Antibióticos versus antisépticos tópicos para la otitis media supurativa crónica
Resumen
Antecedentes
La otitis media supurativa crónica (OMSC), a veces denominada otitis media crónica (OMC), es una inflamación e infección crónica del oído medio y la cavidad mastoidea, caracterizada por la secreción del oído (otorrea) a través de una membrana timpánica perforada. Los síntomas predominantes de la OMSC son la secreción ótica y la pérdida de audición.
Los antibióticos y los antisépticos eliminan o inhiben los microorganismos que pueden ser responsables de la infección. Los antibióticos pueden aplicarse de forma tópica o sistémica por vía oral o mediante inyección. Los antisépticos siempre se aplican directamente en el oído (de forma tópica).
Objetivos
Evaluar la efectividad de los antibióticos versus antisépticos para los pacientes con otitis media supurativa crónica (OMSC).
Métodos de búsqueda
El Especialista en Información del Grupo Cochrane de Enfermedades de Oído, Nariz y Garganta (Cochrane ENT Group Information Specialist) realizó búsquedas en el Registro de Ensayos del Grupo Cochrane de Enfermedades de Oído, Nariz y Garganta; el Registro Cochrane de Ensayos Controlados (CENTRAL; 2019; Número 4, a través del Registro de Estudios Cochrane [Cochrane Register of Studies]); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP y fuentes adicionales para obtener ensayos publicados y no publicados. La fecha de la búsqueda fue el 1 de abril 2019.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados (ECA) con al menos una semana de seguimiento que incluyeron a pacientes (adultos y niños) con secreción ótica crónica de causa desconocida u OMSC, en que la secreción del oído había continuado durante más de dos semanas.
La intervención fue cualquier agente antibiótico único o combinación de agentes, ya sea aplicado de forma tópica (sin corticosteroides) o sistémica. La comparación fue con cualquier agente antiséptico tópico, o una combinación de los mismos, aplicado como gotas para los oídos, polvo o irrigaciones, o como parte de un procedimiento de lavado ótico.
Dos comparaciones fueron los antisépticos tópicos comparados con: a) antibióticos tópicos o b) antibióticos sistémicos. Dentro de cada comparación se separaron los casos en que ambos grupos de pacientes habían recibido antibióticos tópicos a) solos o con lavado ótico y b) además del tratamiento de base (como los antibióticos sistémicos).
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos Cochrane estándar. Se utilizaron los criterios GRADE para evaluar la certeza de la evidencia de cada desenlace.
Los resultados primarios fueron: resolución de la secreción del oído u «oído seco» (confirmada o no otoscópicamente), medida entre una y dos semanas, entre dos y cuatro semanas y después de cuatro semanas; calidad de vida relacionada con la salud mediante un instrumento validado; y dolor de oído (otalgia) o malestar o irritación local. Los resultados secundarios incluyeron la audición, las complicaciones graves y la ototoxicidad medida de varias maneras.
Resultados principales
Se identificaron siete estudios (935 participantes) a través de cuatro comparaciones con antibióticos comparados con ácido acético, acetato de aluminio, ácido bórico y povidona yodada.
Ninguno de los estudios incluidos informó de los resultados de la calidad de vida o las complicaciones graves.
A. Antiséptico tópico (ácido acético) versus antibióticos tópicos (quinolonas o aminoglucósidos)
No se sabe si hay una diferencia en la resolución de la secreción del oído con ácido acético en comparación con aminoglucósidos a la semana o dos semanas (riesgo relativo [RR] 0,88; intervalo de confianza [IC] del 95%: 0,72 a 1,08; 1 estudio; 100 participantes; evidencia de certeza muy baja). Ningún estudio informó de los resultados para la secreción del oído después de cuatro semanas. No se sabe si hubo más dolor de oído, malestar o irritación local con ácido acético o con antibióticos tópicos debido al escaso número de participantes que informaron sobre los eventos (RR 0,16; IC del 95%: 0,02 a 1,34; 2 ECA; 189 participantes; evidencia de certeza muy baja). No se informaron diferencias entre los grupos en cuanto a la audición (quinolonas) o la presunta ototoxicidad (aminoglucósidos) (evidencia de certeza muy baja).
B. Antiséptico tópico (acetato de aluminio) versus antibióticos tópicos
No fue posible utilizar en la revisión los resultados del único estudio que comparó los antibióticos tópicos con acetato de aluminio.
C. Antiséptico tópico (ácido bórico) versus antibióticos tópicos (quinolonas)
Un estudio informó de más participantes con resolución de la secreción del oído cuando se utilizaron antibióticos tópicos (quinolonas) en comparación con gotas óticas de ácido bórico entre una y dos semanas (riesgo relativo [RR] 1,86; intervalo de confianza [IC] del 95%: 1,48 a 2,35; un estudio; 411 participantes; evidencia de certeza moderada). Lo anterior significa que una persona adicional presentará la resolución de la secreción del oído por cada cuatro que reciban antibióticos tópicos (en comparación con ácido bórico) a las dos semanas. Ningún estudio informó de los resultados para la secreción del oído después de cuatro semanas. Hubo una mejoría mayor de la audición en el grupo de antibióticos tópicos en comparación con el grupo de antisépticos tópicos (diferencia de medias [DM] 2,79 decibeles [dB], IC del 95%: 0,48 a 5,10; 1 estudio; 390 participantes; evidencia de certeza baja) pero esta diferencia puede no ser clínicamente significativa.
Puede haber más dolor de oído, malestar o irritación con el ácido bórico comparado con las quinolonas (RR 0,56; IC del 95%: 0,32 a 0,98; 2 estudios; 510 participantes; evidencia de certeza baja). No se informó de la presunta ototoxicidad.
D. Antiséptico tópico (povidona yodada) versus antibióticos tópicos (quinolonas)
No se sabe si hay una diferencia entre las quinolonas y la povidona yodada con respecto a la resolución de la secreción del oído a la semana o dos semanas (RR 1,02; IC del 95%: 0,82 a 1,26; 1 ECA, 39 participantes; evidencia de certeza muy baja). El estudio informó de manera cualitativa que no hubo diferencias entre los grupos para la audición y ningún paciente desarrolló efectos ototóxicos (evidencia de certeza muy baja). No se informaron resultados para la resolución de la secreción del oído más allá de las cuatro semanas, ni para el dolor, el malestar o la irritación del oído.
E. Antiséptico tópico (ácido acético) + lavado ótico versus antibióticos tópicos + sistémicos (quinolonas)
Un estudio informó que los participantes que recibieron antibióticos tópicos y orales tuvieron una menor resolución de la secreción del oído en comparación con las gotas óticas de ácido acético y el lavado ótico (aspiración cada dos días) al mes (RR 0,69; IC del 95%: 0,53 a 0,90; 100 participantes). El estudio no presentó resultados para la resolución de la secreción del oído entre una semana y dos semanas, el dolor de oído, el malestar o la irritación, la audición o la sospecha de ototoxicidad.
Conclusiones de los autores
El tratamiento de la OMSC con antibióticos tópicos (quinolonas) probablemente resulte en un aumento de la resolución de la secreción del oído en comparación con el ácido bórico hasta las dos semanas. Hubo evidencia limitada de la eficacia de otros antibióticos tópicos o antisépticos tópicos, por lo que no es posible establecer conclusiones. Los eventos adversos no se informaron de manera adecuada.
PICO
Resumen en términos sencillos
Antisépticos tópicos en comparación con antibióticos para pacientes con otitis media supurativa crónica
¿Cuál era el objetivo de esta revisión?
El objetivo de esta revisión Cochrane es averiguar si los antisépticos tópicos son más efectivos que los antibióticos en el tratamiento de la otitis media supurativa crónica. Los autores de la revisión recopilaron y analizaron todos los estudios relevantes para responder a esta pregunta.
Mensajes clave
No hay mucha evidencia que compare los antisépticos tópicos con los antibióticos tópicos. La evidencia es muy incierta en cuanto a si los antibióticos o los antisépticos tópicos son más efectivos para reducir la secreción del oído, excepto en cuanto a la probabilidad de que los antibióticos tópicos sean más efectivos que el ácido bórico.
¿Qué se estudió en la revisión?
La otitis media supurativa crónica (OMSC) es una inflamación e infección a largo plazo (crónica) del oído medio, con secreción del oído (otorrea) a través de una membrana timpánica (tímpano) perforada. Los síntomas principales de la OMSC son la secreción ótica y la pérdida de audición.
Los antibióticos son el tratamiento utilizado más comúnmente para la OMSC. Los antibióticos pueden ser «tópicos» (colocados en el canal auditivo en forma de gotas, ungüentos, aerosoles o cremas) o «sistémicos» (administrados por boca o mediante una inyección en un músculo o una vena). Los antisépticos tópicos (antisépticos que se colocan directamente en el oído en forma de gotas o polvo) son un posible tratamiento para la OMSC. Tanto los antibióticos como los antisépticos tópicos eliminan o detienen el crecimiento de los microorganismos que pueden ser responsables de la infección.
Los antibióticos y los antisépticos tópicos se pueden utilizar solos o se pueden agregar a otros tratamientos para la OMSC, como los antibióticos o la limpieza de los oídos (lavado ótico). En esta revisión fue importante examinar si había efectos adversos con el uso de antibióticos y antisépticos. Los posibles eventos adversos podrían incluir irritación de la piel dentro del oído externo, lo cual puede causar malestar, dolor o prurito. Algunos antibióticos y antisépticos (como el alcohol) también pueden ser tóxicos para el oído interno (ototoxicidad), lo que significa que pueden causar una pérdida de audición irreparable (neurosensorial), mareos o zumbidos en el oído (acúfenos).
¿Cuáles son los principales resultados de la revisión?
Se encontraron siete estudios con 935 participantes. Se encontró evidencia para cuatro tipos diferentes de antisépticos tópicos: ácido acético, acetato de aluminio, ácido bórico y povidona yodada.
Comparación de antibióticos con ácido acético, acetato de aluminio o povidona yodada
En comparación con el ácido acético, el acetato de aluminio y la povidona yodada, no se sabe si los antibióticos tópicos o los antibióticos sistémicos mejoran la resolución de la secreción del oído en los pacientes con OMSC debido a que la certeza de la evidencia es muy baja. No es posible saber si hay una diferencia entre los grupos para cualquier otro resultado.
Comparación de los antibióticos con ácido bórico
Se incluyeron dos estudios (532 participantes), que mostraron evidencia de que los antibióticos tópicos (quinolonas) probablemente sean mejores que el ácido bórico para resolver la secreción del oído a la semana o dos semanas. También puede haber menos malestar en el oído (dolor, irritación y hemorragia) y una mejoría mayor en la audición con los antibióticos tópicos en comparación con el ácido bórico.
¿Cómo de actualizada está esta revisión?
La evidencia está actualizada hasta abril 2019.
