اینفیوژن داخل وریدی میدازولام برای آرامسازی نوزادان در بخش مراقبتهای ویژه نوزادان
چکیده
پیشینه
القای مناسب آرامسازی، برای کودکانی که تحت پروسیجرهای ناخوشایند قرار میگیرند، ممکن است اضطراب را کاهش داده و از بروز عوارض پیشگیری کند. میدازولام (midazolam) یک بنزودیازپین کوتاهاثر است که بهطور فزایندهای از آن در بخشهای مراقبتهای ویژه نوزادان (NICUs) استفاده میشود. با این حال، اثربخشی آن به عنوان یک آرامبخش در نوزادان، به طور سیستماتیک ارزیابی نشده است.
اهداف
هدف اولیه
ارزیابی اثربخشی اینفیوژن داخل وریدی میدازولام در القای آرامسازی، که با معیارهای رفتاری و/یا فیزیولوژیکی سطوح آرامسازی، در نوزادان بهشدت بیمار بستری در NICU ارزیابی شدند.
اهداف ثانویه
بررسی اثرات استفاده از اینفیوژن داخل وریدی میدازولام برای آرامسازی، بر عوارض شامل موارد زیر:
1. بروز هموراژی داخل بطنی (IVH)/لوکومالاسی اطراف بطنی (PVL).
2. مورتالیتی.
3. بروز عوارض جانبی مرتبط با استفاده از میدازولام (افت فشار خون، ناهنجاریهای نورولوژیکی).
4. تعداد روزهای استفاده از ونتیلاسیون.
5. تعداد روزهای استفاده از اکسیژن مکمل.
6. بروز پنوموتوراکس.
7. طول مدت اقامت در NICU (تعداد روزها).
8. پیامدهای طولانیمدت مرتبط با تکامل سیستم عصبی.
روشهای جستوجو
ما از استراتژی جستوجوی استاندارد گروه نوزادان در کاکرین برای جستوجو در پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL؛ شماره 5؛ 2016)؛ MEDLINE via PubMed (1966 تا 16 جون 2016)؛ Embase (1980 تا 16 جون 2016) و Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 تا 16 جون 2016) استفاده کردیم. ما بانکهای اطلاعاتی کارآزمایی بالینی، مجموعه مقالات کنفرانس، و فهرست منابع مقالات بازیابی شده را برای یافتن کارآزماییهای تصادفیسازی و کنترل شده و کارآزماییهای شبه‐تصادفیسازی شده جستوجو کردیم.
معیارهای انتخاب
ما برای مرور، کارآزماییهای تصادفیسازی و شبه‐تصادفیسازی و کنترل شده را از اینفیوژن داخل وریدی میدازولام برای القای آرامسازی در نوزادان 28 روزه یا کوچکتر انتخاب کردیم.
گردآوری و تجزیهوتحلیل دادهها
ما با توجه به پیامد اولیه سطح آرامسازی، دادهها را خلاصه کردیم. ما پیامدهای ثانویه را مانند هموراژی داخل بطنی، لوکومالاسی اطراف بطنی، مرگومیر، طول مدت اقامت در NICU و عوارض جانبی مرتبط با میدازولام ارزیابی کردیم. در موارد لازم، متاآنالیزهایی را با استفاده از خطرهای نسبی (RRs) و تفاوتهای خطر (RDs) انجام دادیم و اگر RD از نظر آماری معنیدار بود، تعداد افراد مورد نیاز جهت درمان تا حصول یک پیامد مثبت اضافی (NNTB) یا یک پیامد مضر اضافی (NNTH) را همراه با 95% فاصله اطمینان (95% CI) برای متغیرهای طبقهبندی شده و تفاوتهای میانگین وزندهی شده (WMDs) برای متغیرهای پیوسته محاسبه کردیم. ناهمگونی را با انجام آزمون I‐square (I2) ارزیابی کردیم.
نتایج اصلی
ما در این مرور، 3 کارآزمایی را شامل 148 نوزاد وارد کردیم. ما هیچ کارآزمایی جدیدی را برای این بهروزرسانی شناسایی نکردیم. با استفاده از مقیاسهای مختلف آرامسازی، هر مطالعه سطح بالاتری را از حالت آرامسازی در گروه میدازولام نسبت به گروه دارونما (placebo) نشان داد که از نظر آماری معنیدار بود. با این حال، هیچ کدام از مقیاسهای آرامسازی مورد استفاده، در نوزادان پرهترم تایید نشده؛ بنابراین، ما نمیتوانیم اثربخشی میدازولام را در این جمعیت مشخص کنیم. طول مدت اقامت در NICU در گروه میدازولام، بهطور معنیداری طولانیتر از گروه دارونما بود (WMD؛ 5.4 روز؛ 95% CI؛ 0.40 تا 10.5؛ I2 = 0%؛ 2 مطالعه، 89 نوزاد). یک مطالعه (43 نوزاد) نمرات پروفایل درد نوزاد نارس (PIPP) را به طور قابل توجهی در طول اینفیوژن میدازولام نسبت به تزریق دکستروز (دارونما) پایینتر گزارش کرد (MD: ‐3.80؛ 95% CI؛ 5.93‐ تا 1.67‐). مطالعه دیگر (46 نوزاد) بروز بیشتر عوارض نورولوژیکی را در سن 28 روزگی پس از تولد در گروه میدازولام در مقایسه با گروه مورفین نشان داد (مرگومیر، IVH یا PVL درجه III یا IV) (RR: 7.64؛ 95% CI؛ 1.02 تا 57.21؛ RD: 0.28؛ 95% CI؛ 0.07 تا 0.49؛ NNTH: 4؛ 95% CI؛ 2 تا 14) (تستها برای ناهمگونی قابل اجرا نبود). ما کیفیت این کارآزماییها را طبق ارزیابی GRADE بر اساس پیامدهای زیر، با کیفیت متوسط در نظر گرفتیم: مرگومیر طی بستری در بیمارستان، طول مدت اقامت در NICU، کفایت داروهای ضددرد طبق نمرات PIPP و پیامدهای نورولوژیکی ضعیف تا سن 28 روزگی پس از تولد.
نتیجهگیریهای نویسندگان
یافتهها برای توصیه به استفاده از اینفیوژن داخل وریدی میدازولام، بهعنوان یک داروی آرامبخش برای نوزادان تحت مراقبت ویژه، کافی نیستند. این مرور نگرانیهایی را در مورد ایمنی استفاده از میدازولام در نوزادان ایجاد میکند. تحقیقات بیشتری روی اثربخشی و ایمنی میدازولام در نوزادان لازم است.
