Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb
Abstract
Background
Standard treatment for deep vein thrombosis (DVT) aims to reduce immediate complications. Use of thrombolytic clot removal strategies (i.e. thrombolysis (clot dissolving drugs), with or without additional endovascular techniques), could reduce the long‐term complications of post‐thrombotic syndrome (PTS) including pain, swelling, skin discolouration, or venous ulceration in the affected leg. This is the fourth update of a Cochrane Review first published in 2004.
Objectives
To assess the effects of thrombolytic clot removal strategies and anticoagulation compared to anticoagulation alone for the management of people with acute deep vein thrombosis (DVT) of the lower limb.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and AMED and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registries to 21 April 2020. We also checked the references of relevant articles to identify additional studies.
Selection criteria
We considered randomised controlled trials (RCTs) examining thrombolysis (with or without adjunctive clot removal strategies) and anticoagulation versus anticoagulation alone for acute DVT.
Data collection and analysis
We used standard methodological procedures as recommended by Cochrane. We assessed the risk of bias in included trials with the Cochrane 'Risk of bias' tool. Certainty of the evidence was evaluated using GRADE. For dichotomous outcomes, we calculated the risk ratio (RR) with the corresponding 95% confidence interval (CI). We pooled data using a fixed‐effect model, unless we identified heterogeneity, in which case we used a random‐effects model. The primary outcomes of interest were clot lysis, bleeding and post thrombotic syndrome.
Main results
Two new studies were added for this update. Therefore, the review now includes a total of 19 RCTs, with 1943 participants. These studies differed with respect to the thrombolytic agent, the doses of the agent and the techniques used to deliver the agent. Systemic, loco‐regional and catheter‐directed thrombolysis (CDT) strategies were all included. For this update, CDT interventions also included those involving pharmacomechanical thrombolysis. Three of the 19 included studies reported one or more domain at high risk of bias. We combined the results as any (all) thrombolysis interventions compared to standard anticoagulation.
Complete clot lysis occurred more frequently in the thrombolysis group at early follow‐up (RR 4.75; 95% CI 1.83 to 12.33; 592 participants; eight studies) and at intermediate follow‐up (RR 2.42; 95% CI 1.42 to 4.12; 654 participants; seven studies; moderate‐certainty evidence). Two studies reported on clot lysis at late follow‐up with no clear benefit from thrombolysis seen at this time point (RR 3.25, 95% CI 0.17 to 62.63; two studies). No differences between strategies (e.g. systemic, loco‐regional and CDT) were detected by subgroup analysis at any of these time points (tests for subgroup differences: P = 0.41, P = 0.37 and P = 0.06 respectively).
Those receiving thrombolysis had increased bleeding complications (6.7% versus 2.2%) (RR 2.45, 95% CI 1.58 to 3.78; 1943 participants, 19 studies; moderate‐certainty evidence). No differences between strategies were detected by subgroup analysis (P = 0.25).
Up to five years after treatment, slightly fewer cases of PTS occurred in those receiving thrombolysis; 50% compared with 53% in the standard anticoagulation (RR 0.78, 95% CI 0.66 to 0.93; 1393 participants, six studies; moderate‐certainty evidence). This was still observed at late follow‐up (beyond five years) in two studies (RR 0.56, 95% CI 0.43 to 0.73; 211 participants; moderate‐certainty evidence).
We used subgroup analysis to investigate if the level of DVT (iliofemoral, femoropopliteal or non‐specified) had an effect on the incidence of PTS. No benefit of thrombolysis was seen for either iliofemoral or femoropopliteal DVT (six studies; test for subgroup differences: P = 0.29). Systemic thrombolysis and CDT had similar levels of effectiveness. Studies of CDT included four trials in femoral and iliofemoral DVT, and results from these are consistent with those from trials of systemic thrombolysis in DVT at other levels of occlusion.
Authors' conclusions
Complete clot lysis occurred more frequently after thrombolysis (with or without additional clot removal strategies) and PTS incidence was slightly reduced. Bleeding complications also increased with thrombolysis, but this risk has decreased over time with the use of stricter exclusion criteria of studies. Evidence suggests that systemic administration of thrombolytics and CDT have similar effectiveness. Using GRADE, we judged the evidence to be of moderate‐certainty, due to many trials having small numbers of participants or events, or both. Future studies are needed to investigate treatment regimes in terms of agent, dose and adjunctive clot removal methods; prioritising patient‐important outcomes, including PTS and quality of life, to aid clinical decision making.
PICOs
Plain language summary
Thrombolysis for treatment of acute deep vein thrombosis
Background
Deep vein thrombosis (DVT) occurs when a blood clot forms in a leg vein. The clot can break up and move to the lungs, leading to a potentially serious blockage in blood flow (pulmonary embolism or PE). Because of the damage to the leg vein, post‐thrombotic syndrome (PTS) may develop any time over the next couple of years. Symptoms include leg pain, swelling, skin pigmentation and leg ulcers, leading to loss of mobility. Anticoagulants are the standard treatment for DVT or a clot in a leg vein. These medications thin the blood to reduce further clots from forming and prevent PE; yet PTS can still develop. Another way of treating DVT is by thrombolysis. Thrombolysis breaks down the blood clot, and drugs such as streptokinase, urokinase and tissue plasminogen activator are infused into a vein in the arm or foot. In some cases, these drugs may be directly delivered to the site of the clot, using a catheter and X‐ray control. Additional surgical techniques can also be used to help remove the clot. Possible harmful side effects that can happen after both anticoagulation and thrombolysis include bleeding complications, stroke or intracerebral haemorrhage.
To find out whether thrombolytic clot removal strategies and anticoagulation might be better than anticoagulation alone for the management of people with acute DVT of the leg, we reviewed the evidence from research studies.
How did we identify and evaluate the evidence?
First, we searched the medical literature for randomised controlled trials (RCTs), clinical studies where people are randomly put into one of two or more treatment groups. This type of study provides the most robust evidence about the effects of a treatment. We then compared the results, and summarised the evidence from all the studies. Finally, we assessed how certain the evidence was. To do this, we considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we categorised the evidence as being of very low, low, moderate or high certainty.
What did we find?
We found 19 RCTs that included a total of 1943 people with acute DVT to receive either thrombolysis or anticoagulant treatment. Trials were conducted in Belgium, Canada, Denmark, Egypt, France, Germany, the Netherlands, Norway, South Africa, Sweden, Switzerland, Turkey, the UK and the USA. All trials included men and women ranging in age from 18 to 75 years, with more older adults.
Our review found moderate‐certainty evidence that thrombolysis effectively dissolved the clot so that complete clot breakdown occurred more often with thrombolysis than with standard anticoagulant therapy. Those receiving thrombolysis had more bleeding complications than with standard anticoagulation (6.7% versus 2.2%). Most bleeding episodes occurred in the older studies. Six trials (1393 participants) continued for over six months and found that slightly fewer people developed PTS when treated with thrombolysis; 50% compared with 53% in the standard anticoagulation treatment group. Two trials (211 participants) that continued for over five years showed that fewer people developed PTS when treated with thrombolysis. Use of strict eligibility criteria appears to have improved the safety of this treatment, which is effective delivered directly to the clot by catheter or via the bloodstream from another vein. We did not find any evidence that the position of the clot within the leg made it more or less likely for people to get PTS. Future studies are needed to investigate what clot removal method is most beneficial to patient important outcomes including PTS, bleeding and quality of life.
How up‐to date is this review?
The evidence in this Cochrane Review is current to 21 April 2020.
