroup analysis. | ||||
| 10.1 Botox | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.30] |
| 10.2 Dysport | 5 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.02, 1.66] |
| 10.3 Xeomin | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.62] |
| 11 Adverse events ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| Analysis 1.11 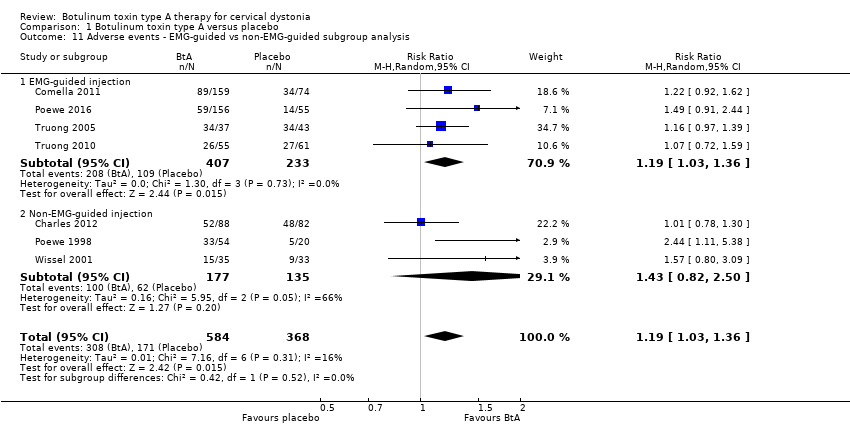 Comparison 1 Botulinum toxin type A versus placebo, Outcome 11 Adverse events ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis. | ||||
| 11.1 EMG‐guided injection | 4 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 11.2 Non‐EMG‐guided injection | 3 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.82, 2.50] |
| 12 Dysphagia Show forest plot | 8 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [1.68, 5.50] |
| Analysis 1.12 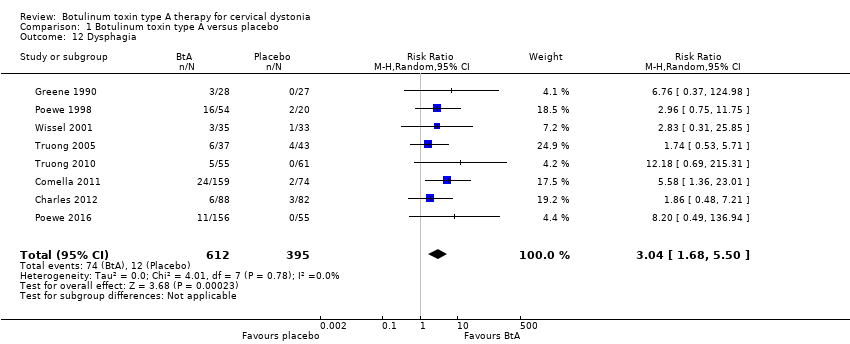 Comparison 1 Botulinum toxin type A versus placebo, Outcome 12 Dysphagia. | ||||
| 13 Diffuse weakness/tiredness Show forest plot | 6 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.08, 2.94] |
| Analysis 1.13  Comparison 1 Botulinum toxin type A versus placebo, Outcome 13 Diffuse weakness/tiredness. | ||||
| 14 Neck weakness Show forest plot | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [0.95, 10.91] |
| Analysis 1.14 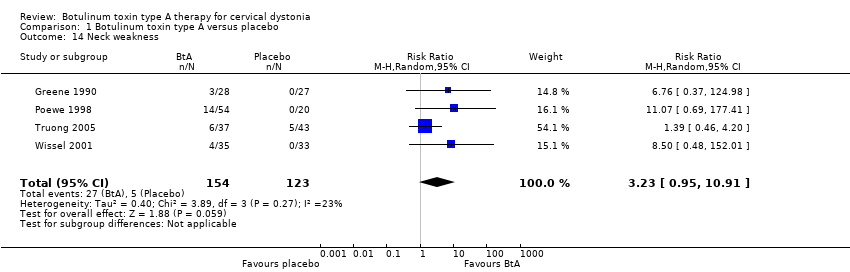 Comparison 1 Botulinum toxin type A versus placebo, Outcome 14 Neck weakness. | ||||
| 15 Voice change/hoarseness Show forest plot | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.37, 8.95] |
| Analysis 1.15  Comparison 1 Botulinum toxin type A versus placebo, Outcome 15 Voice change/hoarseness. | ||||
| 16 Sore throat/dry mouth Show forest plot | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.78, 3.51] |
| Analysis 1.16 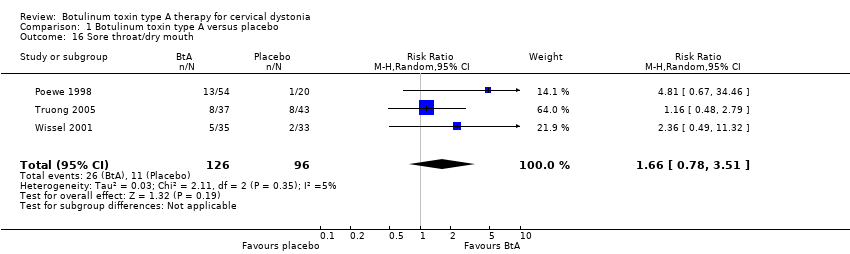 Comparison 1 Botulinum toxin type A versus placebo, Outcome 16 Sore throat/dry mouth. | ||||