PICOs
خلاصه به زبان ساده
اینفیوژن داخل وریدی میدازولام برای آرامسازی نوزادان در بخش مراقبتهای ویژه نوزادان
سوال مطالعه مروری: برای نوزادان بیمار بستری در بخش مراقبتهای ویژه نوزادان (NICU)، میدازولام (midazolam) تجویزی از راه دریپ (drip) پیوسته داخل وریدی، به عنوان یک آرامبخش برای کاهش استرس، که با تغییر در رفتار و علائم حیاتی اندازهگیری میشود، تا چه اندازه مؤثر است؟
پیشینه: القای مناسب حالت آرامسازی (sedation) برای کودکانی که حین دریافت مراقبتهای ویژه، تحت پروسیجرهای ناخوشایند قرار میگیرند، ممکن است اضطراب را کاهش داده و از عوارض پیشگیری کند. میدازولام آرامبخشی است که به طور فزایندهای در NICUها استفاده میشود. با این حال، پژوهشگران بهطور سیستماتیک به مرور شواهد نپرداختهاند تا ببینند که این دارو برای نوزادان در این شرایط مؤثر و بیخطر است یا خیر.
ویژگیهای مطالعه: برای ورود در این مرور، کارآزماییهای تصادفیسازی و کنترل شدهای را انتخاب کردیم که تجویز دریپ پیوسته داخل وریدی میدازولام را به عنوان یک آرامبخش در نوزادان 28 روزه یا کوچکتر بررسی کردند.
نتایج کلیدی: ما 3 کارآزمایی بالینی را در این مرور لحاظ کردیم. با استفاده از مقیاسهای مختلف اندازهگیری سطح آرامسازی، هر مطالعه نشان داد که میدازولام در القای آرامسازی در نوزادان مؤثر است. با این حال، اعتبار مقیاسهای آرامسازی مورد استفاده در این مطالعات در نوزادان اثبات نشده است؛ بنابراین، ما نمیتوانیم مطمئن باشیم که میدازولام در واقع یک آرامبخش موثر برای نوزادان است. بهعلاوه، یک مطالعه نشان داد که کودکان دریافتکننده میدازولام، به طور قابلتوجهی با خطر بیشتر مرگومیر یا آسیبدیدگی مغزی روبهرو هستند و ترکیب نتایج حاصل از 2 مطالعه نشان داد که استفاده از میدازولام ممکن است طول مدت اقامت را در NICU طولانیتر کند.
صنعت: یکی از مطالعات وارد شده در این مرور، از حمایت بخش صنعت استفاده کرده و در 2 مطالعه دیگر، صنعت تمام داروهای مورد استفاده را در مطالعه تامین کرد.
کیفیت شواهد: ما کیفیت شواهد را در مورد پیامدهای مرگومیر طی اقامت بیمارستانی، طول مدت اقامت در NICU، درد و پیامدهای نورولوژیکی در 28 روزگی ارزیابی کرده و شواهد را با کیفیت متوسط یافتیم، زیرا شواهد کافی در دسترس نبودند. بنابراین، نتیجه میگیریم که شواهد کافی به نفع استفاده از میدازولام بهعنوان یک داروی آرامبخش در نوزادانی که تحت مراقبتهای ویژه قرار میگیرند، وجود ندارد. برای اطلاع از ایمنی و اثربخشی میدازولام در این جمعیت، انجام پژوهش بیشتری لازم است.
Authors' conclusions
Summary of findings
| Midazolam infusion compared with placebo for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: placebo infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Midazolam | |||||
| Mortality during hospital stay | High‐risk population | RR 0.79 (0.40 to 1.56) | 122 | ⊕⊕⊕⊝ | Risk of bias for these 3 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 3 studies were conducted in the target population of newborn infants. | |
| 220 per 1000 | 165 per 1000 | |||||
| Length of NICU stay (days) | Mean length of NICU stay ranged across control groups from 9 to 37.5 days. | WMD of NICU stay for intervention groups was 5.4 days longer. | WMD 5.4 days (0.4 to 10.5) | 89 | ⊕⊕⊕⊝ | Risk of bias for these 2 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 2 studies were conducted in the target population of newborn infants. |
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 12.7. | Mean PIPP score in the intervention group was lower at 8.9. | MD ‐3.80 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 1.34 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 230 per 1000 | 310 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| Midazolam infusion compared with morphine infusion for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: morphine infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Morphine | Midazolam | |||||
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 7.9. | Mean PIPP score in the intervention group was 8.9. | MD 1.00 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 7.64 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 318 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
Background
Description of the condition
Term and preterm infants are capable of perceiving pain and stress (Anand 1987). In the neonatal intensive care unit (NICU), supportive and investigative management of sick infants frequently requires painful and uncomfortable procedures. However, because pain and stress are subjective phenomena and are difficult to evaluate in preverbal infants, use of appropriate analgesia and sedatives is often overlooked by care providers. It has been suggested that responses to pain may compromise clinical conditions (Anand 1992), and that adequate sedation during mechanical ventilation may decrease stress (Quinn 1993) and facilitate effective ventilation, so that complications such as pneumothoraces and intraventricular haemorrhage (IVH) may be prevented (Greenough 1983; Perlman 1985).
Description of the intervention
Benzodiazepines, administered as intravenous infusions or as intravenous boluses, can be used to provide sedation, but not analgesia, in many clinical settings. Midazolam is a short‐acting benzodiazepine that has been used increasingly in the NICU.
How the intervention might work
Benzodiazepines are a class of sedatives that act on specific receptors in the central nervous system. These receptors, which are present in the foetus from seven weeks' gestation (Hebebrand 1988), potentiate the neuronal inhibitory pathways mediated by gamma‐aminobutyric acid (GABA) (Jacqz‐Aigrain 1996). Researchers have studied the pharmacokinetics of midazolam in neonates. Midazolam is preferred over other benzodiazepines because of its water solubility and rapid clearance (Jacqz‐Aigrain 1992). Although its elimination half‐life is significantly shorter than that of other benzodiazepines, such as diazepam, its elimination is delayed in preterm neonates compared with older infants and children (Lee 1999). Functional immaturity of hepatic and renal systems in preterm neonates probably accounts for the slower elimination of midazolam. In a recent very large cohort study conducted in Europe, midazolam was given to 576 (9%) of the total cohort of 6680 neonates and to 536 (25%) of 2142 neonates who were tracheally ventilated (Carbajal 2015). Overall, midazolam was by far the sedative most commonly used. It was given to 25% of neonates who had tracheal ventilation, and its use ranged from 0% to 73% across European countries, despite few clinical data to lend support for midazolam sedation in neonates.
It is of note that in an animal model, Koch 2008 reported paradoxical effects of midazolam on nociception and sedation in rats between postnatal days 3 and 10. Midazolam failed to sedate young rats and instead caused an excitatory effect by sensitising their flexor reflex activity. Investigators did not observe the sedative effects of midazolam in supraspinal centres until later in life, after maturation. These results highlight the need for better understanding of the ontogeny of pharmacological effects of drugs such as midazolam that are used routinely in NICUs.