Authors' conclusions
Summary of findings
| Treatment with any thrombolysis clot removal strategy for acute DVT | ||||||
| Patient or population: patients diagnosed with acute DVT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard anticoagulation | Risk with thrombolysis | |||||
| Complete clot lysis (intermediate, 6 months to under 5 years after treatment) | Study population | RR 2.42 (1.42 to 4.12) | 654 | ⊕⊕⊕⊝ | 78 of 244 patients treated with standard anticoagulation had complete clot lysis compared to 198 of 410 in the thrombolysis group | |
| 320 per 1000 | 774 per 1000 (454 to 1000) | |||||
| Bleeding (early, up to 1 month after treatment) | Study population | RR 2.45 | 1943 | ⊕⊕⊕⊝ | ||
| 23 per 1000 | 56 per 1000 (36 to 87) | |||||
| Post‐thrombotic syndrome (intermediate, 6 months to under 5 years after treatment) | Study population | RR 0.78 | 1393 | ⊕⊕⊕⊝ | 329 of 622 patients treated with standard anticoagulation developed PTS compared to 383 of 771 treated with thrombolysis. Additional subgroup analysis did not show any differences in PTS incidence between iliofemoral and femoropopliteal DVTs | |
| 529 per 1000 | 413 per 1000 (349 to 492) | |||||
| Post‐thrombotic syndrome (late, 5 years follow‐up after treatment) | Study population | RR 0.56 | 211 | ⊕⊕⊕⊝ | 75 of 107 patients treated with standard anticoagulation developed PTS compared to 41 of 104 treated with thrombolysis | |
| 701 per 1000 | 393 per 1000 (301 to 512) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Thrombolysis includes delivery of thrombolytics either systemically, loco‐regionally or by CDT. CDT may include the use of additional endovascular techniques | ||||||
Background
Description of the condition
Deep vein thrombosis (DVT) is a major health problem, with between 2.5% to 5% of the population affected at some time in their lives (Browse 1999; White 2006). Its main complications are pulmonary embolism (PE) in the short term and post‐thrombotic syndrome (PTS) in the long term. Standard treatment is with anticoagulation (thinning the blood to reduce formation of further clots) and is aimed mainly at the prevention of PE and recurrent DVT (Kearon 2016; NICE guidelines CG144). Despite treatment, over 50% of patients may suffer post‐thrombotic symptoms in the long term, manifested by some degree of pain, swelling, skin pigmentation or venous ulceration of the affected leg (Kahn 2006; Schulman 2006). This usually becomes apparent in the first two years after the thrombotic event (Brandjes 1997; Kahn 2004; Kahn 2008). Most studies report eventual venous ulceration in at least 6% of DVT patients, despite treatment (Johnson 1995; Schulman 2006). The prevalence of venous ulcers in the general population is around 1 in 1000, and between 40% to 50% of patients with venous ulcers have evidence of post‐thrombotic damage (Browse 1999; Kahn 2004). Complications including venous ulcers may result in significant disability and may be difficult to manage in both the community and secondary care. Because complications develop after hospital admission, there is a low level of awareness of these complications amongst the clinicians who dealt with the acute DVT admission.
Description of the intervention
Standard anticoagulation does not actively remove blood clots (Kearon 2012), whereas thrombolytic drugs act to dissolve blood clots by activating plasminogen. This forms an enzyme called plasmin that breaks links between the fibrin molecules, which make up blood clots. The drugs can be administered systemically through a peripheral vein, loco‐regionally via a vein close to the clot or directly via a catheter to the occluding thrombus (Sharafuddin 2003). The latter method, commonly known as catheter‐directed thrombolysis (CDT), more directly targets plasminogen within the clot and is less affected by potential inhibitors in the circulation (Comerota 1993). Thrombolysis may be used alongside a variety of endovascular (catheter‐based) techniques devices to increase drug penetration and speed clot removal. This is known as endovascular or pharmacomechanical catheter‐directed thrombolysis (PCDT) (Vedantham 2009a; Vedantham 2013). These adjunctive techniques may involve mechanical thrombectomy to aid removal of the clot (suction, rotation, rheolysis), and/or balloon angioplasty or stenting, or a combination of these techniques, aiming to preserve venous function (Sharafuddin 2003; Vedantham 2009a).
How the intervention might work
Dissolving the thrombus in the acute phase may reduce the risk of more permanent damage to the structure and function of the vein, in particular venous valvular function, thus lowering the risk of post‐thrombotic complications in the long term. Combining thrombolysis with adjunctive techniques may allow faster and more complete clot removal (Sharafuddin 2003; Vedantham 2009b).
Why it is important to do this review
This systematic review is the fourth update of a previously published Cochrane Review (Armon 2000 (protocol); Watson 2004; Watson 2010; Watson 2014; Watson 2016). Our review draws together previous comparative trials of thrombolysis and anticoagulation to reassess the advantages and disadvantages of thrombolytic therapy in the context of acute lower limb DVT, and to identify areas for future research. Our review now includes evidence from recent trials, including pharmacomechanical thrombectomy, to reflect current clinical practice and to aid decision‐making for professionals and patients.
Objectives
To assess the effects of thrombolytic clot removal strategies and anticoagulation compared to anticoagulation alone for the management of people with acute DVT of the lower limb.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) of thrombolysis (with or without adjunctive clot removal strategies) and anticoagulation, versus anticoagulation alone, for acute lower limb DVT. Any method of randomisation was eligible, and differences in methodological quality were taken into account in the analysis. Trials that were not analysed on an intention‐to‐treat (ITT) basis were included, provided investigators accounted for all randomised participants.
Types of participants
We included trials of adult (aged 18 and over) participants with acute DVT, defined as onset of symptoms within seven days and confirmed by objective testing with, for example, venography or duplex ultrasonography. Trials including participants with chronic or recurrent venous thrombosis were excluded, as were those with participants commencing treatment after a maximum of 21 days from the onset of symptoms. Trials including participants with arm vein thrombosis were included in the update when the majority of cases affected the lower limb.
Types of interventions
We included trials with the use of any thrombolytic agent, the principal ones being streptokinase, urokinase and tissue plasminogen activator (tPA); other agents were included if used for the treatment of acute DVT. All routes of drug lysis administration were considered, as were different dosing regimens of lytic agents. These included systemic and catheter‐directed thrombolysis (CDT) methods. As combinations of clot removal strategies are now frequently used in clinical practice, we also included studies where adjunctive thrombus removal techniques such as thrombectomy, balloon maceration, balloon venoplasty, aspiration, stenting etc, were used in combination with thrombolysis, provided they were compared to standard anticoagulation alone.
Types of outcome measures
Outcome assessments were classified as early (up to one month); intermediate (after six months to five years) or late (more than five years), from the time of intervention (see Included studies). When data were reported between one and six months, we planned to discuss and reassess the definition of our time points as required.
Primary outcomes
-
Complete clot lysis (defined as achievement of full patency of the affected vein, or complete dissolution of the clot, by objective measures)
-
Bleeding complications, excluding stroke or intracerebral haemorrhage (defined as bleeding causing treatment to be stopped, requiring transfusion or surgery, or causing chronic or fatal sequelae)
-
PTS
Secondary outcomes
-
Any improvement in venous patency (assessed by objective measures such as venography, where pre‐ and post‐comparative data on the degree of restoration of the lumen were available)
-
Stroke, and in particular haemorrhagic stroke (preferably documented by objective means such as a computerised tomography scan or autopsy)
-
Venous ulceration rates
-
Mortality
-
Recurrent DVT
-
PE
-
Venous function (assessed by duplex ultrasound or other objective means such as foot volumetry or ambulatory venous pressure measurements)
-
Quality of life (QoL)
-
Cost comparisons
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year or publication status restrictions:
-
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched on 21 April 2020);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2020, Issue 3);
-
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 21 April 2020);
-
Embase Ovid (searched from 1 January 2017 to 21 April 2020);
-
CINAHL Ebsco (searched from 1 January 2017 to 21 April 2020);
-
AMED Ovid (searched from 1 January 2017 to 21 April 2020).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, strategies were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6 (Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 21 April 2020:
-
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
-
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
The reference lists of articles retrieved by electronic searches were searched for additional citations.
Data collection and analysis
Data were collected from the original papers and authors were contacted for clarification where necessary.
Selection of studies
One review author (CB) screened the titles and abstracts of all articles identified by the search, using Covidence. Non‐relevant articles were removed. Full‐text articles were obtained for the remaining references. Following this, pairs of review authors (from among CB, LW and MA), or one of these review authors and a member of the Cochrane Vascular editorial support team (MS; see Contributions of authors), then independently assessed these articles against the inclusion criteria. Any conflicts were resolved by discussion.
Data extraction and management
Two review authors (CB, LW), or one of these review authors and MS, independently extracted and checked data using pro formas designed by Cochrane Vascular. We contacted authors of ongoing trials to check for available data.
Assessment of risk of bias in included studies
Two review authors (from among CB, LW and MA), or one of these review authors and MS, independently assessed the risk of bias in included studies, following Cochrane Vascular guidelines and using the Cochrane 'Risk of bias' tool (Higgins 2011). Any disagreements were resolved by discussion.
