Infusión intravenosa de midazolam para sedar a niños en la unidad de cuidados intensivos neonatales
Resumen
Antecedentes
La sedación adecuada para los recién nacidos que son sometidos a procedimientos incómodos puede reducir el estrés y evitar las complicaciones. El midazolam es una benzodiacepina de acción corta que se utiliza cada vez más en las unidades de cuidados intensivos neonatales (UCIN). Sin embargo, su efectividad como sedante no se ha evaluado de forma sistemática en los recién nacidos.
Objetivos
Objetivo principal
Evaluar la eficacia de la infusión intravenosa de midazolam para la sedación, evaluada mediante mediciones conductuales o fisiológicas de los niveles de sedación, en recién nacidos en estado crítico en la UCIN.
Objetivos secundarios
Evaluar los efectos de la infusión intravenosa de midazolam para la sedación en las complicaciones, que incluyen las siguientes:
1. Incidencia de hemorragia intraventricular (Hiv)/leucomalacia periventricular (LPV).
2. Mortalidad.
3. Aparición de efectos adversos asociados con la administración de midazolam (hipotensión, alteraciones neurológicas).
4. Días con ventilación.
5. Días con administración de oxígeno.
6. Incidencia de neumotórax.
7. Duración de la estancia en la UCIN (días).
8. Resultados del neurodesarrollo a largo plazo.
Métodos de búsqueda
Se utilizó la estrategia de búsqueda estándar del Grupo Cochrane de Neonatología (Cochrane Neonatal Review Group) para buscar en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2016, número 5); MEDLINE vía PubMed (de 1966 al 16 de junio 2016); Embase (de 1980 al 16 de junio 2016); y en Cumulative Index to Nursing and Allied Health Literature (CINAHL; de 1982 al 16 de junio 2016). Se buscaron ensayos controlados aleatorizados y cuasialeatorizados en las bases de datos de ensayos clínicos, en actas de congresos y en las listas de referencias de los artículos recuperados.
Criterios de selección
Se seleccionaron para la revisión los ensayos controlados aleatorizados y cuasialeatorizados de la infusión intravenosa de midazolam para la sedación en niños de hasta 28 días de edad.
Obtención y análisis de los datos
Se extrajeron datos sobre el resultado primario del nivel de sedación. Se evaluaron los resultados secundarios como la hemorragia intraventricular, la leucomalacia periventricular, la muerte, la duración de la estancia en la UCIN y los efectos adversos asociados con el midazolam. Cuando fue apropiado, se realizaron metanálisis utilizando el riesgo relativo (RR) y las diferencias de riesgos (DR), y cuando la DR era estadísticamente significativa, se calculó el número necesario a tratar para lograr un resultado beneficioso adicional (NNTB) o un resultado perjudicial adicional (NNTD), junto con sus intervalos de confianza del 95% (IC del 95%) para las variables categóricas, y las diferencias de medias ponderadas (DMP) para las variables continuas. Se evaluó la heterogeneidad mediante la realización de la prueba de I‐cuadrado (I2).
Resultados principales
La revisión incluyó tres ensayos con 148 recién nacidos. Para esta actualización, no se identificaron ensayos nuevos. Mediante el uso de diferentes escalas de sedación, cada estudio mostró un nivel de sedación estadísticamente significativo más alto en el grupo de midazolam en comparación con el grupo de placebo. Sin embargo, ninguna de las escalas de sedación utilizadas ha sido validada en niños prematuros; por lo tanto, no fue posible determinar la efectividad del midazolam en esta población. La duración de la estancia en la UCIN fue significativamente mayor en el grupo de midazolam que en el grupo de placebo (DMP 5,4 días, IC del 95%: 0,40 a 10,5; I2 = 0%; dos estudios, 89 niños). Un estudio (43 niños) informó de que las puntuaciones Premature Infant Pain Profile (PIPP) fueron significativamente más bajas durante la infusión de midazolam que durante la infusión de dextrosa (placebo) (DM ‐3,80; IC del 95%: ‐5,93 a ‐1,67). Otro estudio (46 niños) observó una mayor incidencia de eventos neurológicos adversos a los 28 días de edad posnatal (muerte, Hiv grado III o IV o LPV) en el grupo de midazolam en comparación con el grupo de morfina (RR 7,64, IC del 95%: 1,02 a 57,21; DR 0,28, IC del 95%: 0,07 a 0,49; NNTD 4; IC del 95%: 2 a 14) (las pruebas de heterogeneidad no son aplicables). Se considera que estos ensayos eran de calidad moderada según la evaluación con criterios GRADE basado en los siguientes resultados: mortalidad durante la estancia hospitalaria, duración de la estancia en la UCIN, adecuación de la analgesia según las puntuaciones del PIPP y resultados neurológicos deficientes a los 28 días de edad posnatal.
Conclusiones de los autores
Los datos son insuficientes para promover el uso de la infusión intravenosa de midazolam como sedante para los recién nacidos sometidos a cuidados intensivos. Esta revisión plantea preocupación en cuanto a la seguridad del midazolam en los recién nacidos. Es necesario seguir investigando la efectividad y la seguridad del midazolam en los recién nacidos.
PICOs
Resumen en términos sencillos
Infusión intravenosa de midazolam para sedar a niños en la unidad de cuidados intensivos neonatales
Pregunta de la revisión: En el caso de los bebés enfermos que ingresan a una unidad de cuidados intensivos neonatales (UCIN), ¿cuál es la efectividad del midazolam, administrado por goteo intravenoso continuo, como sedante para reducir el estrés, medida según los cambios de comportamiento y los signos vitales?
Antecedentes: La sedación adecuada para los lactantes que son sometidos a procedimientos incómodos mientras reciben cuidados intensivos puede reducir el estrés y evitar complicaciones. El midazolam es un sedante que se usa cada vez más en las UCIN. Sin embargo, los investigadores no han revisado sistemáticamente la evidencia para observar si es efectivo y seguro para los lactantes en este entorno.
Características de los estudios: Se han seleccionado para su inclusión en esta revisión ensayos controlados aleatorizados del goteo intravenoso continuo de midazolam como sedante en lactantes de hasta 28 días de edad.
Resultados clave: Se incluyeron tres ensayos clínicos en esta revisión. Mediante el uso de diferentes escalas para medir el nivel de sedación, cada estudio demostró que el midazolam fue efectivo para proporcionar sedación a los lactantes. Sin embargo, la validez de las escalas de sedación utilizadas en estos estudios no se ha demostrado en los lactantes; por lo tanto, no es posible tener seguridad en cuanto si el midazolam es, de hecho, un sedante efectivo para los lactantes. Además, un estudio demostró que los lactantes que recibieron midazolam presentaron un riesgo significativamente mayor de muerte o lesión cerebral, y los resultados combinados de dos estudios demostraron que el uso de midazolam puede prolongar la duración de la estancia en la UCIN.
Industria: Uno de los estudios incluidos en esta revisión recibió el apoyo de la industria, y para los otros dos estudios, la industria proporcionó todos los fármacos del estudio.