| 17 Vertigo/dizziness Show forest plot | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.38, 5.73] |
| Analysis 1.17 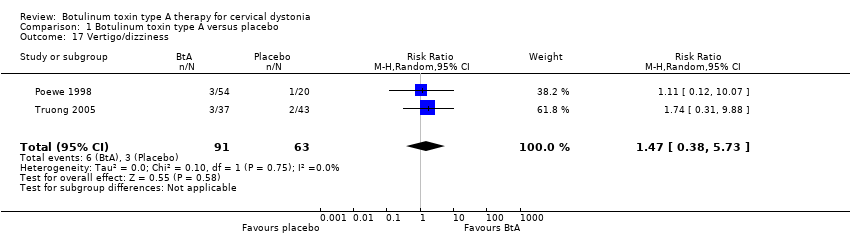 Comparison 1 Botulinum toxin type A versus placebo, Outcome 17 Vertigo/dizziness. | ||||
| 18 Malaise/upper respiratory infection Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.63, 2.64] |
| Analysis 1.18  Comparison 1 Botulinum toxin type A versus placebo, Outcome 18 Malaise/upper respiratory infection. | ||||
| 19 Local pain (injection site) Show forest plot | 7 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.88, 2.02] |
| Analysis 1.19 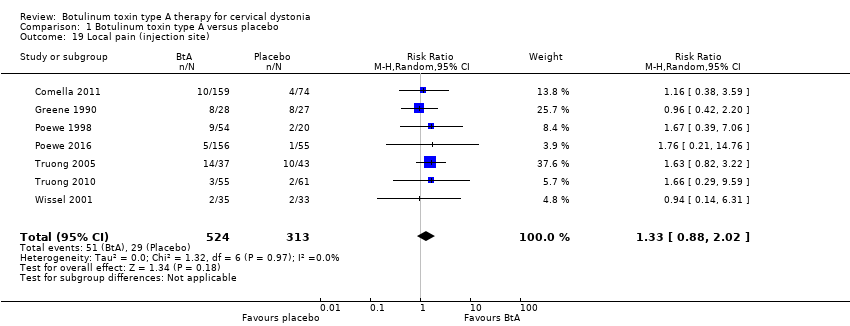 Comparison 1 Botulinum toxin type A versus placebo, Outcome 19 Local pain (injection site). | ||||
| 20 Headache Show forest plot | 6 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.59, 1.86] |
| Analysis 1.20 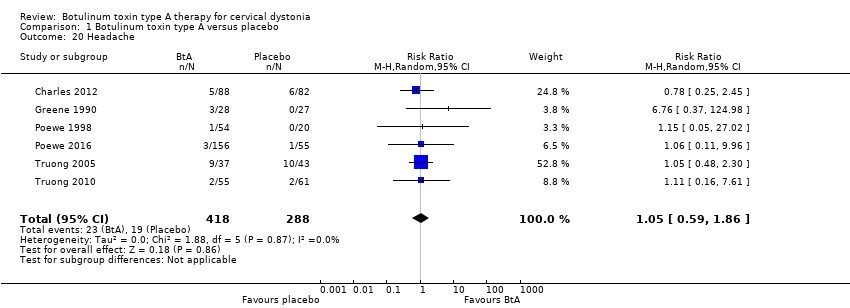 Comparison 1 Botulinum toxin type A versus placebo, Outcome 20 Headache. | ||||
| 21 Any improvement by subjective clinician assessment Show forest plot | 4 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.47, 2.49] |
| Analysis 1.21  Comparison 1 Botulinum toxin type A versus placebo, Outcome 21 Any improvement by subjective clinician assessment. | ||||
| 22 Any improvement by subjective participant assessment Show forest plot | 5 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.83, 2.90] |
| Analysis 1.22 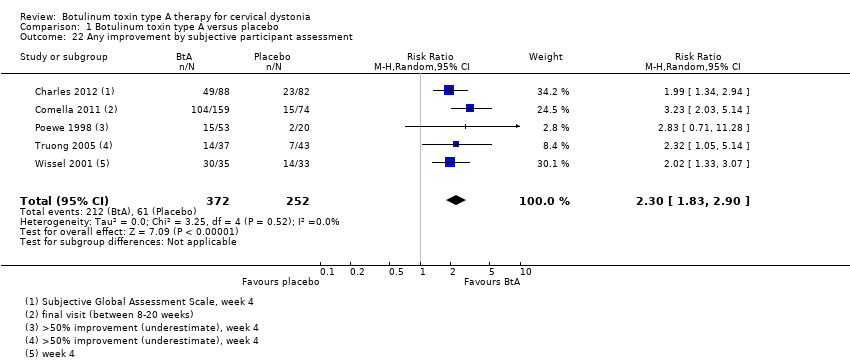 Comparison 1 Botulinum toxin type A versus placebo, Outcome 22 Any improvement by subjective participant assessment. | ||||
| 23 Any improvement by subjective participant assessment ‐ doses subgroup analysis Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.23  Comparison 1 Botulinum toxin type A versus placebo, Outcome 23 Any improvement by subjective participant assessment ‐ doses subgroup analysis. | ||||
| 23.1 Low dose | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.30, 8.43] |