Measures of treatment effect
We performed statistical analyses according to the statistical guidelines for review authors provided by Cochrane Vascular. If appropriate, we calculated a summary statistic for each dichotomous outcome using the risk ratio (RR) and its corresponding 95% confidence interval (CI). We present QoL statistics as reported by the respective studies in terms of means and standard deviations (SD) or standard errors (SE).
Unit of analysis issues
Individual participants were the unit of analysis. If appropriate, the control groups in the multiple arm trials were divided up to avoid double counting in the meta‐analysis.
Dealing with missing data
We conducted ITT analysis where possible. We recalculated any missing statistics from original data, where available. We contacted study authors to request data where it was not possible to identify specific event numbers from the data reported.
Assessment of heterogeneity
Heterogeneity was assessed clinically from descriptions of studies, visually from forest plots and statistically using the Chi2 test. If P < 0.05 for the Chi2 test, we used a random‐effects model; otherwise, we used a fixed‐effect model. We also considered heterogeneity by clinical judgements of differences in participant populations, interventions and outcome assessments.
Assessment of reporting biases
We assessed reporting bias through a review of the identified studies, and considered using funnel plots for outcomes when more than 10 studies with available data were included in a meta‐analysis.
Data synthesis
We pooled studies for meta‐analysis when the interventions, patient groups, outcome measures and timing of outcome assessment were sufficiently similar (determined by consensus). We calculated the pooled RRs and corresponding 95% CIs for dichotomous outcomes. We used a fixed‐effect model unless we identified statistical heterogeneity (as described above), in which case we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We analysed trials together and in subgroups, according to route of administration. We commented on other sources of heterogeneity, such as participant selection, type of DVT, drug or dose, where relevant. Where information was provided, we carried out subgroup analysis by location of DVT (iliofemoral, femoropopliteal or non‐specified).
Sensitivity analysis
We carried out sensitivity analyses for all outcomes where the meta‐analysis included trials judged to have any domain at high risk of bias. To determine if results were robust, we repeated meta‐analyses, excluding these studies.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the GRADEpro GDT software (Gradepro GDT 2020). This summarised the evidence comparing thrombolysis to standard anticoagulation for study populations consisting of patients with acute DVT (summary of findings Table 1). The most important and clinically relevant outcomes (both desirable and undesirable) that were thought to be essential for decision‐making were the outcomes 'complete clot lysis', 'bleeding' and 'post‐thrombotic syndrome'. Assumed control intervention risks were calculated by the mean number of events in the control groups of the selected studies for each outcome. We used the system developed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group for grading the certainty of evidence. We judged the certainty of evidence for each outcome to be high, moderate, low or very low, depending on within‐study risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Atkins 2004).
Results
Description of studies
Results of the search
For this update, two new studies met the criteria for inclusion; both were previously listed as ongoing studies (ATTRACT; CAVA 2020). Seventeen new studies were excluded (Ageno 2016; Ansari 2016; Bulatov 2019; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Fan 2015; Jiang 2017; Kim 2017; Kuo 2017; Liu 2013; NCT02414802; NCT02767232; Righini 2016; Song 2019; Yang 2016); two new studies were assessed as ongoing (ChiCTR‐INR‐16009090; NCT02959801); and two new studies were placed in studies awaiting classification (Gong 2018; Su 2017). See Figure 1.
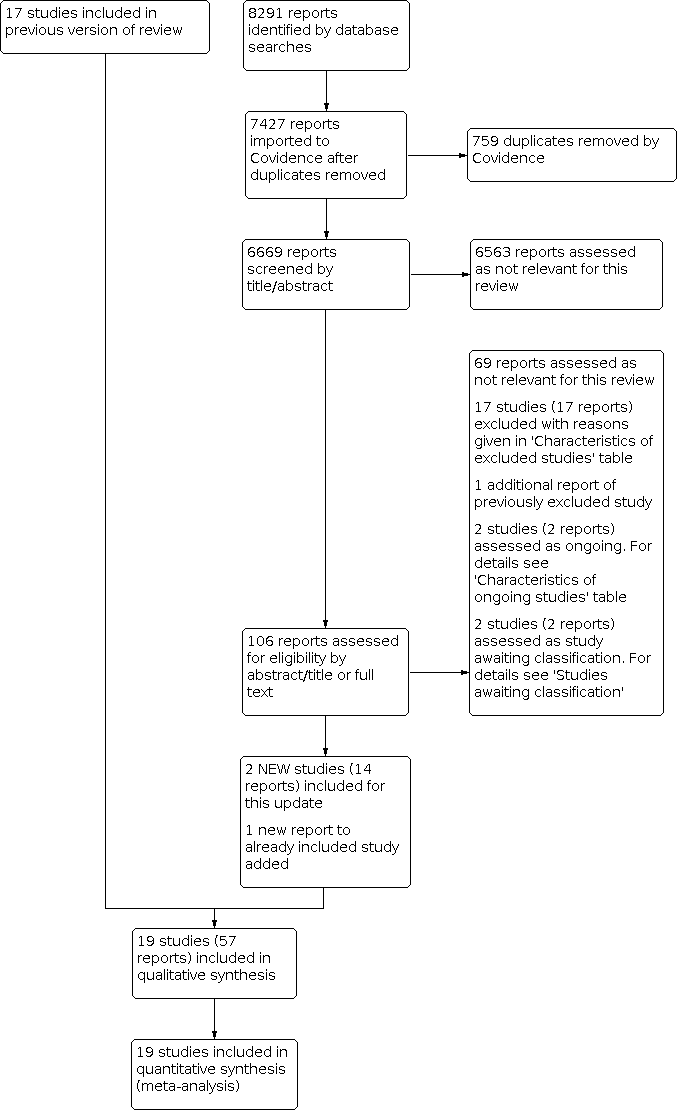
Study flow diagram.
Included studies
In total, we included 19 trials, with 1943 participants (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Studies were carried out from 1969 to 2017.
Participants
Trials were conducted in Belgium, Canada, Denmark, Egypt, France, Germany, the Netherlands, Norway, South Africa, Sweden, Switzerland, Turkey, the UK and the USA. All trials included men and women, with an age range from 18 to 75 years and with a preponderance of older adults. The participants had diverse underlying causes for developing DVT, and varying degrees of level and extent of occlusion. Both Elsharawy 2002 and CAVA 2020 included DVTs affecting femoral and iliofemoral veins; CAVENT included pelvic, femoral and iliofemoral veins; ATTRACT included proximal DVT; other trials included thrombosis affecting different combinations of levels, including popliteal. The only study to include calf vein thrombosis only was Schulman 1986. See Table 1,
| Study | Potential levels of leg vein included |
|---|---|
| proximal to calf | |
| proximal (femoral, common femoral, iliac vein with or without other involved ipsilateral veins) | |
| femoral and iliofemoral | |
| pelvic, iliofemoral, femoral | |
| not specified | |
| proximal | |
| femoral and iliofemoral | |
| popliteal or more proximal | |
| proximal | |
| not specified | |
| not specified | |
| calf up to iliac vein | |
| calf vein thrombosis only | |
| not specified | |
| leg or pelvic (popliteal or more proximal) | |
| not specified | |
| proximal | |
| popliteal up to inferior vena cava | |
| popliteal or more proximal |
Participant inclusion criteria
Participant inclusion criteria have become more restrictive over time. In the earliest study (Kakkar 1969), there were only four contra‐indications: surgery within three days, an unhealed wound, peptic ulcer and hypertension. By the time of Schweizer 2000, a more comprehensive list of contra‐indications had been developed including: surgery or head trauma within the previous three months, malignancy, renal and hepatic disorders, and bleeding disorders. In these later studies, the more comprehensive list reduces the proportion of eligible participants.
Interventions
Interventions included systemic, loco‐regional, catheter‐directed and pharmacomechanical thrombolysis. Systemic and loco‐regional techniques differ only in the veins used to deliver an infusion: the arm or foot respectively. Catheter‐directed thrombolysis (CDT) is a more invasive procedure in which a catheter is inserted percutaneously into a vein using imaging to guide to the clot location. The thrombolytic agent is infused through the catheter into the blood clot itself and the position of the catheter is altered according to the progress made in lysing the blood clot. The majority of trials assessed systemic thrombolysis, with streptokinase the most common agent used. The dose used varied: Schulman 1986 used a low‐dose regime of systemic streptokinase, Tsapogas 1973 used loco‐regional streptokinase and Elsharawy 2002 used catheter‐directed streptokinase with frequent radiological assessment, a technique used again in CAVENT. ATTRACT used mechanical therapy in addition to CDT (pharmacomechanical thrombolysis). These adjunctive therapies could include balloon maceration, catheter aspiration, thrombectomy, percutaneous transluminal balloon venoplasty, stent placement, or combination of the above (ATTRACT; Vedantham 2017). CAVA 2020 used ultrasound accelerated CDT to deliver urokinase.