Calidad de la evidencia: Se evaluó la calidad de la evidencia sobre los resultados de la mortalidad durante la estancia hospitalaria, la duración de la estancia en la UCIN, el dolor y los resultados neurológicos a los 28 días de vida y se encontró que la evidencia era de calidad moderada, ya que no hubo evidencia suficiente disponible. Por lo tanto, se establece la conclusión de que no hay evidencia suficiente que apoye el uso de midazolam como sedante para los lactantes sometidos a cuidados intensivos. Se necesita más investigación para considerar la seguridad y la efectividad del midazolam en esta población.
Conclusiones de los autores
Summary of findings
| Midazolam infusion compared with placebo for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: placebo infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Midazolam | |||||
| Mortality during hospital stay | High‐risk population | RR 0.79 (0.40 to 1.56) | 122 | ⊕⊕⊕⊝ | Risk of bias for these 3 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 3 studies were conducted in the target population of newborn infants. | |
| 220 per 1000 | 165 per 1000 | |||||
| Length of NICU stay (days) | Mean length of NICU stay ranged across control groups from 9 to 37.5 days. | WMD of NICU stay for intervention groups was 5.4 days longer. | WMD 5.4 days (0.4 to 10.5) | 89 | ⊕⊕⊕⊝ | Risk of bias for these 2 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 2 studies were conducted in the target population of newborn infants. |
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 12.7. | Mean PIPP score in the intervention group was lower at 8.9. | MD ‐3.80 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 1.34 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 230 per 1000 | 310 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| Midazolam infusion compared with morphine infusion for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: morphine infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Morphine | Midazolam | |||||
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 7.9. | Mean PIPP score in the intervention group was 8.9. | MD 1.00 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 7.64 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 318 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
Antecedentes
Descripción de la afección
Los niños a término y prematuros son capaces de percibir el dolor y el estrés (Anand 1987). En la unidad de cuidados intensivos neonatales (UCIN), el tratamiento de apoyo e investigación de los niños enfermos suele requerir procedimientos dolorosos e incómodos. Sin embargo, como el dolor y el estrés son fenómenos subjetivos y difíciles de evaluar en los niños en etapa preverbal, los profesionales de atención médica suelen pasar por alto el uso de analgésicos y sedantes apropiados. Se ha sugerido que las respuestas al dolor pueden comprometer las condiciones clínicas (Anand 1992), y que una sedación adecuada durante la ventilación mecánica puede disminuir el estrés (Quinn 1993) y facilitar una ventilación efectiva, de modo que se puedan prevenir complicaciones como el neumotórax y la hemorragia intraventricular (Hiv) (Greenough 1983; Perlman 1985).
Descripción de la intervención
Las benzodiacepinas, administradas en forma de infusiones intravenosas o de bolos intravenosos, pueden utilizarse para proporcionar sedación, pero no analgesia, en muchos entornos clínicos. El midazolam es una benzodiacepina de acción corta que se ha utilizado cada vez más en la UCIN.
De qué manera podría funcionar la intervención
Las benzodiacepinas son una clase de sedantes que actúan sobre receptores específicos del sistema nervioso central. Estos receptores, presentes en el feto desde las siete semanas de gestación (Hebebrand 1988), potencian las vías de inhibición neuronal mediadas por el ácido gamma‐aminobutírico (GABA, por sus siglas en inglés) (Jacqz‐Aigrain 1996). Los investigadores han estudiado la farmacocinética del midazolam en los recién nacidos. El midazolam es la opción preferida en comparación con otras benzodiacepinas debido a su solubilidad en agua y a su rápida eliminación (Jacqz‐Aigrain 1992). Aunque su vida media de eliminación es significativamente más corta que la de otras benzodiacepinas, como el diazepam, su eliminación se retrasa en los niños prematuros en comparación con los recién nacidos y los niños mayores (Lee 1999). La inmadurez funcional del sistema hepático y renal en los recién nacidos prematuros probablemente explica la eliminación más lenta del midazolam. En un estudio de cohortes muy amplio reciente realizado en Europa, se administró midazolam a 576 (9%) de la cohorte total de 6680 neonatos y a 536 (25%) de 2142 neonatos sometidos a ventilación traqueal (Carbajal 2015). En general, el midazolam fue, por mucho, el sedante más utilizado. Se administró al 25% de los recién nacidos sometidos a ventilación traqueal, y su uso osciló entre el 0% y el 73% en los países europeos, a pesar de que se dispone de pocos datos clínicos que respalden la sedación con midazolam en los recién nacidos.
Cabe señalar que en un modelo animal, Koch 2008 informó de efectos paradójicos del midazolam sobre la nocicepción y la sedación en ratas entre los días tres y 10 posparto. El midazolam no logró sedar a las ratas jóvenes y en cambio causó un efecto excitador al sensibilizar su actividad de reflejos del flexor. Los investigadores no observaron los efectos sedantes del midazolam en los centros supraespinales hasta etapas posteriores de la vida, después de la maduración. Estos resultados destacan la necesidad de comprender mejor la ontogenia de los efectos farmacológicos de medicamentos como el midazolam que se utilizan habitualmente en las UCIN.
Por qué es importante realizar esta revisión
No se ha examinado sistemáticamente la efectividad del midazolam intravenoso como sedante en los recién nacidos. Además, no se ha establecido bien su seguridad en la dosis recomendada actualmente en los recién nacidos con enfermedades graves.
Objetivos
Objetivo primario
Evaluar la eficacia de la infusión intravenosa de midazolam para la sedación, evaluada mediante mediciones conductuales o fisiológicas de los niveles de sedación, en recién nacidos en estado crítico en la UCIN.
Objetivos secundarios
Evaluar los efectos de la infusión intravenosa de midazolam para la sedación en las complicaciones, que incluyen las siguientes:
-
Incidencia de hemorragia intraventricular (Hiv)/leucomalacia periventricular (LPV).
-
Mortalidad.
-
Aparición de efectos adversos asociados con la administración de midazolam (hipotensión, alteraciones neurológicas).
-
Días con ventilación.
-
Días con administración de oxígeno.
-
Incidencia de neumotórax.
-
Duración de la estancia en la UCIN (días).
-
Resultados del neurodesarrollo a largo plazo.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se buscaron ensayos controlados aleatorizados y cuasialeatorizados en los que el uso de la infusión intravenosa de midazolam se comparó con placebo u otros sedantes en los recién nacidos sometidos a cuidados intensivos.
Tipos de participantes
Se incluyó a niños de hasta 28 días de edad que fueron ingresados en la UCIN y que necesitaban sedación para intervenciones médicas.
Tipos de intervenciones
Las intervenciones incluyeron la infusión intravenosa continua de midazolam administrada a una dosis de 20 microgramos/kg/h a 60 microgramos/kg/h durante al menos 24 horas para llevar a cabo la sedación durante la ventilación mecánica y los procedimientos de investigación radiológica.
