Corticoesteroides inhalados para la bronquiectasia
Resumen
Antecedentes
La bronquiectasia se está diagnosticando cada vez más y se reconoce como un importante factor que contribuye a la enfermedad pulmonar crónica tanto en adultos como en niños en países de altos y bajos ingresos. Se caracteriza por la dilatación irreversible de las vías respiratorias y se asocia generalmente con la inflamación de las vías respiratorias y la infección bacteriana crónica. El control médico tiene como objetivo principal reducir la morbilidad mediante el control de los síntomas, reducir la frecuencia de las exacerbaciones, mejorar la calidad de vida y prevenir la progresión de la bronquiectasia. Ésta es una actualización de una revisión publicada por primera vez en el año 2000.
Objetivos
Evaluar la eficacia y la seguridad de los corticoesteroides inhalados (CSI) en niños y adultos con bronquiectasia en estado estable, específicamente para evaluar si el uso de CSI: 1) reduce la gravedad y la frecuencia de las exacerbaciones respiratorias agudas; o 2) afecta al declive de la función pulmonar a largo plazo.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane de Ensayos Controlados (Cochrane Register of Controlled Trials, CENTRAL), en el Registro de ensayos del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group Register of trials), en las bases de datos MEDLINE y Embase. La última búsqueda de literatura se realizó en junio de 2017.
Criterios de selección
Todos los ensayos controlados aleatorizados (ECA) que comparan los CSI con un placebo o ninguna medicación. Se incluyeron los niños y adultos con evidencia clínica o radiográfica de bronquiectasias, pero fueron excluidos los pacientes con fibrosis quística (FQ).
Obtención y análisis de los datos
Se revisaron los resultados de la búsqueda según criterios predeterminados para la inclusión. En esta actualización, dos revisores independientes evaluaron la calidad metodológica y el riesgo de sesgo en los ensayos utilizando criterios establecidos y extrajeron los datos mediante un formulario estándar. Se analizó el tratamiento como "tratamiento recibido" y se realizó análisis de sensibilidad.
Resultados principales
La revisión incluyó siete estudios, con 380 adultos. De 380 participantes asignados al azar, 348 completaron los ensayos.
Debido a las diferencias en los resultados informados entre los siete estudios, sólo se pudo realizar un metanálisis limitado tanto para el uso de CSI a corto plazo (6 meses o menos) como para el uso de CSI a más largo plazo (> 6 meses).
Durante el estado estable en el grupo de corto plazo (CSI durante seis meses o menos), según los dos estudios de los que se pudieron incluir datos, no hubo diferencias significativas con respecto a los valores iniciales del volumen espiratorio forzado en el primer segundo (VEF1) al final del estudio (diferencia de medias (DM) ‐0,09; intervalo de confianza (IC) del 95%: ‐0,26 a 0,09) y la capacidad vital forzada (CVF) (DM 0,01 L, IC del 95%: ‐0,16 a 0,17) en adultos con CSI (en comparación con ningún CSI). De manera similar, no se encontró ninguna diferencia significativa en la frecuencia promedio de exacerbación (DM 0,09; IC del 95%: ‐0,61 a 0,79) o en las puntuaciones totales de la calidad de vida relacionada con la salud (CVRS) en adultos con CSI en comparación con adultos sin CSI, aunque los datos disponibles eran limitados. En base a un único estudio no controlado con placebo del que no se pudo extraer datos clínicos, hubo una mejora marginal, aunque estadísticamente significativa, en el volumen de esputo y las puntuaciones de disnea con CSI.
El único estudio sobre los resultados a largo plazo (más de 6 meses) que examinó la función pulmonar y otros resultados clínicos, no mostró ningún efecto significativo de los CSI en ninguno de los resultados. No se pudo sacar ninguna conclusión sobre los efectos adversos debido a los limitados datos disponibles.
A pesar de que los autores de los siete estudios declararon que eran doble ciego, juzgamos que un estudio (CSI de corta duración) tenía un alto riesgo de sesgo basado en el cegamiento, el desgaste y la notificación de los resultados. La calidad de la evidencia según GRADE fue baja para todos los resultados (debido al ensayo no controlado por placebo, a comparaciones indirectas y a la imprecisión con un número reducido de participantes y estudios).
Conclusiones de los autores
Esta revisión actualizada indica que no hay evidencia suficiente para apoyar el uso rutinario de los CSI en adultos con bronquiectasias en estado estable. Además, no se puede sacar ninguna conclusión sobre el uso de los CSI en adultos durante una exacerbación aguda o en niños (para cualquier estado), ya que no se han realizado estudios al respecto.
PICOs
Resumen en términos sencillos
El papel de los corticoesteroides inhalados (ICS) en el manejo de la bronquiectasia
Antecedentes
La bronquiectasia es una enfermedad pulmonar. Las personas con bronquiectasia suelen experimentar síntomas a largo plazo, como tos productiva o húmeda, brotes repetidos (exacerbaciones) y mala calidad de vida. Las personas con bronquiectasias tienen inflamación de las vías respiratorias y muchas tienen síntomas parecidos al asma (como tos y sibilancias). Debido a esto, los corticoesteroides inhalados (CSI), comúnmente usados en el asma, también podrían mejorar los síntomas, reducir los brotes y/o reducir el empeoramiento de la función pulmonar en personas con bronquiectasia. Sin embargo, el uso rutinario de los CSI también puede causar efectos secundarios no deseados.
Pregunta de la revisión
¿Cuáles son los beneficios de usar el CSI regularmente en el manejo de adultos y niños con bronquiectasia?
Características de los estudios
Se incluyeron estudios que compararon los CSI con ningún CSI, o con un placebo (es decir, una medicación hecha para que se vea igual a los CSI pero sin ingredientes activos). Sólo se incluyeron estudios en los que se decidió al azar quién recibiría el CSI y quién no. Los participantes incluidos en los siete estudios fueron 380 adultos a los que se les diagnosticó bronquiectasia por los síntomas o por una detallada exploración pulmonar (tomografía computarizada (TC)). No se incluyeron estudios que involucraran a participantes con fibrosis quística, que también puede causar bronquiectasias. Aunque se planeó incluir estudios que involucraran a niños con bronquiectasia, no se encontraron tales estudios.
¿Qué evidencia se encontró?
A partir de la evidencia disponible hasta junio de 2017, se encontraron siete estudios elegibles con participantes adultos que examinaron el papel de los CSI en la bronquiectasia. Los adultos tenían bronquiectasias estables, no tenían un brote al comienzo del estudio.
Se pudo incluir los resultados de dos estudios que dieron CSI por menos de seis meses a adultos con bronquiectasias estables. Los CSI no marcaron una diferencia en la función pulmonar, el número de exacerbaciones durante el estudio o la calidad de vida. En otro estudio, que también dio CSI por menos de seis meses, se encontró una pequeña reducción en el esputo (flema) y una mejora en la falta de aire. Sin embargo, como estos resultados fueron de un estudio que no utilizó un placebo no se pudo tener seguridad de ellos.
El único estudio sobre el uso a largo plazo de los CSI (es decir, durante más de 6 meses) no mostró ningún beneficio significativo de los CSI para ninguno de los resultados.
No hubo estudios realizados cuando los participantes tenían un brote de su bronquiectasia. Tampoco hubo estudios que involucraran a niños con bronquiectasia. Es importante señalar que no se sabe si los CSI están vinculados a más efectos secundarios no deseados, porque los estudios no proporcionaron mucha información al respecto.
Conclusión
La revisión encontró que no hay suficiente evidencia para el uso rutinario de los CSI en adultos con bronquiectasias estables. No es posible sacar conclusiones sobre el uso de los CSI para los brotes de bronquiectasia, o sobre su uso en niños, porque no se encontró ningún estudio.