| 23.2 Medium dose | 4 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.82, 3.25] |
| 23.3 High dose | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 3.39 [2.16, 5.33] |
| 24 Any improvement by subjective participant assessment ‐ BtA formulation subgroup analysis Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.24 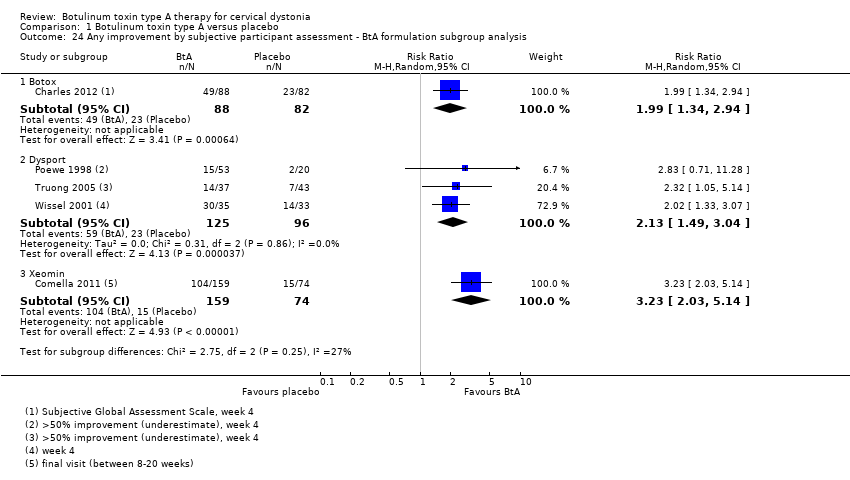 Comparison 1 Botulinum toxin type A versus placebo, Outcome 24 Any improvement by subjective participant assessment ‐ BtA formulation subgroup analysis. | ||||
| 24.1 Botox | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.34, 2.94] |
| 24.2 Dysport | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.49, 3.04] |
| 24.3 Xeomin | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [2.03, 5.14] |
| 25 Any improvement by subjective participant assessment ‐ EMG guided vs non‐EMG‐guided subgroup analysis Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.25 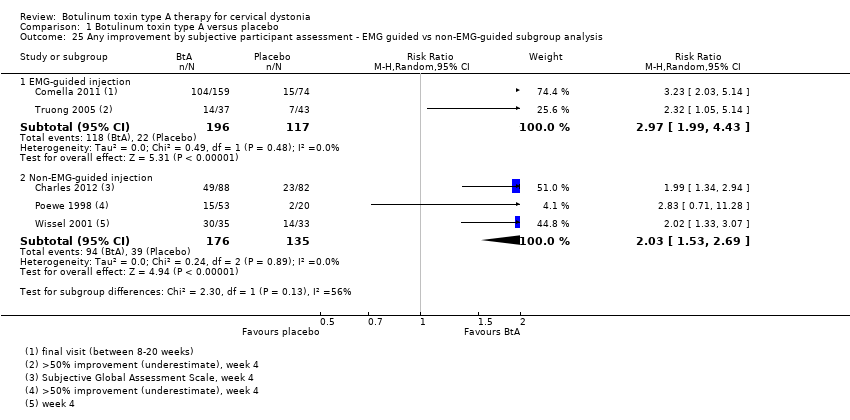 Comparison 1 Botulinum toxin type A versus placebo, Outcome 25 Any improvement by subjective participant assessment ‐ EMG guided vs non‐EMG‐guided subgroup analysis. | ||||
| 25.1 EMG‐guided injection | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [1.99, 4.43] |
| 25.2 Non‐EMG‐guided injection | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.53, 2.69] |
| 26 Cervical dystonia‐specific pain Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] | |
| Analysis 1.26  Comparison 1 Botulinum toxin type A versus placebo, Outcome 26 Cervical dystonia‐specific pain. | ||||
| 27 Cervical dystonia‐specific pain ‐ TWSTRS pain subscale subgroup analysis Show forest plot | 3 | Mean Difference (Random, 95% CI) | 2.11 [1.38, 2.83] | |
| Analysis 1.27  Comparison 1 Botulinum toxin type A versus placebo, Outcome 27 Cervical dystonia‐specific pain ‐ TWSTRS pain subscale subgroup analysis. | ||||
| 28 Cervical dystonia‐specific pain ‐ BtA formulation subgroup analysis Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] | |
