Antibiotics versus topical antiseptics for chronic suppurative otitis media
Information
- DOI:
- https://doi.org/10.1002/14651858.CD013056.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 05 January 2020see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane ENT Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Karen Head: scoped the review, and designed and wrote the protocol. Screened the search results and selected studies, carried out data extraction, 'Risk of bias' assessment and statistical analyses, and wrote the text of the review.
Lee Yee Chong: scoped the review, and designed and wrote the protocol. Screened the search results and selected studies, carried out data extraction, 'Risk of bias' assessment and statistical analyses, and reviewed and edited the text of the review.
Mahmood F Bhutta: helped to scope, design and write the protocol; reviewed the analyses of results and provided clinical guidance at all stages of the review. Reviewed and edited the text of the review.
Peter S Morris: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review.
Shyan Vijayasekaran: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review.
Martin J Burton: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review. Wrote the abstract for the review.
Anne GM Schilder: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review.
Christopher G Brennan‐Jones: helped to scope, design and write the protocol; clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT
-
NHMRC Centre of Research Excellence in Ear and Hearing Health of Aboriginal and Torres Strait Islander Children, Australia
Declarations of interest
Karen Head: none known.
Lee Yee Chong: none known.
Mahmood F Bhutta: Mahmood Bhutta has received an honorarium from Novus Therapeutics for advice on an experimental treatment for otitis media (not related to any treatment in this review).
Peter S Morris: Peter Morris has contributed to an Expert Advisory Group on chronic suppurative otitis media and conjugate pneumococcal vaccines in Australia for GlaxoSmithKline. He has also been a Chief Investigator on project grants from National Health and Medical Research Council of Australia addressing treatments for chronic suppurative otitis media.
Shyan Vijayasekaran: none known.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported in part by the National Institute of Health Research (NIHR) University College London Hospitals Biomedical Research Centre. The research is funded by the NIHR and EU Horizon2020. She is the national chair of the NIHR Clinical Research Network ENT Specialty. She is the Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she acts as an advisor on clinical trial design and delivery to a range of biotech companies, most currently Novus Therapeutics.
Christopher G Brennan‐Jones: none known.
Acknowledgements
This project was funded by the NHMRC Centre of Research Excellence in Ear and Hearing Health of Aboriginal and Torres Strait Islander Children (NHMRC CRE_ICHEAR). The contents of the publications arising from this work are solely the responsibility of the authors and do not reflect the views of NHMRC.
We are grateful to Mr Iain Swan for peer reviewing this protocol, and to consumer referee Joan Blakely for her helpful comments. We would also like to thank Dr. Adrian James, as Acting Co‐ordinating Editor for Cochrane ENT, for his insightful comments and advice, and the other members of the Cochrane ENT editorial board for their input and encouragement.
We would like to sincerely thank Jenny Bellorini and Samantha Cox from the Cochrane ENT team for their invaluable help, which has enabled the completion of this suite of reviews, and Jessica Daw for assisting with the preparation and collation of the final reviews.
We would also like to thank the following clinicians, scientists and consumers who provided comments on the initial scoping review and prioritisation exercise for this suite of reviews into CSOM: Amanda Leach, Chris Perry, Courtney McMahen, De Wet Swanepoel, Deborah Lehmann, Eka Dian Safitri, Francis Lannigan, Harvey Coates, Has Gunasekera, Ian Williamson, Jenny Reath, Kathy Brooker, Kathy Currie, Kelvin Kong, Matthew Brown, Pavanee Intakorn, Penny Abbot, Samantha Harkus, Sharon Weeks, Shelly Chadha, Stephen O'Leary, Victoria Stroud and Yupitri Pitoyo.
We are indebted to Therese Dalsbø, Artur Gevorgyan, Nathan Gonik, Anna Kashchuk, Esther Martin, Stefano Morettini, Jussi Mustonen, Irina Telegina, Yu‐Tian Xiao, Ibrahim Ethem Yayali, Francine Choi, Chiara Arienti, Maria Paula Garcia, Karen Sagomonyants and Elizabeth Weeda for translating and identifying primary studies for inclusion or exclusion for this suite of reviews.
We thank Carolyn McFadyen for her help and support in providing documents from the previous Cochrane Reviews.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jan 05 | Antibiotics versus topical antiseptics for chronic suppurative otitis media | Review | Karen Head, Lee-Yee Chong, Mahmood F Bhutta, Peter S Morris, Shyan Vijayasekaran, Martin J Burton, Anne GM Schilder, Christopher G Brennan-Jones | |
| 2018 Jun 22 | Antibiotics versus topical antiseptics for chronic suppurative otitis media | Protocol | Karen Head, Lee‐Yee Chong, Mahmood F Bhutta, Peter S Morris, Shyan Vijayasekaran, Martin J Burton, Anne GM Schilder, Christopher G Brennan‐Jones | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs
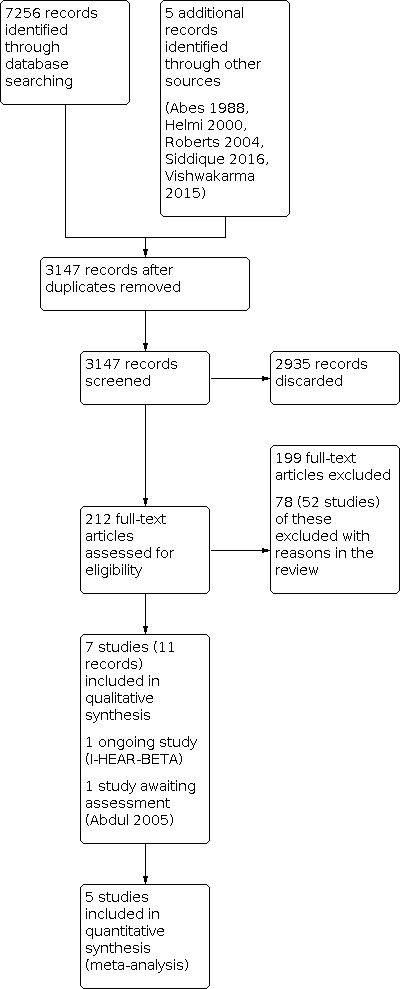
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Topical antibiotics versus acetic acid, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 1: Topical antibiotics versus acetic acid, Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 1: Topical antibiotics versus acetic acid, Outcome 3: Ear pain, discomfort, irritation