Calidad de la evidencia
En general, la calidad de la evidencia se consideró baja. Hubo preocupación porque el estudio más grande, que mostró algunos beneficios, no usó un placebo. Esto significa que los participantes y el personal del estudio habrían sabido quiénes recibían CSI y quiénes no, lo que podría afectar los resultados. Además, la confianza en los resultados disminuyó porque sólo se encontró un pequeño número de estudios para incluir en la revisión y algunos de los estudios pueden haber incluido a personas con otro tipo de enfermedades pulmonares, además de la bronquiectasia.
Authors' conclusions
Summary of findings
| Inhaled corticosteroids compared to placebo for bronchiectasis (< 6 months) | ||||||
| Patient or population: people with bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function (spirometry indices) ‐ FEV1 (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.038 to 0.805 | MD 0.09 lower | ‐ | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (spirometry indices) ‐ FVC (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.062 to 0.0218 | MD 0.01 higher | ‐ | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ diffusion capacity % predicted (end of study) | Mean end of study value 84.2 | MD 2.70 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ RV % predicted (end of study values) | Mean end of study value 106 | MD 2.00 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ TLC % predicted (end of study values) | Mean end of study value 86.4 | MD 3.20 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Average number of exacerbations per participant | Average number of exacerbations per patient ranged from 0.97 to 1.31 | MD 0.17 lower | ‐ | 127 | ⊕⊕⊝⊝ Low1,2,3 | |
| Pseudomonas aeruginosa (P aeruginosa) colonisation | 410 per 1000 | 395 per 1000 | OR 0.94 | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The largest study was not a placebo controlled trial (Martinez‐Garcia 2006). | ||||||
| Inhaled corticosteroids compared to placebo for bronchiectasis (medium‐ to long‐term outcomes) | ||||||
| Patient or population: people with bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function indices ‐ FEV1% predicted (end study minus baseline values) | Mean change from baseline 0 | MD 0.30 higher | ‐ | 86 | ⊕⊕⊝⊝ Low1,2 | |
| Lung function indices ‐ FVC % predicted (end study minus baseline values) | Mean change from baseline 0.9 | MD 0.90 lower | ‐ | 86 | ⊕⊕⊝⊝ Low1,2 | |
| Number of participants improved | 628 per 1000 | 490 per 1000 | OR 0.57 | 86 | ⊕⊕⊝⊝ Low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Only a single study with small participant numbers; we downgraded outcome one point for imprecision. | ||||||
Background
Description of the condition
Bronchiectasis, previously termed an 'orphan disease' is increasingly recognised as a major cause of respiratory morbidity, especially in low‐income countries (Karadag 2005; Karakoc 2001), and in some ethnic populations of affluent countries (Chang 2002; Edwards 2003; Singleton 2000). More recently, it has also been increasingly reported from some high‐income countries (Sanchez‐Munoz 2016). Bronchiectasis is the end result of a variety of airway insults and predisposing conditions that culminate in airway injury, recurrent or persistent airway infection and destruction (Chang 2010). The underlying aetiology of bronchiectasis varies from being a consequence of recurrent respiratory infections to rare immune deficiencies. Other causes include primary ciliary dyskinesia, allergic bronchopulmonary aspergillosis and mycobacterial infection (Olveira 2017; Shoemark 2007). A common feature of most patients is the impaired local and/or systemic host defences to infection.
The dominant symptoms and signs of bronchiectasis are productive or wet cough, dyspnoea on exertion and presence of other respiratory signs (clubbing, chest wall deformity, respiratory noises such as wheeze or crepitations on auscultation). In the long‐term, pulmonary function decline may occur (Keistinen 1997; Twiss 2006). In studies involving both children and adult cohorts, asthma‐like symptoms in people with bronchiectasis have been described and when present, are associated with accelerated pulmonary function decline when compared to those with bronchiectasis, but without asthma‐like symptoms (Field 1969; Keistinen 1997).
Like people with chronic obstructive pulmonary disease (COPD) and cystic fibrosis, children and adults with bronchiectasis also suffer from recurrent acute exacerbations, some of whom require hospitalised treatment. Pulmonary exacerbations present with a worsening of the baseline clinical state (Kapur 2012a), and are associated with increased morbidity (Kapur 2009), progressive deterioration in lung function (Ellerman 1997; Kapur 2010), and poor quality of life in both adults and children with bronchiectasis (Kapur 2012; Wilson 1997). Effective management regimes for bronchiectasis would reduce the frequency or severity of respiratory exacerbations, and/or the long‐term pulmonary function decline.
The hallmarks of bronchiectasis are stasis of infected airway secretions; reduced airway mucus clearance; and regional or diffuse airway wall dilatation, thickening and destruction with loss of airway structural integrity (Mandal 2013). Based on Cole's 'vicious circle hypothesis', microbial colonisation/infection is important in the pathophysiology of bronchiectasis, as it leads to bronchial obstruction and a normal or exaggerated inflammatory response (Cole 1986). As in COPD, chronic airway obstruction and bronchial hyper‐responsiveness is known to occur in bronchiectasis. A variety of proinflammatory airway cells such as neutrophils, lymphocytes, macrophages and eosinophils have been implicated in the pathogenesis of both bronchiectasis and COPD (Mandal 2013; Kim 2008).
Although inflammation is considered an important driver of bronchial hyper‐responsiveness and airway instability in airway diseases such as COPD and asthma (Berend 2008), evidence directly implicating it in bronchial hyper‐responsiveness in bronchiectasis is unclear. Since bronchiectasis involves similar airway inflammatory mediators, suppression of this inflammatory process could potentially slow the rate of airway damage.
Description of the intervention
The anti‐inflammatory effect of corticosteroids on the airways has been well documented in inflammatory airway diseases, such as asthma (Booth 1995), and COPD (Renkema 1996; Sobieraj 2008). Inhaled preparation of corticosteroids provide a non‐specific, local anti‐inflammatory effect with minimal systemic side effects. ICS are available as metered dose inhalers, dry powder devices or nebulised, and can be used for short or long durations. Short duration use of ICS likely has different effects compared to longer‐term ICS use for our primary outcome of interest (lung function), as well as for other adverse event outcomes, such as bone metabolism (Kerstjens 1994), and children's growth (Pruteanu 2014), further discussed in the last paragraph of the 'Data synthesis' section.
Inhaled corticosteroids (ICS) are beneficial in some (but not all) people with COPD (Ernst 2015). However, use of ICS is associated with adverse events in children and adults that range from mild (candidiasis) to serious (adrenal insufficiency (Holme 2008), osteoporosis, cataracts, pneumonia) events. Recent evidence indicates increased risk of pneumonia and lower respiratory tract infections with use of ICS in adults with COPD (Wang 2016). Since bronchiectasis involves similar disruption of the host airway defences, it is plausible that this cohort is also at higher risk of respiratory infections with the use of ICS.
How the intervention might work
Airway eosinophilia and bronchial hyper‐responsiveness has been reported in adults (Ip 1991;Tsikrika 2017), and children with bronchiectasis (Goyal 2016;Pizzutto 2013), hence ICS may be potentially beneficial for this group of patients, as ICS is usually efficacious in people with airway eosinophilia.
Why it is important to do this review
The role of ICS in bronchiectasis remains unclear. An update of the original systematic review on the efficacy of ICS in the management of children and adults with bronchiectasis (Kapur 2009) could help guide clinical practice.