Se excluyeron los estudios que utilizaban una combinación de midazolam y un analgésico para los recién nacidos sometidos a procedimientos dolorosos. También se excluyeron los estudios que investigaban el uso de dosis de midazolam en bolo intravenoso, a menos que el bolo fuera seguido de una infusión; y los estudios que examinaban el uso de midazolam como agente de inducción anestésica o como anticonvulsivo.
Tipos de medida de resultado
Resultados primarios
El resultado primario fue el nivel de sedación, evaluado de acuerdo a:
-
medidas de comportamiento: acciones faciales, excitación, tono muscular, movimientos físicos y comportamiento respiratorio, que pueden evaluarse mediante sistemas de puntuación adecuados para la edad; y
-
parámetros fisiológicos: cambios en la frecuencia cardíaca, la frecuencia respiratoria, la presión sanguínea, la saturación de oxígeno y los niveles plasmáticos de cortisol o catecolamina, medidos al inicio y a intervalos regulares durante la administración de midazolam.
Resultados secundarios
-
Hiv (definido por la clasificación de Papile et al [Papile 1978]).
-
LPV (definido como quistes periventriculares en la imagenología del cerebro, con exclusión de los quistes del plexo subependimario o coroideo).
-
Mortalidad (muerte en el plazo de los 28 días de vida).
-
Efectos adversos asociados con el uso de midazolam: hipotensión (disminución significativa desde el inicio en comparación con los controles), anormalidades neurológicas (actividades epileptiformes, trastornos del movimiento, mioclonía, hipertonía, hipotonía).
-
Días de asistencia ventilación mecánica.
-
Días de tratamiento con administración de oxígeno.
-
Neumotórax.
-
Días de estancia en la UCIN.
-
Resultados del desarrollo neurológico, evaluados con un instrumento validado de evaluación del desarrollo.
-
Neurobehavioural Assessment of Preterm Infants (NAPI) (Snider 2005).
Métodos de búsqueda para la identificación de los estudios
Búsquedas electrónicas
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1966 to 16 June 2016); Embase (1980 to 16 June 2016); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 16 June 2016), using the following search term: (midazolam), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); the ISRCTN Registry).
For previous editions of this review, we identified randomised and quasi‐randomised controlled trials of intravenous midazolam in infants from the Cochrane Central Register of Controlled Trials (CENTRAL; 2012, Issue 3) in the Cochrane Library; MEDLINE (from 1985 to March 2012); Embase (1980 to 2012) and CINAHL (1980 to 2012), using the medical subject headings (MeSH): midazolam; infant; newborn. We searched for abstracts published in Pediatric Academic Societies Meetings Abstract Archives from 1990 to 2011. We imposed no language restrictions. We attempted to contact investigators of studies meeting the inclusion criteria to gather additional data for analysis. We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; controlled‐trials.com; who.int/ictrp), and we searched the Web of Science to identify any trial that quoted the earliest study that we identified (Jacqz‐Aigrain 1994).
Búsqueda de otros recursos
In addition, we manually searched bibliographies of articles and personal files and imposed no language restrictions. We attempted to contact investigators of studies meeting the inclusion criteria to gather additional data for analysis. We did not attempt to identify unpublished studies. We excluded studies involving neonates and older infants and children if we could not extract data for neonates.
Obtención y análisis de los datos
We used the standard method of the Cochrane Neonatal Review Group for performing systematic reviews (http://neonatal.cochrane.org/resources‐review‐authors).
Selección de los estudios
We included randomised and quasi‐randomised controlled trials involving neonates aged 28 days or younger that included a treatment group and a placebo group. We accepted for the review studies that reported outcome measures including physiological, behavioural and hormonal changes, as well as adverse neurological outcomes.
We excluded studies involving neonates and older infants and children if we could not extract data for neonates.
Two review authors (EN, AT) independently decided to include or exclude a specific study. When discrepancies occurred, the three review authors (EN, AT, AO) made the decision by consensus.
Extracción y manejo de los datos
We created a data collection form on which we abstracted the following data from the included studies: demographics of participants, age at enrolment into the study, inclusion and exclusion criteria, sample size, treatment and control group regimens and outcomes. Two review authors (EN, AT) independently abstracted the data and resolved differences by consensus.
Evaluación del riesgo de sesgo de los estudios incluidos
Two review authors (EN, AO) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains: selection bias, performance bias, attrition bias, reporting bias and any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 2 for a detailed description of risks of bias for each domain.
Medidas del efecto del tratamiento
We performed statistical analyses using Review Manager 5.1 software. We analysed categorical data using risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial (NNTB) or harmful outcome (NNTH). We analysed continuous data by using weighted mean difference (WMD) and reported the 95% confidence interval (CI) for all estimates.
Evaluación de la heterogeneidad
We examined heterogeneity between trials by inspecting forest plots (if we included at least 10 trials in one analysis) and quantified the impact of heterogeneity by using the I2 statistic. If we detected statistical heterogeneity, we planned to explore possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments) by performing post hoc subgroup analyses.
Síntesis de los datos
When we identified at least two RCTs that evaluated the effectiveness of intravenous midazolam infusions by examining the same outcome measures, we pooled the results to obtain an overall estimate of effect size using RevMan 5.1.4 (RevMan 2011). We used the Mantel‐Haenszel method for estimates of typical RR and RD, and the inverse variance method for measured quantities. We used the fixed‐effect model for all meta‐analyses.
Quality of the evidence
For the 2016 update, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schunemann 2013), to assess the quality of evidence for the following clinically relevant outcomes: mortality during hospital stay, length of NICU stay, adequacy of analgesia as measured by the Premature Infant Pain Profile (PIPP) (Stevens 1996) and poor neurological outcomes by 28 days' postnatal age. In terms of the primary outcome of level of sedation, none of the sedation scales used in these studies had been validated in preterm infants; therefore, we could not ascertain the effectiveness of midazolam in this population as reported by these studies and did not subject this outcome to GRADE assessment.
We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (and two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates and presence of publication bias.
The GRADE approach assesses the quality of a body of evidence and assigns one of four grades.
-
High: We are very confident that the true effect lies close to the estimate of effect.
-
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
-
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
-
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Two review authors (EN, AO) independently assessed the quality of the evidence for each of the outcomes above. We used the GRADEpro Guideline Development Tool to create ‘Summary of findings’ tables to report the quality of evidence. See Appendix 3 for details on assessment of quality of the evidence.
Análisis de subgrupos e investigación de la heterogeneidad
We prospectively planned no subgroup analyses.
Results
Description of studies
Results of the search
We presented results of the search in the study flow diagram for the review update provided in Figure 1. We identified six RCTs on the use of intravenous midazolam in infants; we included three of these and excluded three.
Literature searches conducted in September 2009, March 2012 and June 2016 identified no additional trials.