Goldhaber 1990, Turpie 1990 and Verhaeghe 1989 used systemic tPA. While doses of tPA varied, there was no obvious cut‐off for high or low doses. Goldhaber 1996 randomised two regimes of tPA, with and without heparin, compared to heparin alone. The two treatment arms were combined for the purposes of this review. Schweizer 1998 had two treatment arms, loco‐regional tPA and urokinase; and Schweizer 2000 had four treatment arms: systemic streptokinase, systemic urokinase, loco‐regional urokinase and loco‐regional tPA. Kiil 1981 used low‐dose systemic urokinase.
Co‐treatments
Monitoring regimes for heparinisation varied, and length of anticoagulation after the initial phase may be limited to a few months or continued for over a year. In some trials, especially the more recent ones, the use of compression bandages and elevation were reported; and for longer follow‐up, some participants were required to use compression stockings with rigorous ascertainment of compliance with the continued treatment.
Size
Nine studies had fewer than 50 participants (Arnesen 1978; Elsharawy 2002; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Tsapogas 1973; Verhaeghe 1989), and four studies had more than 100 participants (ATTRACT; CAVA 2020; CAVENT; Schweizer 2000). Most studies therefore lacked power to detect statistically significant effects. A power calculation was described in four studies (CAVA 2020; CAVENT; Elsharawy 2002; Schweizer 2000). ATTRACT was the largest trial, with 692 participants.
Outcomes
One trial (Verhaeghe 1989) reported results for randomised participants together with non‐randomised participants, but we have used only the data from randomised participants. Some studies reported outcomes using scales that could not be combined (Marder 1977). Removal of the clot was reported using various categorisations. In order to capture as much information as possible, we report in this review both complete clot dissolution or lysis, indicating that the venous patency was 100% restored, and any degree of venographic improvement in patency. Tsapogas 1973 reported partial or complete clearance (75% to 100%), a measure not used in any other study, and others reported partial clearance (50% to 100%). The more recent trials report primarily on PTS and QoL (ATTRACT; CAVA 2020; CAVENT), with CAVENT also reporting cost comparisons.
Length of follow‐up
All trials assessed outcomes in the period immediately after treatment. This was usually at one week, although the range was 36 hours to one month. We collectively grouped these as early outcomes. Intermediate outcomes have been classified as those determined after six months and under five years. No data were reported between this early and intermediate phase (i.e. after one month and before six months). Late outcomes were those reported five years or more after the intervention. PTS was assessed between one and six years. The longest follow‐up (six years) was in the Arnesen 1978 study.
Excluded studies
Seventeen additional trials were excluded for this update (Ageno 2016; Ansari 2016; Bulatov 2019; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Fan 2015; Jiang 2017; Kim 2017; Kuo 2017; Liu 2013; NCT02414802; NCT02767232; Righini 2016; Song 2019; Yang 2016). This brings the total number of excluded studies to 40 (Ageno 2016; Ansari 2016; Ansell 1990; Bashir 2014; Bieger 1976; Browse 1968; Bulatov 2019; Cakir 2014; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Engelberger 2015; Fan 2015; Jiang 2017; Johansson 1979; Kim 2017; Kuo 2017; Liu 2013; Marini 1991; Markevicius 2004; NCT02414802; NCT02767232; Patra 2014; Persson 1977; Pinto 1997; Righini 2016; Robertson 1967; Santiago 2014; Sas 1985; Schweizer 1996; Silistreli 2004; Song 2019; Sui 2013; Tibbutt 1974; Tibbutt 1977; TORPEDO 2012; Yang 2016; Zhang 2014; Zimmermann 1986). Eight trials did not satisfy the criteria for randomisation (Bashir 2014; Browse 1968; Bulatov 2019; Johansson 1979; Markevicius 2004; Robertson 1967; Santiago 2014; Schweizer 1996). Twenty‐four studies did not include a comparison of thrombolysis versus anticoagulation (Ageno 2016; Ansari 2016; Cakir 2014; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Engelberger 2015; Fan 2015; Jiang 2017; Kim 2017; Kuo 2017; Liu 2013; Marini 1991; NCT02414802; Pinto 1997; Righini 2016; Song 2019; Sui 2013; Tibbutt 1974; Tibbutt 1977; Yang 2016; Zhang 2014; Zimmermann 1986); one study was withdrawn due to lack of funding (NCT02767232); DVT was not confirmed objectively in one study (Bieger 1976); and onset of symptoms was beyond 21 days in two studies (Patra 2014; Silistreli 2004). In three cases, insufficient information was obtained despite attempts to contact the authors (Ansell 1990; Persson 1977; Sas 1985). TORPEDO 2012 was excluded as only 33 out 90 participants received thrombolysis. See the Characteristics of excluded studies table for further information.
Ongoing studies
For this update, we identified two new ongoing studies (ChiCTR‐INR‐16009090; NCT02959801). Two previously ongoing studies are now included (ATTRACT; CAVA 2020). We contacted the study investigators of the three ongoing studies, but no data were available (ChiCTR‐INR‐16009090; IRCT201108035625N3; NCT02959801). See Characteristics of ongoing studies for further details.
Studies awaiting classification
For this update, two potential new studies were identified and placed into Studies awaiting classification until a thorough assessment of the full text can be made (Gong 2018; Su 2017).
Risk of bias in included studies
The quality of reporting of most trials was high, see Figure 2 and Figure 3. See the Characteristics of included studies table for detailed information. Minor protocol violations were reported in several studies, and losses to follow‐up were more common in the later phases.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Many studies reported random allocation from a random numbers table or computer‐generated sequence (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Elsharawy 2002; Goldhaber 1990; Schulman 1986; Schweizer 1998; Tsapogas 1973; Ugurlu 2002; Verhaeghe 1989), although sometimes this detail was lacking (Common 1976; Elliot 1979; Goldhaber 1996; Kiil 1981; Marder 1977; Schweizer 2000; Turpie 1990; Verhaeghe 1989). Many older studies did not give details on allocation concealment, and this remained a possible risk of bias (Common 1976; Elliot 1979; Elsharawy 2002; Kiil 1981; Marder 1977; Schweizer 1998; Schweizer 2000; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Studies with good allocation concealment also found significant effects. In some cases, insufficient detail was reported on whether envelopes were sequentially numbered, sealed or opaque (Common 1976; Elliot 1979; Goldhaber 1996; Schulman 1986; Tsapogas 1973).
Blinding
With the exception of Tsapogas 1973, all studies used blinding for the assessment of venograms. Turpie 1990 and Verhaeghe 1989 used identical placebo infusions and therefore were double‐blind. Where participants were not blinded to the treatment group (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Marder 1977; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Ugurlu 2002), an assessment was made that this introduced a low risk of bias where the assessor was blinded and using objective measures, which was the case in most studies (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Schulman 1986; Schweizer 1998; Schweizer 2000; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Blinding participants would be more difficult with more interventional approaches. However, this lack of blinding of participants may have introduced bias in the longer term as participants in receipt of thrombolysis may be more likely to have impressed upon them, or to heed advice given on, the importance of complying with co‐treatments such as compression stockings. For example, compliance was higher in the treatment group in CAVENT. In Kakkar 1969 neither the participants nor outcome assessors were blinded, and this study was therefore judged to have a high risk of bias.
Incomplete outcome data
Most studies did not demonstrate any major differences in follow‐up between the treatment and control groups for the main outcomes, in the early or intermediate follow‐up periods and so were judged to have a low risk of attrition bias. Marder 1977 was assessed as having high risk of bias for this category as it was not possible to separate the data from the three patients who were added non‐randomly after randomisation took place. In ATTRACT, a total of 80 patients missed all PTS assessments. Fifty‐two of these were in the control group (14%), compared to 28 (8%) in the intervention group. Sensitivity analysis carried out by the study authors did not demonstrate a difference in the PTS outcome compared to primary analysis so this was judged to not impact the risk of bias assessment in this domain.
Selective reporting
In some cases subgroups were reported that did not include all trial participants, for example, PTS in those with complete clot lysis, but these were not included in the review. As results including non‐randomised participants were reported in Marder 1977, this was judged to be at high risk of bias. Duplicate reports of studies were identified in the selection process and multiple sources were searched, with no language restriction.