Why it is important to do this review
The effectiveness of intravenous midazolam as a sedative in neonates has not been systematically reviewed. Moreover, its safety at the currently recommended dosage in critically ill neonates has not been well established.
Objectives
Primary objective
To assess the effectiveness of intravenous midazolam infusion for sedation, as evaluated by behavioural and/or physiological measurements of sedation levels, in critically ill neonates in the NICU.
Secondary objectives
To assess effects of intravenous midazolam infusion for sedation on complications including the following.
-
Incidence of intraventricular haemorrhage (IVH)/periventricular leukomalacia (PVL).
-
Mortality.
-
Occurrence of adverse effects associated with the use of midazolam (hypotension, neurological abnormalities).
-
Days of ventilation.
-
Days of supplemental oxygen.
-
Incidence of pneumothorax.
-
Length of NICU stay (days).
-
Long‐term neurodevelopmental outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials and quasi‐randomised trials in which the use of intravenous midazolam infusion was compared with placebo or other sedatives in neonates undergoing intensive care.
Types of participants
We included infants aged 28 days or younger who were admitted to the NICU requiring sedation for medical interventions.
Types of interventions
Interventions included continuous intravenous infusion of midazolam administered at a dose of 20 microgram/kg/h to 60 microgram/kg/h for at least 24 hours for sedation during mechanical ventilation and radiological investigative procedures.
We excluded studies that used a combination of midazolam and an analgesic for neonates undergoing painful procedures. We also excluded studies that investigated the use of intravenous bolus doses of midazolam, unless the bolus was followed by an infusion; and studies examining use of midazolam as an anaesthetic induction agent or as an anticonvulsant.
Types of outcome measures
Primary outcomes
The primary outcome was level of sedation, evaluated by:
-
behavioural measures: facial actions, excitability, muscle tone, physical movements and respiratory behaviour, which may be evaluated by age‐appropriate scoring systems; and
-
physiological parameters: changes in heart rate, respiratory rate, blood pressure, oxygen saturation and plasma cortisol or catecholamine levels, measured at baseline and at regular intervals during midazolam administration.
Secondary outcomes
-
IVH (defined by classification of Papile et al (Papile 1978)).
-
PVL (defined as periventricular cysts on brain imaging, with exclusion of subependymal or choroid plexus cysts).
-
Mortality (death within 28 days of life).
-
Adverse effects associated with use of midazolam: hypotension (significant drop from baseline compared with controls), neurological abnormalities (epileptiform activities, movement disorders, myoclonus, hypertonia, hypotonia).
-
Days of mechanical ventilation.
-
Days of supplemental oxygen use.
-
Pneumothorax.
-
Days of NICU stay.
-
Neurodevelopmental outcomes, as evaluated by a validated developmental assessment tool.
-
Neurobehavioural Assessment of Preterm Infants (NAPI) (Snider 2005).
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1966 to 16 June 2016); Embase (1980 to 16 June 2016); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 16 June 2016), using the following search term: (midazolam), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); the ISRCTN Registry).
For previous editions of this review, we identified randomised and quasi‐randomised controlled trials of intravenous midazolam in infants from the Cochrane Central Register of Controlled Trials (CENTRAL; 2012, Issue 3) in the Cochrane Library; MEDLINE (from 1985 to March 2012); Embase (1980 to 2012) and CINAHL (1980 to 2012), using the medical subject headings (MeSH): midazolam; infant; newborn. We searched for abstracts published in Pediatric Academic Societies Meetings Abstract Archives from 1990 to 2011. We imposed no language restrictions. We attempted to contact investigators of studies meeting the inclusion criteria to gather additional data for analysis. We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; controlled‐trials.com; who.int/ictrp), and we searched the Web of Science to identify any trial that quoted the earliest study that we identified (Jacqz‐Aigrain 1994).
Searching other resources
In addition, we manually searched bibliographies of articles and personal files and imposed no language restrictions. We attempted to contact investigators of studies meeting the inclusion criteria to gather additional data for analysis. We did not attempt to identify unpublished studies. We excluded studies involving neonates and older infants and children if we could not extract data for neonates.
Data collection and analysis
We used the standard method of the Cochrane Neonatal Review Group for performing systematic reviews (http://neonatal.cochrane.org/resources‐review‐authors).
Selection of studies
We included randomised and quasi‐randomised controlled trials involving neonates aged 28 days or younger that included a treatment group and a placebo group. We accepted for the review studies that reported outcome measures including physiological, behavioural and hormonal changes, as well as adverse neurological outcomes.
We excluded studies involving neonates and older infants and children if we could not extract data for neonates.
Two review authors (EN, AT) independently decided to include or exclude a specific study. When discrepancies occurred, the three review authors (EN, AT, AO) made the decision by consensus.
Data extraction and management
We created a data collection form on which we abstracted the following data from the included studies: demographics of participants, age at enrolment into the study, inclusion and exclusion criteria, sample size, treatment and control group regimens and outcomes. Two review authors (EN, AT) independently abstracted the data and resolved differences by consensus.
Assessment of risk of bias in included studies
Two review authors (EN, AO) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains: selection bias, performance bias, attrition bias, reporting bias and any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 2 for a detailed description of risks of bias for each domain.
Measures of treatment effect
We performed statistical analyses using Review Manager 5.1 software. We analysed categorical data using risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial (NNTB) or harmful outcome (NNTH). We analysed continuous data by using weighted mean difference (WMD) and reported the 95% confidence interval (CI) for all estimates.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting forest plots (if we included at least 10 trials in one analysis) and quantified the impact of heterogeneity by using the I2 statistic. If we detected statistical heterogeneity, we planned to explore possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments) by performing post hoc subgroup analyses.
Data synthesis
When we identified at least two RCTs that evaluated the effectiveness of intravenous midazolam infusions by examining the same outcome measures, we pooled the results to obtain an overall estimate of effect size using RevMan 5.1.4 (RevMan 2011). We used the Mantel‐Haenszel method for estimates of typical RR and RD, and the inverse variance method for measured quantities. We used the fixed‐effect model for all meta‐analyses.
Quality of the evidence
For the 2016 update, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schunemann 2013), to assess the quality of evidence for the following clinically relevant outcomes: mortality during hospital stay, length of NICU stay, adequacy of analgesia as measured by the Premature Infant Pain Profile (PIPP) (Stevens 1996) and poor neurological outcomes by 28 days' postnatal age. In terms of the primary outcome of level of sedation, none of the sedation scales used in these studies had been validated in preterm infants; therefore, we could not ascertain the effectiveness of midazolam in this population as reported by these studies and did not subject this outcome to GRADE assessment.
We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (and two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates and presence of publication bias.
The GRADE approach assesses the quality of a body of evidence and assigns one of four grades.