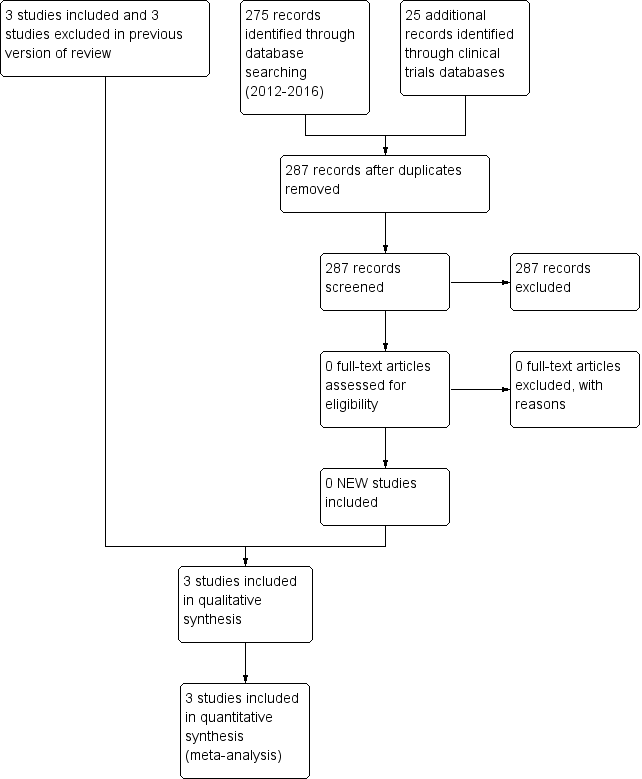
Study flow diagram: review update.
Included studies
For details, see Characteristics of included studies.
Jacqz‐Aigrain 1994 randomly assigned 46 preterm infants (25 at < 33 weeks' gestation and 21 at ≥ 33 weeks' gestation) ≤ 48 hours of age to receive midazolam infusion or manufactured placebo for five days while mechanically ventilated for respiratory distress syndrome. Twenty‐four infants received midazolam and 22 received placebo infusions. Researchers withdrew one infant in the midazolam group because of major neurological abnormalities at 24 hours of age; and two infants from the midazolam group and two from the placebo group within 72 hours owing to rapid clinical improvement. They noted contamination in one infant in the placebo group (midazolam was detectable in the serum at 24 hours). Baseline characteristics did not differ between groups. Severity of illness, as measured by mean airway pressure (MAP) while ventilated and fraction of inspired oxygen (FiO2) from the time of enrolment to the end of the study, was not significantly different between groups. Investigators administered midazolam as an infusion at 60 microgram/kg/h for up to five days in infants at ≥ 33 weeks' gestation, and at 60 microgram/kg/h for one day followed by 30 microgram/kg/h for up to a total of five days in infants at < 33 weeks' gestation. They did not report the duration of the infusion. Weaning of sedatives was allowed after at least 48 hours of administration, but investigators did not specify a weaning protocol. The primary outcome was adequacy of sedation as measured by a behavioural score adapted from the clinical neurological and behavioural scoring system of Barrier (Barrier 1989) and by changes in physiological variables (heart rate and blood pressure). The sedation score consisted of five items that assessed facial expression, sucking, spontaneous motor activity, excitability/responsiveness to stimulation and excessive flexion, with scores ranging from 0 (sedation) to 5 (inadequate sedation). Care providers measured the sedation score four times per day during treatment (nurses twice, physicians twice). Secondary outcomes included days of ventilation support, days of supplemental oxygen, surfactant use, duration of NICU stay and common complications of preterm birth (e.g. pneumothorax, pulmonary interstitial emphysema, hypotension, chronic lung disease, necrotising enterocolitis, intracranial haemorrhage, persistent pulmonary hypertension of the newborn, death). Researchers reported outcomes for all 46 infants.
In a multi‐centre randomised pilot study (Anand 1999), investigators assigned 67 preterm infants of 24 to 32 weeks' gestation who were ≤ 72 hours of age and who were ventilated for less than eight hours to receive midazolam infusion, morphine infusion or dextrose placebo infusion for as long as sedation was considered necessary up to a maximum of 14 days. Twenty‐two infants received midazolam infusion, 24 received morphine infusion and 21 received dextrose placebo. The three groups did not differ significantly in baseline characteristics. Severity of illness at birth, as assessed by the Clinical Risk Index for Babies (CRIB) score (The International Neonatal Network), showed no significant differences among groups at birth (P = 0.24). However, severity of illness as measured by the Neonatal Medical Index (NMI) (Korner 1993) on the basis of response variables during the hospital stay showed significant differences in the distribution of risk categories among the three groups at discharge (P = 0.01). Researchers administered midazolam at 200 microgram/kg loading dose followed by an infusion of 20 microgram/kg/h, 40 microgram/kg/h or 60 microgram/kg/h for infants of gestational age 24 to 26 weeks, 27 to 20 weeks or 30 to 33 weeks, respectively. They administered morphine at 100 microgram/kg loading dose followed by an infusion of 10 microgram/kg/h, 20 microgram/kg/h or 30 microgram/kg/h for infants of gestational age 24 to 26 weeks, 27 to 29 weeks or 30 to 33 weeks, respectively. Duration of the infusion was not different among groups (5.1 days vs 3.4 days vs 5.0 days in the midazolam, morphine and placebo groups, respectively; P = 0.37). If necessary, they provided additional boluses of morphine and documented the frequency and amount given as measures of inadequate sedation. Investigators used a standardised protocol in weaning sedatives. The primary outcome was the incidence of adverse neurological events (defined as neonatal death, grade III or IV IVH or PVL). Researchers measured the adequacy of sedation by obtaining the COMFORT score, an eight‐item behavioural and physiological measurement of distress in the paediatric intensive care unit (Ambuel 1992). This score includes assessment of alertness, calmness/agitation, respiratory response, physical movement, mean arterial blood pressure, heart rate, muscle tone and facial tension, with scores ranging from 8 (sedated) to 40 (not adequately sedated). They measured adequacy of analgesia by obtaining the Premature Infant Pain Profile (PIPP) (Stevens 1996) in response to tracheal suctioning. The PIPP score includes assessment of gestational age, behavioural state, heart rate, oxygen saturation, brow bulge, eye squeeze and nasolabial furrow, with scores ranging from 0 (adequate analgesia) to 21 (inadequate analgesia). They obtained the two scores on all infants at baseline, after 24 hours of infusion and at 10 to 12 hours after discontinuation of the infusion. Other secondary outcomes included days of mechanical ventilation, continuous positive airway pressure, supplemental oxygen use, incidence of pneumothorax, duration of NICU and hospital stay, days to full enteral (full strength, full gavage, full oral) feeds, daily weight gain and neurodevelopmental outcomes at 36 weeks' corrected age as measured by Neurobehavioral Assessment of the Premature Infant (NAPI) examination cluster scores (Korner 1991). Researchers reported outcomes for all 67 infants.