Authors' conclusions
Summary of findings
| Topical antibiotics compared to acetic acid for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) Aminoglycosides | 100 | RR 0.88 | Study population | ⊕⊝⊝⊝ | It is very uncertain whether acetic acid is more effective at resolving ear discharge compared with topical aminoglycoside antibiotics at 14 days | ||
| 84.0% | 73.9% | 10.1% fewer | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study reported this outcome |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study reported this outcome |
| Ear pain, discomfort, irritation | 189 | RR 0.16 | Study population | ⊕⊝⊝⊝ | Acetic acid may cause more ear pain, discomfort and/or irritation than topical antibiotics (aminoglycosides and quinolones) but we are very uncertain about the results | ||
| 5.3% | 0.9% | 4.5% fewer | |||||
| Hearing | 107 | One study reports that "audiometric tests showed no detectable overall, isolated not idiosyncratic hearing loss from any treatment". No numeric results were provided. | very low3 | It is uncertain whether there is a difference in hearing between topical quinolones and topical acetic acid | |||
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity | 100 | One study (100 participants) reported: "… none of the patients had any kind of ear damage or toxicity" | very low4 | It is uncertain if there is a difference in ototoxicity between topical aminoglycosides and topical acetic acid | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low‐certainty evidence: downgraded by one level due to study limitations (risk of bias) as the study had unclear randomisation, allocation concealment and blinding. Downgraded by one level due to indirectness as the outcome used was 'clinical cure' rather than resolution of ear discharge. Downgraded by one level due to suspected publication bias as one 'unpublished' study was identified indicating the possibility of unreported trials. 3Downgraded to very low‐certainty evidence: downgraded by two levels due to imprecision as no numeric results were provided and the result came from a small study (107 participants). Downgraded by one level due to suspected publication bias as one 'unpublished' study was identified indicating the possibility of unreported trials. 4Downgraded to very low‐certainty evidence: downgraded by one level due to study limitations (risk of bias) as the study had unclear randomisation, allocation concealment and blinding. Downgraded by one level due to imprecision as the result came from a small study (100 participants). Downgraded by one level due to suspected publication bias (one 'unpublished' study identified indicating the possibility of unreported trials). | |||||||
| Topical quinolones compared to boric acid for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical quinolones | With topical quinolones | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) Quinolones | 411 | RR 1.86 | Study population | ⊕⊕⊕⊝ | Topical quinolones are likely to increase the number of people with resolution of ear discharge at 2 weeks compared with topical boric acid | ||
| 31.9% | 59.3% | 27.4% more | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study measured resolution of ear discharge at 4 weeks |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study measured quality of life |
| Ear pain, discomfort, irritation | 510 | RR 0.56 | Study population | ⊕⊕⊝⊝ | Topical quinolones may result in less ear pain, discomfort or irritation at 4 weeks compared to topical boric acid | ||
| 11.8% | 6.6% | 5.2% fewer | |||||
| Average change in hearing from baseline | 390 | — | The mean average change in hearing from baseline without topical quinolones was 2.69 dB | The mean average change in hearing from baseline with topical quinolones was 5.42 dB | MD 2.79 dB higher | ⊕⊕⊝⊝ | Topical quinolones may result in greater improvement in mean hearing from baseline compared with topical boric acid; however this effect size may not be clinically important |
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by one level to moderate‐certainty evidence due to suspected publication bias. We identified two unpublished studies comparing antibiotics and antiseptics, which indicates that there may be more unpublished studies. | |||||||
| Topical antibiotics compared to povidone‐iodine for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) | 39 | RR 1.02 | Study population | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the resolution of ear discharge at 2 weeks between topical antibiotics and topical povidone‐iodine | ||
| 88.9% | 90.7% | 1.8% more | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Ear pain, discomfort, irritation ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Hearing | 40 (1 RCT) | "There was no deterioration of hearing as assessed by pure tone audiometry" | very low2 | — | |||
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity | 40 | "No patient developed allergic manifestations or ototoxic effects" | very low3 | — | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low‐certainty evidence. Downgraded by one level due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting). Downgraded by one level due to imprecision (small study size: 39 participants, confidence interval crosses the line of minimally clinical important difference). Downgraded by one level due to suspected publication bias (one study referred to long‐term results that appear to be unpublished and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). 2Downgraded to very low‐certainty evidence. Downgraded by one level due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting). Downgraded by two levels due to imprecision (no numeric results were presented and very small study size (39 participants)). Downgraded by one level due to suspected publication bias (one study referred to long‐term results, which appear to be unpublished, and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). 3Downgraded to very low‐certainty evidence. Downgraded by two levels due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting as it is unclear how the outcome was defined). Downgraded by two levels due to imprecision (no numeric results were presented and very small study size (39 participants). Downgraded by one level due to suspected publication bias (one study referred to long‐term results that appear to be unpublished and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). | |||||||
Background
This is one of a suite of Cochrane Reviews evaluating the comparative effectiveness of non‐surgical interventions for chronic suppurative otitis media (CSOM) using topical antibiotics, topical antibiotics with corticosteroids, systemic antibiotics, topical antiseptics and aural toileting (ear cleaning) methods (Table 1).
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
|---|---|---|---|---|---|
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018a).
CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a).
CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b).
CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018b).
CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a).
CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b).
CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018).
This review compares the effectiveness of topical antibiotics (without steroids), or systemic antibiotics, against topical antiseptics for CSOM.
Description of the condition
Chronic suppurative otitis media (CSOM), which is also often referred to as chronic otitis media (COM), is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane.
The predominant symptoms of CSOM are ear discharge and hearing loss. Ear discharge can be persistent or intermittent, and many sufferers find it socially embarrassing (Orji 2013). Some patients also experience discomfort or earache. Most patients with CSOM experience temporary or permanent hearing loss with average hearing levels typically between 10 and 40 decibels (Jensen 2013). The hearing loss can be disabling, and it can have an impact on speech and language skills, employment prospects, and on children’s psychosocial and cognitive development, including academic performance (Elemraid 2010; Olatoke 2008; WHO 2004). Consequently, quality of life can be affected. CSOM can also progress to serious complications in rare cases (and more often when cholesteatoma is present): both extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) have been reported (Dubey 2007; Yorgancılar 2013).
CSOM is estimated to have a global incidence of 31 million episodes per year, or 4.8 new episodes per 1000 people (all ages), with 22% of cases affecting children under five years of age (Monasta 2012; Schilder 2016). The prevalence of CSOM varies widely between countries, but it disproportionately affects people at socio‐economic disadvantage. It is rare in high‐income countries, but common in many low‐ and middle‐income countries (Mahadevan 2012; Monasta 2012; Schilder 2016; WHO 2004).
Definition of disease
There is no universally accepted definition of CSOM. Some define CSOM in patients with a duration of otorrhoea of more than two weeks but others may consider this an insufficient duration, preferring a minimum duration of six weeks or more than three months (Verhoeff 2006). Some include diseases of the tympanic membrane within the definition of CSOM, such as tympanic perforation without a history of recent ear discharge, or the disease cholesteatoma (a growth of the squamous epithelium of the tympanic membrane).
In accordance with a consensus statement, here we use CSOM only to refer to tympanic membrane perforation, with intermittent or continuous ear discharge (Gates 2002). We have used a duration of otorrhoea of two weeks as an inclusion criterion, in accordance with the definition used by the World Health Organization, but we have used subgroup analyses to explore whether this is a factor that affects observed treatment effectiveness (WHO 2004).
Many people affected by CSOM do not have good access to modern primary healthcare, let alone specialised ear and hearing care, and in such settings health workers may be unable to view the tympanic membrane to definitively diagnose CSOM. It can also be difficult to view the tympanic membrane when the ear discharge is profuse. Therefore we have also included, as a subset for analysis, studies where participants have had chronic ear discharge for at least two weeks, but where the diagnosis is unknown.
At‐risk populations
Some populations are considered to be at high risk of CSOM. There is a high prevalence of disease among Indigenous people such as the Aboriginal and Torres Strait Islander Australian, Native American and Inuit populations. This is likely due to an interplay of factors, including socio‐economic deprivation and possibly differences resulting from population genetics (Bhutta 2016). Those with primary or secondary immunodeficiency are also susceptible to CSOM. Children with craniofacial malformation (including cleft palate) or chromosomal mutations such as Down syndrome are prone to chronic non‐suppurative otitis media ('glue ear'), and by extrapolation may also be at greater risk of suppurative otitis media. The reasons for this association with craniofacial malformation are not well understood, but may include altered function of the Eustachian tube, coexistent immunodeficiency, or both. These populations may be less responsive to treatment and more likely to develop CSOM, recurrence or complications.
Children who have a grommet (ventilation tube) in the tympanic membrane to treat glue ear or recurrent acute otitis media may be more prone to develop CSOM; however, their pathway to CSOM may differ and therefore they may respond differently to treatment. Children with grommets who have chronic ear discharge meeting the CSOM criteria are therefore considered to be a separate high‐risk subgroup (van der Veen 2006).
Treatment
Treatments for CSOM may include topical antibiotics (administered into the ear) with or without steroids, systemic antibiotics (given either by mouth or by injection), topical antiseptics and ear cleaning (aural toileting), all of which can be used on their own or in various combinations. Whereas primary healthcare workers or patients themselves can deliver some treatments (for example, some aural toileting and antiseptic washouts), in most countries antibiotic therapy requires prescription by a doctor. Surgical interventions are an option in cases where complications arise or in patients who have not responded to pharmacological treatment; however, there is a range of practice in terms of the type of surgical intervention that should be considered and the timing of the intervention. In addition, access to or availability of surgical interventions is setting‐dependent. This series of Cochrane Reviews therefore focuses on non‐surgical interventions. In addition, most clinicians consider cholesteatoma to be a variant of CSOM, but acknowledge that it will not respond to non‐surgical treatment (or will only respond temporarily) (Bhutta 2011). Therefore, studies in which more than half of the participants were identified as having cholesteatoma are not included in these reviews.
Description of the intervention
Antibiotics are the most commonly used treatment for CSOM. They can be administered topically (as drops, ointments, sprays or creams to the affected area) or systemically (either by mouth or by injection into a vein (intravenous) or muscles (intramuscular)).
Topical application of antibiotics has the advantage of potentially delivering high concentrations of antibiotic to the affected area, whereas systemic antibiotics are absorbed and distributed throughout the body. However, the penetration of topical antibiotics into the middle ear may be compromised if the perforation in the tympanic membrane is small or there is copious mucopurulent discharge in the ear canal that cannot be cleaned. It may also be difficult to achieve compliance with topical dosing in young children. In these cases, systemic antibiotics may have an advantage.
Antiseptics are substances that kill or inhibit the growth and development of micro‐organisms. Agents that have been used for treating CSOM include povidone‐iodine, aluminium acetate, boric acid, chlorhexidine, alcohol, acetic acid and hydrogen peroxide. Antiseptics can be delivered as drops or as washes using a syringe. The frequency of administration and duration of treatment can vary. Syringing may bring additional benefit by flushing out debris or pus, thus reducing the overall bacterial load. Antiseptics can be used alone or in addition to other treatments for CSOM, such as antibiotics or aural toileting.
How the intervention might work
CSOM is a chronic and often polymicrobial (involving more than one micro‐organism) infection of the middle ear. Broad‐spectrum antibiotics such as second‐generation quinolones and aminoglycosides, which are often active against the most frequently cultured micro‐organisms (Pseudomonas aeruginosa and Staphylococcus aureus), are therefore commonly used (Mittal 2015) (Table 2). It is possible that antibiotics for CSOM that target Pseudomonas aeruginosa may have an advantage over antibiotics that do not. Dose and duration of treatment are also important factors but are less likely to affect relative effectiveness if given within the therapeutic range. Generally, treatment for at least five days is necessary and a duration of one to two weeks is sufficient to resolve uncomplicated infections. However, in some cases it may take more than two weeks for the ear to become dry and therefore longer follow‐up (more than four weeks) may be needed to monitor for recurrence of discharge.
| Class of antibiotics | Examples | Route of administration |
|---|---|---|
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
Topical antiseptics are administered to the ear to inhibit the micro‐organisms that may be responsible for the condition. Although the mechanism of action of most antiseptics is thought to relate to disruption of the bacterial cell wall followed by penetration into the cell and action at the target site(s), different groups of antiseptics have different properties (e.g. iodines, alcohols, acids) (Table 3). We therefore analysed these groups separately and pooling only occurred where there was no evidence of a difference in effect.
| Antiseptic agent used aurally | Target and mechanism of action |
|---|---|
| Rubbing alcohol (ethanol, isopropanol) | Penetrating agents that cause loss of cellular membrane function, leading to release of intracellular components, denaturing of proteins, and inhibition of DNA, RNA, protein and peptidoglycan synthesis. |
| Povidone‐iodine | Highly active oxidising agents that destroy cellular activity of proteins. Disrupts oxidative phosphorylation and membrane‐associated activities. Iodine reacts with cysteine and methionine thiol groups, nucleotides and fatty acids, resulting in cell death. |
| Chlorhexidine | Membrane‐active agents that damage cell wall and outer membrane, resulting in collapse of membrane potential and intracellular leakage. Enhanced passive diffusion mediates further uptake, causing coagulation of cytosol. |
| Hydrogen peroxide | Produces hydroxyl free radicals that function as oxidants, which react with lipids, proteins and DNA. Sulfhydryl groups and double bonds are targeted in particular, thus increasing cell permeability. |
| Boric acid | It is likely that the change in the pH media of the ear canal interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. |
| Aluminium acetate/acetic acid | Acetic acid changes the pH media of the ear canal and interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. Aluminium acetate is an astringent that helps reduce itching, stinging and inflammation. |
Sources: Gupta 2015; McDonnell 1999; Sheldon 2005.