| Analysis 1.28 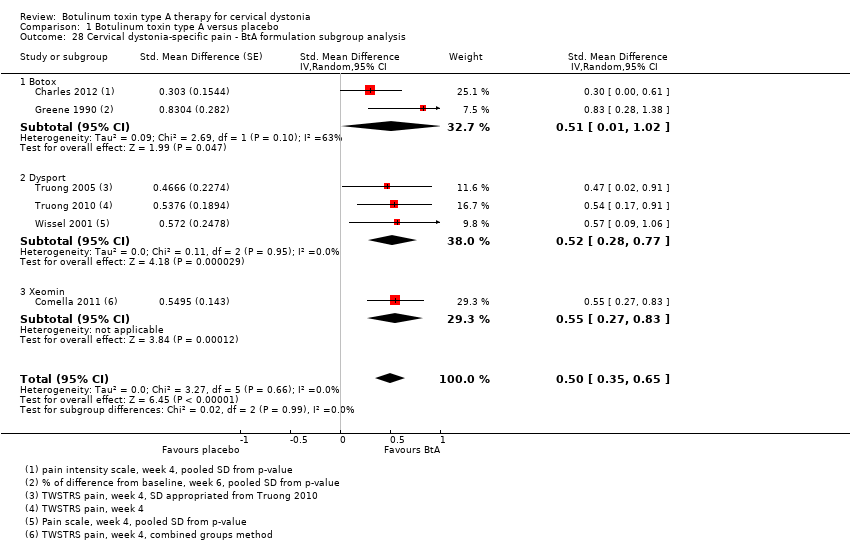 Comparison 1 Botulinum toxin type A versus placebo, Outcome 28 Cervical dystonia‐specific pain ‐ BtA formulation subgroup analysis. | ||||
| 28.1 Botox | 2 | Std. Mean Difference (Random, 95% CI) | 0.51 [0.01, 1.02] | |
| 28.2 Dysport | 3 | Std. Mean Difference (Random, 95% CI) | 0.52 [0.28, 0.77] | |
| 28.3 Xeomin | 1 | Std. Mean Difference (Random, 95% CI) | 0.55 [0.27, 0.83] | |
| 29 Cervical dystonia‐specific pain ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis Show forest plot | 6 | 654 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] |
| Analysis 1.29 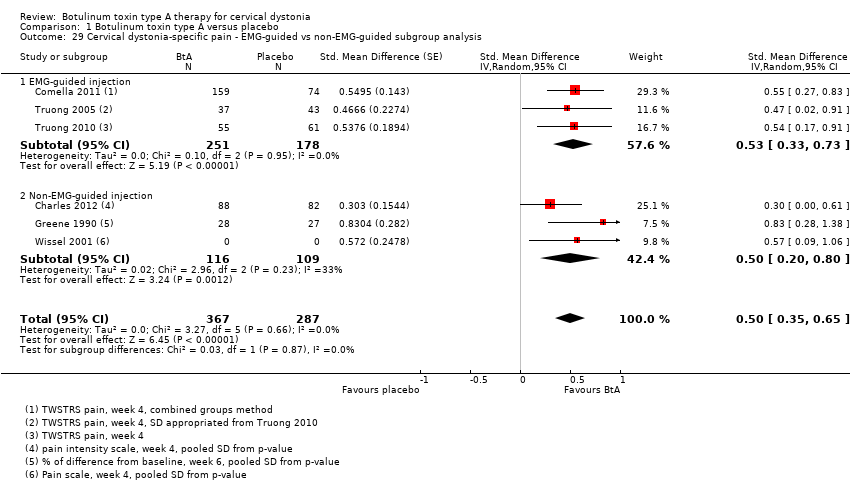 Comparison 1 Botulinum toxin type A versus placebo, Outcome 29 Cervical dystonia‐specific pain ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis. | ||||
| 29.1 EMG‐guided injection | 3 | 429 | Std. Mean Difference (Random, 95% CI) | 0.53 [0.33, 0.73] |
| 29.2 Non‐EMG‐guided injection | 3 | 225 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.20, 0.80] |
| 30 Tolerability ‐ withdrawals Show forest plot | 4 | 574 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.23, 0.62] |
| Analysis 1.30  Comparison 1 Botulinum toxin type A versus placebo, Outcome 30 Tolerability ‐ withdrawals. | ||||
| 31 Tolerability ‐ withdrawals due lack of efficacy subgroup analysis Show forest plot | 3 | 519 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.17, 0.53] |
| Analysis 1.31  Comparison 1 Botulinum toxin type A versus placebo, Outcome 31 Tolerability ‐ withdrawals due lack of efficacy subgroup analysis. | ||||
| 32 Tolerability ‐ withdrawals due to adverse events subgroup analysis Show forest plot | 2 | 288 | Risk Ratio (IV, Random, 95% CI) | 3.10 [0.36, 26.74] |
| Analysis 1.32  Comparison 1 Botulinum toxin type A versus placebo, Outcome 32 Tolerability ‐ withdrawals due to adverse events subgroup analysis. | ||||
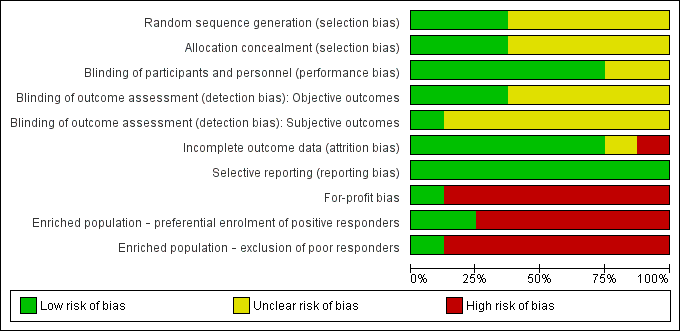