Objectives
To evaluate the efficacy and safety of inhaled corticosteroids (ICS) in children and adults with stable state bronchiectasis, specifically to assess whether the use of ICS: (1) reduces the severity and frequency of acute respiratory exacerbations; or (2) affects long‐term pulmonary function decline.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) using inhaled corticosteroids (ICS) in patients with bronchiectasis, in comparison to placebo.
Types of participants
Children or adults with bronchiectasis (defined clinically or radiologically) not related to cystic fibrosis.
Types of interventions
All types of ICS.
Types of outcome measures
Primary outcomes
-
Change in objective measures of lung function.
Secondary outcomes
(A) for short‐term effectiveness (6 months or less)
-
Mean difference in bronchiectasis severity control (wheeze, dyspnoea, cough diary, etc).
-
Mean of respiratory exacerbations, or hospitalisations per participant, or both.
-
Sputum volume.
-
Mean difference in other objective indices (airway markers of inflammation, exhaled nitric oxide, etc).
-
Mean difference in quality of life indices.
-
Proportions experiencing adverse effects of the intervention, (e.g. pharyngeal candidiasis, voice change, pneumonia, etc).
(B) for medium‐ to long‐term outcomes (greater than 6 months)
-
Clinical indices of bronchiectasis severity control (quality of life, cough diary, Likert scale, visual analogue scale, level of interference of cough, etc).
-
Relevant airway markers of inflammation.
-
Proportions experiencing adverse effects of the intervention, (e.g. adrenal insufficiency, cataracts, linear growth, etc).
-
Frequency of exacerbation per subject.
Search methods for identification of studies
Electronic searches
This is an update of a previous Cochrane Review (Kapur 2009).
For this update, we identified studies from the following sources.
-
The Cochrane Airways Group Trials Register (updated June 2017).
-
The Cochrane Central Register of Controlled Trials (CENTRAL; Issue 5, 2016.
-
MEDLINE (1950 to June 2017).
-
Embase (1980 to June 2017).
-
The list of references in relevant publications.
-
Written communication with the study authors of the studies included in the review, when necessary.
For the full database topic search strategies, see Appendix 1. We searched all databases with no restriction on language of publication. We searched the CENTRAL database for handsearched conference abstracts and grey literature.
We also conducted searches of ClinicalTrials.gov (www.ClinicalTrials.gov), and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/), using the search strategy in Appendix 2. We searched all databases from their inception to 5 February 2018, and we imposed no restriction on language of publication.
Searching other resources
We wrote to the lead authors of all the studies included and three study authors replied; we incorporated any information provided.
Data collection and analysis
Selection of studies
From the title, abstract, or descriptors, two review authors (NK, AC) independently reviewed the literature searches to identify potentially relevant studies for full review. There was no disagreement between the review authors as to which studies should be included. We recorded the selection process in sufficient detail to complete the PRISMA (PRISMA 2009) flow diagram (Figure 1) and Characteristics of excluded studies table.
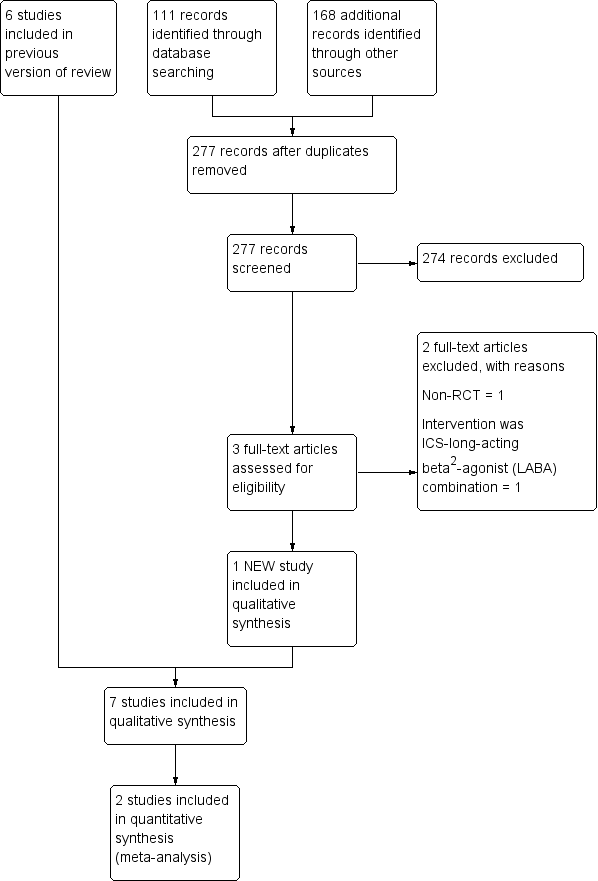
Study flow diagram.
Data extraction and management
We reviewed studies that satisfied the inclusion criteria and recorded the following information: study setting, year of study, source of funding, participant recruitment details (including number of eligible participants), inclusion and exclusion criteria, other symptoms, randomisation and allocation concealment method, numbers of participants randomised, blinding (masking) of participants, care providers and outcome assessors, dose and type of intervention, duration of therapy, cointerventions, numbers of participants not followed up, reasons for withdrawals from study protocol (clinical, side effects, refusal and other), details on side effects of therapy, and whether intention‐to‐treat analyses were possible. We extracted data independently on the outcomes described previously. We requested further information from the study authors, but only three responded (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006); we incorporated the information received.
Assessment of risk of bias in included studies
We included all assessments in the 'Characteristics of included studies' table. We measured inter‐reviewer reliability for the identification of high quality studies for each component using the Kappa statistic. Agreement between the two review authors was excellent (weighted kappa score for quality assessment scores was 0.81).
Two review authors (NK, AC) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreement by discussion. We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We carried out blinding separately for different key outcomes, where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
The statistical analysis proposed, included calculation of a pooled estimate of treatment effect for each dichotomous outcome across all studies (odds of outcome in participants allocated to receive treatment compared with odds of outcome in participants allocated to control group); and calculation of a pooled estimate of treatment effect for each continuous outcome across all studies in the form of a mean difference (MD). For continuous variables, we recorded mean absolute change from baseline for each group and standard deviation (SD) for each group.
Unit of analysis issues
We used the participant as the unit of analysis.
Dealing with missing data
We sought data on a number of participants with each outcome event listed above, by allocated treatment group in order for us to conduct an intention‐to‐treat (ITT) analysis. We also contacted the study investigators for clarification and further information where necessary.
Assessment of heterogeneity
We described and tested any heterogeneity between the study results to see if it reached statistical significance using a chi2 test. We estimated the 95% confidence interval (CI) using a random‐effects model whenever there were concerns about statistical heterogeneity.
Assessment of reporting biases
We checked all reports of the included studies to check that all the stated outcomes were reported and the results were presented in the 'Risk of bias table' in the 'Characteristics of included studies' table.
Data synthesis
We calculated odds ratio (OR) using a modified ITT analysis for the dichotomous outcome variables of each individual study. This analysis assumes that participants not available for outcome assessment have not improved (and probably represents a conservative estimate of effect). An initial qualitative comparison of all the individually analysed studies examined whether pooling of results (meta‐analysis) is reasonable. This took into account differences in study populations, inclusion/exclusion criteria, interventions, outcome assessment, and estimated effect size.
We included results from studies that met the inclusion criteria and reported any of the outcomes of interest in the subsequent meta‐analyses. We calculated the summary weighted risk ratio (RR) and 95% CI (fixed‐effect model) using Review Manager 5 (Review Manager 2014). For cross‐over studies, we calculated mean treatment differences from raw data. We extracted or imputed and entered the date as fixed‐effect generic inverse variance outcomes to provide summary weighted differences and 95% CIs. In cross‐over trials, we only included data from the first arm in meta‐analysis when the data were combined with parallel studies (Elbourne 2002). We calculated numbers needed to treat to benefit (NNTB) were calculated from the pooled OR and applied its 95% CI to a specified baseline risk using an online calculator (Cates 2008). If studies had reported outcomes using different measurement scales, we planned to use the standardised mean difference (SMD).