Some antibiotics (such as aminoglycosides) and antiseptics (such as chlorhexidine or alcohol) can be toxic to the inner ear (ototoxicity), which might be experienced as sensorineural hearing loss, dizziness or tinnitus. For antibiotics, ototoxicity is less likely to be a risk when applied topically in patients with CSOM (Phillips 2007).
For both topical antibiotics and antiseptics, local discomfort, ear pain or itching may occur through the action of putting ear drops into the ear or because the topical antibiotics/antiseptics or their excipients cause chemical or allergic irritation of the skin of the outer ear.
Systemic antibiotics can have off‐target side effects, for example diarrhoea or nausea. However, the risk or incidence of these events is not expected to be different from other common infections since the doses and duration of treatment used are similar in CSOM. A broader concern is the association of the overuse of antibiotics with increasing resistance among community‐ and hospital‐acquired pathogens.
Why it is important to do this review
Although antibiotics are widely recommended as first‐line treatment for CSOM, topical antiseptic agents generally cost less. They are also more readily available, do not require prescription by a doctor and do not need refrigerated transport. These factors make them an attractive option in resource‐constrained environments. Evidence‐based knowledge of the relative effectiveness of antibiotics and topical antiseptics could help to optimise their use.
Objectives
To assess the effectiveness of antibiotics versus antiseptics for people with chronic suppurative otitis media (CSOM).
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
-
Randomised controlled trials (including cluster‐randomised trials where the unit of randomisation is the setting or operator) and quasi‐randomised trials.
-
Patients were followed up for at least one week.
We excluded studies with the following design characteristics:
-
Cross‐over trials, because CSOM is not expected to be a stable chronic condition. Unless data from the first phase were available, we excluded such studies.
-
Studies that randomised participants by ear (within‐patient controlled) because, by definition, the effects of systemic treatments are not localised. This applies to studies that compared systemic antibiotics versus topical antiseptics. Note: we did not exclude studies comparing topical antibiotics with topical antiseptics that randomised participants by ear but we analysed these using the methods outlined in Unit of analysis issues.
Types of participants
We included studies with patients (adults and children) who had:
-
chronic ear discharge of unknown cause; or
-
chronic suppurative otitis media.
We defined patients with chronic ear discharge as patients with at least two weeks of ear discharge, where the cause of the discharge was unknown.
We defined patients with chronic suppurative otitis media (CSOM) as patients with:
-
chronic or persistent ear discharge for at least two weeks; and
-
a perforated tympanic membrane.
We did not exclude any populations based on age, risk factors (cleft palate, Down syndrome), ethnicity (e.g. Australian Aboriginal or Torres Strait Islanders) or the presence of ventilation tubes (grommets). Where available, we recorded these factors in the patient characteristics section during data extraction from the studies. If any of the included studies recruited these patients as a majority (80% or more), we analysed them in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We excluded studies where the majority (more than 50%) of participants:
-
had an alternative diagnosis to CSOM (e.g. otitis externa);
-
had underlying cholesteatoma;
-
had ear surgery within the last six weeks.
We did not include studies designed to evaluate interventions in the immediate peri‐surgical period, which were focused on assessing the impact of the intervention on the surgical procedure or outcomes.
Types of interventions
Antibiotics
We included all topical and systemic antibiotics. Topical antibiotics were applied directly into the ear canal. The most common formulations are ear drops but other formulations such as sprays have also been included.
Systemic antibiotics are administered orally or parenterally (intramuscular or intravenous).
We excluded studies that conducted swabs and tests for antimicrobial sensitivity and then based the choice of antibiotics for each participant on the results of the laboratory test.
Duration
At least five days of treatment with antibiotics was required, except for antibiotics where a shorter duration has been suggested as equivalent (e.g. azithromycin for systemic antibiotics).
Dose
There was no limitation on the dose or frequency of administration.
Topical antiseptics
Any single, or combination of, topical antiseptic agent of any class including (but not limited to) povidone‐iodine, aluminium acetate, boric acid, chlorhexidine, alcohol and hydrogen peroxide. The topical antiseptics could be applied directly into the ear canal as ear drops, powders or irrigations, or as part of an aural toileting procedure.
Dose/duration
There was no limitation on the dose, duration or frequency of application.
Comparisons
We analysed topical antibiotics and systemic antibiotics as separate comparisons:
-
Topical antibiotics versus topical antiseptics.
-
Systemic antibiotics versus topical antiseptics.
We analysed these as three main scenarios depending on which common therapy was applied in the background:
-
Topical or systemic antibiotics versus topical antiseptics as a single treatment (main therapy): this included studies where all participants in both treatment groups either received no other treatment or only received aural toileting. This also included situations where antiseptics were applied only once (e.g. as part of microsuction at the start of treatment).
-
Topical or systemic antibiotics versus topical antiseptics as an add‐on therapy to antiseptics: this included studies where all participants in both treatment groups also used a daily antiseptic, which was a different type to the antiseptic under investigation, with or without aural toileting.
-
Topical or systemic antibiotics versus topical antiseptics as an add‐on therapy to other systemic or topical antibiotics: this included studies where all participants in both treatment groups also received a systemic or topical antibiotic, which was a different type to the antibiotic under investigation, with or without aural toileting or antiseptics.
Many comparison pairs were possible in this review. The main comparisons of interest that we have summarised and presented in the 'Summary of findings' tables are:
-
topical antibiotics versus topical antiseptics as single therapies (main treatments); and
-
systemic antibiotics versus topical antiseptics as single therapies (main treatments).
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
We extracted and reported data from the longest available follow‐up for all outcomes.
Primary outcomes
-
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at:
-
between one week and up to two weeks;
-
two weeks to up to four weeks; and
-
after four weeks.
-
-
Health‐related quality of life using a validated instrument for CSOM (e.g. Chronic Otitis Media Questionnaire (COMQ)‐12 (Phillips 2014a; Phillips 2014b; van Dinther 2015), Chronic Otitis Media Outcome Test (COMOT)‐15 (Baumann 2011), Chronic Ear Survey (CES) (Nadol 2000)).
-
Ear pain (otalgia) or discomfort or local irritation.
Secondary outcomes
-
Hearing, measured as the pure‐tone average of air conduction thresholds across four frequencies tested (500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) of the affected ear. If this was not available, we reported the pure‐tone average of the thresholds measured.
-
Serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy), and death.
-
Ototoxicity; this was measured as 'suspected ototoxicity' as reported by the studies where available, and as the number of people with the following symptoms that may be suggestive of ototoxicity:
-
sensorineural hearing loss;
-
balance problems/dizziness/vertigo;
-
tinnitus.
-
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 1 April 2019.
Electronic searches
The Information Specialist searched:
-
the Cochrane ENT Register (searched via the Cochrane Register of Studies to 1 April 2019);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 4) (searched via the Cochrane Register of Studies Web to 1 April 2019);
-
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 1 April 2019);
-
Ovid EMBASE (1974 to 1 April 2019);
-
EBSCO CINAHL (1982 to 1 April 2019);
-
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (search to 1 April 2019);
-
Web of Knowledge, Web of Science (1945 to 1 April 2019);
-
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies to 1 April 2019);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search to 1 April 2019).
We also searched:
-
IndMed (search to 22 March 2018);
-
African Index Medicus (search to 22 March 2018).
The search strategies for major databases are detailed in Appendix 1. The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. The strategies were designed to identify all relevant studies for a suite of reviews on various interventions for chronic suppurative otitis media (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b). Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011).
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered adverse effects described in included studies only.
We contacted original authors for clarification and further data if trial reports were unclear and we arranged translations of papers where necessary.
Data collection and analysis
Selection of studies
At least two review authors (KH/LYC) independently screened all titles and abstracts of the references obtained from the database searches to identify potentially relevant studies. At least two review authors (KH/LYC) evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
At least two review authors (KH/LYC/CBJ/MB) independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved any differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the included studies, such as study design, setting (including location), year of study, sample size, age and sex of participants, and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers (see Appendix 2). For this review, this included the following information whenever available:
-
duration of ear discharge at entry to the study;
-
diagnosis of ear discharge (where known);
-
number people who may have been at higher risk of CSOM, including those with cleft palate or Down syndrome;
-
ethnicity of participants including the number who were from Indigenous populations;
-
number who had previously had ventilation tubes (grommets) inserted (and, where known, the number who had tubes still in place);
-
number who had previous ear surgery;
-
number who had previous treatments for CSOM (non‐responders, recurrent versus new cases).
We recorded concurrent treatments alongside the details of the interventions used. See the 'Data extraction form' in Appendix 2 for more details.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis, i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
-
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from disease‐specific quality of life scales such as COMQ‐12, COMOT‐15 and CES as continuous data.
-
For binary data: the number of participants who experienced an event and the number of patients assessed at the time point.
-
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted it into binary data.
-
Time‐to‐event outcomes: we were not expecting any outcomes to be measured as time‐to‐event data. However, if outcomes such as resolution of ear discharge were measured in this way, we would have reported the hazard ratios.
For resolution of ear discharge, we extracted the longest available data within the time frame of interest, defined as from one week up to (and including) two weeks (7 days to 14 days), from two weeks up to (and including) four weeks (15 to 28 days), and after four weeks (28 days or one month).
For other outcomes, we reported the results from the longest available follow‐up period.
Extracting data for pain/discomfort and adverse effects
For these outcomes, there were variations in how studies had reported the outcomes. For example, some studies reported both 'pain' and 'discomfort' separately whereas others did not. Prior to the commencement of data extraction, we agreed and specified a data extraction algorithm for how data should be extracted.
We extracted data for serious complications as a composite outcome. If a study reported more than one complication and we could not distinguish whether these occurred in one or more patients, we extracted the data with the highest incidence to prevent double counting.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper, we attempted to contact the study authors to try to obtain the raw values. When the raw values were not provided, we extracted information from the graphs using an online data extraction tool, using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
At least two review authors (KH/LYC/CBJ/MB) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), using the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
-
sequence generation;
-
allocation concealment;
-
blinding of participants, personnel and outcome assessment;
-
incomplete outcome data;
-
selective reporting;
-
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with complete resolution of ear discharge) as risk ratios (RR) with confidence intervals (CIs). For the key outcomes that are presented in the 'Summary of findings' table, we expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also calculated the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk was typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies, which is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we would have also attempted to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD). If different scales were used to measure the same outcome, we used the standardised mean difference (SMD) and provided a clinical interpretation of the SMD values.
Unit of analysis issues
Cross‐over studies
This review did not use data from phase II of cross‐over studies.
The ear as the unit of randomisation: within‐patient randomisation in patients with bilateral ear disease
For data from studies where 'within‐patient' randomisation was used (i.e. studies where both ears (right versus left) were randomised) we adjusted the analyses for the paired nature of the data (Elbourne 2002; Stedman 2011), as outlined in section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
The ear as the unit of randomisation: non‐paired randomisation in patients with bilateral ear disease
Some patients with bilateral disease may have received the same treatment in both ears, whereas others received a different treatment in each ear. We did not exclude these studies, but we only reported the data if specific pairwise adjustments were completed or if sufficient data were obtained to be able to make the adjustments.