Risk of bias of included studies: review authors' judgements about each risk of bias item presented as percentages across all included studies
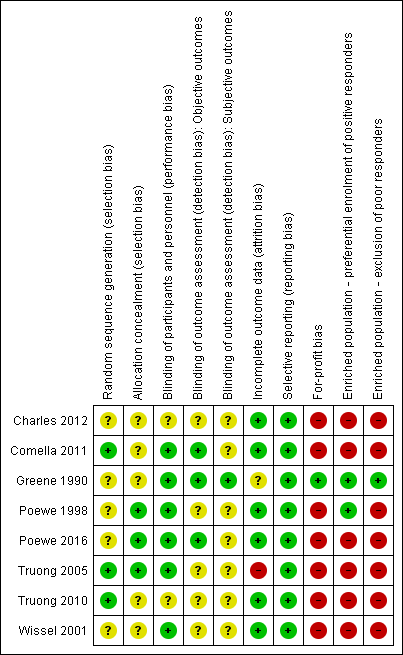
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Botulinum toxin type A versus placebo, Outcome 1 Cervical dystonia‐specific improvement.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 2 Cervical dystonia‐specific improvement ‐ TWSTRS subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 3 Cervical dystonia‐specific severity ‐ as assessed with TWSTRS subscale.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 4 Cervical dystonia‐specific disability ‐ as assessed with TWSTRS subscale.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 5 Cervical dystonia‐specific improvement ‐ doses subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 6 Cervical dystonia‐specific improvement ‐ BtA formulation subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 7 Cervical dystonia‐specific improvement ‐ EMG‐guided versus non‐EMG‐guided subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 8 Adverse events.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 9 Adverse events ‐ doses subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 10 Adverse events ‐ BtA formulation subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 11 Adverse events ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 12 Dysphagia.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 13 Diffuse weakness/tiredness.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 14 Neck weakness.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 15 Voice change/hoarseness.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 16 Sore throat/dry mouth.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 17 Vertigo/dizziness.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 18 Malaise/upper respiratory infection.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 19 Local pain (injection site).