Arya 2001 randomised 33 infants with birth weight < 2000 grams and requiring mechanical ventilation during the first week of life to receive midazolam or placebo infusion for sedation. Seventeen infants received midazolam and 16 received placebo. The two groups were similar in baseline characteristics. Severity of respiratory illness, as measured by peak inspiratory pressure (PIP), MAP, oxygenation index (OI) and the alveolar‐arterial oxygen gradient (AaDO2), was similar between the two groups at the time of enrolment. Investigators administered midazolam intravenously at 200 microgram/kg loading dose followed by an infusion of 60 microgram/kg/h. Infants in both groups also received morphine infusion at 10 microgram/kg/h during the study period. The study concentrated on the first 48 hours of midazolam infusion and did not report on duration of benzodiazepine use nor on method of weaning. Three infants in each group did not complete the first 24 hours of the study, and four in each group did not complete 48 hours of the study. Reasons for withdrawal were death (13 infants) and extubation (one infant). Researchers included these infants in the analyses on an intention‐to‐treat basis. The primary outcome was adequacy of sedation as measured by a behavioural score adapted from the clinical neurological and behavioural scoring system of Barrier (Barrier 1989). This is the same scoring system used in Jacqz‐Aigrain 1994. Study authors assessed infants for adequacy of sedation before midazolam administration, then every six hours over the 48‐hour study period. Other measured outcomes included changes in physiological variables (heart rate and blood pressure), changes in oxygen requirement (FiO2) and ventilation requirement (PIP, positive end‐expiratory pressure (PEEP), ventilator rate) and arterial blood gas as measured by mean daily values. Investigators documented complications related to mechanical ventilation (air leak, IVH) and potential adverse effects of midazolam (epileptiform movements, hypotension, tachycardia and oliguria) while reporting the duration of ventilation. They reported no long‐term outcomes but described outcomes for all 33 infants in the study.
Excluded studies
For details, see Characteristics of excluded studies. We excluded one trial that used a single bolus dose of intravenous midazolam (McCarver‐May 1996) and another trial that used intravenous midazolam for anaesthetic induction (Kawakami 1998). The third excluded trial (Parkinson 1997) used midazolam for sedation in individuals from one day to 15 years of age, and we could not extract data pertaining to neonates. The three trials included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) reported on the effectiveness of midazolam infusion and included a total of 146 infants.
Risk of bias in included studies
For details, see the Risk of bias in included studies table, Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
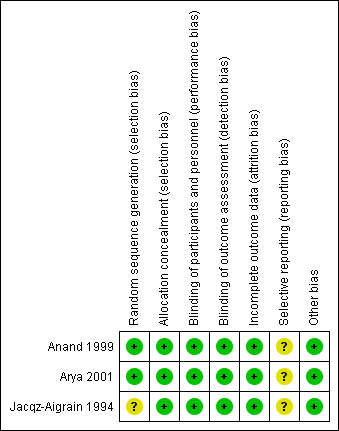
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of bias for random sequence generation was low in two studies (Anand 1999; Arya 2001) and unclear in one study (Jacqz‐Aigrain 1994). All three studies adequately concealed allocation (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994).
Blinding
Risk of performance and detection was low in all three studies (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994).
Incomplete outcome data
Investigators reported outcomes for all randomised infants.
Selective reporting
A protocol was not available for any of the three studies, so we could not judge whether deviations from the protocol occurred.
Other potential sources of bias
Included studies appeared free of other sources of bias. Anand 1999 received support from industry, and Arya 2001 and Jacqz‐Aigrain 1994 received midazolam and placebo from a pharmaceutical company.
Only Arya 2001 performed sample size calculation, and Anand 1999 stated that the study was a pilot trial. All three studies performed statistical analyses using an intention‐to‐treat approach.
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2
Midazolam infusion versus placebo (comparison 1)
Anand 1999 evaluated outcomes by performing analysis of variance to detect statistically significant differences among midazolam, morphine and placebo groups. For this review, we performed comparisons between the midazolam group and the placebo group on relevant continuous outcome variables using information provided by the publication (sample size, mean, standard deviation (SD), standard error of the mean). For the current update of this review, we included an additional comparison of midazolam versus morphine infusion for the outcome of PIPP during drug infusion, as well as for poor neurological outcome by 28 days' postnatal age.
Primary outcomes
Primary outcomes involved level of sedation, as evaluated by:
-
behavioural measures: facial actions, excitability, muscle tone, physical movements and respiratory behaviour, which may be evaluated by age‐appropriate scoring systems; and
-
physiological parameters: changes in heart rate, respiratory rate, blood pressure, oxygen saturation and plasma cortisol or catecholamine levels, measured at baseline and at regular intervals during midazolam administration.
In Additional Table 1 ('Sedation scores used in included studies'), we present some basic information about measures of sedation used. All investigators applied the scores to infants and children, most of whom were older than one month, and did not include preterm newborn infants. None of the tools used to assess sedation had been validated for use in newborn infants. We therefore reported the results of each included study separately, as did the authors of the included trials. We did not enter data for sedation scores into RevMan analyses. For the same reasons, we did not include sedation scores in the 'Summary of findings' tables.
| Study reference for included trial | Name of score used | Reference for the score | Age of infants/children subjected to the score | Score validated in newborns? |
| COMFORT Scale | Ambuel 1992 ‐ 37 participants (age newborn to 204 months (mean 37.1; SD 52.7)) Marx 1994 ‐ children (age 0 to 102 months (mean age > 1 year) | No | ||
| Sedation score | Barrier 1989 ‐ 23 infants (age 1 to 7 months) | No | ||
| Behaviour score | Craig 1984 ‐ 30 children (age 2 to 24 months) Barrier 1989; Robieux 1991 ‐ 41 infants and toddlers (age 3 to 36 months) | No |
Sedation scores (behavioural measures)
Jacqz‐Aigrain 1994 enrolled and reported on 46 newborn infants. Sedation scores (behavioural measures) (Barrier 1989; Craig 1984; Robieux 1991) were not different between groups at baseline. The midazolam group had consistently lower scores (more sedated) than the placebo group on all days, as assessed by both nurses and physicians (P < 0.05). Investigators observed significant decreases in sedation scores from baseline (mean (SD) score 1.9 (0.4)) to day 1 (score 1.1 (0.3); P < 0.01), day 2 (score 0.8 (0.2); P < 0.010) and day 3 (score 1.1 (0.3); P < 0.05) in the midazolam group (per nurses' scores) and significant increases in the placebo group from baseline (mean (SD) score 1.7 (0.3)) to day 1 (score 2.6 (0.3); P < 0.01) (per physicians' scores).
Anand 1999 enrolled and reported on 67 preterm infants and found statistically significantly lower COMFORT scores (more sedated) in the midazolam group during the infusion (mean (SD) score 14.9 (4.6) vs 17.5 (4.2); P = 0.04), although they noted no statistically significant differences in scores between the two groups before infusion and 12 hours after the infusion was stopped (mean (SD) score 15.9 (3.8) vs 15.6 (3.2); P = 0.8, before the infusion; and 15.8 (4.7) vs 16.2 (4.1); P = 0.76, after the infusion). In response to tracheal suctioning, the midazolam group had significantly lower PIPP scores (more sedated) during the infusion compared with the placebo group (mean (SD) score 8.9 (3.3) vs 12.7 (3.8); P < 0.001). The requirement for additional morphine was not statistically different between midazolam and placebo groups, but midazolam groups tended to require fewer additional morphine doses than placebo groups.