-
High: We are very confident that the true effect lies close to the estimate of effect.
-
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
-
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
-
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Two review authors (EN, AO) independently assessed the quality of the evidence for each of the outcomes above. We used the GRADEpro Guideline Development Tool to create ‘Summary of findings’ tables to report the quality of evidence. See Appendix 3 for details on assessment of quality of the evidence.
Subgroup analysis and investigation of heterogeneity
We prospectively planned no subgroup analyses.
Results
Description of studies
Results of the search
We presented results of the search in the study flow diagram for the review update provided in Figure 1. We identified six RCTs on the use of intravenous midazolam in infants; we included three of these and excluded three.
Literature searches conducted in September 2009, March 2012 and June 2016 identified no additional trials.
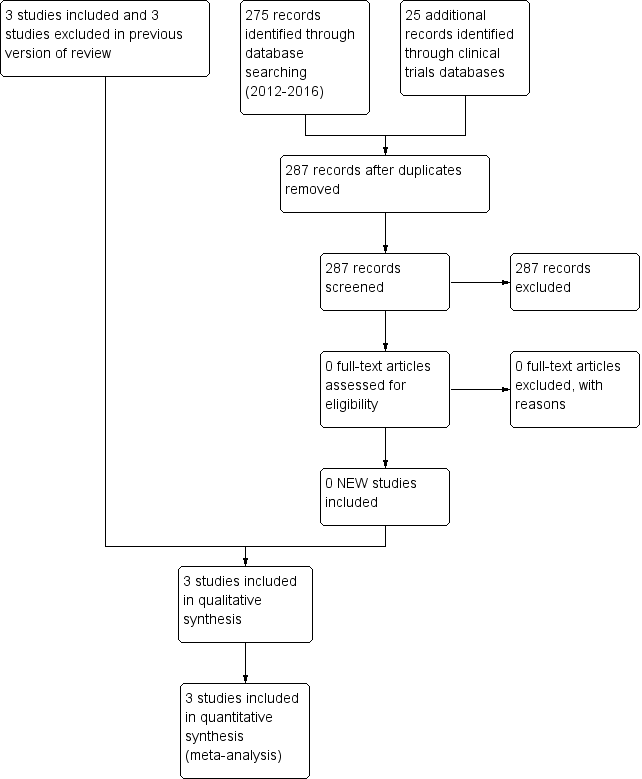
Study flow diagram: review update.
Included studies
For details, see Characteristics of included studies.
Jacqz‐Aigrain 1994 randomly assigned 46 preterm infants (25 at < 33 weeks' gestation and 21 at ≥ 33 weeks' gestation) ≤ 48 hours of age to receive midazolam infusion or manufactured placebo for five days while mechanically ventilated for respiratory distress syndrome. Twenty‐four infants received midazolam and 22 received placebo infusions. Researchers withdrew one infant in the midazolam group because of major neurological abnormalities at 24 hours of age; and two infants from the midazolam group and two from the placebo group within 72 hours owing to rapid clinical improvement. They noted contamination in one infant in the placebo group (midazolam was detectable in the serum at 24 hours). Baseline characteristics did not differ between groups. Severity of illness, as measured by mean airway pressure (MAP) while ventilated and fraction of inspired oxygen (FiO2) from the time of enrolment to the end of the study, was not significantly different between groups. Investigators administered midazolam as an infusion at 60 microgram/kg/h for up to five days in infants at ≥ 33 weeks' gestation, and at 60 microgram/kg/h for one day followed by 30 microgram/kg/h for up to a total of five days in infants at < 33 weeks' gestation. They did not report the duration of the infusion. Weaning of sedatives was allowed after at least 48 hours of administration, but investigators did not specify a weaning protocol. The primary outcome was adequacy of sedation as measured by a behavioural score adapted from the clinical neurological and behavioural scoring system of Barrier (Barrier 1989) and by changes in physiological variables (heart rate and blood pressure). The sedation score consisted of five items that assessed facial expression, sucking, spontaneous motor activity, excitability/responsiveness to stimulation and excessive flexion, with scores ranging from 0 (sedation) to 5 (inadequate sedation). Care providers measured the sedation score four times per day during treatment (nurses twice, physicians twice). Secondary outcomes included days of ventilation support, days of supplemental oxygen, surfactant use, duration of NICU stay and common complications of preterm birth (e.g. pneumothorax, pulmonary interstitial emphysema, hypotension, chronic lung disease, necrotising enterocolitis, intracranial haemorrhage, persistent pulmonary hypertension of the newborn, death). Researchers reported outcomes for all 46 infants.
In a multi‐centre randomised pilot study (Anand 1999), investigators assigned 67 preterm infants of 24 to 32 weeks' gestation who were ≤ 72 hours of age and who were ventilated for less than eight hours to receive midazolam infusion, morphine infusion or dextrose placebo infusion for as long as sedation was considered necessary up to a maximum of 14 days. Twenty‐two infants received midazolam infusion, 24 received morphine infusion and 21 received dextrose placebo. The three groups did not differ significantly in baseline characteristics. Severity of illness at birth, as assessed by the Clinical Risk Index for Babies (CRIB) score (The International Neonatal Network), showed no significant differences among groups at birth (P = 0.24). However, severity of illness as measured by the Neonatal Medical Index (NMI) (Korner 1993) on the basis of response variables during the hospital stay showed significant differences in the distribution of risk categories among the three groups at discharge (P = 0.01). Researchers administered midazolam at 200 microgram/kg loading dose followed by an infusion of 20 microgram/kg/h, 40 microgram/kg/h or 60 microgram/kg/h for infants of gestational age 24 to 26 weeks, 27 to 20 weeks or 30 to 33 weeks, respectively. They administered morphine at 100 microgram/kg loading dose followed by an infusion of 10 microgram/kg/h, 20 microgram/kg/h or 30 microgram/kg/h for infants of gestational age 24 to 26 weeks, 27 to 29 weeks or 30 to 33 weeks, respectively. Duration of the infusion was not different among groups (5.1 days vs 3.4 days vs 5.0 days in the midazolam, morphine and placebo groups, respectively; P = 0.37). If necessary, they provided additional boluses of morphine and documented the frequency and amount given as measures of inadequate sedation. Investigators used a standardised protocol in weaning sedatives. The primary outcome was the incidence of adverse neurological events (defined as neonatal death, grade III or IV IVH or PVL). Researchers measured the adequacy of sedation by obtaining the COMFORT score, an eight‐item behavioural and physiological measurement of distress in the paediatric intensive care unit (Ambuel 1992). This score includes assessment of alertness, calmness/agitation, respiratory response, physical movement, mean arterial blood pressure, heart rate, muscle tone and facial tension, with scores ranging from 8 (sedated) to 40 (not adequately sedated). They measured adequacy of analgesia by obtaining the Premature Infant Pain Profile (PIPP) (Stevens 1996) in response to tracheal suctioning. The PIPP score includes assessment of gestational age, behavioural state, heart rate, oxygen saturation, brow bulge, eye squeeze and nasolabial furrow, with scores ranging from 0 (adequate analgesia) to 21 (inadequate analgesia). They obtained the two scores on all infants at baseline, after 24 hours of infusion and at 10 to 12 hours after discontinuation of the infusion. Other secondary outcomes included days of mechanical ventilation, continuous positive airway pressure, supplemental oxygen use, incidence of pneumothorax, duration of NICU and hospital stay, days to full enteral (full strength, full gavage, full oral) feeds, daily weight gain and neurodevelopmental outcomes at 36 weeks' corrected age as measured by Neurobehavioral Assessment of the Premature Infant (NAPI) examination cluster scores (Korner 1991). Researchers reported outcomes for all 67 infants.