Other potential sources of bias
There were no other specific concerns about bias except for Marder 1977, who added three non‐randomised participants to the study post‐randomisation.
Effects of interventions
See: Summary of findings 1 Treatment with any thrombolysis strategy for acute deep vein thrombosis
Thrombolysis versus standard anticoagulation
Nineteen studies were included in the meta‐analysis (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Schweizer 2000 had two treatment groups, one systemic thrombolysis and one loco‐regional thrombolysis group. We carried out subgroup analysis to investigate any overall effect of thrombolytics and also to compare the different thrombolysis strategies.
Complete clot lysis
Eight trials with 592 participants reported on the occurrence of early complete clot lysis (up to one month follow‐up) (Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Kakkar 1969; Schulman 1986; Schweizer 2000; Ugurlu 2002). In all trials this was more likely in the treatment group, although the extent of the effect varied and the results were statistically heterogeneous. A random‐effects model demonstrated a benefit from thrombolysis treatment (RR 4.75, 95% CI 1.83 to 12.33; P = 0.001; Analysis 1.1). No differences between thrombolysis strategy used were seen with subgroup analysis (test for subgroup differences: P = 0.41).
Seven trials with a total of 654 participants reported intermediate clot lysis (after six months) and in all cases this was more likely in the groups treated with thrombolysis (Common 1976; CAVENT; Elliot 1979; Elsharawy 2002; Schulman 1986; Schweizer 1998; Schweizer 2000). There was a benefit seen with thrombolysis treatment, (RR 2.42, 95% CI 1.42 to 4.12; P = 0.001 using a random‐effects model (moderate‐certainty evidence; Analysis 1.2). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.37). We downgraded the certainty of the evidence from high to moderate due to imprecision.
Two trials with a total of 206 participants reported clot lysis at five years and over (Arnesen 1978; CAVENT). There was no clear benefit of clot lysis with thrombolysis seen at this time point (RR 3.25, 95% CI 0.17 to 62.63; P = 0.44; Analysis 1.3). No differences between thrombolysis strategies used were seen with subgroup analysis (test for subgroup differences: P = 0.06).
CAVA 2020 data on clot lysis are to be published in subsequent papers.
Bleeding
This category excluded cerebral bleeding and minor bleeds, for example, oozing from venepuncture sites and superficial haematomas. All 19 trials reported on the occurrence of bleeding episodes (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Overall, 6.7% (72/1073) of participants in the thrombolysis group experienced a bleeding complication compared to 2.2% (20/870) of participants in the standard anticoagulation group (RR 2.45, 95% CI 1.58 to 3.78; 1943 participants; 19 studies; P < 0.0001; moderate‐certainty evidence; Analysis 1.4). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.25). We downgraded the certainty of the evidence from high to moderate due to imprecision. We detected no indication of reporting bias (Figure 4).

Funnel plot of comparison: 1 Thrombolysis versus standard anticoagulation, outcome: 1.4 Bleeding (early, subgrouped by thrombolysis strategy).
PTS
Six studies reported clinically assessed PTS at six months to 5 years (intermediate) (ATTRACT; CAVA 2020; CAVENT; Elliot 1979; Schweizer 1998; Schweizer 2000), in a format that could be combined, with a total of 1393 participants. Fewer cases of PTS were reported in those participants receiving thrombolysis compared to those in the control group (RR 0.78, 95% CI 0.66 to 0.93; 1393 participants; six studies; P = 0.006; moderate‐certainty evidence; Analysis 1.5). We downgraded the certainty of the evidence from high to moderate due to imprecision and small number of participants in the majority of the included studies. The incidence in the thrombolysis group compared with the control group was 383/771 (49.6%) versus 329/622 (52.8%, ranging from 35% to 96% in different trials, which may reflect definitions, treatment doses and adjunctive treatments). We detected heterogeneity (P = 0.01), so we used a random‐effects model. No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.22; Analysis 1.5). Two studies provided data for systemic thrombolysis and these showed a reduction in the incidence of PTS (RR 0.54, 95% CI 0.31 to 0.92; 170 participants; P = 0.02). It is of note that both studies used a much higher dose of thrombolytic than those used in other studies (Elliot 1979; Schweizer 2000; see Characteristics of included studies). No clear benefit for CDT was shown by pooling results from the ATTRACT, CAVA 2020 and CAVENT trials (RR 0.89, 95% CI 0.74 to 1.05; 1032 participants; P = 0.17).
We also carried out subgroup analysis by DVT level (iliofemoral, femoropopliteal or non‐specified). We have included all participants of CAVENT in the iliofemoral group as this population was similar to the ATTRACT iliofemoral group (personal communication with the study authors). In keeping with reports above, overall the thrombolysis group experienced less PTS (RR 0.82, 95% CI 0.71 to 0.94; 1393 participants; six studies, P = 0.006). No differences in PTS incidence between level of DVT were detected between subgroups (test for subgroup differences: P = 0.29; Analysis 1.6).
Two studies with 211 participants (Arnesen 1978; CAVENT), reported reduced incidence of clinically assessed PTS at over five years (late follow‐up) following thrombolysis (RR 0.56, 95% CI 0.43 to 0.73; 211 participants; two studies; P < 0.0001; moderate‐certainty evidence; Analysis 1.7). We downgraded the certainty of the evidence from high to moderate due to the small number of studies and participants. In the control group, the incidence of PTS was 75/107 (70%) and in the thrombolysis group 41/104 (39%). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.28).
Any improvement in venous patency
Nine trials reported on improvements in venous patency defined by a change in occlusion of the affected segment after treatment (Arnesen 1978; Common 1976; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Turpie 1990; Ugurlu 2002). All of these studies used systemic thrombolysis strategies except for Elsharawy 2002. Out of a total of 421 participants, improvement was more likely in those receiving thrombolysis (RR 2.48; 95% CI 1.35 to 4.57, P = 0.004; Analysis 1.8). Statistical heterogeneity was noted (P < 0.0001), and a random‐effects model was used. The study by Marder 1977, which showed a difference in mean change from venograms, could not be included due to the reporting format used. A greater improvement was noted but for randomised participants this was not reported to be significantly different. Similarly the Verhaeghe 1989 data could not be included in the meta‐analysis. No clear differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.05).
Stroke or intracerebral haemorrhage
Three trials reported the occurrence of stroke or intracerebral haemorrhage (Common 1976; Goldhaber 1990; Marder 1977). All trials described bleeding complications, therefore the absence of mention of any serious neurological complications or cerebral bleeds was taken to indicate that none were detected. Out of a total of 1943 participants three events occurred in the treatment group (3/1943) and none in the control group. The pooled RR was 1.92 (95% CI 0.34 to 10.86; P = 0.46; 19 studies; Analysis 1.9). All studies where stroke occurred were from before 1990.
Leg ulceration
Five studies described ulceration of the leg occurring more than six months from trial entry (ATTRACT; CAVENT; Elliot 1979; Schulman 1986; Schweizer 1998). Fifteen events occurred in the treatment group (15/513) and 19/520 in the control group (RR 0.76, 95% CI 0.39 to 1.49; 1033 participants; five studies; P = 0.43; Analysis 1.10). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.72).
Arnesen 1978 reported at a mean of 6.5 years and so fell within the definition of late ulceration. This study involved a small number of participants, with 3/18 control participants experiencing ulceration after six years compared to 0/17 in the thrombolysis participants (RR 0.15, 95% CI 0.01 to 2.72; P = 0.20; Analysis 1.11).
Mortality
Ten trials reported on deaths occurring up to one month after treatment (ATTRACT; Arnesen 1978; Common 1976; Elliot 1979; Elsharawy 2002; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 2000); three trials reported that no deaths occurred in this period (ATTRACT; Elsharawy 2002; Schweizer 2000). A total of five events occurred in the treatment group (5/677) and seven in the control group (7/543), out of a total of 1220 participants. There were relatively few events and no clear difference between the groups (RR 0.76; 95% CI 0.31 to 1.89; P = 0.56; Analysis 1.12). All the recorded events occurred within the systemic thrombolysis subgroup, a test for subgroup differences was not estimable. All trials where deaths occurred at this time point were from before 1987.