Arya 2001 enrolled and reported on 33 preterm newborns. Sedation scores were not significantly different between the two groups at baseline. The midazolam group had statistically significantly lower sedation scores (more sedated) compared with the placebo group from 18 hours after the start of infusion (median (range) score 0 (0 to 3) vs 1 (0 to 4); P < 0.05). This trend continued for the study duration (up to 48 hours), with statistically significant differences noted at 36 (median (range) score 0 vs 1 (0 to 3); P < 0.05), 42 (0 (0‐3) vs 1 (0‐3); P < 0.05) and 48 hours (0 (0 to 2) vs 1 (0 to 3); P < 0.05) of study drug infusion.
Even though Jacqz‐Aigrain 1994 and Arya 2001 used the same sedation score, we could not combine results on adequacy of sedation by meta‐analysis, as Arya 2001 presented sedation scores as median values and ranges, whereas Jacqz‐Aigrain 1994 presented results as means and SDs.
Sedation scores (physiological parameters)
Jacqz‐Aigrain 1994 enrolled and reported on 46 newborn infants. The physiological parameters heart rate and blood pressure did not differ between groups at baseline but were significantly lower in the midazolam group than in the placebo group on days 1 and 2. These trends continued through to day 5, although they were not statistically significant. One infant in the midazolam group and seven in the placebo group (P < 0.05) were inadequately sedated and required fentanyl and muscle relaxants within 72 hours. Two infants in the midazolam group received fentanyl within 72 hours (Jacqz‐Aigrain 1994).
Secondary outcomes
Intraventricular haemorrhage (outcome 1.1)
Neither Jacqz‐Aigrain 1994 nor Anand 1999 found a statistically significant difference between midazolam and placebo groups in the incidence of IVH. Arya 2001 observed no intracranial haemorrhage during the 48‐hour study period in all enrolled neonates. Meta‐analysis of results of the three studies (n = 122) showed no statistically significant differences in the incidence of IVH of any grade (typical RR 1.68, 95% CI 0.87 to 3.24; typical RD 0.12, 95% CI ‐0.02 to 0.26; I2 = 0% (none) for RR but 64% (moderate) for RD; Analysis 1.1).
Mortality (outcome 1.2)
Neither Jacqz‐Aigrain 1994 nor Anand 1999 found a statistically significant difference in mortality between midazolam and placebo groups. Arya 2001 did not report mortality as an outcome measure. However, six infants in the midazolam group and seven in the placebo group died before completing the 48‐hour study period. Meta‐analysis of results of the three studies showed no evidence of effect (typical RR 0.79, 95% CI 0.40 to 1.56; typical RD ‐0.05, 95% CI ‐0.18 to 0.09; I2 = 0% (none for both RR and RD); Analysis 1.2).
Days on ventilation (outcome 1.3)
Combined results of Jacqz‐Aigrain 1994 and Anand 1999 showed no statistically significant difference in days of ventilation (WMD 3.6 days, 95% CI ‐0.2 to 7.4 days; I2 = 0% (none); Analysis 1.3).
Arya 2001 presented data on days of ventilation as median values and ranges, so we could not combine these data with data from the other two studies. Median duration of ventilation (range) was 53 hours (7 to 216) in the midazolam group and 59 hours (13 to 194) in the placebo group.
Days on supplemental oxygen use (outcome 1.4)
Combined results of Jacqz‐Aigrain 1994 and Anand 1999 showed no statistically significant difference in days of supplemental oxygen use (WMD 0.6 days, 95% CI ‐5.3 to 6.6 days; I2 = 0% (none); Analysis 1.4).
Pneumothorax (outcome 1.5)
All three studies (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) reported on pneumothorax (n = 122) and observed no significant effect of midazolam versus placebo for this outcome (typical RR 1.08, 95% CI 0.41 to 2.84; typical RD 0.01, 95% CI ‐0.10 to 0.12; I2 = 0% (none for both); Analysis 1.5).
Length of NICU stay (outcome 1.6)
Jacqz‐Aigrain 1994 and Anand 1999 reported that length of NICU stay was not statistically significantly different between midazolam and placebo groups. Meta‐analysis of these data showed that the midazolam group had a statistically significantly longer length of stay in the NICU than the placebo group (WMD 5.4 days, 95% CI 0.4 to 10.5 days; I2 = 0% (none); Analysis 1.6; Figure 4).

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.6 Length of NICU stay (days).
Arya 2001 did not report on length of NICU stay.
Average NAPI score at 36 weeks' postmenstrual age (outcome 1.7)
Anand 1999 reported average NAPI score at 36 weeks' postmenstrual age and found no significant difference between midazolam and placebo (dextrose) groups (MD ‐2.10, 95% CI ‐14.38 to 10.18; tests for heterogeneity not applicable; Analysis 1.7).
Poor neurological outcome up to 28 days' postnatal age (IVH grade III or IV, PVL or death at 28 days or sooner without discharge from the NICU) (outcome 1.8)
Anand 1999 reported on this outcome and described no statistically significant difference between midazolam and placebo (dextrose) groups (RR 1.34, 95% CI 0.50 to 3.56; RD 0.08, 95% CI ‐0.19 to 0.35; tests for heterogeneity not applicable; Analysis 1.8).
PIPP score during drug infusion (outcome 1.9)
Anand 1999 reported on this outcome and found a statistically significant difference between midazolam and placebo (dextrose) groups favouring the midazolam group (MD ‐3.80, 95% CI ‐5.93 to ‐1.67; tests for heterogeneity not applicable; Analysis 1.9; Figure 5).

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.9 PIPP score during drug infusion.
Occurrence of adverse effects associated with midazolam administration
Jacqz‐Aigrain 1994 observed no adverse neurological effects, but investigators excluded one infant in the midazolam group from the study within 24 hours owing to major neurological abnormalities. Researchers provided no details of this case and reported no statistically significant differences between groups in the incidence of hypotension requiring albumin or vasoactive drugs (8/24 vs 6/22).
Anand 1999 noted no adverse neurological effects associated with midazolam administration and did not report the incidence of hypotension.
Arya 2001 described no adverse neurological effects associated with midazolam administration. Researchers noted epileptiform movements of unknown cause in two infants in the placebo group and noted no significant hypotension in any infant during the study period.
Neurodevelopmental outcome
Jacqz‐Aigrain 1994 and Arya 2001 did not report long‐term neurodevelopmental outcomes.
Midazolam infusion versus morphine (comparison 2)
PIPP score during drug infusion (outcome 2.1)
Anand 1999 reported on this outcome and described no statistically significant differences between midazolam and morphine groups (MD 1.00, 95% CI ‐0.66 to 2.66; tests for heterogeneity not applicable).
Poor neurological outcome by 28 days' postnatal age (IVH grade III or IV, PVL or death at 28 days or sooner without discharge from the NICU) (outcome 2.2)
Arya 2001 reported on this outcome in 46 infants. Investigators observed statistically significantly increased risk of poor neurological outcome by 28 days' postnatal age compared with infants treated with morphine (RR 7.64, 95% CI 1.02 to 57.21; RD 0.28, 95% CI 0.07 to 0.49; NNTH 4, 95% CI 2 to 14; test for heterogeneity not applicable; Analysis 2.2; Figure 6).