'Summary of findings' table
We created 'Summary of findings' tables using the following outcomes.
Short‐term ICS use (≤ 6 months)
-
Lung function indices forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) (in L, end of study minus baseline values)
-
Lung function indices diffusion capacity % predicted (end of study values)
-
Average number of exacerbations per participant
-
Pseudomonas aeruginosa (P aeruginosa) colonisation
There are various types of lung function abnormality in people with bronchiectasis (Guan 2014). As this was our primary outcome measure, we included the various indices that reflect these abnormalities.
Longer‐term ICS use (> 6 months)
-
Lung function indices FEV1 and FVC (in L, end of study minus baseline values)
-
Average number of exacerbations per participant
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We then used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
We evaluated the outcomes described above based on short (6 months or less) and long duration (> 6 months) use of ICS, as done previously in our Cochrane Review (Kapur 2009). This arbitrary cut‐off is a commonly used time frame in studies (Daniel 2017; Kerstjens 1994; Lee 2012), when evaluating the effects of ICS, as duration of ICS use may impact on clinical and structural pulmonary outcomes (e.g. airway remodelling (Barnes 2010)), and adverse events (e.g. growth (Pruteanu 2014)), and bone metabolism (Kerstjens 1994). Lung function in people with bronchiectasis generally declines slowly over time and as this was our a priori defined primary outcome, the effect of short duration ICS theoretically could differ from longer duration ICS. Outcomes such as bronchial hyper‐responsiveness takes months of ICS therapy to plateau (Barnes 2010). An example with respect to adverse events: in the Cochrane Review on the effect of ICS on height in growing children with asthma, the authors found no difference between groups (ICS versus placebo) in the first six months of ICS use, but a significant difference between groups was present in ICS use at the 12 months time point (Pruteanu 2014).
Subgroup analysis and investigation of heterogeneity
We planned the following a priori subgroup analysis.
-
Children (aged 18 years or less) and adults (> 18 years).
-
Dose of ICS; low (< 400 µg), moderate (400 µg to 800 µg), high (> 800 µg) of beclomethasone equivalent.
-
Severity of bronchiectasis (based on lung function).
Sensitivity analysis
We also planned sensitivity analyses to assess the impact of the following potentially important factors on the overall outcomes.
-
Study quality.
-
Variation in the inclusion criteria.
-
Differences in the medications used in the intervention and comparison groups.
-
Analysis using random‐effects model.
-
Analysis by 'treatment received'.
Results
Description of studies
Results of the search
For the updated review in 2009 the Airways Group Register identified 341 potentially relevant titles. After assessing the abstracts, we obtained nine papers for consideration for inclusion in the review. We excluded three studies as inhaled corticosteroids (ICS) were not compared to placebo/no treatment or were non‐randomised studies or included participants with pneumonia (Ghosh 2002; Monton 1999; ONeil 2004; see Characteristics of excluded studies table).
For this 2018 update, we conducted searches from 2011 to 30 June 2017 and the Cochrane Airways Group's Register identified 111 new references; we identified an additional 277 titles through searches of ClinicalTrials.gov (www.ClinicalTrials.gov), and the WHO trials portal (www.who.int/ictrp/en/). After assessing the abstracts, we obtained three articles for consideration to be included in the review; we excluded two studies because ICS was compared to an ICS‐long‐acting beta2‐agonist (LABA) combination (Martinez‐Garcia 2012), or was a non‐randomised study (Guran 2008). We included one new study in this update (Hernando 2012) (Figure 1).
Included studies
See Characteristics of included studies and Table 1 'Summary of included studies characteristics'.
| Study ID | Intervention | Control | Duration of intervention |
| Beclomethasone dipropionate 750 µg twice daily by MDI | Placebo | 6 weeks | |
| Budesonide 400 µg twice daily | Placebo | 6 months | |
| Beclomethasone 800 µg per day over 2 doses | Placebo | 4 weeks | |
| Fluticasone 500 µg twice daily by MDI or 250 µg fluticasone twice daily | No treatment | 6 months | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 4 weeks | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks |
MDI: metered dose inhaler
We identified one new study for this review update (Hernando 2012), including 77 participants. A total of seven studies met the inclusion criteria for this review update (Elborn 1992; Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). All included studies identified were published in English. We requested additional data from all study authors but only three authors responded (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006), and provided additional data. We also requested data on outcomes at 24 weeks for the Tsang 2005 study as well as the lung function values in actual volumes (instead of percentage predicted) from the study authors.
Study design and population
All seven studies were single centre studies. The studies included participants with bronchiectasis diagnosed on bronchography (Elborn 1992), or high resolution computed tomography (HRCT) of the chest (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). All studies excluded participants with cystic fibrosis, with six studies excluding participants with bronchial asthma also (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). Participants with allergic bronchopulmonary aspergillosis were also excluded from the Elborn 1992 study and the Martinez‐Garcia 2006 study. None of the studies included any children.
The Joshi 2004 and Elborn 1992 studies were cross‐over studies and the others were parallel group studies. All were double‐blind placebo controlled trials except the Martinez‐Garcia 2006 study, which did not use placebo in the control group.
Participants with bronchiectasis were recruited during stable state, defined as "free from exacerbation for four weeks" (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006), or "stable 24‐hour sputum volume, FEV1 and FVC" (Tsang 1998; Tsang 2004; Tsang 2005). No study was performed during an acute respiratory exacerbation.
Interventions
Moderate to high doses of ICS were used: budesonide 800 µg/day (Hernando 2012), beclomethasone 800 µg/day (Joshi 2004), beclomethasone 1500 µg/day in the Elborn 1992 study and fluticasone 1000 µg/day (2000 µg/day budesonide equivalent) (Martinez‐Garcia 2006; Tsang 1998;Tsang 2004;Tsang 2005). The Martinez‐Garcia 2006 study had a third arm with inhaled fluticasone 500 µg/day and we combined this data with the 1000 µg/day arm and compared them as a single group with the control group wherever possible.
The study duration ranged from a short duration of four to six weeks in the Elborn 1992, Joshi 2004 and Tsang 1998 studies to six months in the Martinez‐Garcia 2006 and Hernando 2012 studies. Two studies were of one year duration (Tsang 2004;Tsang 2005), with visits at four, 12, 24, 36 and 52 weeks.
Outcomes
Lung functions were available as an outcome variable in all studies except the Tsang 2004 study, which reported only fractional exhaled nitric oxide (FeNO) levels. Forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) (in litres or % predicted) were available from all studies. Peak expiratory flow rate (PEFR) was reported in the Elborn 1992, Joshi 2004 and Tsang 1998 studies, with the Martinez‐Garcia 2006 and Tsang 1998 studies giving details about total lung capacity, residual volume and diffusion capacity. We did not include spirometric parameters from the Joshi 2004 study in the final analysis since the 'pre‐treatment' values were reported for the whole 20 group together and these values were not available separately for the two groups at baseline. We also did not include data from the Tsang 1998 and Tsang 2004 studies in the final analysis, since geometric mean was used instead of arithmetic mean. We did not include any of the data from the Elborn 1992 study, since pre‐cross‐over data were not available separately.