Four trials with a total of 1144 participants reported mortality occurring up to five years after treatment (ATTRACT; CAVA 2020; Elliot 1979; Schweizer 2000). No deaths were reported in either group in Schweizer 2000; CAVA 2020, ATTRACT and Elliot 1979 reported similar numbers of deaths in each group (RR 0.81, 95% CI 0.39 to 1.69; 1144 participants; four studies; P = 0.58; Analysis 1.13). Most deaths were unrelated to the clot or treatment but rather to underlying conditions. ATTRACT reported one death due to PE in the thrombolysis group. No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.76).
Two trials with a total of 230 participants reported mortality after five years follow‐up (Arnesen 1978; CAVENT). Seven deaths occurred in the thrombolysis group (7/111) and 12/119 in the control group with a RR of 0.61 (95% CI 0.25 to 1.50; P = 0.28; Analysis 1.14). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.17).
Recurrent venous thromboembolism (DVT/VTE)
At intermediate time points, one trial reported on recurrent DVT (Arnesen 1978), while ATTRACT, CAVA 2020 and CAVENT reported recurrent VTE. Seventy‐three events (including one fatal PE event in the ATTRACT study) occurred in the treatment group (73/520), compared to 58/547 in the control group. The RR was 1.32, 95% CI 0.96 to 1.83; 1067 participants; four studies; P = 0.09); Analysis 1.15. No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.92).
At five year follow‐up, CAVENT reported 13/87 and 21/89 VTE events in the thrombolysis and anticoagulation groups respectively (RR 0.63, 95% CI 0.34 to 1.18; Analysis 1.16). Thirteen events were in the ipsilateral leg, 10 in the contralateral leg, nine were PE and two were unknown. Six patients with chronic iliac vein occlusions (one in the CDT group and five in the control group), were referred and had endovascular recanalisation with stenting. Although randomised to the treatment group, the CDT patient had not received CDT as planned, due to technical failure (Haig 2016).
PE
Six trials reported the occurrence of a PE in the early phase (Arnesen 1978; Elliot 1979; Elsharawy 2002; Kakkar 1969; Schulman 1986; Schweizer 2000). One study noted the absence of any PE (Schulman 1986). The diagnostic criteria used were variable. With the exception of participants who died from PE (one in the treatment group, two in the control group), transient clinical symptoms often occurred but with no objective diagnostic confirmation described. Where deaths were attributed to PE, postmortem examinations were not mentioned. For this reason, the results should be interpreted with caution. The RR was 1.01 (95% CI 0.33 to 3.05; 443 participants; six studies; P = 0.98; Analysis 1.17). No differences were detected between subgroups (P = 0.43). CAVENT did not measure this outcome at this time point. ATTRACT reported that there were 6/336 recurrent VTE events in the thrombolysis group compared to 4/355 (P = 0.5) within the first 10 days; it is not specified if these were PE or DVT.
Venous function (intermediate)
Three trials reported on presence of normal venous function (CAVENT; Elsharawy 2002; Schulman 1986). Overall, no clear benefit to venous function with thrombolysis was shown, (RR 2.18; 95% CI 0.86 to 5.54; 255 participants, 3 studies, P = 0.10, Analysis 1.18). Heterogeneity was detected (P = 0.009) so a random‐effects model was used. Subgroup analysis suggests a difference between use of systemic and CDT strategies (P = 0.03) with increased normal venous function being seen in the CDT group (RR 3.18, 95% CI 1.41 to 7.19; 224 participants; two studies; P = 0.005).
QoL
Only ATTRACT, CAVA 2020 and CAVENT measured QoL. All three studies used the Venous Insufficiency Epidemological and Economic Study Quality of Life (VEINES‐QOL) measure, which includes both an overall score and a symptom score. In addition, CAVENT and CAVA 2020 used the generic instrument EQ‐5D; and ATTRACT and CAVA 2020 used the Medical Outcomes Study 36‐Item Short Form Health Survey (SF‐36). This includes both a physical component score (PCS) and mental component score (MCS). CAVA 2020 also used the Pain Disability Index (PDI), which reports limitations in daily activities due to pain (scored from 0 to 10, 0 no limitations, 10 fully disabled). VEINES‐QoL, EQ‐5D and SF‐36 scores range from 0 to 100, higher scores indicating better QoL or health perception. A difference of 3 to 4 points is considered clinically meaningful.
After 12 months, CAVA 2020 reported that there were no differences between the CDT and standard treatment group for any health‐related and disease‐specific QoL assessment (mean (SD) VEINES‐Sym score for CDT was 50.1 (11.1) compared to 49.7 (9) in the standard treatment group; mean (SD) SF36 general scores were 65.6 (17.8) in the CDT group compared to 64.9 (22.8) in the standard treatment group; mean (SD) EQ5D score for the CDT group was 85.7 (15.0) compared to 82.3 (21.0) in the standard treatment group; and using the PDI, the mean (SD) CDT group score was 8.7 (12.4) compared to 13.1 (16.3) in the standard treatment group.
After 24 months CAVENT reported there were no differences in QoL between the additional CDT and standard treatment arms; mean difference for the EQ‐5D index was 0.04 (95% CI ‐0.10 to 0.17), for the VEINES‐QOL score 0.2 (95% CI ‐2.8 to 3.0) and for the VEINES‐Sym score 0.5 (95% CI ‐2.4 to 3.4; P > 0.37). After five years, CAVENT reported no difference in mean generic QoL scores, disease specific QoL scores, or symptom severity score between the groups (Enden 2012; Enden 2013a).
Independent of treatment arms, patients with PTS had poorer outcomes than patients without PTS after 24 months; mean difference for EQ‐5D was 0.09 (95% CI 0.03 to 0.15), for VEINES‐QOL score 8.6 (95% CI 5.9 to 11.2) and for VEINES‐Sym score 9.8 (95% CI 7.3 to 12.3; P < 0.001). After five years the EQ‐5D, VEINES‐QOL and VEINES‐Sym scores for patients with PTS were lower than for those without PTS (Enden 2012; Enden 2013a).
After 24 months, ATTRACT reported no difference between the groups with the SF‐36 form (mean (SE) 11.18 (0.91) in the thrombolysis group compared to 10.06 (0.97) in the control group (P = 0.37)); with the VEINES‐QOL score (mean (SE) 27.67 (1.71) in the thrombolysis group compared to 23.47 (1.83) in the control group (P = 0.37)); or the VEINES‐Sym score (mean (SE) 20.58 (1.70) in the thrombolysis group compared to 17.31 (1.81) in the control group (P = 0.17)). A report of a secondary analysis of the ATTRACT study by Kahn 2018 reported VEINES‐QOL scores were better in the thrombolysis group compared to the control group at 30 days (mean (SE) 64.9 (1.4) versus 60.3 (1.4); P = 0.018) and six months (77.0 (1.4) versus 73.1 (1.4); P = 0.044), respectively. This improvement was also detected in the iliofemoral subgroup but not in the femoropopliteal subgroup (Kahn 2018).
Cost comparisons
Only CAVENT has reported on this outcome (Enden 2013b). Additional CDT accumulated 32.31 quality‐adjusted life years (QALYs) compared with 31.68 QALYs after standard treatment. The lifetime cost of CDT was USD 64,709 compared to USD 51,866 with standard treatment. The incremental cost‐effectiveness ratio was USD 20,429 per QALY gained, and the study authors concluded that the probability that CDT was cost‐effective was 82% at a willingness to pay threshold of USD 50,000 per QALY gained (Enden 2013b). CDT may have additional costs compared to systemic administration.
Sensitivity analyses
We carried out sensitivity analyses for all outcomes where the meta‐analysis included trials judged to have any domain at high risk of bias. To determine if results were robust, meta‐analyses were repeated excluding the following studies: Kakkar 1969; Marder 1977; Tsapogas 1973. Forest plots and summary figures were visually assessed and for all outcomes the results remained consistent.