Forest plot of comparison: 2 Midazolam versus morphine, outcome: 2.2 Poor neurological outcome up to 28 days' postnatal age.
Discusión
Resumen de los resultados principales
Desde que se introdujo el midazolam en la unidad de cuidados intensivos neonatales (UCIN) en la década de 1980 se ha publicado poca información sobre su efectividad y seguridad cuando se lo administra a los recién nacidos con enfermedades graves. La mayoría de los informes presentados hasta la fecha son series de casos e informes de casos del uso de midazolam en pacientes de diversos grupos etarios (de tres días a 18 años de edad), administrado en dosis variables (de 0,025 mg/kg a 0,3 mg/kg administrados en bolo, a 24 microgramos/kg/h a 400 microgramos/kg/h administrados como infusión) (Hartwig 1991; Pellier 1999; Rosen 1991; Stenhammar 1994). Los tres estudios incluidos en esta revisión (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) son los únicos ensayos controlados aleatorizados (ECA) realizados hasta la fecha sobre el uso de la infusión de midazolam para la sedación en los niños. Las búsquedas repetidas en la literatura en septiembre 2009; marzo 2012 y junio 2016 no dieron lugar a ensayos adicionales.
Las herramientas para medir el nivel de sedación en los niños prematuros son pocas (AAP/CPS 2000). En la actualidad el nivel de sedación en dichos niños se mide mediante escalas previamente validadas en niños de mayor edad. Se desconoce si estas escalas son apropiadas para los niños prematuros. Por lo tanto, en los tres ECA incluidos en esta revisión (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994), aunque la infusión intravenosa de midazolam pareció proporcionar un sedante efectivo en comparación con placebo, los investigadores no pudieron establecer conclusiones definitivas sobre su efectividad como sedante en los niños prematuros. Anand 1999 evaluó el nivel de sedación utilizando la puntuación COMFORT, una escala compuesta basada en ocho ítems conductuales y fisiológicos utilizados para evaluar la angustia (Ambuel 1992). Aunque estos ítems son aplicables a los niños prematuros, esta puntuación se ha validado solo en los niños de más edad (media de edad 37,1 meses). Jacqz‐Aigrain 1994 y Arya 2001 utilizaron una escala de sedación adaptada del sistema de puntuación de Barrier (Barrier 1989), que no había sido validada en niños prematuros, y seleccionaron cinco de 10 ítems del sistema de puntuación. Se desconoce la validez de una puntuación tan adaptada para evaluar el nivel de sedación en los recién nacidos.
Jacqz‐Aigrain 1994 mostró incidencias similares de hemorragia intracraneal entre los grupos de midazolam y de control. Sin embargo, el recién nacido tratado con midazolam que fue excluido dentro de las 24 horas por anormalidades neurológicas importantes plantea la preocupación sobre la seguridad del midazolam. En Anand 1999 la incidencia de resultados neurológicos deficientes (muerte, hemorragia intraventricular [Hiv] grave, leucomalacia periventricular [LPV]) fue mayor en el grupo de midazolam que en los grupos de placebo y morfina (32% vs 24% vs 4%, respectivamente; P = 0,03). Cabe señalar, sin embargo, que el grupo de morfina incluyó un mayor porcentaje de niños de sexo femenino con un peso ligeramente superior al nacer y una edad gestacional más madura. Estas características iniciales pueden haber contribuido a las diferencias en los resultados neurológicos observados en estos grupos.
Compleción y aplicabilidad general de las pruebas
Hoy en día, solo 146 recién nacidos han sido reclutados en tres ensayos que comparan el midazolam versus placebo o morfina. Los estudios incluidos en esta revisión observaron eventos neurológicos adversos con mayor frecuencia, aunque posiblemente de origen multifactorial, entre los niños tratados con midazolam.
Calidad de la evidencia
Los tres ensayos incluidos en esta revisión (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) tuvieron tamaños de la muestra pequeños pero un riesgo bajo de sesgo para la mayoría de los ítems incluidos en las tablas de riesgo de sesgo. El riesgo de sesgo se calificó como poco claro para el informe selectivo (sesgo de informe), ya que no se disponía de los protocolos de los tres estudios. Se evaluaron estos ensayos como de calidad moderada según los grados de evidencia del GRADE Working Group. En consecuencia, es probable que la realización de estudios de investigación adicionales tenga una repercusión importante sobre la confianza en la estimación del efecto y que pueda modificarla. Lo más importante es que ninguna de las escalas de sedación utilizadas en estos ensayos había sido validada en recién nacidos, y solo fue posible incluir algunos resultados en las tablas de «Resumen de resultados» (Resumen de resultados, Tabla 1; Resumen de resultados, Tabla 2).
Sesgos potenciales en el proceso de revisión
Se tiene conocimiento de que no hay sesgos en el proceso de revisión. Sin embargo, aunque se utilizó un método de búsqueda robusto, no es posible excluir la posibilidad de que se pudiera haber pasado por alto evidencia de la literatura.
Acuerdos y desacuerdos con otros estudios o revisiones
La literatura ha informado de efectos neurológicos adversos asociados con el midazolam en los recién nacidos a término y prematuros (Adams 1997; Bergman 1991; Collins 1991; Magny 1994; Ng 2002; van den Anker 1992). Los investigadores han informado de una variedad de efectos neurológicos transitorios después de la administración de midazolam en bolos o infusiones, o ambos, entre los que se incluye un deterioro del nivel de conciencia, una falta de seguimiento visual, hipertonía, hipotonía, movimientos coreicos, movimientos discinéticos, actividad mioclónica y actividad epileptiforme. Se han observado anormalidades en los electroencefalogramas en algunos casos. En todos los casos, los efectos fueron transitorios, aunque los investigadores no han informado de resultados del neurodesarrollo a largo plazo. En dos estudios (Harte 1997; van Straaten 1992) se encontró una disminución significativa de la velocidad del flujo sanguíneo de la arteria cerebral media entre los niños prematuros a los que se les administró una única inyección en bolo de midazolam. Este efecto duró hasta una hora y estuvo directamente relacionado con una disminución en la presión arterial media. Por lo tanto, parece que los efectos neurológicos del midazolam pueden estar relacionados, al menos en parte, con la hipoperfusión cerebral transitoria. Se desconocen las secuelas a largo plazo de estos efectos.
Se creía que el mecanismo de hipotensión inducida por el midazolam era la vasodilatación relacionada con los niveles de prostanoides extravasculares y de calcio (Modanlou 1997). Jacqz‐Aigrain 1994 descubrió que el número de niños con inestabilidad hemodinámica no era significativamente diferente entre los grupos de midazolam y de placebo (ocho versus seis, respectivamente), aunque los niños del grupo de midazolam tenían una presión arterial significativamente más baja que los del grupo de placebo. Otros investigadores (Burtin 1991; Ng 2002; van den Anker 1992) observaron una hipotensión significativa en varios niños prematuros después de dosis en bolo e infusiones de midazolam que requirió la restauración del volumen o fármacos vasoactivos. En la mayoría de los casos, los profesionales habían administrado fentanilo de manera concomitante.