The patient as the unit of randomisation
Some studies randomised by patient and those with bilateral CSOM received the same intervention for both ears. In some studies the results may be reported as a separate outcome for each ear (the total number of ears is used as the denominator in the analysis). The correlation of response between the left ear and right ear when given the same treatment was expected to be very high, and if both ears were counted in the analysis this was effectively a form of double counting, which may be especially problematic in smaller studies if the number of people with bilateral CSOM was unequal. We did not exclude these studies, but we only reported the results if the paper presented the data in such a way that we could include the data from each participant only once (one data point per participant) or if we had enough information to reliably estimate the effective sample size or inflated standard errors as presented in chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If this was not possible, we attempted to contact the authors for more information. If there was no response from the authors, then we did not include data from these studies in the analysis.
If we found cluster‐randomised trials by setting or operator, we analysed these according to the methods in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We attempted to contact the study authors via email whenever the outcome of interest was not reported but the methods of the study had suggested that the outcome had been measured. We did the same if not all of the data required for the meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we did not conduct any other imputations. We extracted and analysed data for all outcomes using the available case analysis method.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included studies for potential differences in the types of participants recruited, interventions or controls used, and the outcomes measured. We did not pool studies where the clinical heterogeneity made it unreasonable to do so.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculated the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis was likely to occur. We tried to find further information from the study authors, but if no further information could be obtained, we noted this as being a high risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an unclear risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We intended to create funnel plots if sufficient studies (more than 10) were available for an outcome. If we had observed asymmetry of the funnel plot, we would have conducted a more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we pooled mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the SMD had to be used as an effect measurement, we did not pool change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies where most participants (80% or more) met the criteria stated below in order to determine whether the effect of the intervention was different compared to other patients. Due to the risks of reporting and publication bias with unplanned subgroup analyses of trials, we only analysed subgroups reported in studies if these were prespecified and stratified at randomisation.
We planned to conduct subgroup analyses regardless of whether statistical heterogeneity was observed for studies that included patients identified as high‐risk (i.e. thought to be less responsive to treatment and more likely to develop CSOM, recurrence or complications) and patients with ventilation tubes (grommets). 'High‐risk' patients include Indigenous populations (e.g. Australian Aboriginal and Torres Strait Islanders, Native Americans and Inuit populations of Alaska, Canada and Greenland), people with craniofacial malformation (e.g. cleft palate), Down syndrome and people with known immunodeficiency.
We planned to present the main analyses of this review in the form of forest plots based on this main subgroup analysis.
-
For the high‐risk group, this applied to the outcomes resolution of ear discharge (dry ear), quality of life, pain/discomfort, development of complications and hearing loss.
For patients with ventilation tubes, this applied to the outcome resolution of ear discharge (dry ear) for the time point of four weeks or more because this group was perceived to be at lower risk of treatment failure and recurrence than other patient groups. If statistical heterogeneity was observed, we also conducted subgroup analysis for the effect modifiers below. If there were statistically significant subgroup effects, we presented these subgroup analysis results as forest plots.
For this review, effect modifiers included:
-
Diagnosis of CSOM: it was likely that some studies would include patients with chronic ear discharge but who had not had a diagnosis of CSOM. Therefore, we subgrouped studies where most patients (80% or more) met the criteria for CSOM diagnosis in order to determine whether the effect of the intervention was different compared to patients where the precise diagnosis was unknown and inclusion into the study was based purely on chronic ear discharge symptoms.
-
Duration of ear discharge: there is uncertainty about whether the duration of ear discharge prior to treatment has an impact on the effectiveness of treatment and whether more established disease (i.e. discharge for more than six weeks) is more refractory to treatment compared with discharge of a shorter duration (i.e. less than six weeks).
-
Patient age: patients who were younger than two years old versus patients up to six years old versus adults. Patients under two years are widely considered to be more difficult to treat.
We presented the results as subgroups regardless of the presence of statistical heterogeneity based on these three factors:
-
Class of antibiotics. We grouped by pharmacological class, e.g. quinolones, aminoglycosides, penicillins etc. The rationale for this was that different classes may have had different effectiveness and side effect profiles.
-
Spectrum of activity against Pseudomonas aeruginosa (groups with known activity against Pseudomonas aeruginosa versus groups without activity against Pseudomonas aeruginosa. This is the most commonly found bacteria in patients with CSOM and its presence is associated with tissue damage.
-
Type of antiseptic used in the comparison arm (e.g. iodines, alcohols, acids). This is because different types of antiseptic have different mechanisms of action and therefore the treatment effects and adverse effect profiles are likely to be different.
When other antibiotics were also used as a common treatment in both the intervention and comparison group, we investigated the class and antipseudomonal activity when statistical heterogeneity was present and could not be explained by the other subgroup analyses.
No other subgroups based on the pharmacological properties of antibiotics were planned, but we considered the method and frequency of aural toileting if there was remaining unexplained heterogeneity despite conducting the other subgroup analyses.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
-
Impact of model chosen: fixed‐effect versus random‐effects model.
-
Risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed)).
-
Where there was statistical heterogeneity, studies that only recruited patients who had previously not responded to one of the treatments under investigation in the RCT. Studies that specifically recruited patients who did not respond to a treatment could potentially have reduced the relative effectiveness of an agent.
If any of these investigations found a difference in the size of the effect or heterogeneity, we mentioned this in the Effects of interventions section and/or presented the findings in a table.
GRADE and 'Summary of findings' table
Using the GRADE approach, at least two review authors (KH/LYC) independently rated the overall certainty of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The certainty of evidence reflects the extent to which we were confident that an estimate of effect was correct and we applied this in the interpretation of results. There were four possible ratings: 'high', 'moderate', 'low' and 'very low' (Handbook 2011). A rating of 'high' certainty evidence implies that we were confident in our estimate of effect and that further research was very unlikely to change our confidence in the estimate of effect. A rating of 'very low' certainty implies that any estimate of effect obtained was very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors could lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading was determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision;
-
publication bias.
The 'Summary of findings' tables present the following outcomes:
-
resolution of ear discharge or 'dry ear':
-
at between one week and up to two weeks;
-
after four weeks;
-
-
health‐related quality of life;
-
ear pain (otalgia) or discomfort or local irritation;
-
hearing;
-
serious complications;
-
suspected ototoxicity.
Results
Description of studies
Results of the search
The searches retrieved a total of 7256 references and we identified five additional references from other sources. This reduced to 3147 after removal of duplicates. We screened the titles and abstracts and subsequently removed 2935 references. We assessed 212 full texts for eligibility of which we discarded 199 references; we excluded 78 of these references (52 studies) with reasons recorded in the review (see Excluded studies).
We included seven studies (11 references) (Fradis 1997; Gupta 2015; Jaya 2003; Loock 2012; Macfadyen 2005; van Hasselt 1997; Vishwakarma 2015). The Characteristics of included studies table and Table 4 provide further details of the included studies.
| Ref ID (no. participants) | Setting | Population | Antibiotic | Topical antiseptic | Treatment | Follow‐up | Background treatment | Notes |
|---|---|---|---|---|---|---|---|---|
| Topical antibiotics versus acetic acid | ||||||||
| (159 participants) | South Africa, city (secondary care) | Patients with otorrhoea because of active mucosal COM Age over 6 years (90% between 20 and 34 years) | Ciprofloxacin, ear drops, (no concentration), 6 drops/8 hours | 1% acetic acid 6 drops/12 hours | 4 weeks | Up to 8 weeks | Aural cleaning at 1st visit | Part of a 3‐arm trial; third arm used boric acid (see below) |
| (58 participants in relevant arms) | Malawi (community setting) | CSOM (no details) "Children" ‐ no age information provided | 0.3% ofloxacin 3 drops/8 hours | 2% acetic acid in spirit 25% and glycerine 30% 3 drops/8 hours | 2 weeks | 8 weeks | Suction cleaning at the start of trial, at 1‐week and 2‐week follow‐up | Part of a 3‐arm trial; third arm used topical antiseptics + steroids |
| Neomycin 0.5%/polymixin B 0.1%, 3 drops/8 hours | ||||||||
| (100 participants) | India (secondary care) | Tubotympanic (safe) type of CSOM Mean age 69 years (range: 10 to 60 years) | Gentamicin (0.3%), ear drops, 3 drops every 8 hours | Acetic acid (1.5%), ear drops, 3 drops every 8 hours | 2 weeks | 2 weeks | None listed | Resolution of ear discharge measured as symptom score |
| Topical antibiotics versus aluminium acetate (Burow's solution) | ||||||||
| (51 participants, 60 ears) | Israel (ENT outpatient clinic) | Chronic otitis media Mean: 44.4 years (range 18 to 73 years) | Ciprofloxacin (no concentration), 15 drops per day | 1% aluminium acetate solution 5 drops/8 hours | 3 weeks | 3 weeks | None mentioned | Randomisation by ear Not possible to use results 3‐arm trial |
| Tobramycin (no concentration), 15 drops per day | ||||||||
| Topical antibiotics versus boric acid | ||||||||
| (159 participants) | South Africa, city (secondary care) | Patients with otorrhoea because of active mucosal COM Age over 6 years (90% between 20 and 34 years) | Ciprofloxacin, ear drops, (no concentration), 6 drops/8 hours | Boric acid powder Single administration | 4 weeks (antibiotics) | Up to 8 weeks | Aural cleaning at 1st visit | Part of a 3‐arm trial; third arm used acetic acid (see above) |
| Macfadyen 2005 (427 participants) | Kenya, rural (community, school setting) | Children (aged over 5 years) with CSOM Mean age 11.1 ± 3.15 years | 0.3% ciprofloxacin, ear drops, no volume given every 12 hours | 2% boric acid in 45% alcohol, ear drops, no volume given every 12 hours | School days only for 2 weeks | 4 weeks | Daily dry mopping before application | — |
| Topical antibiotics versus povidone‐iodine | ||||||||
| Jaya 2003 (40 participants) | India, city (ENT outpatient clinic) | Actively discharging CSOM with moderate to large central perforation Age over 10 years (50% between 21 to 21 to 40) | Ciprofloxacin 0.3% ear drops, 3 drops 3 times daily | Povidone‐iodine 5% solution, 3 drops 3 times daily | 10 days | 4 weeks | Suction cleaning before trial and then daily dry mopping | — |
| Systemic and topical antibiotics versus acetic acid and aural toileting | ||||||||
| (100 participants) | India (secondary care) | CSOM Mean age: 36.4 years (range: 6 to 72 years) | Topical ciprofloxacin (no concentration/volume) daily for 3 months, plus oral ciprofloxacin, 500 mg twice daily for 15 days | Diluted acetic acid (2 mL) daily. Every second day this was completed at the hospital with suction ear cleaning. Continued until no further discharge | See details for each treatment arm | 3 months | Dry mopping at 1st visit | — |
We identified one ongoing study (I‐HEAR‐BETAa; see Characteristics of ongoing studies). One reference is awaiting classification (Abdul 2005; see Characteristics of studies awaiting classification).
A flow chart of study retrieval and selection is provided in Figure 1.
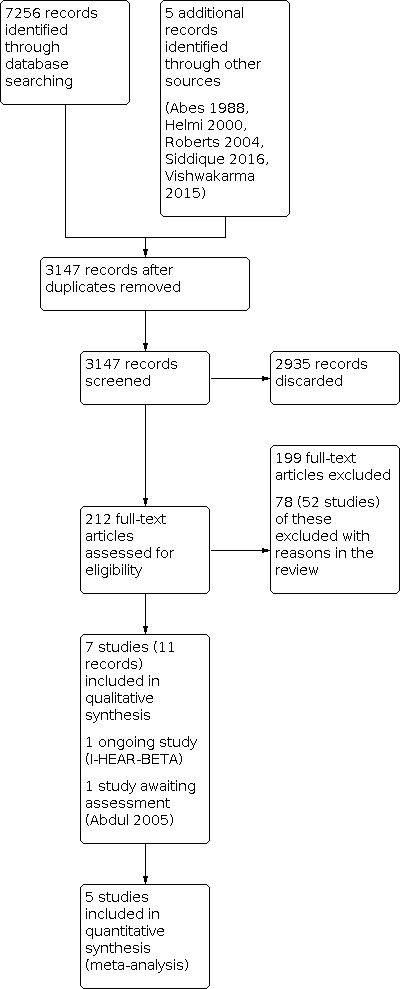
Process for sifting search results and selecting studies for inclusion.