Discussion
Summary of main results
Complete clot lysis was more likely following thrombolysis at both early (RR 4.75, 95% CI 1.83 to 12.33), and intermediate time points (RR 2.42, 95% CI 1.42 to 4.12; moderate‐certainty evidence). The use of objective classification of the degree of lysis would assist, in the future, with quantifying this outcome and the patency of the veins. This benefit is off set by the increased incidence of major bleeding (RR 2.45, 95% CI 1.58 to 3.78; moderate‐certainty evidence). The rationale for the use of thrombolysis for DVT is to prevent long‐term complications related to poor venus function including PTS and ulceration. In this meta‐analysis involving 19 studies, 53% of control participants at intermediate time points and 70% at late follow‐up experienced PTS, which is in line with other estimates. Pooling all types of thrombolysis, the results showed a slight reduction in the risk of PTS with use of thrombolysis at the intermediate time point (RR 0.78, 95% CI 0.66 to 0.93; moderate‐certainty evidence); and at late follow‐up (RR 0.56, 95% CI 0.43 to 0.73; moderate‐certainty evidence). The clinical importance of this reduction is difficult to interpret. The overall benefit of thrombolysis is reduced compared to the previous version of our review (Watson 2016), due to the inclusion of one and two year data from the CAVA 2020 trial and a large multi‐centre trial (ATTRACT), which, in contrast to CAVENT, reported no benefit on incidence of PTS following thrombolysis. Differences between ATTRACT, CAVA 2020 and CAVENT include size (692 participants in ATTRACT versus 209 participants in CAVENT), and a greater use of mechanical adjunctive therapies, versus the longer thrombolytic infusion times in ATTRACT and CAVA 2020 compared to CAVENT. CAVENT primarily recruited patients with iliofemoral DVT, as did CAVA 2020; while in ATTRACT, 43% of participants had femoropopliteal DVT, a population less likely to develop PTS (Kahn 2008). However, subgroup analysis by the ATTRACT study authors did not indicate a difference in PTS incidence between these two levels of DVT. For this update, we carried out subgroup analysis to investigate any effect on PTS incidence by DVT level. This failed to demonstrate any clear effect between iliofemoral, femoropopliteal or non‐specified level of DVT. The authors of ATTRACT highlighted an increased number of participants in the control group who did not attend PTS assessments, suggesting this may have lead to an underestimation of treatment effect. Sensitivity analysis carried out by them did not support this possibility. The ATTRACT authors reported a decrease in the severity of PTS in the pharmacomechanical group compared to the anticoagulant group (RR 0.73 95% CI 0.54 to 0.98; P = 0.04). Subsequent publications from the ATTRACT study reporting on stratified analysis highlight that patients with iliofemoral DVT experienced less severe PTS and improved venous disease‐specific QoL (Comerota 2019). The CAVA 2020 trial reported a higher number of recurrent thrombotic events in the CDT group as a result of in‐stent‐thrombosis. Recurrent thrombosis is one of the main risk factors for PTS and may partly explain why the results did not favour CDT in this iliofemoral population, as had been expected (Prandoni 2004).
This updated meta‐analysis indicates that thrombolysis improved venous patency, with the majority of studies reporting on this outcome using systemic delivery routes (RR 2.48, 95% CI 1.35 to 4.57). The risk of inducing unwanted bleeding with thrombolytics has been the most important factor limiting its use for patients with DVT. Most bleeding episodes and deaths occurred in the earlier studies. Bleeding episodes (excluding stroke) causing interruption of therapy, interventions such as transfusion, or chronic sequelae (a condition following as a consequence of a disease) occurred more often with thrombolysis than with standard anticoagulation. There is no strong evidence that one particular route of administration or agent was excessively hazardous in this respect, although it is notable that no bleeding occurred in the Elsharawy 2002 study. This may have been due to strict exclusion criteria and the close radiological monitoring and dose titration depending upon clot lysis. A high proportion of patients with DVT are, however, unsuitable for thrombolytic treatment because of extensive contra‐indications. Three intracerebral bleeds occurred in these trials (Common 1976; Goldhaber 1990; Marder 1977). Adoption of current contra‐indications may have prevented these events in more recent trials. A stroke occurred in a participant with polycythaemia rubra vera who received streptokinase (Common 1976), an intracranial bleed in a participant with controlled hypertension treated with tPA (Goldhaber 1990), and a fatal intracranial haemorrhage in a patient with a remote history of cerebrovascular accident (Marder 1977). Two of the early deaths in the treatment groups may also have been prevented with the use of current contra‐indications to thrombolysis: a participant with metastatic carcinoma (Common 1976), and a participant with recent surgery (Kakkar 1969).
Four trials with a total of 1144 participants reported on mortality occurring up to five years after treatment (ATTRACT; CAVA 2020; Elliot 1979; Schweizer 2000). No deaths were reported in either group in Schweizer 2000; ATTRACT; CAVA 2020 and Elliot 1979 reported similar numbers of deaths in each group. One trial (Schweizer 2000), reported the absence of further PE episodes at one year, and ATTRACT reported one death due to PE in the thrombolysis group. Results relating to PE were inconclusive due to uncertainty surrounding diagnosis. Two PE were reported in the CAVA 2020 standard anticoagulation group compared to none in the CDT group. There was no clear evidence of any differences between the groups in ulceration beyond six months, or recurrent VTE or DVT. While overall no benefit was seen in the thrombolysis group on venous function, increased venous function was suggested in the CDT group, though this should be interpreted with caution due to the limited number of participants.
CAVENT examined both QoL and cost‐effectiveness. ATTRACT and CAVA 2020 also reported QoL. For QoL, there was no clear difference between CDT and standard treatment although PTS was associated with a lower QoL (ATTRACT). The incremental cost‐effectiveness ratio was USD 20,429 per QALY gained (Enden 2013b). This incremental cost‐effectiveness ratio for CDT is within the range for approval by bodies making recommendation for implementation (Dakin 2014; NICE PMG9).
Overall completeness and applicability of evidence
The evidence presented is highly relevant to determining the effect of thrombolysis for DVT. The effectiveness of newer catheter‐directed methods appears to be similar to that of systemic administration. Evidence suggests effectiveness at levels not limited to iliofemoral. As there is a degree of consistency in the results of trials over time, as well as in different settings, it is likely that the findings have external validity. Further evidence is desirable to confirm the effect of newer methods, and the factors predicting more successful outcomes. For this update, we have been able to include data from ATTRACT and CAVA 2020, where clinicians used a combination of invasive procedures, which reflects newer available strategies to remove clots. With respect to standard treatment with anticoagulation, selected patients with extensive DVT may benefit from systemic thrombolysis or by endovascular interventions such as catheter‐directed and percutaneous mechanical thrombectomy if this were considered safe. This is consistent with the current 'Recommendations and link to evidence' from National Institute for Clinical Excellence (NICE) guidelines (NICE guidelines CG144). There are implications for inpatient treatment, where anticoagulation for DVT is now delivered in outpatient settings, and for the resourcing of more invasive procedures.
No comparisons between thrombolysis and subcutaneous low molecular weight heparin, administered at home, for DVT were identified.
There were not enough data in this review to make any definitive comparison between the different agents or doses of thrombolytics used. Strepokinase and urokinase are more common in older studies, with tPA typically used more recently. Streptokinase appears to have been most widely studied but treatment doses varied widely, as did doses of other thrombolytics and control anticoagulant regimes. ATTRACT mentions the option of using the newer non‐vitamin K oral anticoagulant drugs (rivaroxaban) but it is not known what percentage of participants received this. CAVA 2020 reported use of acenocoumarol, phenprocoumon, DOAC and LMWH. We do not have enough information to draw any conclusions over the question of whether use of the newer anticoagulants has improved outcomes in the standard treatment groups.
Quality of the evidence
This evidence is based on 19 trials involving 1943 participants from a range of countries and settings. The key limitation of the studies is the paucity of long‐term follow‐up. The methodological quality of the studies was mostly high, and the results were consistent across a range of settings and patient groups. Using GRADE assessment, the body of evidence relating to complete clot lysis (intermediate), bleeding (early) and PTS (intermediate and late) was judged to be of moderate certainty, downgraded due to many trials having low numbers of participants or events, or both (See summary of findings Table 1). There were obvious differences between the inclusion criteria and the conduct of studies completed over 40 years ago compared to more recent studies. However, the results across studies were consistent and we have reasonable confidence in the results.
Potential biases in the review process
It is likely that all relevant studies were identified and included. Relevant data were requested or obtained from study authors, although for older studies this was less likely to be successful. Efforts were made to reduce bias in the review process by ensuring double independent data extraction and quality assessment of studies.