Clinical parameters of cough, wheeze and dyspnoea were measured differently in different studies. The Elborn 1992 study used a visual analogue scale (VAS) to quantify these symptoms, the Martinez‐Garcia 2006 study defined significant cough as that persisting for > 50% of days. Dyspnoea was measured by using the transition dyspnoea index in the Martinez‐Garcia 2006 study. The Hernando 2012 study measured cough, sputum production and dyspnoea on a scale of 0 to 3 and combined these three as a total symptom score. Clinical parameters of cough, dyspnoea and wheezing, although reported by the Tsang 2005 study, were not defined properly and we did not include them in the analysis. Twenty‐four‐hour sputum volume was included as an outcome variable in the Elborn 1992, Martinez‐Garcia 2006, Tsang 1998 and Tsang 2005 studies.
Quality of life was included as an outcome parameter in the Martinez‐Garcia 2006 and Hernando 2012 studies, with both using the St. George Respiratory Questionnaire to calculate total scores as well as symptoms, activity and impact score.
The Martinez‐Garcia 2006, Tsang 1998 and Tsang 2005 studies defined exacerbation as persistent (> 24 hours) deterioration in at least three respiratory symptoms (including cough, dyspnoea, haemoptysis, increased sputum purulence or volume, and chest pain), with or without fever, radiographic deterioration, systemic disturbances, or deterioration in chest signs. The Hernando 2012 study defined exacerbation as worsening of more than 48 hours duration of at least three of the four symptoms of cough, sputum production, dyspnoea and fever.
The study characteristics are described in the 'Characteristics of included studies' table.
Excluded studies
We excluded five studies from the review. The reason for their exclusion is discussed in the 'Characteristics of excluded studies' table. The most common reason for exclusion was the study not being a RCT (two studies).
Risk of bias in included studies
Risk of bias judgements for included studies are summarised in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The generation of randomisation sequence was not reported in six studies and so we judged risk of bias to be unclear (Elborn 1992; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). Only one study stated how the random sequence was generated and we judged this to have low risk of bias (Hernando 2012). Allocation concealment was unclear in all seven studies (Elborn 1992; Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005).
Blinding
We judged all studies as low risk as they were double‐blind, except Martinez‐Garcia 2006, which we judged as high risk as they did not have a placebo arm, and the blinding was done only for comparing two dosages of ICS.
Incomplete outcome data
Two studies reported the progress of all randomised participants in each group (Tsang 1998; Tsang 2004), resulting in low risk of bias, whereas in Joshi 2004, the bias was unclear as there was no mention of withdrawals or dropouts. We judged four studies as high risk of bias as they did not describe where their dropouts occurred (Elborn 1992; Hernando 2012; Martinez‐Garcia 2006; Tsang 2005). Furthermore, we could not extract pre‐cross‐over arm data from Elborn 1992 or include it in any of the meta‐analysis. Follow‐up was between 80% to 90% in Tsang 2005 and was unclear in Joshi 2004. The remaining studies reported outcomes in > 90% of the participants (Hernando 2012; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004).
Selective reporting
We judged six studies to have low risk of bias (Elborn 1992; Hernando 2012; Joshi 2004; Tsang 1998; Tsang 2004; Tsang 2005), as they reported all data for outcomes. One study did not report all the outcome data for the 500 µg/day arm (Martinez‐Garcia 2006), therefore we judged it at high risk of bias.
Other potential sources of bias
We judged six studies as potentially having high risk of bias in other areas (Elborn 1992; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). The baseline values for lung functions, sputum amount and sputum inflammatory markers were significantly different clinically between the arms in three studies (Tsang 1998; Tsang 2004; Tsang 2005). We judged Elborn 1992 as high risk due to the cross‐over design with no washout period. Joshi 2004 included participants with significant post‐bronchodilator response which indicates ICS would be beneficial. Martinez‐Garcia 2006 did not complete ITT analysis.
Effects of interventions
See: Summary of findings for the main comparison Inhaled corticosteroids compared to placebo for bronchiectasis (short‐term use of 6 months or less); Summary of findings 2 Inhaled corticosteroids compared to placebo for bronchiectasis (longer‐term use of > 6 months)
The seven studies involved 380 participants, with 348 completing the studies (Elborn 1992; Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). The data that we could include in the meta‐analysis were very limited, with just two studies contributing most data (Hernando 2012; Martinez‐Garcia 2006).
Stable state bronchiectasis: short‐term (≤ 6 months) outcomes
We included data from the Hernando 2012 and Martinez‐Garcia 2006 studies in the short‐term stable state analysis. See summary of findings Table for the main comparison for the main comparisons.
Primary outcome: lung function
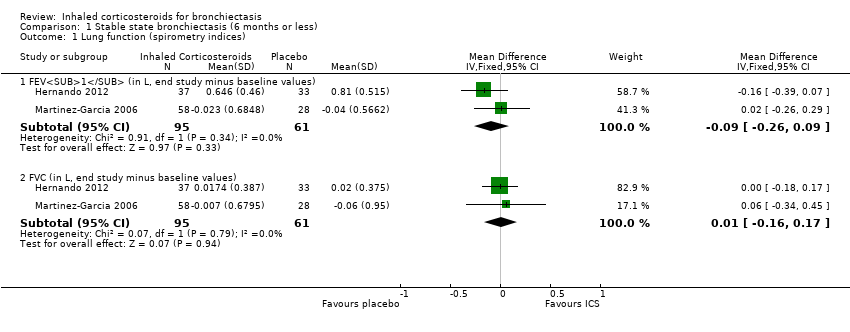
We included date from two studies in this meta‐analysis (Hernando 2012; Martinez‐Garcia 2006). We used change from baseline data to remove bias in differences at baseline. For both FEV1 and FVC, we combined data of the two steroid dose arms for the Martinez‐Garcia 2006 study into a single intervention arm when comparing against the no steroid arm. For FEV1, the pooled data showed no difference between the groups (mean difference (MD) ‐0.09, 95% confidence interval (CI) ‐0.26 to 0.09; participants = 156; Analysis 1.1). A similar result was seen for FVC (MD 0.01, 95% CI ‐0.16 to 0.17; participants = 156; Analysis 1.1), with no significant heterogeneity between studies. The Elborn 1992 study also reported an improvement in the FEV1 in the ICS group compared to the placebo group (P = 0.03), but not in FVC; as the values were not reported, we were unable to pool this.
Based on a single study from which we could include data in the final analysis, ICS showed no difference in the following outcomes: diffusing capacity of the lungs for carbon monoxide (DLCO) (MD 2.70, 95% CI ‐2.49 to 7.89), residual volume (MD 2.00, 95% CI ‐9.41 to 13.41) and total lung capacity (MD 3.20, 95% CI ‐1.99 to 8.39) . We only used the 500 µg twice daily arm, since data on the lower dose ICS arm were not available (Martinez‐Garcia 2006).
Secondary outcome: clinical severity
We could only display data from a single non‐placebo study for these clinical parameters (Martinez‐Garcia 2006). We combined data from the two ICS dose arms for wheeze and sputum production, but not for dyspnoea, since lower dose steroid data were unavailable. The number of participants with sputum reduction as well as with improvement in dyspnoea was significantly higher in the ICS arm compared to the control arm (Analysis 1.3). There were no differences between groups for the clinical parameters of cough and wheeze. Although the Martinez‐Garcia 2006 study described a significant difference between groups for the number of participants experiencing reduced cough, we found no difference between groups when we performed ITT analyses. Also, as the methodology of subjective cough measures was not a validated method, we did not display this data as a forest plot. Although we did not include date from the Elborn 1992 study in the final analysis, the study reported that the ICS group had a significant improvement in cough (P = 0.02) but not wheeze and dyspnoea. Again, only the P value was reported. The data from the Hernando 2012 study showed no significant improvement in the total symptom score in the group on ICS compared to control (MD ‐3.22, 95% CI ‐8.15 to 1.71), but we could not include the data since the subdivision of the score was not available. None of the other studies reported these clinical outcomes.