Comparison 1 Botulinum toxin type A versus placebo, Outcome 20 Headache.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 21 Any improvement by subjective clinician assessment.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 22 Any improvement by subjective participant assessment.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 23 Any improvement by subjective participant assessment ‐ doses subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 24 Any improvement by subjective participant assessment ‐ BtA formulation subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 25 Any improvement by subjective participant assessment ‐ EMG guided vs non‐EMG‐guided subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 26 Cervical dystonia‐specific pain.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 27 Cervical dystonia‐specific pain ‐ TWSTRS pain subscale subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 28 Cervical dystonia‐specific pain ‐ BtA formulation subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 29 Cervical dystonia‐specific pain ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 30 Tolerability ‐ withdrawals.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 31 Tolerability ‐ withdrawals due lack of efficacy subgroup analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 32 Tolerability ‐ withdrawals due to adverse events subgroup analysis.
| Botulinum toxin type A compared to placebo for cervical dystonia | ||||||
| Patient or population: adults with cervical dystonia | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Certainty in the evidence | What happens | ||
| With placebo | With botulinum toxin type A | Difference | ||||
| Cervical dystonia‐specific impairment Assessed 3 to 6 weeks post‐injection using TWSTRS total score | ‐ | 3.9 TWSTRS units decrease | 12.45 TWSTRS units decrease | The mean change from baseline was 8.06 TWSTRS units higher (6.08 higher to 10.05 higher) in the BtA group compared to the placebo group | ⊕⊕⊕⊝ | BtA treatment probably improves cervical dystonia‐specific impairment |
| Adverse events Assessed at any point during follow‐up | RR 1.19 | 46.5% | 55.3% | 8.8% more | ⊕⊕⊕⊝ | BtA treatment probably increases the risk of adverse events |
| Subjective participant assessment Assessed 3 to 6 weeks post‐injection | RR 2.30 | 24.2% | 55.7% | 31.5% more | ⊕⊕⊕⊝ | BtA treatment probably increases the likelihood that patients will detect any form of improvement |
| Pain relief Assessed 3 to 6 weeks post‐injection using TWSTRS pain subscore | ‐b | ‐b | ‐b | The mean change from baseline was 2.11 TWSTRS units higher (1.38 higher to 2.83 higher) in the BtA group compared to the placebo group | ⊕⊕⊕⊝ | BtA treatment probably improves cervical dystonia‐related pain |
| Tolerability Assessed at any point during follow‐up | RR 0.38 | 20.5% | 7.8% (4.7 to 12.7) | 12.7% fewer (15.8 to 7.8) | ⊕⊕⊕⊝ | BtA treatment probably slightly decreases the risk of withdrawal of clinical trials |
| Dysphagia Assessed at any point during follow‐up | RR 3.04 | 3.0% | 9.2% | 6.2% more | ⊕⊕⊕⊝ | BtA treatment probably increases the risk of dysphagia |
| Diffuse weakness/tiredness Assessed at any point during follow‐up | RR 1.78 | 5.6% | 10.1% | 4.4% more | ⊕⊕⊕⊝ | BtA treatment probably increases the risk of diffuse weakness/tiredness |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded one level due to serious study limitations, namely concerns with randomisation procedures and other biases such as 'for‐profit' bias. | ||||||
| Term | Definition |
| BtA‐non‐responsive | People who do not experience the expected benefit from treatment with botulinum toxin type A |
| Cervical dystonia or spasmodic torticollis | A common movement disorder in which people have abnormal movements or postures of the head and neck that they cannot control. It is frequently accompanied by social embarrassment and pain. |
| Chemodenervation | The process by which botulinum toxin causes muscular paralysis. Although all the anatomical elements necessary for muscular control are intact (i.e. nerve, synapse and muscle), there is a chemical process that disables the transmission of the electrical signal from the nerve to the muscle. |