Agreements and disagreements with other studies or reviews
The evidence presented here is consistent with findings of other reviews, which have included a broader range of evidence than RCTs. A review of the literature by Patterson 2010 concluded that in carefully selected patients, CDT offered benefits in treatment, although further trial evidence was needed. Vedantham 2010 indicated benefits in CDT for people with extensive acute iliofemoral DVT, low expected bleeding risk and good functional status, although Comerota 2008 also emphasised a need for further research. A meta‐analysis by Du 2015 included both randomised and non‐randomised studies and had similar findings. Systemic thrombolysis is not current practice, although this review suggests that it has similar effectiveness to CDT, possibly due to its higher dosing regimes (Characteristics of included studies). More recent reviews, which also include data from ATTRACT, report that although a benefit from CDT/PMT (in terms of PTS incidence or severity) may be seen in selected patients (iliofemoral DVT), it is unclear if this benefit outweighs the increased bleeding risk and costs (ten Cate‐Hoek 2018; Chiasakul 2018; Poston 2018).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Thrombolysis versus standard anticoagulation, outcome: 1.4 Bleeding (early, subgrouped by thrombolysis strategy).

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 1: Complete clot lysis (early, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 2: Complete clot lysis (intermediate, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 3: Complete clot lysis (late, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 4: Bleeding (early, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 5: PTS (intermediate, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 6: PTS by iliofemoral/fempop (intermediate, subgrouped by location)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 7: PTS (late, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 8: Any improvement in venous patency (early)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 9: Stroke (early, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 10: Leg ulceration (intermediate, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 11: Leg ulceration (late)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 12: Mortality (early, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 13: Mortality (intermediate, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 14: Mortality (late, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 15: Recurrent DVT (intermediate, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 16: Recurrent DVT (late, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 17: Pulmonary embolism (early, subgrouped by thrombolysis strategy)

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 18: Venous function (intermediate, subgrouped by thrombolysis strategy)
| Treatment with any thrombolysis clot removal strategy for acute DVT | ||||||
| Patient or population: patients diagnosed with acute DVT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard anticoagulation | Risk with thrombolysis | |||||
| Complete clot lysis (intermediate, 6 months to under 5 years after treatment) | Study population | RR 2.42 (1.42 to 4.12) | 654 | ⊕⊕⊕⊝ | 78 of 244 patients treated with standard anticoagulation had complete clot lysis compared to 198 of 410 in the thrombolysis group | |
| 320 per 1000 | 774 per 1000 (454 to 1000) | |||||
| Bleeding (early, up to 1 month after treatment) | Study population | RR 2.45 | 1943 | ⊕⊕⊕⊝ | ||
| 23 per 1000 | 56 per 1000 (36 to 87) | |||||
| Post‐thrombotic syndrome (intermediate, 6 months to under 5 years after treatment) | Study population | RR 0.78 | 1393 | ⊕⊕⊕⊝ | 329 of 622 patients treated with standard anticoagulation developed PTS compared to 383 of 771 treated with thrombolysis. Additional subgroup analysis did not show any differences in PTS incidence between iliofemoral and femoropopliteal DVTs | |
| 529 per 1000 | 413 per 1000 (349 to 492) | |||||
| Post‐thrombotic syndrome (late, 5 years follow‐up after treatment) | Study population | RR 0.56 | 211 | ⊕⊕⊕⊝ | 75 of 107 patients treated with standard anticoagulation developed PTS compared to 41 of 104 treated with thrombolysis | |
| 701 per 1000 | 393 per 1000 (301 to 512) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Thrombolysis includes delivery of thrombolytics either systemically, loco‐regionally or by CDT. CDT may include the use of additional endovascular techniques | ||||||
| Study | Potential levels of leg vein included |
|---|---|
| proximal to calf | |
| proximal (femoral, common femoral, iliac vein with or without other involved ipsilateral veins) | |
| femoral and iliofemoral | |
| pelvic, iliofemoral, femoral | |
| not specified | |
| proximal | |
| femoral and iliofemoral | |
| popliteal or more proximal | |
| proximal | |
| not specified | |
| not specified | |
| calf up to iliac vein | |
| calf vein thrombosis only | |
| not specified | |
| leg or pelvic (popliteal or more proximal) | |
| not specified | |
| proximal | |
| popliteal up to inferior vena cava | |
| popliteal or more proximal |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Complete clot lysis (early, subgrouped by thrombolysis strategy) Show forest plot | 8 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [1.83, 12.33] |
| 1.1.1 Systemic | 7 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 3.65 [1.40, 9.56] |
| 1.1.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 10.55 [0.66, 168.79] |
| 1.1.3 CDT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 21.79 [1.38, 343.26] |
| 1.2 Complete clot lysis (intermediate, subgrouped by thrombolysis strategy) Show forest plot | 7 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.42, 4.12] |
| 1.2.1 Systemic | 4 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [1.46, 9.93] |
| 1.2.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.03, 2.97] |
| 1.2.3 CDT | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [0.52, 12.17] |
| 1.3 Complete clot lysis (late, subgrouped by thrombolysis strategy) Show forest plot | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.17, 62.63] |
| 1.3.1 Systemic | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 16.76 [1.03, 272.11] |
| 1.3.2 Loco‐regional | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3.3 CDT | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.33] |
| 1.4 Bleeding (early, subgrouped by thrombolysis strategy) Show forest plot | 19 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.58, 3.78] |
| 1.4.1 Systemic | 14 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.24, 3.19] |
| 1.4.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.41, 23.05] |
| 1.4.3 CDT | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.30 [1.67, 31.98] |
| 1.5 PTS (intermediate, subgrouped by thrombolysis strategy) Show forest plot | 6 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.66, 0.93] |
| 1.5.1 Systemic | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.31, 0.92] |
| 1.5.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.07] |
| 1.5.3 CDT | 3 | 1032 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.05] |
| 1.6 PTS by iliofemoral/fempop (intermediate, subgrouped by location) Show forest plot | 6 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 1.6.1 Iliofemoral DVT | 4 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.01] |
| 1.6.2 Femoropopliteal DVT | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.76, 1.27] |
| 1.6.3 Unspecified DVT | 2 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.92] |
| 1.7 PTS (late, subgrouped by thrombolysis strategy) Show forest plot | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.43, 0.73] |
| 1.7.1 Systemic | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.88] |
| 1.7.2 loco‐regional | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7.3 CDT | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.79] |
| 1.8 Any improvement in venous patency (early) Show forest plot | 9 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.35, 4.57] |
| 1.8.1 Systemic | 8 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.28, 3.70] |
| 1.8.2 CDT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 35.05 [2.28, 539.63] |
| 1.9 Stroke (early, subgrouped by thrombolysis strategy) Show forest plot | 19 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.34, 10.86] |
| 1.9.1 Systemic | 14 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.34, 10.86] |
| 1.9.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.9.3 CDT | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.10 Leg ulceration (intermediate, subgrouped by thrombolysis strategy) Show forest plot | 5 | 1033 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.39, 1.49] |
| 1.10.1 Systemic | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.53] |
| 1.10.2 Loco‐regional | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.17, 13.60] |
| 1.10.3 CDT | 2 | 880 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.54] |
| 1.11 Leg ulceration (late) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12 Mortality (early, subgrouped by thrombolysis strategy) Show forest plot | 10 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.31, 1.89] |
| 1.12.1 Systemic | 8 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.31, 1.89] |
| 1.12.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.12.3 CDT | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13 Mortality (intermediate, subgrouped by thrombolysis strategy) Show forest plot | 4 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.39, 1.69] |
| 1.13.1 Systemic | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.27, 3.43] |
| 1.13.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13.3 CDT | 2 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.31, 1.86] |
| 1.14 Mortality (late, subgrouped by thrombolysis strategy) Show forest plot | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.50] |
| 1.14.1 Systemic | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.24] |
| 1.14.2 CDT | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.30] |
| 1.15 Recurrent DVT (intermediate, subgrouped by thrombolysis strategy) Show forest plot | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.83] |
| 1.15.1 Systemic | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.37, 5.40] |
| 1.15.2 Loco‐regional | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.15.3 CDT | 3 | 1032 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.94, 1.84] |
| 1.16 Recurrent DVT (late, subgrouped by thrombolysis strategy) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 Systemic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.16.2 CDT | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.34, 1.18] |
| 1.17 Pulmonary embolism (early, subgrouped by thrombolysis strategy) Show forest plot | 6 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.33, 3.05] |
| 1.17.1 Systemic | 5 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.36, 4.10] |
| 1.17.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.17.3 CDT | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 1.18 Venous function (intermediate, subgrouped by thrombolysis strategy) Show forest plot | 3 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.86, 5.54] |
| 1.18.1 Systemic | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.59, 1.83] |
| 1.18.2 Loco‐regional | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.18.3 CDT | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [1.41, 7.19] |