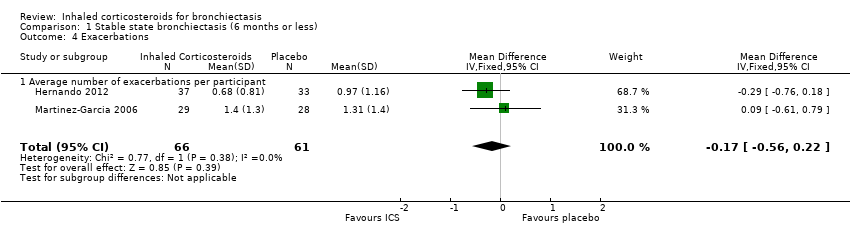
Secondary outcome: exacerbations
Data on average number of exacerbations per participant were available from two studies (Hernando 2012; Martinez‐Garcia 2006). The combined data showed no difference between the two groups (MD ‐0.17, 95% CI ‐0.56 to 0.22; participants = 127; Analysis 1.4). In the Tsang 1998 study, one participant in the fluticasone group experienced an exacerbation compared with three participants in the placebo group. In the Hernando 2012 study, the mean numbers of exacerbations in the ICS group was 0.68 compared to 0.97 in the placebo group, though this was not reported to be statistically significant. In the same study, a much higher proportion of participants (12%) needed hospital admission in the placebo group compared to the ICS group (2.7%), but this was not statistically significant. We only used the 500 µg twice daily arm for the Martinez‐Garcia 2006 study, since data on the lower dose ICS arm were not available for this parameter.
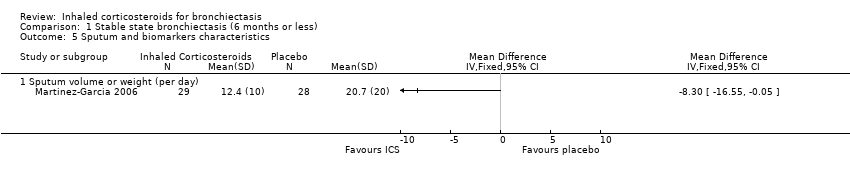
Secondary outcome: sputum and biomarker characteristics
The Martinez‐Garcia 2006 study was the only study included in this analysis which showed a trend towards reduction in the sputum volume (MD ‐8.30, 95% CI ‐16.55 to ‐0.05; participants = 57; Analysis 1.5). We only used the 500 µg twice daily arm for the Martinez‐Garcia 2006 study, since data on the low dose ICS arm were not available. We did not include data on sputum volume and sputum inflammatory markers from the Tsang 1998 study in the final analysis due to clinically significant differences in the baseline value between the two groups. The Elborn 1992 study also described a significant improvement in the 24‐hour sputum volume in the ICS group (P = 0.003), but neither the size of the effect nor the CI was provided. The other studies did not include this as an outcome variable.
Secondary outcome: fractional exhaled nitric oxide (FeNO)
The Tsang 2004 study showed no change in the FeNO between the two groups at 24 weeks. Since the data were skewed and geometric means were reported, we did not include this data in the analysis.
The proportion of eosinophils as a percentage of total cells were significantly less in the sputum at the end of ICS therapy compared to the end of placebo therapy in the Hernando 2012 study, although all other inflammatory sputum markers, including IL‐8, did not show any significant difference.
Secondary outcome: Pseudomonas colonisation
Data on proportion of participants colonised with P aeruginosa at the end of treatment were available from two studies (Hernando 2012; Martinez‐Garcia 2006), with pooled data showing no increased risk of P aeruginosa colonisation with ICS therapy. We used combined data for the two steroid dose arms for the Martinez‐Garcia 2006 study for this parameter. We included extra data provided by the Hernando 2012 study in this analysis.
The Tsang 1998 study explained that the density of P aeruginosa in the sputum was similar between groups at the end of treatment (median density of 1.0 x 10E7 cfu/mL (inter‐quartile range (IQR) 0.33 to 5.15) in the ICS group and 1.4 x 10E7 cfu/mL (IQR 0.7 to 4.67) in the placebo group.
However, the density of total bacteria and commensal bacteria in sputum increased after four weeks of therapy with ICS when compared to baseline (median baseline value was 2.6 x 10E7 cfu/mL (IQR 0.94 to 5.28) and median post‐treatment value was 11.6 x 10E7 cfu/mL (IQR 0.34 to 33.8) whilst the corresponding value in the placebo group was lower post‐treatment (median baseline value was 2.6 x 10E7 cfu/mL (IQR 0.34 to 4.3) and median post‐treatment value was 1.3 x 10E7 cfu/mL (IQR 0.55 to 2.75). The post‐treatment between‐group comparisons P value did not reach statistical significance (P = 0.06) (Tsang 1998).
Secondary outcome: quality of life
Data were available from two studies (Hernando 2012; Martinez‐Garcia 2006). When data of the two ICS dose arms were combined in the Martinez‐Garcia 2006 study, and clinical improvement in health‐related quality of life (HRQoL) analysed as a dichotomous variable, significantly more people in the ICS group experienced a clinically important improvement in their HRQoL score compared to the no treatment group (Analysis 1.3.4). The Hernando 2012 study reported worsening in total quality of life score in both the ICS and placebo group when compared to baseline, but the between‐group difference was not statistically significant (MD ‐3.22, 95% CI ‐8.15 to 1.71; participants = 70; Analysis 1.7). When we pooled the data from the two included studies, there was a statistically significant improvement in activity score but not for total scores or symptom score (Analysis 1.7). Only 500 µg twice daily ICS data were available for these parameters. Impact score data were only available from the Hernando 2012 study.
Secondary outcome: adverse events
Only one study reported adverse events (Martinez‐Garcia 2006), and it was more frequent in the treatment group receiving 1000 µg versus 500 µg (19 participants versus 7, P = 0.04). The most common side effects were dry mouth, local irritation and transient dysphonia.
Stable state bronchiectasis: long‐term (> 6 months) outcomes
We included data from the Tsang 2004 study and Tsang 2005 study in the long‐term stable state analysis, although the Tsang 2004 study had only FeNO as the outcome variable. See summary of findings Table 2 for the main comparisons.
Primary outcome: lung function
For FEV1 % predicted (end study minus baseline values), data from the single study, Tsang 2005, showed no difference between the two groups (MD 0.30, 95% CI ‐17.43 to 18.03; participants = 86; Analysis 2.1).
For FVC % predicted (end study minus baseline values), data from the single study, Tsang 2005, showed no difference between the two groups (MD ‐0.90, 95% CI ‐14.59 to 12.79; participants = 86; Analysis 2.1).
Secondary outcome: clinical severity
We did not include any data for the clinical parameters from the two studies of more than six months duration (Tsang 2004; Tsang 2005).
Secondary outcome: exacerbations
The Tsang 2005 study showed a non‐significant improvement in exacerbation frequency in the ICS group compared to the control group (odds ratio (OR) 0.57, 95% CI 0.24 to 1.34; participants = 86; Analysis 2.2).
Secondary outcome: sputum and biomarker characteristics
As an overall effect, ICS had no effect on the 24‐hour sputum volume when given for a period of 52 weeks, although we did not include the data for this outcome in the analysis because only median and interquartile ranges were available and the data were very skewed. As a subgroup analysis, the Tsang 2005 study reported a significant improvement in the amount of sputum volume/day in the subgroup of participants with sputum volume < 30 mL/ day, exacerbation frequency ≤ two/year, and sputum purulence score > 5 (data not available).