Arya 2001 randomised 33 infants with birth weight < 2000 grams and requiring mechanical ventilation during the first week of life to receive midazolam or placebo infusion for sedation. Seventeen infants received midazolam and 16 received placebo. The two groups were similar in baseline characteristics. Severity of respiratory illness, as measured by peak inspiratory pressure (PIP), MAP, oxygenation index (OI) and the alveolar‐arterial oxygen gradient (AaDO2), was similar between the two groups at the time of enrolment. Investigators administered midazolam intravenously at 200 microgram/kg loading dose followed by an infusion of 60 microgram/kg/h. Infants in both groups also received morphine infusion at 10 microgram/kg/h during the study period. The study concentrated on the first 48 hours of midazolam infusion and did not report on duration of benzodiazepine use nor on method of weaning. Three infants in each group did not complete the first 24 hours of the study, and four in each group did not complete 48 hours of the study. Reasons for withdrawal were death (13 infants) and extubation (one infant). Researchers included these infants in the analyses on an intention‐to‐treat basis. The primary outcome was adequacy of sedation as measured by a behavioural score adapted from the clinical neurological and behavioural scoring system of Barrier (Barrier 1989). This is the same scoring system used in Jacqz‐Aigrain 1994. Study authors assessed infants for adequacy of sedation before midazolam administration, then every six hours over the 48‐hour study period. Other measured outcomes included changes in physiological variables (heart rate and blood pressure), changes in oxygen requirement (FiO2) and ventilation requirement (PIP, positive end‐expiratory pressure (PEEP), ventilator rate) and arterial blood gas as measured by mean daily values. Investigators documented complications related to mechanical ventilation (air leak, IVH) and potential adverse effects of midazolam (epileptiform movements, hypotension, tachycardia and oliguria) while reporting the duration of ventilation. They reported no long‐term outcomes but described outcomes for all 33 infants in the study.
Excluded studies
For details, see Characteristics of excluded studies. We excluded one trial that used a single bolus dose of intravenous midazolam (McCarver‐May 1996) and another trial that used intravenous midazolam for anaesthetic induction (Kawakami 1998). The third excluded trial (Parkinson 1997) used midazolam for sedation in individuals from one day to 15 years of age, and we could not extract data pertaining to neonates. The three trials included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) reported on the effectiveness of midazolam infusion and included a total of 146 infants.
Risk of bias in included studies
For details, see the Risk of bias in included studies table, Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
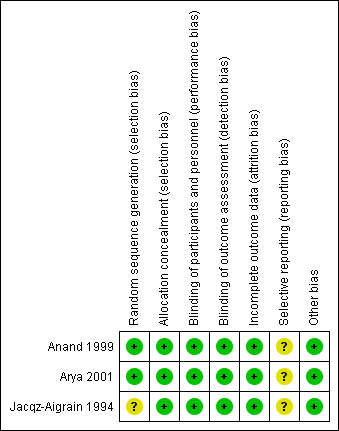
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of bias for random sequence generation was low in two studies (Anand 1999; Arya 2001) and unclear in one study (Jacqz‐Aigrain 1994). All three studies adequately concealed allocation (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994).
Blinding
Risk of performance and detection was low in all three studies (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994).
Incomplete outcome data
Investigators reported outcomes for all randomised infants.
Selective reporting
A protocol was not available for any of the three studies, so we could not judge whether deviations from the protocol occurred.
Other potential sources of bias
Included studies appeared free of other sources of bias. Anand 1999 received support from industry, and Arya 2001 and Jacqz‐Aigrain 1994 received midazolam and placebo from a pharmaceutical company.
Only Arya 2001 performed sample size calculation, and Anand 1999 stated that the study was a pilot trial. All three studies performed statistical analyses using an intention‐to‐treat approach.
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2
Midazolam infusion versus placebo (comparison 1)
Anand 1999 evaluated outcomes by performing analysis of variance to detect statistically significant differences among midazolam, morphine and placebo groups. For this review, we performed comparisons between the midazolam group and the placebo group on relevant continuous outcome variables using information provided by the publication (sample size, mean, standard deviation (SD), standard error of the mean). For the current update of this review, we included an additional comparison of midazolam versus morphine infusion for the outcome of PIPP during drug infusion, as well as for poor neurological outcome by 28 days' postnatal age.
Primary outcomes
Primary outcomes involved level of sedation, as evaluated by:
-
behavioural measures: facial actions, excitability, muscle tone, physical movements and respiratory behaviour, which may be evaluated by age‐appropriate scoring systems; and
-
physiological parameters: changes in heart rate, respiratory rate, blood pressure, oxygen saturation and plasma cortisol or catecholamine levels, measured at baseline and at regular intervals during midazolam administration.
In Additional Table 1 ('Sedation scores used in included studies'), we present some basic information about measures of sedation used. All investigators applied the scores to infants and children, most of whom were older than one month, and did not include preterm newborn infants. None of the tools used to assess sedation had been validated for use in newborn infants. We therefore reported the results of each included study separately, as did the authors of the included trials. We did not enter data for sedation scores into RevMan analyses. For the same reasons, we did not include sedation scores in the 'Summary of findings' tables.
| Study reference for included trial | Name of score used | Reference for the score | Age of infants/children subjected to the score | Score validated in newborns? |
| COMFORT Scale | Ambuel 1992 ‐ 37 participants (age newborn to 204 months (mean 37.1; SD 52.7)) Marx 1994 ‐ children (age 0 to 102 months (mean age > 1 year) | No | ||
| Sedation score | Barrier 1989 ‐ 23 infants (age 1 to 7 months) | No | ||
| Behaviour score | Craig 1984 ‐ 30 children (age 2 to 24 months) Barrier 1989; Robieux 1991 ‐ 41 infants and toddlers (age 3 to 36 months) | No |
Sedation scores (behavioural measures)
Jacqz‐Aigrain 1994 enrolled and reported on 46 newborn infants. Sedation scores (behavioural measures) (Barrier 1989; Craig 1984; Robieux 1991) were not different between groups at baseline. The midazolam group had consistently lower scores (more sedated) than the placebo group on all days, as assessed by both nurses and physicians (P < 0.05). Investigators observed significant decreases in sedation scores from baseline (mean (SD) score 1.9 (0.4)) to day 1 (score 1.1 (0.3); P < 0.01), day 2 (score 0.8 (0.2); P < 0.010) and day 3 (score 1.1 (0.3); P < 0.05) in the midazolam group (per nurses' scores) and significant increases in the placebo group from baseline (mean (SD) score 1.7 (0.3)) to day 1 (score 2.6 (0.3); P < 0.01) (per physicians' scores).