Included studies
We included seven studies (11 references) (Fradis 1997; Gupta 2015; Jaya 2003; Loock 2012; Macfadyen 2005; van Hasselt 1997; Vishwakarma 2015). The Characteristics of included studies table and Table 4 provide a further details of the included studies.
Study design
Three studies were three‐arm trials (Fradis 1997; Loock 2012; van Hasselt 1997). In each case, all three study arms were included in the comparison. Details of the other study arms can be found in the Characteristics of included studies table.
One study was presented as a non‐peer reviewed report where no mention of randomisation was made (van Hasselt 1997). However, the same study author refers to the study as "randomised" in the introduction of a later publication. The remaining six studies indicated that they were "randomised".
Unit of randomisation
There were no cluster‐randomised trials identified. Fradis 1997 randomised participants by ear, rather than by person, meaning that the 9 (of 51) included participants with bilateral disease may have been given different topical antibiotics for each ear. It is not possible to separate out these participants and so the results cannot be used.
Sample size
A total of 935 participants were included in the seven studies. The number of participants in the studies ranged from 40 to 427.
Location
Three studies were conducted in India (Gupta 2015; Jaya 2003; Vishwakarma 2015) and three studies were conducted in different African countries: Malawi (van Hasselt 1997), Kenya (Macfadyen 2005), and South Africa (Loock 2012). The final study was conducted in Israel (Fradis 1997).
Setting
Five studies were based in secondary care in the ENT departments of hospitals (Fradis 1997; Gupta 2015; Jaya 2003; Loock 2012; Vishwakarma 2015). Macfadyen 2005 was completed in primary schools and van Hasselt 1997 was a community study.
Population
Age and sex
The ages of participants are reported in Table 4. One study recruited only children (van Hasselt 1997) and six studies included both adults and children although randomisation did not appear to be stratified by age in any study (Gupta 2015; Jaya 2003; Loock 2012; Macfadyen 2005; Vishwakarma 2015). Six studies reported that they included both males and females. The percentage of females in the studies ranged from 39% to 65%.
Diagnosis
Main diagnosis
Chronic suppurative otitis media (CSOM) was the main diagnosis in all studies (Table 4). None of the studies reported any of the participants having an alternative cause of ear discharge.
Duration of discharge
Three studies did not list the duration of discharge (Loock 2012; van Hasselt 1997; Vishwakarma 2015). Where reported, the information was provided in different ways making comparison difficult. The minimum duration of discharge in Gupta 2015 was four weeks, Macfadyen 2005 reported a median duration of eight weeks and the mean duration of discharge in Fradis 1997 was 24 months. Jaya 2003 provided the most information and identified that 15 participants (37.5%) had symptoms for less than one week, 20 participants (50%) for between one and four weeks, and five participants (12.5%) had symptoms for longer than four weeks. The paper noted that 27 participants (67.5%) had CSOM for more than five years.
Intervention
Details of the interventions, background treatments and treatment durations for each of the included studies are summarised in Table 4. The treatment durations lasted between 10 days and 4 weeks.
Comparisons
The included studies presented information for five comparisons:
-
Topical antibiotics versus acetic acid: two studies used quinolones (Loock 2012; van Hasselt 1997) and one study used aminoglycosides (Vishwakarma 2015).
-
Topical antibiotics versus aluminium acetate: one study arm used quinolones and the other used aminoglycosides (Fradis 1997).
-
Topical antibiotics versus boric acid (either ear drops or a single administration of boric acid powder): two studies both used quinolones (Loock 2012; Macfadyen 2005).
-
Topical antibiotics versus povidone‐iodine: one study used quinolones (Jaya 2003).
-
Topical antibiotics and systemic antibiotics (quinolones) versus acetic acid and aural toileting: one study (Gupta 2015).
Outcomes
Resolution of ear discharge
All seven studies reported resolution of ear discharge as an outcome, although the definitions, methods and timing of assessment differed between studies. These are summarised in Table 5
| Reference | Unit of randomisation | Discharge results reported by | Definition | Otoscopically confirmed? | Time points | Notes |
|---|---|---|---|---|---|---|
| Ear | Ear | "Clinical success" defined as cessation of otorrhoea and eradication of the micro‐organisms in the post‐treatment culture | Unclear | 2 to 4 weeks: 21 days | Not possible to use these results as randomisation by ear (9/51 patients had bilateral disease) | |
| Person | Person | "Absence of discharge" | Otoscopically confirmed | 2 to 4 weeks: 15 days After 4 weeks: 1 month | — | |
| Person | Person | "Inactive" ear | Microscopic examination | 1 to 2 weeks: 2 weeks 2 to 4 weeks: 4 weeks | — | |
| Person | Person | "Inactive" ear (dry) | Otoscopically confirmed | 2 to 4 weeks: 4 weeks | Also measured patient satisfaction, which asked patients whether their ears were 'completely dry', 'better but not completely dry', 'no better, still running' | |
| Person | Both by person | Resolution of aural discharge | Otoscopically confirmed | 1 to 2 weeks: 2 weeks 2 to 4 weeks: 4 weeks | For bilateral disease results were reported for when either ear was dry and when both ears were dry. For this review we have used the 'both ears' results. | |
| Unclear | Results reported by ear | "Dry ear" | Unclear | 1 to 2 weeks: 1 week 2 to 4 weeks: 2 weeks | Results not used as it was not possible to account for correlation between ears due to bilateral disease | |
| Person | Person | "Clinical cure" defined as a score of < 3 on a symptom scale1 | Unclear | 1 to 2 weeks: 14 days | — |
1Symptom scale; tinnitus: absent (0), mild (1), moderate (2), severe (3); amount of discharge: absent (0), mild (1), moderate (2), severe (3); type of discharge: absent (0), mucoid (1), mucopurulent (2), purulent (3). Sum scores in each category to give range of 0 to 9.
Health‐related quality of life using a validated instrument
No studies reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
Three studies reported this outcome, although the definitions are different and the methods of assessment are not always clear. Loock 2012 gave the number of participants who reported unpleasant taste and burning sensation, Vishwakarma 2015 reported one case of "mild irritability" with the use of acetic acid and Macfadyen 2005 recorded "ear pain, irritation, and bleeding".
Hearing
One study presented the average change in hearing (air conduction) from baseline at four weeks as decibels (dB) averaged over 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz (Macfadyen 2005). Four studies noted that audiometry was completed as part of the study but results were either not presented (Fradis 1997; Gupta 2015) or only presented narratively (Jaya 2003; Loock 2012). No hearing outcomes were measured or reported in two studies (van Hasselt 1997; Vishwakarma 2015).
Serious complications (including intracranial complications, extracranial complications and death)
Serious complications were not consistently reported. One study reported that no serious complications occurred (Loock 2012).
Suspected ototoxicity
This outcome was not consistently reported. Two studies attempted to record ototoxicity (Jaya 2003; Vishwakarma 2015).
Excluded studies
We excluded 52 studies (78 papers) after reviewing the full text. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table. The main reasons for exclusion were as follows:
We excluded 47 studies (73 papers) because the comparisons were not appropriate for this review, but were relevant to another review in this suite:
-
Topical antibiotics (CSOM‐1): Asmatullah 2014; de Miguel 1999; Esposito 1990; Gyde 1978; Jamallulah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1998a.
-
Systemic antibiotics (CSOM‐2): de Miguel 1999; Eason 1986; Esposito 1990; Fliss 1990; Ghosh 2012; Legent 1994; Nwokoye 2015; Onali 2018; Picozzi 1983; Ramos 2003; Renuknanada 2014; Rotimi 1990; Sanchez Gonzales 2001; Somekh 2000; van der Veen 2007.
-
Topical versus systemic antibiotics (CSOM‐3): de Miguel 1999; Esposito 1990; Esposito 1992; Povedano 1995; Ramos 2003; Yuen 1994.
-
Topical antibiotics with steroids (CSOM‐4): Boesorire 2000; Browning 1988; Couzos 2003; Crowther 1991; Eason 1986; Gendeh 2001; Helmi 2000; Indudharan 2005; Kaygusuz 2002; Lazo Saenz 1999; Leach 2008; Miro 2000; Panchasara 2015; Ramos 2003; Subramaniam 2001; Tong 1996.
-
Topical antiseptics (CSOM‐5): Eason 1986; Minja 2006; Papastavros 1989.
-
Aural toileting (CSOM‐7): Eason 1986; Kiris 1998; Smith 1996.
We excluded the remaining five studies (five papers) for the following reasons:
-
Browning 1983: although the comparison was antibiotics compared with topical antiseptics, the antibiotics were prescribed based on the results of the culture and so no standard antibiotic treatment was given.
-
Clayton 1990: less than 20% of participants within the study had CSOM.
-
Roydhouse 1981: the intervention was a mucolytic agent (bromhexine), which was not classified as an antiseptic.
-
Thorpe 2000: compared three concentrations of the same topical antiseptic (aluminium acetate), which is not a question included in this review.
-
van Hasselt 1998b: although the comparison was topical antibiotics with topical antiseptics, the antibiotics were given as a single dose, which does not meet the inclusion criteria for this review.
Risk of bias in included studies
See Figure 2 for the 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for the 'Risk of bias' summary (our judgements about each risk of bias item for each included study).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We judged two studies to be at high risk of bias (Gupta 2015; van Hasselt 1997). Neither study reported methods for sequence generation and Gupta 2015 indicated that participants who were already using antibiotics were allocated to the antiseptic treatment group, which may have created biases between the groups but the baseline characteristics are not provided. For van Hasselt 1997, the original report did not mention randomisation and this was only mentioned in passing as part of the introduction to a different study.
We judged three studies as at unclear risk of bias as they did not provide clear data on how the randomisation schedule was generated (Fradis 1997; Jaya 2003; Vishwakarma 2015). We judged two studies as at low risk as they reported randomisation well (Loock 2012; Macfadyen 2005).
Allocation concealment
We judged the same two studies that are at high risk of selection bias to be at high risk of allocation concealment bias (Gupta 2015; van Hasselt 1997). In Gupta 2015, as there was selection of participants to one of the groups (those using antibiotics to the acetic acid group), we have to assume allocation concealment to have been broken. For van Hasselt 1997, as there were an unequal number of people between the groups (46 versus 38 versus 12) without any explanation, there is a risk of bias due to selective allocation.
We judged Vishwakarma 2015 as at unclear risk of bias as it does not provide information on allocation concealment. We judged three studies as at low risk of bias because they reported measures to protect bias from allocation concealment well (Fradis 1997; Loock 2012; Macfadyen 2005).
Blinding
Performance bias
We assessed two studies to be at high risk of performance bias: Gupta 2015 did not mention blinding and Vishwakarma 2015 reported that the study was an "open study".
We assessed two studies to be at unclear risk of bias. Although Loock 2012 made some attempts to blind participants to treatment, because one of the treatments (boric acid) was given as a powder and the other two as ear drops blinding was not likely to be effective. van Hasselt 1997 did not mention blinding and as one of the treatments was acetic acid ear drops, which have a distinctive smell, blinding would have been difficult.
We assessed three studies as at low risk of bias because they provided sufficient descriptions of how they kept participants and professionals blinded the allocated treatments (Fradis 1997; Jaya 2003; Macfadyen 2005).
Detection bias
Similar to performance bias, we assessed two studies to be at high risk of bias (Gupta 2015; Vishwakarma 2015), two studies as at unclear risk (Loock 2012; van Hasselt 1997) and three studies as at low risk of detection bias (Fradis 1997; Jaya 2003; Macfadyen 2005).
Incomplete outcome data
We assessed one study to be at high risk of attrition bias: van Hasselt 1997 reported a high loss to follow‐up (27/96; 28%) despite being a short trial. No reasons were provided and the loss is not balanced across groups, ranging from 8% to 40% of participants by group.