Sputum purulence was scored as 0, 1, 2, 3, 4, 5, 6, 7 or 8 (absence of, completely transparent, almost transparent, translucent but colourless, opaque, milky white grey, pale green, moderately green, and dark green sputum, respectively) in the Tsang 2005 study. After 52 weeks, there was no significant difference in the purulence scores between the ICS and placebo groups (MD 0.2, 95% CI ‐0.94 to 1.34; participants = 86; Analysis 2.3).
Secondary outcome: FeNO
The Tsang 2004 study showed no change in the FeNO between the two groups at 52 weeks, although we did not include the data due to its skewed nature.
Secondary outcome: adverse events
None of the studies reported adverse events.
Sensitivity analysis
Removing the study with a poor quality score (no placebo) made no difference to the results for FEV1 and FVC. The activity subscore of the St. George Respiratory Questionnaire (a measure of HRQoL) became non‐significant when we removed the non‐placebo controlled study (Martinez‐Garcia 2006).
For the clinical data, when we analysed separately the data from the fluticasone 1000 µg arm of the Martinez‐Garcia 2006 study and the 500 µg arm, in both arms in the meta‐analysis, those on ICS still had a higher proportion of participants with sputum volume reduction of > 50%. For the outcomes of wheeze and cough there was still no difference between the groups. For the outcome of dyspnoea, actual data were not provided for the 500 µg arm but the study authors mentioned that there was no difference between groups. Hence it is assumed that the significant effect present for the 1000 µg/day group (outcome 1.3.3) was no longer present for the 500 µg/day group.
Analysis using random‐effects did not alter the significance of any of the outcomes. None of the other planned sensitivity analysis were relevant.
Discussion
Summary of main results
We did not identify any studies involving children in our searches. Seven studies involving adult participants fulfilled the inclusion criteria. Of the 380 randomised, 348 participants completed the trials. In the short‐term group ( inhaled corticosteroids (ICS) for ≤ 6 months), use of high dose ICS in adults with bronchiectasis did not lead to any statistical or clinically significant change in the lung function indices of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC). Further, there was no statistically significant improvement in the overall health‐related quality of life (HRQoL) indices except in the activity score. There was improvement in sputum production and dyspnoea scores in the ICS group from the single study with available data for this outcome (Martinez‐Garcia 2006), but the result should be interpreted with caution as the study was not placebo controlled. When we only included placebo controlled studies, there were no significant differences between groups in all outcomes examined (spirometry, clinical outcomes of exacerbation or sputum volume, etc.). The single study on long‐term outcomes showed no significant benefit of ICS in any of the outcomes.
Overall completeness and applicability of evidence
This Cochrane review is limited to seven studies involving adult participants with variable designs, variable doses and length of study. Also, data that could be combined for the meta‐analyses were limited to only two studies for the various outcomes examined. As there were no paediatric studies, data from this review cannot be extrapolated to children. Further, in children, the adverse effect of high dose, or long‐term ICS, or both, is likely to be more serious than in adults, in particular to the detrimental effect on linear growth (Philip 2014).
The meta‐analyses derived from two studies of the short‐term effect of ICS in bronchiectasis showed no benefit of ICS on lung function parameters of FEV1 and FVC (compared to controls), though the data included were limited (Hernando 2012; Martinez‐Garcia 2006). There was improvement in clinical parameters of dyspnoea and sputum production in the ICS group, based on data from the single study which was not placebo controlled. Similar improvement was also seen in the activity score of HRQoL in the ICS group, though the effect was marginal. We could not include data from Joshi 2004 and Tsang 1998 in the final analysis, but both studies had not reported any improvement in the lung function parameters between the steroid and placebo groups. On the contrary, data from the Elborn 1992 study (which we could not include in the meta‐analysis) showed a significant improvement in the FEV1 in the ICS group compared to the placebo group. Thus, it remains uncertain whether ICS confers any benefit in people with bronchiectasis.
Data on the short‐term effect of ICS on clinical parameters of cough, wheeze, dyspnoea and sputum volume were available from two studies (Hernando 2012; Martinez‐Garcia 2006). The Martinez‐Garcia 2006 study showed a significant improvement in dyspnoea in the 1000 µg/day fluticasone group and sputum volume for the combined 500 µg/day and 1000 µg/day group compared to the control group, although no such effect was seen in the total symptom score from the Hernando 2012 study. We did not include data on sputum volume from the Tsang 1998 study in the analysis in view of the clinically significant difference in the baseline values. The Martinez‐Garcia 2006 study showed a significant improvement in the quality of life score in the ICS group using the St. George Respiratory Questionnaire, though no such improvement was reported in the Hernando 2012 study. On pooling these data, the effect favoured ICS use largely due to the large effect of the non‐placebo controlled study (Martinez‐Garcia 2006).
Recurrent acute pulmonary exacerbations form part of the disease progression in patients with bronchiectasis and many of these exacerbations require hospital admission. Recurrent exacerbations are associated with progressive deterioration of lung functions (Kapur 2010), and are also one of the strong predictors of poor quality of life in bronchiectasis (Wilson 1997). In this review, based on limited data, short‐term use of ICS did not influence frequency of exacerbations. Prolonged ICS administration also did not influence exacerbation frequency (Tsang 2005). This becomes more relevant with the fact that ICS actually increased the bacterial density in the airways and most exacerbations in bronchiectasis are likely to be infective in origin (Tsang 1998).
Administration of ICS for a longer duration significantly increased the number of participants who had a more than 20% reduction in the 24‐hour sputum volume, but did not have any beneficial effect in the other clinical or spirometric parameters (Tsang 2004; Tsang 2005). This lack of effect again suggests that infection and not pure inflammation, is probably the more relevant underlying pathogenic mechanism of disease progression in bronchiectasis.
The ICS doses used in several studies were very high (up to 2000 µg/day budesonide equivalent). This limits applicability of the data to most patients in our current era where ICS doses used are generally lower, in light of the increased appreciation of adverse events related to prolonged use of high ICS doses, such as the effect on linear growth (Philip 2014). High dose ICS use is also associated with adverse events in children and adults that range from mild events (candidiasis) to serious events (pneumonia, adrenal insufficiency, osteoporosis, cataracts) (Sobieraj 2008). The Martinez‐Garcia 2006 study reported dry mouth, local irritation and transient dysphonia as the most common adverse effects. None of the other studies reported any adverse effects. While there was no significant difference in adverse events between groups, the total sample size was small, rendering a likely type‐1 error. The potential harm associated with prolonged ICS use, or high ICS doses, or both, need to be considered along with its potential benefit.
Bronchiectasis is a heterogenous condition, with a substantial overlap in people with chronic obstructive pulmonary disease (COPD). Not all the studies in this review excluded smokers and thus, whether data in our review can be universally applied is unknown. Nearly 50% of adults with COPD have bronchiectasis (Chang 2008). A Cochrane Review concluded that ICS administration reduces the rate of exacerbations as well as the rate of decline in quality of life in patients with stable state COPD (Yang 2012). As the Martinez‐Garcia 2006 study included participants who were smokers, there is a possibility that some of these participants had COPD and this may influence the positive findings in that study. It is possible that COPD‐associated bronchiectasis may respond differently to treatment modalities when compared to bronchiectasis associated with other causes.
Persistent inflammation plays a role in deterioration of lung function in bronchiectasis (Ip 1993). Studies in adults with cystic fibrosis suggest that ICS treatment improves bronchial hyper‐responsiveness and spirometric parameters (Van Haren 1995). Thus, it is theoretically possible that ICS may improve lung function, and with it clinical parameters in non‐cystic fibrosis bronchiectasis as well, although this review has shown that any benefit from ICS is inconsistent. However, given the increased presence of airway hyper‐responsiveness in patients with non‐cystic fibrosis bronchiectasis (Ip 1991), it is possible that ICS may have a role in this subgroup.