| Dysphagia | A discomfort or difficulty when swallowing. |
| Electromyography | An examination that displays the electrical activity of muscles using pieces of metal attached to the skin or inserted into the muscle. |
| Non‐naive | People who have been treated in the past with botulinum toxin. |
| Voluntary action | Movements that people are able to control, start and stop when they want to. |
| Study ID | Number of participants | Total dropouts | Age mean, range (years) | Baseline CD impairment BtA/placebo | % participants naive to Bt | BtA formulation | Total dose per participant | EMG‐guidance | Study duration (weeks) |
| Charles 2012 | 170 | 35 (11 in BtA) | 55, 31‐76 | 9.2/9.3 (CDSS) | 0 | Botox (OnaBtA) | 236 | No | 10 |
| Comella 2011 | 233 | 14 (8 in BtA) | 53 | 42.4/41.8 (TWSTRS) | 39 | Xeomin (IncoBtA) | 120 or 240 | At discretion | 20 |
| Greene 1990 | 55 | 3 (3 in BtA) | 50 | 21% severe/ 41% severe | 100 | Botox (OnaBtA) | 150 to 165 | No | 12 |
| Poewe 1998 | 75 | 2 (2 in BtA) | 47, 26‐82 | 13.9/14.4 (Tsui scale) | 100 | Dysport (AboBtA) | 250, 500 or 1000 | No | 8 |
| Poewe 2016 | 213 | N/A | 49 | 46/47 (TWSTRS) | 10 | Dysport (AboBtA) | 500 | N/A | 12 |
| Truong 2005 | 80 | 56 (22 in BtA) | 54, 27‐78 | 45.1/46.2 (TWSTRS) | 26 | Dysport (AboBtA) | 500 | At discretion | 20 |
| Truong 2010 | 116 | 33 (10 in BtA) | 53, 20‐79 | 43.8/45.8 (TWSTRS) | 17 | Dysport (AboBtA) | 500 | At discretion | 12 |
| Wissel 2001 | 68 | 0 | 48, 18‐75 | 11.1/11.5 (Tsui scale) | 31 | Dysport (AboBtA) | 500 | No | 16 |
| Bt: botulinum toxin; CD: cervical dystonia; CDSS: Cervical Dystonia Severity Scale; EMG: electromyography; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cervical dystonia‐specific improvement Show forest plot | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| 2 Cervical dystonia‐specific improvement ‐ TWSTRS subgroup analysis Show forest plot | 4 | 522 | Mean Difference (IV, Random, 95% CI) | 8.06 [6.08, 10.05] |
| 3 Cervical dystonia‐specific severity ‐ as assessed with TWSTRS subscale Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 3.13 [2.15, 4.11] |
| 4 Cervical dystonia‐specific disability ‐ as assessed with TWSTRS subscale Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 2.52 [1.72, 3.31] |
| 5 Cervical dystonia‐specific improvement ‐ doses subgroup analysis Show forest plot | 6 | 777 | Std. Mean Difference (Random, 95% CI) | 0.84 [0.68, 1.00] |
| 5.1 Low dose | 1 | 39 | Std. Mean Difference (Random, 95% CI) | 1.24 [0.55, 1.94] |
| 5.2 Medium dose | 6 | 545 | Std. Mean Difference (Random, 95% CI) | 0.76 [0.59, 0.94] |
| 5.3 High dose | 2 | 193 | Std. Mean Difference (Random, 95% CI) | 1.08 [0.53, 1.63] |
| 6 Cervical dystonia‐specific improvement ‐ BtA formulation subgroup analysis Show forest plot | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| 6.1 Botox | 1 | 170 | Std. Mean Difference (Random, 95% CI) | 0.38 [0.08, 0.69] |
| 6.2 Dysport | 5 | 430 | Std. Mean Difference (Random, 95% CI) | 0.75 [0.54, 0.96] |
| 6.3 Xeomin | 1 | 233 | Std. Mean Difference (Random, 95% CI) | 0.82 [0.53, 1.10] |
| 7 Cervical dystonia‐specific improvement ‐ EMG‐guided versus non‐EMG‐guided subgroup analysis Show forest plot | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| 7.1 EMG‐guided injection | 4 | 522 | Std. Mean Difference (Random, 95% CI) | 0.71 [0.52, 0.89] |
| 7.2 Non‐EMG‐guided injection | 3 | 311 | Std. Mean Difference (Random, 95% CI) | 0.79 [0.27, 1.31] |
| 8 Adverse events Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 9 Adverse events ‐ doses subgroup analysis Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Low dose | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.56, 3.85] |
| 9.2 Medium dose | 6 | 664 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.06, 1.44] |
| 9.3 High dose | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.72, 5.02] |
| 10 Adverse events ‐ BtA formulation subgroup analysis Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 10.1 Botox | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.30] |
| 10.2 Dysport | 5 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.02, 1.66] |