Study flow diagram: review update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.6 Length of NICU stay (days).

Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.9 PIPP score during drug infusion.

Forest plot of comparison: 2 Midazolam versus morphine, outcome: 2.2 Poor neurological outcome up to 28 days' postnatal age.

Comparison 1 Midazolam versus placebo, Outcome 1 Intraventricular haemorrhage (any grade).
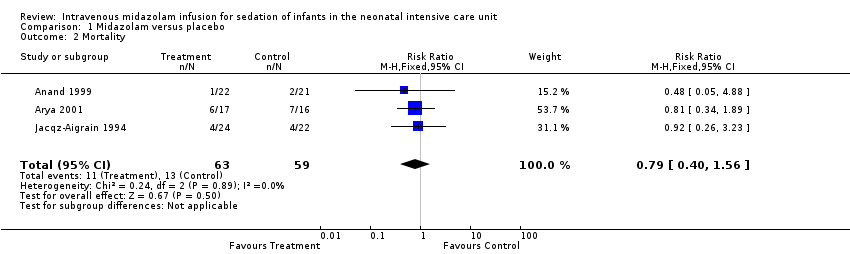
Comparison 1 Midazolam versus placebo, Outcome 2 Mortality.
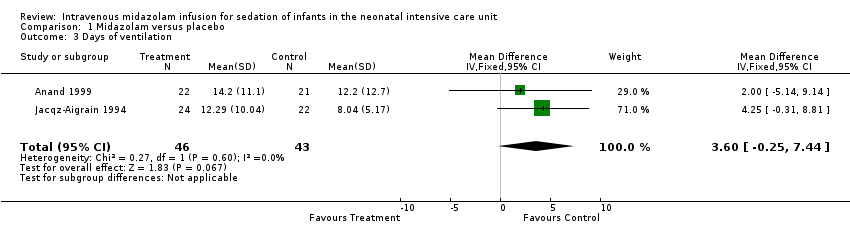
Comparison 1 Midazolam versus placebo, Outcome 3 Days of ventilation.

Comparison 1 Midazolam versus placebo, Outcome 4 Days of supplemental oxygen use.

Comparison 1 Midazolam versus placebo, Outcome 5 Pneumothorax.

Comparison 1 Midazolam versus placebo, Outcome 6 Length of NICU stay (days).

Comparison 1 Midazolam versus placebo, Outcome 7 Average NAPI scores at 36 weeks' PMA.

Comparison 1 Midazolam versus placebo, Outcome 8 Poor neurological outcome by 28 days' postnatal age.
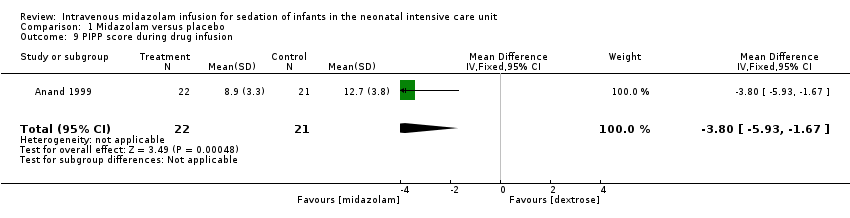
Comparison 1 Midazolam versus placebo, Outcome 9 PIPP score during drug infusion.
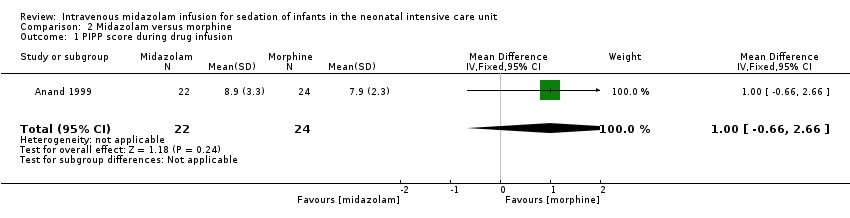
Comparison 2 Midazolam versus morphine, Outcome 1 PIPP score during drug infusion.

Comparison 2 Midazolam versus morphine, Outcome 2 Poor neurological outcome up to 28 days' postnatal age.
| Midazolam infusion compared with placebo for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: placebo infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Midazolam | |||||
| Mortality during hospital stay | High‐risk population | RR 0.79 (0.40 to 1.56) | 122 | ⊕⊕⊕⊝ | Risk of bias for these 3 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 3 studies were conducted in the target population of newborn infants. | |
| 220 per 1000 | 165 per 1000 | |||||
| Length of NICU stay (days) | Mean length of NICU stay ranged across control groups from 9 to 37.5 days. | WMD of NICU stay for intervention groups was 5.4 days longer. | WMD 5.4 days (0.4 to 10.5) | 89 | ⊕⊕⊕⊝ | Risk of bias for these 2 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 2 studies were conducted in the target population of newborn infants. |
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 12.7. | Mean PIPP score in the intervention group was lower at 8.9. | MD ‐3.80 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 1.34 | 43 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 230 per 1000 | 310 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| Midazolam infusion compared with morphine infusion for sedation in neonates | ||||||
| Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: morphine infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Morphine | Midazolam | |||||
| PIPP score during drug infusion Range of scale 0‐21 for infants | Mean PIPP score in the control group was 7.9. | Mean PIPP score in the intervention group was 8.9. | MD 1.00 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 7.64 | 46 | ⊕⊕⊕⊝ | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. | |
| 318 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| Study reference for included trial | Name of score used | Reference for the score | Age of infants/children subjected to the score | Score validated in newborns? |
| COMFORT Scale | Ambuel 1992 ‐ 37 participants (age newborn to 204 months (mean 37.1; SD 52.7)) Marx 1994 ‐ children (age 0 to 102 months (mean age > 1 year) | No | ||
| Sedation score | Barrier 1989 ‐ 23 infants (age 1 to 7 months) | No | ||
| Behaviour score | Craig 1984 ‐ 30 children (age 2 to 24 months) Barrier 1989; Robieux 1991 ‐ 41 infants and toddlers (age 3 to 36 months) | No |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraventricular haemorrhage (any grade) Show forest plot | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.87, 3.24] |
| 2 Mortality Show forest plot | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Days of ventilation Show forest plot | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐0.25, 7.44] |
| 4 Days of supplemental oxygen use Show forest plot | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐5.30, 6.57] |
| 5 Pneumothorax Show forest plot | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.41, 2.84] |
| 6 Length of NICU stay (days) Show forest plot | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 5.44 [0.40, 10.49] |
| 7 Average NAPI scores at 36 weeks' PMA Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐14.38, 10.18] |
| 8 Poor neurological outcome by 28 days' postnatal age Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.50, 3.56] |
| 9 PIPP score during drug infusion Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐3.80 [‐5.93, ‐1.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PIPP score during drug infusion Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.66, 2.66] |
| 2 Poor neurological outcome up to 28 days' postnatal age Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.64 [1.02, 57.21] |