We assessed four studies to be at unclear risk of attrition bias:
-
Fradis 1997: presented a loss to follow‐up of 10% (6/60) but no reasons were provided or details of to which group the lost participants were allocated.
-
Gupta 2015: the study does not report any patients dropping out but as the study lasted three months and the participants were advised to visit the hospital every other day its seems unlikely that there was no loss to follow‐up.
-
Jaya 2003: only four participants (10%) did not provide results; the reasons were not given and these missing data points could have affected the efficacy results due to the small sample size. In particular, it may have been important if they withdrew due to adverse events.
-
Loock 2012: provided the loss to follow‐up rates in the three treatment groups as 5.8%, 15.1% and 18.5% but did not provide reasons within the paper.
We assessed two studies as at low risk of attrition bias because they reported low dropout rates (Macfadyen 2005; Vishwakarma 2015).
Selective reporting
We assessed one study to be at high risk of publication bias (van Hasselt 1997). It was unpublished and makes reference to longer‐term results that were not found in our searches.
We assessed five studies to be at unclear risk of selective reporting bias (Fradis 1997; Gupta 2015; Jaya 2003; Loock 2012; Vishwakarma 2015). Four of these studies stated that hearing assessment was completed pre‐ and post‐treatment but either did not report the results or only reported vague narrative statements (Fradis 1997; Gupta 2015; Jaya 2003; Loock 2012). Vishwakarma 2015 used symptom scales as their primary outcome but failed to provide information on the definition or validation of these scales.
We assessed one study as at low risk of selective reporting bias as it was a well‐reported study (Macfadyen 2005).
None of the studies had protocols identified through our searches of clinical trials registries.
Other potential sources of bias
Funding
Three papers did not provide information about funding (Fradis 1997; Gupta 2015; Jaya 2003) and a further paper declared that there were no funding sources (Vishwakarma 2015).
Two studies were funded through national or international research grants: Loock 2012 was funded by the ENT Society of South Africa, National Health Laboratory Service of South Africa (NHLS) but "received no sponsorship or incentive from manufacturers of any of the treatments used"; Macfadyen 2005 was funded by the Wellcome Trust (grant reference number: 056756/Z/99/Z) but the study also declared that "Alcon (Denmark and Belgium) provided the Ciloxan supplies".
It appears that van Hasselt 1997 was funded by the Christian Blind Mission International.
Declaration of interest
Four studies did not make a statement about any interests (Fradis 1997; Gupta 2015; Macfadyen 2005; van Hasselt 1997), whilst the remaining three either declared that they had no interests (Loock 2012; Vishwakarma 2015) or that they had no financial interests (Jaya 2003).
Effects of interventions
See: Summary of findings 1 Topical antibiotics compared to acetic acid for chronic suppurative otitis media; Summary of findings 2 Topical antibiotics (quinolones) compared to boric acid for chronic suppurative otitis media; Summary of findings 3 Topical antibiotics (quinolones) compared to povidone‐iodine for chronic suppurative otitis media
See also summary of findings Table 1; summary of findings Table 2; summary of findings Table 3.
Comparison 1: Topical antibiotics versus acetic acid
Three studies including participants with CSOM compared topical antibiotics to acetic acid (Loock 2012; van Hasselt 1997; Vishwakarma 2015; 303 participants), although van Hasselt 1997 had two relevant comparisons as there were two antibiotic arms each of which used different antibiotics. Two studies compared topical quinolones to acetic acid (Loock 2012; van Hasselt 1997; 165 participants). One study compared aminoglycosides with acetic acid (Vishwakarma 2015; 100 participants). One study compared acetic acid against neomycin/polymyxin B (van Hasselt 1997; 85 participants).
Primary outcomes
Resolution of ear discharge
van Hasselt 1997 only reported the results of ear discharge by ear and it was not possible to determine how many participants were allocated to each group; these results therefore cannot be used in the analysis.
Between one week and up to two weeks
One study using aminoglycosides reported this outcome (Vishwakarma 2015), but it was not clear if there was a difference in resolution of ear discharge (unclear if this was otoscopically confirmed) between the groups receiving acetic acid and gentamicin (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.72 to 1.08; 100 participants; Analysis 1.1). Using GRADE we assessed the evidence as being very low certainty.
Two weeks to up to four weeks
Loock 2012 reported that more participants in the ciprofloxacin group had resolution of ear discharge (otoscopically confirmed) compared with acetic acid (RR 2.93, 95% CI 1.71 to 5.04; 89 participants; Analysis 1.2).
After four weeks
No study reported resolution of ear discharge at this time point.
Health‐related quality of life using a validated instrument
No studies reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
Two studies reported this outcome, although the definitions used were different (Loock 2012; Vishwakarma 2015). Vishwakarma 2015 reported "mild irritability" in one case in the acetic acid group, whereas Loock 2012 reported "unpleasant taste and burning sensation" in four cases in the acetic acid group. No events were reported in the antibiotic group.
There may be more ear pain or discomfort or local irritation with acetic acid compared to topical antibiotics but we are very uncertain about the results (RR 0.16, 95% CI 0.02 to 1.34; 2 studies; 189 participants; I2 = 0%; very low‐certainty evidence; Analysis 1.3).
Secondary outcomes
Hearing
Loock 2012 measured the outcome of hearing but only presented the results narratively in their report: "audiometric tests showed no detectable overall, isolated not idiosyncratic hearing loss from any treatment" (very low‐certainty evidence). None of the other studies reported this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Vishwakarma 2015 noted that "... none of the patients had any kind of ear damage or toxicity" (very low‐certainty evidence). No other study reported this outcome.
Subgroup analyses
No subgroup analyses were possible for this comparison:
-
High‐risk populations: no studies reported high‐risk populations as defined in our protocol.
-
Patients with ventilation tubes: no studies reported the inclusion of participants with ventilation tubes.
-
Diagnosis of CSOM: all included studies used CSOM as the inclusion criterion.
-
Duration of ear discharge: none of the studies reported the duration of discharge.
-
Patient age: all studies that provided age information included both adults and children, but did not stratify between the two populations.
Comparison 2: Topical antibiotics versus aluminium acetate
One study of participants with a diagnosis of CSOM was included in this comparison: Fradis 1997, which used ears as the unit of randomisation. Pairwise adjustments were not completed and sufficient data could not be obtained for us to be able to make the adjustments, therefore none of the outcomes could be used in the analysis (Unit of analysis issues).
Primary outcomes
Resolution of ear discharge
The study reported results for resolution of ear discharge at two to four weeks, but the results were not presented in a useable form. No other results for other time points were available.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not measure this outcome.
Secondary outcomes
Hearing
The study did not measure this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
The study did not measure this outcome.
Comparison 3: Topical antibiotics versus boric acid/boric acid powder
Two studies including participants with CSOM were included in this comparison (Loock 2012; Macfadyen 2005; 532 participants). Both studies used ciprofloxacin (quinolone) but in comparison Macfadyen 2005 used boric acid ear drops whereas Loock 2012 used a single application of boric acid powder.
Primary outcome
Resolution of ear discharge
Between one week and up to two weeks
Macfadyen 2005 reported that topical quinolones are likely to increase the number of people with resolution of ear discharge (otoscopically confirmed) at two weeks compared with topical boric acid ear drops (RR 1.86, 95% CI 1.48 to 2.35; 411 participants; Analysis 3.1). This means that one additional person would have resolution of ear discharge for every four people (95% CI 3 to 7) receiving topical antibiotics (compared with boric acid) at two weeks. The evidence was of moderate certainty.
Two weeks to up to four weeks
Both studies reported that more people had resolution of ear discharge with topical quinolones at between two to four weeks compared with boric acid (RR 1.27, 95% CI 1.07 to 1.49; 488 participants; 2 studies; I2 = 16%; Analysis 3.2) (Loock 2012; Macfadyen 2005).
After four weeks
No study reported the results for this outcome at this time point.
Health‐related quality of life using a validated instrument
Neither study measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
Both studies measured this outcome. Macfadyen 2005 reported "pain, irritation, and bleeding" and Loock 2012 did not report any episodes of ear pain, discomfort or irritation in either group within this comparison. The results showed that topical quinolones may result in less ear pain, discomfort or irritation at four weeks compared to topical boric acid (RR 0.56, 95% CI 0.32 to 0.98; 510 participants; 2 studies; I2 = 0%; low‐certainty evidence; Analysis 3.3).
Secondary outcomes
Hearing
Both studies measured hearing, although Loock 2012 only reported results narratively: "Audiometric tests showed no detectable overall, isolated not idiosyncratic hearing loss from any treatment".Macfadyen 2005 compared the mean change in hearing from baseline between the two treatment groups. Although both groups had mean improvements in hearing, topical antibiotics (quinolones) appeared to result in a greater improvement in mean hearing compared with topical boric acid (mean difference (MD) 2.79 decibels (dB), 95% CI 0.48 to 5.10 Hz; 390 participants; low‐certainty evidence; Analysis 3.4). It is not clear whether this change is clinically meaningful.
Serious complications (including intracranial complications, extracranial complications and death)
No study reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Neither study reported this outcome.
Subgroup analyses
No subgroup analyses were possible for this comparison:
-
High‐risk populations: neither study reported high‐risk populations as defined in our protocol.
-
Patients with ventilation tubes: neither study reported the inclusion of patients with ventilation tubes.
-
Diagnosis of CSOM: both studies used CSOM as the inclusion criterion.
-
Duration of ear discharge: only one study reported the duration of discharge.
-
Patient age: one study included only children and the other included both adults and children, but did not stratify the two populations.
Comparison 4: Topical antibiotics versus povidone‐iodine
One study of participants with CSOM was included for this comparison and compared ciprofloxacin with povidone‐iodine ear drops (Jaya 2003; 40 participants).
Primary outcomes
Resolution of ear discharge
Between one week and up to two weeks
In Jaya 2003 it was uncertain if there was a difference between topical antibiotics (ciprofloxacin) and topical povidone‐iodine in the resolution of ear discharge (otoscopically confirmed) at two weeks (RR 1.02, 95% CI 0.82 to 1.26; 39 participants; very low‐certainty evidence; Analysis 4.1).
Two weeks to up to four weeks
In Jaya 2003 it was uncertain if there was a difference between topical antibiotics (ciprofloxacin) and topical povidone‐iodine in the resolution of ear discharge at four weeks (RR 1.03, 95% CI 0.81 to 1.30; 36 participants; Analysis 4.2).
After four weeks
The study did not report this outcome at this time point.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not measure this outcome.
Secondary outcomes
Hearing
Jaya 2003 measured hearing but only provides narrative results, stating that, "There was no deterioration of hearing as assessed by pure‐tone audiometry" (very low‐certainty evidence).
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Jaya 2003 reported that "No patient developed allergic manifestations or ototoxic effects" (very low‐certainty evidence).
Comparison 5: Topical and systemic antibiotics versus acetic acid and aural toileting
Gupta 2015 (100 participants with CSOM) compared treatment with both topical and systemic antibiotics (ciprofloxacin) with daily acetic acid ear drops alongside an intensive aural toileting regimen, which involved microsuction of the ear every two days.
Primary outcomes
Resolution of ear discharge
Between one week and up to two weeks
No results were available for this outcome at this time point.
Two weeks to up to four weeks
Gupta 2015 reported that fewer people in the group receiving topical and systemic quinolones had resolution of ear discharge (otoscopically confirmed) compared with those receiving acetic acid and intensive aural toileting at 15 days (RR 0.61, 95% CI 0.40 to 0.93; 100 participants; Analysis 5.1).
After four weeks
Gupta 2015 reported that fewer people in the group receiving topical and systemic quinolones had resolution of ear discharge compared with those receiving acetic acid and intensive aural toileting at one month (RR 0.69, 95% CI 0.53 to 0.90; 100 participants; Analysis 5.2).
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not measure this outcome.