Anand 1999 enrolled and reported on 67 preterm infants and found statistically significantly lower COMFORT scores (more sedated) in the midazolam group during the infusion (mean (SD) score 14.9 (4.6) vs 17.5 (4.2); P = 0.04), although they noted no statistically significant differences in scores between the two groups before infusion and 12 hours after the infusion was stopped (mean (SD) score 15.9 (3.8) vs 15.6 (3.2); P = 0.8, before the infusion; and 15.8 (4.7) vs 16.2 (4.1); P = 0.76, after the infusion). In response to tracheal suctioning, the midazolam group had significantly lower PIPP scores (more sedated) during the infusion compared with the placebo group (mean (SD) score 8.9 (3.3) vs 12.7 (3.8); P < 0.001). The requirement for additional morphine was not statistically different between midazolam and placebo groups, but midazolam groups tended to require fewer additional morphine doses than placebo groups.
Arya 2001 enrolled and reported on 33 preterm newborns. Sedation scores were not significantly different between the two groups at baseline. The midazolam group had statistically significantly lower sedation scores (more sedated) compared with the placebo group from 18 hours after the start of infusion (median (range) score 0 (0 to 3) vs 1 (0 to 4); P < 0.05). This trend continued for the study duration (up to 48 hours), with statistically significant differences noted at 36 (median (range) score 0 vs 1 (0 to 3); P < 0.05), 42 (0 (0‐3) vs 1 (0‐3); P < 0.05) and 48 hours (0 (0 to 2) vs 1 (0 to 3); P < 0.05) of study drug infusion.
Even though Jacqz‐Aigrain 1994 and Arya 2001 used the same sedation score, we could not combine results on adequacy of sedation by meta‐analysis, as Arya 2001 presented sedation scores as median values and ranges, whereas Jacqz‐Aigrain 1994 presented results as means and SDs.
Sedation scores (physiological parameters)
Jacqz‐Aigrain 1994 enrolled and reported on 46 newborn infants. The physiological parameters heart rate and blood pressure did not differ between groups at baseline but were significantly lower in the midazolam group than in the placebo group on days 1 and 2. These trends continued through to day 5, although they were not statistically significant. One infant in the midazolam group and seven in the placebo group (P < 0.05) were inadequately sedated and required fentanyl and muscle relaxants within 72 hours. Two infants in the midazolam group received fentanyl within 72 hours (Jacqz‐Aigrain 1994).
Secondary outcomes
Intraventricular haemorrhage (outcome 1.1)
Neither Jacqz‐Aigrain 1994 nor Anand 1999 found a statistically significant difference between midazolam and placebo groups in the incidence of IVH. Arya 2001 observed no intracranial haemorrhage during the 48‐hour study period in all enrolled neonates. Meta‐analysis of results of the three studies (n = 122) showed no statistically significant differences in the incidence of IVH of any grade (typical RR 1.68, 95% CI 0.87 to 3.24; typical RD 0.12, 95% CI ‐0.02 to 0.26; I2 = 0% (none) for RR but 64% (moderate) for RD; Analysis 1.1).
Mortality (outcome 1.2)
Neither Jacqz‐Aigrain 1994 nor Anand 1999 found a statistically significant difference in mortality between midazolam and placebo groups. Arya 2001 did not report mortality as an outcome measure. However, six infants in the midazolam group and seven in the placebo group died before completing the 48‐hour study period. Meta‐analysis of results of the three studies showed no evidence of effect (typical RR 0.79, 95% CI 0.40 to 1.56; typical RD ‐0.05, 95% CI ‐0.18 to 0.09; I2 = 0% (none for both RR and RD); Analysis 1.2).
Days on ventilation (outcome 1.3)
Combined results of Jacqz‐Aigrain 1994 and Anand 1999 showed no statistically significant difference in days of ventilation (WMD 3.6 days, 95% CI ‐0.2 to 7.4 days; I2 = 0% (none); Analysis 1.3).
Arya 2001 presented data on days of ventilation as median values and ranges, so we could not combine these data with data from the other two studies. Median duration of ventilation (range) was 53 hours (7 to 216) in the midazolam group and 59 hours (13 to 194) in the placebo group.
Days on supplemental oxygen use (outcome 1.4)
Combined results of Jacqz‐Aigrain 1994 and Anand 1999 showed no statistically significant difference in days of supplemental oxygen use (WMD 0.6 days, 95% CI ‐5.3 to 6.6 days; I2 = 0% (none); Analysis 1.4).
Pneumothorax (outcome 1.5)
All three studies (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) reported on pneumothorax (n = 122) and observed no significant effect of midazolam versus placebo for this outcome (typical RR 1.08, 95% CI 0.41 to 2.84; typical RD 0.01, 95% CI ‐0.10 to 0.12; I2 = 0% (none for both); Analysis 1.5).
Length of NICU stay (outcome 1.6)
Jacqz‐Aigrain 1994 and Anand 1999 reported that length of NICU stay was not statistically significantly different between midazolam and placebo groups. Meta‐analysis of these data showed that the midazolam group had a statistically significantly longer length of stay in the NICU than the placebo group (WMD 5.4 days, 95% CI 0.4 to 10.5 days; I2 = 0% (none); Analysis 1.6; Figure 4).

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.6 Length of NICU stay (days).
Arya 2001 did not report on length of NICU stay.
Average NAPI score at 36 weeks' postmenstrual age (outcome 1.7)
Anand 1999 reported average NAPI score at 36 weeks' postmenstrual age and found no significant difference between midazolam and placebo (dextrose) groups (MD ‐2.10, 95% CI ‐14.38 to 10.18; tests for heterogeneity not applicable; Analysis 1.7).
Poor neurological outcome up to 28 days' postnatal age (IVH grade III or IV, PVL or death at 28 days or sooner without discharge from the NICU) (outcome 1.8)
Anand 1999 reported on this outcome and described no statistically significant difference between midazolam and placebo (dextrose) groups (RR 1.34, 95% CI 0.50 to 3.56; RD 0.08, 95% CI ‐0.19 to 0.35; tests for heterogeneity not applicable; Analysis 1.8).