The bacteriology of sputum influences future morbidity and mortality; specifically, people with Pseudomonas aeruginosa (P aeruginosa) have more rapid lung function decline (Finch 2015). As the bacteriology in all the included studies were unclear, the applicability of this review in the different subsets of people with various bacteriology such as mycobacteria and P aeruginosa is also uncertain.
In the consideration of using ICS, especially long‐term ICS (> 6 months) in patients, clinicians should be cognisant of the possible effect of corticosteroids on cell immunity dysregulation (Sabroe 2013). Biologically, this effect could lead to an increase in infections that could worsen clinical outcomes, such as lung function and exacerbations (Sabroe 2013). Indeed, adults with COPD on ICS have an increased unadjusted risk of having pneumonia (Festic 2016). Although this adverse event is a clinically important issue in people with bronchiectasis, only one study specifically reported on adverse events and thus we could not assess this in this review. However, in the ICS group of shorter duration (6 months or less), two studies found no increased risk of P aeruginosa in the ICS arm compared to placebo (Analysis 1.6). One study found a non‐significant increase in sputum bacterial density in the ICS group (Tsang 1998), whilst there was a non‐decrease in the placebo group (between‐groups post‐treatment P = 0.06).
Quality of the evidence
The overall quality of evidence was low (summary of findings Table for the main comparison; summary of findings Table 2). The sample size and number of studies were small. The major contributor to the benefit of ICS found in the meta‐analyses was from a non‐placebo controlled study. The studies also did not exclude participants with airway hyper‐responsiveness or coexistent COPD, hence the potential of skewing the results in favour of ICS.
Potential biases in the review process
We conducted this review in accordance with a prespecified protocol. We have outlined changes from the protocol in the Differences between protocol and review section and we did not identify any major sources of bias in the review process.
Agreements and disagreements with other studies or reviews
Our findings are similar to that outlined in bronchiectasis guidelines of the British Thoracic Society (Pasteur 2010), and the Thoracic Society of Australia and New Zealand (Chang 2015). We found no other published systematic reviews on the use of ICS for people with bronchiectasis.
Whilst a separate disease, cystic fibrosis lung disease manifests with bronchiectasis. Studies in cystic fibrosis have also shown no benefits with ICS. A Cochrane Review of ICS in cystic fibrosis concluded that there is insufficient evidence to determine if they are beneficial or harmful (Balfour‐Lynn 2014). In a large prospective, multicentre study, withdrawal of ICS for six months was not associated with significant worsening of cystic fibrosis lung disease (Balfour‐Lynn 2006). Participants in whom ICS were discontinued did not have a change in lung function over time, an increased need for oral or intravenous antibiotics, or a shorter time to pulmonary exacerbation (Balfour‐Lynn 2006).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 1 Lung function (spirometry indices).

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 2 Lung function (other indices).

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 3 Clinical severity indices.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 4 Exacerbations.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 5 Sputum and biomarkers characteristics.

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 6 Pseudomonas aeruginosa colonisation.

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 7 St George HRQoL (end of study minus baseline).

Comparison 2 Stable state (> 6 months), Outcome 1 Lung function indices.

Comparison 2 Stable state (> 6 months), Outcome 2 Exacerbations.
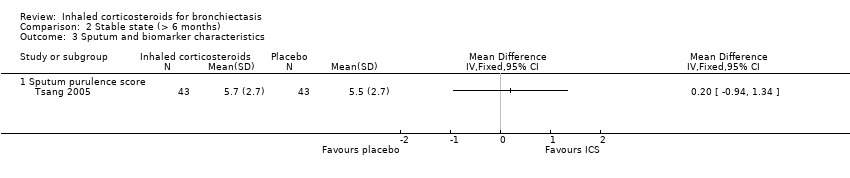
Comparison 2 Stable state (> 6 months), Outcome 3 Sputum and biomarker characteristics.
| Inhaled corticosteroids compared to placebo for bronchiectasis (< 6 months) | ||||||
| Patient or population: people with bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function (spirometry indices) ‐ FEV1 (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.038 to 0.805 | MD 0.09 lower | ‐ | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (spirometry indices) ‐ FVC (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.062 to 0.0218 | MD 0.01 higher | ‐ | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ diffusion capacity % predicted (end of study) | Mean end of study value 84.2 | MD 2.70 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ RV % predicted (end of study values) | Mean end of study value 106 | MD 2.00 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ TLC % predicted (end of study values) | Mean end of study value 86.4 | MD 3.20 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Average number of exacerbations per participant | Average number of exacerbations per patient ranged from 0.97 to 1.31 | MD 0.17 lower | ‐ | 127 | ⊕⊕⊝⊝ Low1,2,3 | |
| Pseudomonas aeruginosa (P aeruginosa) colonisation | 410 per 1000 | 395 per 1000 | OR 0.94 | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The largest study was not a placebo controlled trial (Martinez‐Garcia 2006). | ||||||
| Inhaled corticosteroids compared to placebo for bronchiectasis (medium‐ to long‐term outcomes) | ||||||
| Patient or population: people with bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function indices ‐ FEV1% predicted (end study minus baseline values) | Mean change from baseline 0 | MD 0.30 higher | ‐ | 86 | ⊕⊕⊝⊝ Low1,2 | |
| Lung function indices ‐ FVC % predicted (end study minus baseline values) | Mean change from baseline 0.9 | MD 0.90 lower | ‐ | 86 | ⊕⊕⊝⊝ Low1,2 | |
| Number of participants improved | 628 per 1000 | 490 per 1000 | OR 0.57 | 86 | ⊕⊕⊝⊝ Low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Only a single study with small participant numbers; we downgraded outcome one point for imprecision. | ||||||
| Study ID | Intervention | Control | Duration of intervention |
| Beclomethasone dipropionate 750 µg twice daily by MDI | Placebo | 6 weeks | |
| Budesonide 400 µg twice daily | Placebo | 6 months | |
| Beclomethasone 800 µg per day over 2 doses | Placebo | 4 weeks | |
| Fluticasone 500 µg twice daily by MDI or 250 µg fluticasone twice daily | No treatment | 6 months | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 4 weeks | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks | |
| MDI: metered dose inhaler | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung function (spirometry indices) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 FEV1 (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.26, 0.09] |
| 1.2 FVC (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.16, 0.17] |
| 2 Lung function (other indices) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diffusion capacity % predicted (end of study) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐2.49, 7.89] |
| 2.2 RV % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐9.41, 13.41] |
| 2.3 TLC % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐1.99, 8.39] |
| 3 Clinical severity indices Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Number of participants with regular wheeze (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Number of participants without sputum reduction of > 50% (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Number of participants with no improvement in dyspnoea score > 1 (min important difference) (1000F) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Number of participants with no clinically significant improvement in HRQoL | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Exacerbations Show forest plot | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| 4.1 Average number of exacerbations per participant | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| 5 Sputum and biomarkers characteristics Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Sputum volume or weight (per day) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pseudomonas aeruginosa colonisation Show forest plot | 2 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.96] |
| 7 St George HRQoL (end of study minus baseline) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Total score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐3.54 [‐8.00, 0.92] |
| 7.2 Symptom score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐4.75 [‐10.42, 0.92] |
| 7.3 Activity score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐6.21 [‐12.40, ‐0.01] |
| 7.4 Impact score | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.63 [‐9.35, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung function indices Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 FEV1% predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 FVC % predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exacerbations Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Number of participants improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum and biomarker characteristics Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Sputum purulence score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