Secondary outcomes
Hearing
Although Gupta 2015 mentioned in the methods section that hearing was measured pre‐ and post‐treatment, no results were presented.
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
The study did not measure this outcome.
Discussion
Summary of main results
We identified seven studies that we included in this review (Fradis 1997; Gupta 2015; Jaya 2003; Loock 2012; Macfadyen 2005; van Hasselt 1997; Vishwakarma 2015). All of the studies included patients with CSOM; none included patients with chronic ear discharge. Due to the limited number of included studies, the methods and the choice of outcome measures used in these studies and the incomplete reporting of some results, for many of the comparisons there was not much evidence. Adverse events (ear pain, discomfort or irritation) were not well reported. Only studies using topical antibiotics without steroids were included in this review.
Comparison 1: Topical antibiotics versus acetic acid
See also summary of findings Table 1.
Three studies (303 participants) compared topical antibiotics with acetic acid (Loock 2012; van Hasselt 1997; Vishwakarma 2015). One of these was a three‐arm study comparing two different topical antibiotics with acetic acid. Two studies used quinolones, one used aminoglycosides and one used neomycin/polymyxin B. It is very uncertain if there is a difference in resolution of ear discharge between aminoglycosides and acetic acid at one to two weeks (very low‐certainty evidence). At between two to four weeks the only study with useable results reported a higher rate of people with dry ear with topical antibiotics (quinolones) compared to acetic acid (risk ratio (RR) 2.93, 95% confidence interval (CI) 1.71 to 5.04; 89 participants). No study reported results for ear discharge after four weeks.
More ear pain, discomfort or local irritation was reported with acetic acid compared to topical antibiotics (quinolones or aminoglycosides) but the results were very uncertain (RR 0.16, 95% CI 0.02 to 1.34; 189 participants; 2 studies; I2 = 0%; very low‐certainty evidence). No differences between the groups were found for hearing (quinolones) or suspected ototoxicity (aminoglycoside) in a narrative report. No results were available for quality of life or serious complications.
Comparison 2: Topical antibiotics versus aluminium acetate
The only available study for this comparison randomised participants by ear and did not present the results in a way that allowed for adjustment due to the correlation of results between ears; the results for resolution of ear discharge could therefore not be used. No other results were reported.
Comparison 3: Topical antibiotics versus boric acid
See also summary of findings Table 2.
Two studies (532 participants) compared topical antibiotics (quinolones) with boric acid, although one study used boric acid ear drops (Macfadyen 2005) and the other used a single administration of borax powder (Loock 2012). Results at both between one to two weeks (RR 1.86, 95% CI 1.48 to 2.35; 411 participants; 1 study; moderate‐certainty evidence) and between two to four weeks (RR 1.27, 95% CI 1.07 to 1.49; 488 participants; 2 studies; I2 = 16%) showed that topical antibiotics (quinolones) are likely to increase the number of people with resolution of ear discharge compared with boric acid. Neither study reported results for ear discharge after four weeks.
There was a bigger change in hearing (improvement) in the topical antibiotic group compared to the topical antiseptic group (mean difference (MD) 2.79 dB, 95% CI 0.48 to 5.10; 390 participants; 1 study; low‐certainty evidence) but this difference may not be clinically significant. There may be more adverse events (ear pain, discomfort or irritation) with boric acid compared with quinolones (RR 0.56, 95% CI 0.32 to 0.98; 510 participants; 2 studies; I2 = 0%; low‐certainty evidence). Neither study reported quality of life, serious complications or suspected ototoxicity.
Comparison 4: Topical antibiotics versus povidone‐iodine
See also summary of findings Table 3.
One study (40 participants) compared topical antibiotics (quinolones) with povidone‐iodine. It is uncertain if there is a difference between topical antibiotics and povidone‐iodine with respect to resolution of ear discharge at between one to two weeks (very low‐certainty evidence) and at between two to four weeks. The study reported qualitatively that there were no differences between the groups in hearing and reported that none of the patients developed ototoxic effects. No results for resolution of ear discharge beyond four weeks, adverse effects (ear pain/discomfort/irritation), serious complications or quality of life were reported.
Comparison 5: Topical and systemic antibiotics versus acetic acid and aural toileting
One study (100 participants) compared participants taking both topical and oral antibiotics (quinolone) with participants who received daily acetic acid ear drops and suction aural toileting every other day. Fewer patients receiving topical and oral antibiotics had resolution of ear discharge compared with acetic acid ear drops and aural toileting at both two to four weeks (RR 0.61, 95% CI 0.40 to 0.93; 100 participants) and at one month (RR 0.69, 95% CI 0.53 to 0.90; 100 participants). Although no analysis of the results using GRADE was completed, the study was identified as having a high risk of bias with regards to randomisation sequence generation, allocation concealment and selective reporting, and the study was unblinded (Figure 3). Caution should be taken when interpreting these results.
Short‐term results for resolution of ear discharge (between one to two weeks) were not reported. Health‐related quality of life, ear pain, discomfort or irritation, serious complications, hearing and suspected ototoxicity were not reported.
One interesting finding was the difference in the result between comparison one (topical antibiotics (quinolone) versus acetic acid) and comparison five (topical and systemic antibiotic (quinolone) versus acetic acid and daily aural toileting). Comparison one shows a higher rate of people with dry ear with topical antibiotics (quinolones) compared to acetic acid at between two to four weeks, whereas the results from comparison five indicate that fewer patients receiving topical and oral antibiotics had resolution of ear discharge compared with acetic acid ear drops and suction aural toileting every two days, at the same time point (two to four weeks) (RR 0.61, 95% CI 0.40 to 0.93; 100 participants). This result would appear to indicate that the addition of daily aural toileting to acetic acid had a large impact on the results. However, the absolute resolution rates for the group receiving both topical and systemic antibiotics in Gupta 2015 was very low (38%) compared with the resolution of ear discharge of 73% in Loock 2012, which used topical antibiotics alone. More research into the effects of aural toileting is required.
Overall completeness and applicability of evidence
None of the studies included participants that met the criteria for being a 'high‐risk' population as defined in our Methods section, although the recruitment was from regions that are likely to have a relatively high incidence of CSOM (Monasta 2012). All studies only included participants with CSOM (without alternative diagnoses) and most studies included adults and children.
The available evidence included four different antiseptic agents. This does not represent the full range of antiseptics available and differences in their use (such as method of administration) made it difficult to draw conclusions about specific agents.
Most of the antibiotics used within the studies were quinolones (seven study arms) with aminoglycosides being tested in only three study arms. No data for other antibiotics were available.
Data for many of the outcomes were missing. None of the studies reported health‐related quality of life or serious complications and hearing, ear pain and suspected ototoxicity. The length of follow‐up in most studies was between one to four weeks, meaning that there was limited evidence regarding the long‐term effects of treatment for the resolution of ear discharge in people with CSOM.
Quality of the evidence
Generally the included studies were small (the median sample size was 100 participants) with many being poorly reported, which led to some having an unclear risk of bias, particularly for incomplete data (attrition bias).
We consider that there is a high risk of publication bias in this area: one included study was reported only as a non‐peer reviewed report and made reference to further follow‐up for which we were unable to find any information. We are aware of unpublished data in other comparisons in CSOM (Brennan‐Jones 2018b) and we felt this to also be a risk for this comparison. We know of one study that appears from the abstract to compare antibiotics with topical antiseptics but we are unable to obtain an abstract or full copy of the paper (Abdul 2005).
We noted a large variation in the rates of resolution of ear discharge across all studies. Even between studies where participants were treated with ciprofloxacin the resolution rates varied from 38% at two weeks where patients were receiving both topical and oral ciprofloxacin (Gupta 2015) to 90% at two weeks where participants were only treated with topical ciprofloxacin (Jaya 2003). The reasons for this variation were not clear from the reported characteristics of the studies but could have been due to the baseline characteristics of the population, efficacy, frequency of application or compliance with treatment, local levels of antibiotic resistance or even quality of antibiotic manufacture. This adds to the difficulty in drawing conclusions.
Potential biases in the review process
By only including studies that provided their results by person, there was one study which we could not use for the primary outcome. This reduced the amount of data that we were able to analyse. However, as we know that the correlation of results between ears is likely to be high, we felt that the inclusion of the results of both ears into the analysis would be likely to lead to double counting and results that could generate spurious conclusions.
Agreements and disagreements with other studies or reviews
This review is part of a series of Cochrane Reviews on CSOM (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b). The review that compared topical antiseptics with placebo or no treatment concluded that the effectiveness of antiseptics in the treatment of CSOM (compared with no treatment) is uncertain due to the paucity of the evidence and the very low certainty of that which is available (Head 2018a).
These reviews supersede a pair of previous Cochrane Reviews examining topical antibiotics for CSOM (Macfadyen 2005a; Macfadyen 2006).
There are few previous reviews or guidelines for CSOM. The World Health Organization (WHO) in 2004 suggested that first‐line treatment of CSOM should comprise aural toilet and topical antibiotic drops, with second‐line treatment comprising an alternative topical antibiotic (guided by results of microbiological culture) or parenteral antibiotics (WHO 2004). The Australian government recommendations from 2010 for the treatment of Aboriginal and Torres Strait Islanders gave similar recommendations, with first‐line treatment comprising aural toilet (or antiseptic washout) followed by topical antibiotics, and second‐line treatment with parenteral antibiotics (Morris 2010). An expert panel of the American Academy of Otolaryngology in 2000 came to a similar conclusion (Hannley 2000).
The BMJ Best Evidence series on CSOM, Morris 2012, based their review comparing topical antiseptics with topical antibiotics on the previous Cochrane Review (Macfadyen 2005a). It concluded that for adults it was not possible to tell if topical antiseptics were more effective at resolving otorrhoea than topical antibiotics. In children the review concluded that "topical antibiotics improve resolution of ear discharge compared with topical antiseptics" based on one study. There was not enough information to draw any conclusions about adverse event data for adults or children.