PIPP score during drug infusion (outcome 1.9)
Anand 1999 reported on this outcome and found a statistically significant difference between midazolam and placebo (dextrose) groups favouring the midazolam group (MD ‐3.80, 95% CI ‐5.93 to ‐1.67; tests for heterogeneity not applicable; Analysis 1.9; Figure 5).

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.9 PIPP score during drug infusion.
Occurrence of adverse effects associated with midazolam administration
Jacqz‐Aigrain 1994 observed no adverse neurological effects, but investigators excluded one infant in the midazolam group from the study within 24 hours owing to major neurological abnormalities. Researchers provided no details of this case and reported no statistically significant differences between groups in the incidence of hypotension requiring albumin or vasoactive drugs (8/24 vs 6/22).
Anand 1999 noted no adverse neurological effects associated with midazolam administration and did not report the incidence of hypotension.
Arya 2001 described no adverse neurological effects associated with midazolam administration. Researchers noted epileptiform movements of unknown cause in two infants in the placebo group and noted no significant hypotension in any infant during the study period.
Neurodevelopmental outcome
Jacqz‐Aigrain 1994 and Arya 2001 did not report long‐term neurodevelopmental outcomes.
Midazolam infusion versus morphine (comparison 2)
PIPP score during drug infusion (outcome 2.1)
Anand 1999 reported on this outcome and described no statistically significant differences between midazolam and morphine groups (MD 1.00, 95% CI ‐0.66 to 2.66; tests for heterogeneity not applicable).
Poor neurological outcome by 28 days' postnatal age (IVH grade III or IV, PVL or death at 28 days or sooner without discharge from the NICU) (outcome 2.2)
Arya 2001 reported on this outcome in 46 infants. Investigators observed statistically significantly increased risk of poor neurological outcome by 28 days' postnatal age compared with infants treated with morphine (RR 7.64, 95% CI 1.02 to 57.21; RD 0.28, 95% CI 0.07 to 0.49; NNTH 4, 95% CI 2 to 14; test for heterogeneity not applicable; Analysis 2.2; Figure 6).

Forest plot of comparison: 2 Midazolam versus morphine, outcome: 2.2 Poor neurological outcome up to 28 days' postnatal age.
Discussion
Summary of main results
Since midazolam was introduced into the neonatal intensive care unit (NICU) in the 1980s, little information has been published on its effectiveness and safety when administered to critically ill neonates. Most reports to date are case series and case reports of midazolam use in patients of diverse age groups (from three days to 18 years of age), given at variable doses (from 0.025 mg/kg to 0.3 mg/kg administered as a bolus, to 24 microgram/kg/h to 400 microgram/kg/h administered as an infusion) (Hartwig 1991; Pellier 1999; Rosen 1991; Stenhammar 1994). The three studies included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) are the only randomised controlled trials (RCTs) conducted to date on the use of midazolam infusion for sedation in infants. Repeated literature searches in September 2009, March 2012 and June 2016 yielded no additional trials.
Tools to measure level of sedation in preterm infants are few (AAP/CPS 2000). Sedation level in such infants is currently measured by scales previously validated in older infants and children. Whether these scales are appropriate in preterm infants is unknown. Therefore, in the three RCTs included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994), although intravenous infusion of midazolam appeared to provide an effective sedative compared with placebo, investigators could draw no definitive conclusions on its effectiveness as a sedative in preterm infants. Anand 1999 assessed level of sedation using the COMFORT score, a composite scale based on eight behavioural and physiological items used to assess distress (Ambuel 1992). Although these items are applicable to preterm infants, this score has been validated only in older infants and children (mean age, 37.1 months). Jacqz‐Aigrain 1994 and Arya 2001 used a sedation scale adapted from the scoring system of Barrier (Barrier 1989), which had not been validated in preterm infants, by selecting five of 10 items from the scoring system. The validity of such an adapted score in assessing sedation level in neonates is unknown.
Jacqz‐Aigrain 1994 showed similar incidences of intracranial haemorrhage between midazolam and control groups. However, the midazolam‐treated infant who was excluded within 24 hours for major neurological abnormalities raises concern about the safety of midazolam. In Anand 1999, the incidence of poor neurological outcomes (death, severe intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL)) was higher in the midazolam group than in placebo and morphine groups (32% vs 24% vs 4%, respectively; P = 0.03). It should be noted, however, that the morphine group included a higher percentage of female infants with slightly higher birth weight and more mature gestational age. These baseline characteristics may have contributed to the differences in neurological outcomes noted in these groups.
Overall completeness and applicability of evidence
Today, only 146 neonates have been enrolled in three trials comparing midazolam versus placebo or morphine. The studies included in this review observed adverse neurological events more frequently, although possibly multi‐factorial in origin, among midazolam‐treated infants.
Quality of the evidence
The three trials included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) had small sample sizes but low risk of bias for most of the items included in the risk of bias tables. We rated risk of bias as unclear for selective reporting (reporting bias), as the protocols for all three studies were not available to us. We assessed these trials as having moderate quality according to GRADE Working Group grades of evidence. Thus further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Most important, none of the sedation scales used in these trials had been validated in newborns, and we could include only a few outcomes in the 'Summary of findings' tables (summary of findings Table for the main comparison; summary of findings Table 2).
Potential biases in the review process
We are aware of no biases in our review process. However, although we used a robust search method, we cannot exclude the possibility that we could have missed literature evidence.
Agreements and disagreements with other studies or reviews
The literature has reported adverse neurological effects associated with midazolam in term and preterm neonates (Adams 1997; Bergman 1991; Collins 1991; Magny 1994; Ng 2002; van den Anker 1992). Investigators have reported a variety of transient neurological effects after boluses or infusions, or both, of midazolam, including impaired level of consciousness, lack of visual following, hypertonia, hypotonia, choreic movements, dyskinetic movements, myoclonus and epileptiform activity. They have noted abnormalities on electroencephalograms in some cases. In all cases, effects were transient, although researchers have not reported long‐term neurodevelopmental outcomes. Two studies (Harte 1997; van Straaten 1992) found a significant decrease in middle cerebral artery blood flow velocity among preterm infants administered a single bolus injection of midazolam. This effect lasted up to one hour and was directly related to a drop in mean arterial blood pressure. Thus, it appears that the neurological effects of midazolam may be related at least in part to transient cerebral hypoperfusion. Long‐term sequelae of these effects remain unknown.
The mechanism of midazolam‐induced hypotension was thought to be vasodilation related to levels of extravascular prostanoids and calcium (Modanlou 1997). Jacqz‐Aigrain 1994 found that the number of infants with haemodynamic instability was not significantly different between midazolam and placebo groups (eight vs six, respectively), although infants in the midazolam group had significantly lower blood pressure than infants in the placebo group. Other investigators (Burtin 1991; Ng 2002; van den Anker 1992) observed significant hypotension in several preterm infants after bolus doses and infusions of midazolam that required volume resuscitation or vasoactive drugs. In most cases, providers had administered fentanyl concomitantly.