| 10.3 Xeomin | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.62] |
| 11 Adverse events ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 11.1 EMG‐guided injection | 4 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 11.2 Non‐EMG‐guided injection | 3 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.82, 2.50] |
| 12 Dysphagia Show forest plot | 8 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [1.68, 5.50] |
| 13 Diffuse weakness/tiredness Show forest plot | 6 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.08, 2.94] |
| 14 Neck weakness Show forest plot | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [0.95, 10.91] |
| 15 Voice change/hoarseness Show forest plot | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.37, 8.95] |
| 16 Sore throat/dry mouth Show forest plot | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.78, 3.51] |
| 17 Vertigo/dizziness Show forest plot | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.38, 5.73] |
| 18 Malaise/upper respiratory infection Show forest plot | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.63, 2.64] |
| 19 Local pain (injection site) Show forest plot | 7 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.88, 2.02] |
| 20 Headache Show forest plot | 6 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.59, 1.86] |
| 21 Any improvement by subjective clinician assessment Show forest plot | 4 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.47, 2.49] |
| 22 Any improvement by subjective participant assessment Show forest plot | 5 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.83, 2.90] |
| 23 Any improvement by subjective participant assessment ‐ doses subgroup analysis Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 Low dose | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.30, 8.43] |
| 23.2 Medium dose | 4 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.82, 3.25] |
| 23.3 High dose | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 3.39 [2.16, 5.33] |
| 24 Any improvement by subjective participant assessment ‐ BtA formulation subgroup analysis Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 Botox | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.34, 2.94] |
| 24.2 Dysport | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.49, 3.04] |
| 24.3 Xeomin | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [2.03, 5.14] |
| 25 Any improvement by subjective participant assessment ‐ EMG guided vs non‐EMG‐guided subgroup analysis Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.1 EMG‐guided injection | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [1.99, 4.43] |
| 25.2 Non‐EMG‐guided injection | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.53, 2.69] |
| 26 Cervical dystonia‐specific pain Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] | |
| 27 Cervical dystonia‐specific pain ‐ TWSTRS pain subscale subgroup analysis Show forest plot | 3 | Mean Difference (Random, 95% CI) | 2.11 [1.38, 2.83] | |
| 28 Cervical dystonia‐specific pain ‐ BtA formulation subgroup analysis Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] | |
| 28.1 Botox | 2 | Std. Mean Difference (Random, 95% CI) | 0.51 [0.01, 1.02] | |
| 28.2 Dysport | 3 | Std. Mean Difference (Random, 95% CI) | 0.52 [0.28, 0.77] | |
| 28.3 Xeomin | 1 | Std. Mean Difference (Random, 95% CI) | 0.55 [0.27, 0.83] | |
| 29 Cervical dystonia‐specific pain ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis Show forest plot | 6 | 654 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] |
| 29.1 EMG‐guided injection | 3 | 429 | Std. Mean Difference (Random, 95% CI) | 0.53 [0.33, 0.73] |
| 29.2 Non‐EMG‐guided injection | 3 | 225 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.20, 0.80] |
| 30 Tolerability ‐ withdrawals Show forest plot | 4 | 574 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.23, 0.62] |
| 31 Tolerability ‐ withdrawals due lack of efficacy subgroup analysis Show forest plot | 3 | 519 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.17, 0.53] |
| 32 Tolerability ‐ withdrawals due to adverse events subgroup analysis Show forest plot | 2 | 288 | Risk Ratio (IV, Random, 95% CI) | 3.10 [0.36, 26.74] |