Process for sifting search results and selecting studies for inclusion.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Topical antibiotics versus acetic acid, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 1: Topical antibiotics versus acetic acid, Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 1: Topical antibiotics versus acetic acid, Outcome 3: Ear pain, discomfort, irritation

Comparison 2: Topical antibiotics versus aluminium acetate, Outcome 1: Ototoxicity

Comparison 3: Topical antibiotics versus boric acid, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 3: Topical antibiotics versus boric acid, Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 3: Topical antibiotics versus boric acid, Outcome 3: Ear pain, discomfort, irritation

Comparison 3: Topical antibiotics versus boric acid, Outcome 4: Change in hearing

Comparison 4: Topical antibiotics versus povidone‐iodine, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 4: Topical antibiotics versus povidone‐iodine, Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 5: Topical and systemic antibiotics versus acetic acid, Outcome 1: Resolution of ear discharge (2 to 4 weeks)

Comparison 5: Topical and systemic antibiotics versus acetic acid, Outcome 2: Resolution of ear discharge (after 4 weeks)
| Topical antibiotics compared to acetic acid for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) Aminoglycosides | 100 | RR 0.88 | Study population | ⊕⊝⊝⊝ | It is very uncertain whether acetic acid is more effective at resolving ear discharge compared with topical aminoglycoside antibiotics at 14 days | ||
| 84.0% | 73.9% | 10.1% fewer | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study reported this outcome |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study reported this outcome |
| Ear pain, discomfort, irritation | 189 | RR 0.16 | Study population | ⊕⊝⊝⊝ | Acetic acid may cause more ear pain, discomfort and/or irritation than topical antibiotics (aminoglycosides and quinolones) but we are very uncertain about the results | ||
| 5.3% | 0.9% | 4.5% fewer | |||||
| Hearing | 107 | One study reports that "audiometric tests showed no detectable overall, isolated not idiosyncratic hearing loss from any treatment". No numeric results were provided. | very low3 | It is uncertain whether there is a difference in hearing between topical quinolones and topical acetic acid | |||
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity | 100 | One study (100 participants) reported: "… none of the patients had any kind of ear damage or toxicity" | very low4 | It is uncertain if there is a difference in ototoxicity between topical aminoglycosides and topical acetic acid | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low‐certainty evidence: downgraded by one level due to study limitations (risk of bias) as the study had unclear randomisation, allocation concealment and blinding. Downgraded by one level due to indirectness as the outcome used was 'clinical cure' rather than resolution of ear discharge. Downgraded by one level due to suspected publication bias as one 'unpublished' study was identified indicating the possibility of unreported trials. 3Downgraded to very low‐certainty evidence: downgraded by two levels due to imprecision as no numeric results were provided and the result came from a small study (107 participants). Downgraded by one level due to suspected publication bias as one 'unpublished' study was identified indicating the possibility of unreported trials. 4Downgraded to very low‐certainty evidence: downgraded by one level due to study limitations (risk of bias) as the study had unclear randomisation, allocation concealment and blinding. Downgraded by one level due to imprecision as the result came from a small study (100 participants). Downgraded by one level due to suspected publication bias (one 'unpublished' study identified indicating the possibility of unreported trials). | |||||||
| Topical quinolones compared to boric acid for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical quinolones | With topical quinolones | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) Quinolones | 411 | RR 1.86 | Study population | ⊕⊕⊕⊝ | Topical quinolones are likely to increase the number of people with resolution of ear discharge at 2 weeks compared with topical boric acid | ||
| 31.9% | 59.3% | 27.4% more | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study measured resolution of ear discharge at 4 weeks |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study measured quality of life |
| Ear pain, discomfort, irritation | 510 | RR 0.56 | Study population | ⊕⊕⊝⊝ | Topical quinolones may result in less ear pain, discomfort or irritation at 4 weeks compared to topical boric acid | ||
| 11.8% | 6.6% | 5.2% fewer | |||||
| Average change in hearing from baseline | 390 | — | The mean average change in hearing from baseline without topical quinolones was 2.69 dB | The mean average change in hearing from baseline with topical quinolones was 5.42 dB | MD 2.79 dB higher | ⊕⊕⊝⊝ | Topical quinolones may result in greater improvement in mean hearing from baseline compared with topical boric acid; however this effect size may not be clinically important |
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by one level to moderate‐certainty evidence due to suspected publication bias. We identified two unpublished studies comparing antibiotics and antiseptics, which indicates that there may be more unpublished studies. | |||||||
| Topical antibiotics compared to povidone‐iodine for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) | 39 | RR 1.02 | Study population | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the resolution of ear discharge at 2 weeks between topical antibiotics and topical povidone‐iodine | ||
| 88.9% | 90.7% | 1.8% more | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Ear pain, discomfort, irritation ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Hearing | 40 (1 RCT) | "There was no deterioration of hearing as assessed by pure tone audiometry" | very low2 | — | |||
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity | 40 | "No patient developed allergic manifestations or ototoxic effects" | very low3 | — | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low‐certainty evidence. Downgraded by one level due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting). Downgraded by one level due to imprecision (small study size: 39 participants, confidence interval crosses the line of minimally clinical important difference). Downgraded by one level due to suspected publication bias (one study referred to long‐term results that appear to be unpublished and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). 2Downgraded to very low‐certainty evidence. Downgraded by one level due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting). Downgraded by two levels due to imprecision (no numeric results were presented and very small study size (39 participants)). Downgraded by one level due to suspected publication bias (one study referred to long‐term results, which appear to be unpublished, and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). 3Downgraded to very low‐certainty evidence. Downgraded by two levels due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting as it is unclear how the outcome was defined). Downgraded by two levels due to imprecision (no numeric results were presented and very small study size (39 participants). Downgraded by one level due to suspected publication bias (one study referred to long‐term results that appear to be unpublished and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). | |||||||
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
|---|---|---|---|---|---|
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
| CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018a). CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a). CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b). CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018b). CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a). CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b). CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018). | |||||
| Class of antibiotics | Examples | Route of administration |
|---|---|---|
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
| Antiseptic agent used aurally | Target and mechanism of action |
|---|---|
| Rubbing alcohol (ethanol, isopropanol) | Penetrating agents that cause loss of cellular membrane function, leading to release of intracellular components, denaturing of proteins, and inhibition of DNA, RNA, protein and peptidoglycan synthesis. |
| Povidone‐iodine | Highly active oxidising agents that destroy cellular activity of proteins. Disrupts oxidative phosphorylation and membrane‐associated activities. Iodine reacts with cysteine and methionine thiol groups, nucleotides and fatty acids, resulting in cell death. |
| Chlorhexidine | Membrane‐active agents that damage cell wall and outer membrane, resulting in collapse of membrane potential and intracellular leakage. Enhanced passive diffusion mediates further uptake, causing coagulation of cytosol. |
| Hydrogen peroxide | Produces hydroxyl free radicals that function as oxidants, which react with lipids, proteins and DNA. Sulfhydryl groups and double bonds are targeted in particular, thus increasing cell permeability. |
| Boric acid | It is likely that the change in the pH media of the ear canal interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. |
| Aluminium acetate/acetic acid | Acetic acid changes the pH media of the ear canal and interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. Aluminium acetate is an astringent that helps reduce itching, stinging and inflammation. |
| Sources: Gupta 2015; McDonnell 1999; Sheldon 2005. | |
| Ref ID (no. participants) | Setting | Population | Antibiotic | Topical antiseptic | Treatment | Follow‐up | Background treatment | Notes |
|---|---|---|---|---|---|---|---|---|
| Topical antibiotics versus acetic acid | ||||||||
| (159 participants) | South Africa, city (secondary care) | Patients with otorrhoea because of active mucosal COM Age over 6 years (90% between 20 and 34 years) | Ciprofloxacin, ear drops, (no concentration), 6 drops/8 hours | 1% acetic acid 6 drops/12 hours | 4 weeks | Up to 8 weeks | Aural cleaning at 1st visit | Part of a 3‐arm trial; third arm used boric acid (see below) |
| (58 participants in relevant arms) | Malawi (community setting) | CSOM (no details) "Children" ‐ no age information provided | 0.3% ofloxacin 3 drops/8 hours | 2% acetic acid in spirit 25% and glycerine 30% 3 drops/8 hours | 2 weeks | 8 weeks | Suction cleaning at the start of trial, at 1‐week and 2‐week follow‐up | Part of a 3‐arm trial; third arm used topical antiseptics + steroids |
| Neomycin 0.5%/polymixin B 0.1%, 3 drops/8 hours | ||||||||
| (100 participants) | India (secondary care) | Tubotympanic (safe) type of CSOM Mean age 69 years (range: 10 to 60 years) | Gentamicin (0.3%), ear drops, 3 drops every 8 hours | Acetic acid (1.5%), ear drops, 3 drops every 8 hours | 2 weeks | 2 weeks | None listed | Resolution of ear discharge measured as symptom score |
| Topical antibiotics versus aluminium acetate (Burow's solution) | ||||||||
| (51 participants, 60 ears) | Israel (ENT outpatient clinic) | Chronic otitis media Mean: 44.4 years (range 18 to 73 years) | Ciprofloxacin (no concentration), 15 drops per day | 1% aluminium acetate solution 5 drops/8 hours | 3 weeks | 3 weeks | None mentioned | Randomisation by ear Not possible to use results 3‐arm trial |
| Tobramycin (no concentration), 15 drops per day | ||||||||
| Topical antibiotics versus boric acid | ||||||||
| (159 participants) | South Africa, city (secondary care) | Patients with otorrhoea because of active mucosal COM Age over 6 years (90% between 20 and 34 years) | Ciprofloxacin, ear drops, (no concentration), 6 drops/8 hours | Boric acid powder Single administration | 4 weeks (antibiotics) | Up to 8 weeks | Aural cleaning at 1st visit | Part of a 3‐arm trial; third arm used acetic acid (see above) |
| Macfadyen 2005 (427 participants) | Kenya, rural (community, school setting) | Children (aged over 5 years) with CSOM Mean age 11.1 ± 3.15 years | 0.3% ciprofloxacin, ear drops, no volume given every 12 hours | 2% boric acid in 45% alcohol, ear drops, no volume given every 12 hours | School days only for 2 weeks | 4 weeks | Daily dry mopping before application | — |
| Topical antibiotics versus povidone‐iodine | ||||||||
| Jaya 2003 (40 participants) | India, city (ENT outpatient clinic) | Actively discharging CSOM with moderate to large central perforation Age over 10 years (50% between 21 to 21 to 40) | Ciprofloxacin 0.3% ear drops, 3 drops 3 times daily | Povidone‐iodine 5% solution, 3 drops 3 times daily | 10 days | 4 weeks | Suction cleaning before trial and then daily dry mopping | — |
| Systemic and topical antibiotics versus acetic acid and aural toileting | ||||||||
| (100 participants) | India (secondary care) | CSOM Mean age: 36.4 years (range: 6 to 72 years) | Topical ciprofloxacin (no concentration/volume) daily for 3 months, plus oral ciprofloxacin, 500 mg twice daily for 15 days | Diluted acetic acid (2 mL) daily. Every second day this was completed at the hospital with suction ear cleaning. Continued until no further discharge | See details for each treatment arm | 3 months | Dry mopping at 1st visit | — |
| Reference | Unit of randomisation | Discharge results reported by | Definition | Otoscopically confirmed? | Time points | Notes |
|---|---|---|---|---|---|---|
| Ear | Ear | "Clinical success" defined as cessation of otorrhoea and eradication of the micro‐organisms in the post‐treatment culture | Unclear | 2 to 4 weeks: 21 days | Not possible to use these results as randomisation by ear (9/51 patients had bilateral disease) | |
| Person | Person | "Absence of discharge" | Otoscopically confirmed | 2 to 4 weeks: 15 days After 4 weeks: 1 month | — | |
| Person | Person | "Inactive" ear | Microscopic examination | 1 to 2 weeks: 2 weeks 2 to 4 weeks: 4 weeks | — | |
| Person | Person | "Inactive" ear (dry) | Otoscopically confirmed | 2 to 4 weeks: 4 weeks | Also measured patient satisfaction, which asked patients whether their ears were 'completely dry', 'better but not completely dry', 'no better, still running' | |
| Person | Both by person | Resolution of aural discharge | Otoscopically confirmed | 1 to 2 weeks: 2 weeks 2 to 4 weeks: 4 weeks | For bilateral disease results were reported for when either ear was dry and when both ears were dry. For this review we have used the 'both ears' results. | |
| Unclear | Results reported by ear | "Dry ear" | Unclear | 1 to 2 weeks: 1 week 2 to 4 weeks: 2 weeks | Results not used as it was not possible to account for correlation between ears due to bilateral disease | |
| Person | Person | "Clinical cure" defined as a score of < 3 on a symptom scale1 | Unclear | 1 to 2 weeks: 14 days | — | |
| 1Symptom scale; tinnitus: absent (0), mild (1), moderate (2), severe (3); amount of discharge: absent (0), mild (1), moderate (2), severe (3); type of discharge: absent (0), mucoid (1), mucopurulent (2), purulent (3). Sum scores in each category to give range of 0 to 9. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Aminoglycosides | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.72, 1.08] |
| 1.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Quinolone vs acetic acid | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.71, 5.04] |
| 1.3 Ear pain, discomfort, irritation Show forest plot | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.34] |
| 1.3.1 Quinolones | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.96] |
| 1.3.2 Aminoglycosides | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Ototoxicity Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Quinolones | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.48, 2.35] |
| 3.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Quinolones | 2 | 488 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.07, 1.49] |
| 3.3 Ear pain, discomfort, irritation Show forest plot | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 3.3.1 Quinolones | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 3.4 Change in hearing Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Quinolone | 1 | 390 | Mean Difference (IV, Fixed, 95% CI) | 2.79 [0.48, 5.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Quinolones | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 4.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Quinolone | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.81, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Quinolone | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 5.2 Resolution of ear discharge (after 4 weeks) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.90] |
| 5.2.1 Quinolone | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.90] |