Study flow diagram: review update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.6 Length of NICU stay (days).

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.9 PIPP score during drug infusion.

Forest plot of comparison: 2 Midazolam versus morphine, outcome: 2.2 Poor neurological outcome up to 28 days' postnatal age.

Comparison 1 Midazolam versus placebo, Outcome 1 Intraventricular haemorrhage (any grade).
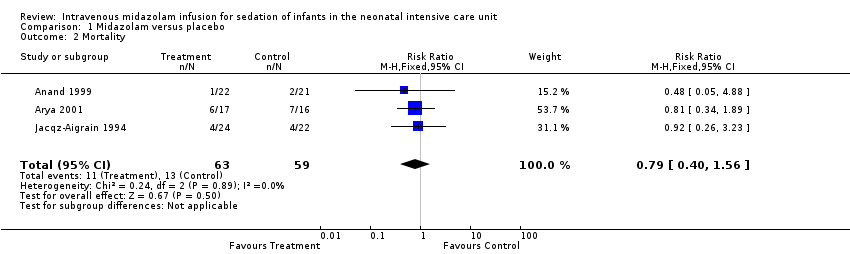
Comparison 1 Midazolam versus placebo, Outcome 2 Mortality.
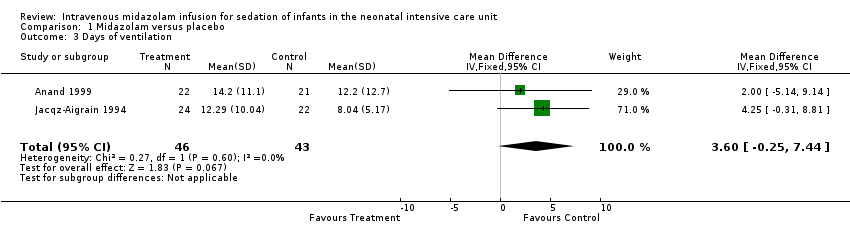
Comparison 1 Midazolam versus placebo, Outcome 3 Days of ventilation.

Comparison 1 Midazolam versus placebo, Outcome 4 Days of supplemental oxygen use.

Comparison 1 Midazolam versus placebo, Outcome 5 Pneumothorax.

Comparison 1 Midazolam versus placebo, Outcome 6 Length of NICU stay (days).

Comparison 1 Midazolam versus placebo, Outcome 7 Average NAPI scores at 36 weeks' PMA.

Comparison 1 Midazolam versus placebo, Outcome 8 Poor neurological outcome by 28 days' postnatal age.
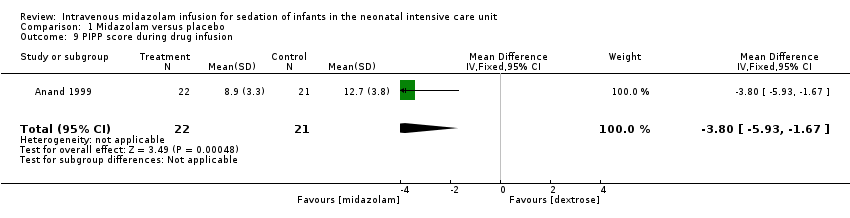
Comparison 1 Midazolam versus placebo, Outcome 9 PIPP score during drug infusion.
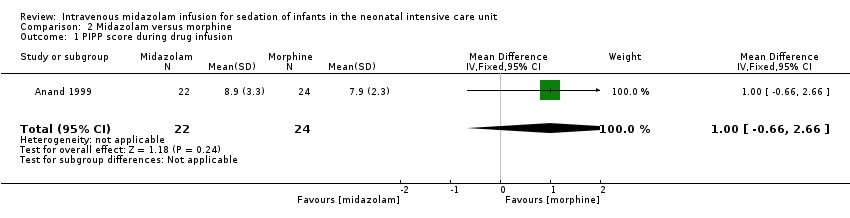
Comparison 2 Midazolam versus morphine, Outcome 1 PIPP score during drug infusion.

Comparison 2 Midazolam versus morphine, Outcome 2 Poor neurological outcome up to 28 days' postnatal age.
| Midazolam infusion compared with placebo for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: placebo infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Midazolam | |||||
| Mortality during hospital stay | High‐risk population | RR 0.79 (0.40 to 1.56) | 122 | ⊕⊕⊕⊝ | Risk of bias for these 3 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 3 studies were conducted in the target population of newborn infants. | |
| 220 per 1000 | 165 per 1000 | |||||
| Length of NICU stay (days) | Mean length of NICU stay ranged across control groups from 9 to 37.5 days. | WMD of NICU stay for intervention groups was 5.4 days longer. | WMD 5.4 days (0.4 to 10.5) | 89 | ⊕⊕⊕⊝ | Risk of bias for these 2 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 2 studies were conducted in the target population of newborn infants. |
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 12.7. | Mean PIPP score in the intervention group was lower at 8.9. | MD ‐3.80 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 1.34 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 230 per 1000 | 310 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| Midazolam infusion compared with morphine infusion for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: morphine infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Morphine | Midazolam | |||||
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 7.9. | Mean PIPP score in the intervention group was 8.9. | MD 1.00 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 7.64 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 318 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| Study reference for included trial | Name of score used | Reference for the score | Age of infants/children subjected to the score | Score validated in newborns? |
| COMFORT Scale | Ambuel 1992 ‐ 37 participants (age newborn to 204 months (mean 37.1; SD 52.7)) Marx 1994 ‐ children (age 0 to 102 months (mean age > 1 year) | No | ||
| Sedation score | Barrier 1989 ‐ 23 infants (age 1 to 7 months) | No | ||
| Behaviour score | Craig 1984 ‐ 30 children (age 2 to 24 months) Barrier 1989; Robieux 1991 ‐ 41 infants and toddlers (age 3 to 36 months) | No |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraventricular haemorrhage (any grade) Show forest plot | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.87, 3.24] |
| 2 Mortality Show forest plot | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Days of ventilation Show forest plot | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐0.25, 7.44] |
| 4 Days of supplemental oxygen use Show forest plot | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐5.30, 6.57] |
| 5 Pneumothorax Show forest plot | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.41, 2.84] |
| 6 Length of NICU stay (days) Show forest plot | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 5.44 [0.40, 10.49] |
| 7 Average NAPI scores at 36 weeks' PMA Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐14.38, 10.18] |
| 8 Poor neurological outcome by 28 days' postnatal age Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.50, 3.56] |
| 9 PIPP score during drug infusion Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐3.80 [‐5.93, ‐1.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PIPP score during drug infusion Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.66, 2.66] |
| 2 Poor neurological outcome up to 28 days' postnatal age Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.64 [1.02, 57.21] |