Comparison 2: Topical antibiotics versus aluminium acetate, Outcome 1: Ototoxicity

Comparison 3: Topical antibiotics versus boric acid, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 3: Topical antibiotics versus boric acid, Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 3: Topical antibiotics versus boric acid, Outcome 3: Ear pain, discomfort, irritation

Comparison 3: Topical antibiotics versus boric acid, Outcome 4: Change in hearing

Comparison 4: Topical antibiotics versus povidone‐iodine, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 4: Topical antibiotics versus povidone‐iodine, Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 5: Topical and systemic antibiotics versus acetic acid, Outcome 1: Resolution of ear discharge (2 to 4 weeks)

Comparison 5: Topical and systemic antibiotics versus acetic acid, Outcome 2: Resolution of ear discharge (after 4 weeks)
| Topical antibiotics compared to acetic acid for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) Aminoglycosides | 100 | RR 0.88 | Study population | ⊕⊝⊝⊝ | It is very uncertain whether acetic acid is more effective at resolving ear discharge compared with topical aminoglycoside antibiotics at 14 days | ||
| 84.0% | 73.9% | 10.1% fewer | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study reported this outcome |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study reported this outcome |
| Ear pain, discomfort, irritation | 189 | RR 0.16 | Study population | ⊕⊝⊝⊝ | Acetic acid may cause more ear pain, discomfort and/or irritation than topical antibiotics (aminoglycosides and quinolones) but we are very uncertain about the results | ||
| 5.3% | 0.9% | 4.5% fewer | |||||
| Hearing | 107 | One study reports that "audiometric tests showed no detectable overall, isolated not idiosyncratic hearing loss from any treatment". No numeric results were provided. | very low3 | It is uncertain whether there is a difference in hearing between topical quinolones and topical acetic acid | |||
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity | 100 | One study (100 participants) reported: "… none of the patients had any kind of ear damage or toxicity" | very low4 | It is uncertain if there is a difference in ototoxicity between topical aminoglycosides and topical acetic acid | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low‐certainty evidence: downgraded by one level due to study limitations (risk of bias) as the study had unclear randomisation, allocation concealment and blinding. Downgraded by one level due to indirectness as the outcome used was 'clinical cure' rather than resolution of ear discharge. Downgraded by one level due to suspected publication bias as one 'unpublished' study was identified indicating the possibility of unreported trials. 3Downgraded to very low‐certainty evidence: downgraded by two levels due to imprecision as no numeric results were provided and the result came from a small study (107 participants). Downgraded by one level due to suspected publication bias as one 'unpublished' study was identified indicating the possibility of unreported trials. 4Downgraded to very low‐certainty evidence: downgraded by one level due to study limitations (risk of bias) as the study had unclear randomisation, allocation concealment and blinding. Downgraded by one level due to imprecision as the result came from a small study (100 participants). Downgraded by one level due to suspected publication bias (one 'unpublished' study identified indicating the possibility of unreported trials). | |||||||
| Topical quinolones compared to boric acid for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical quinolones | With topical quinolones | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) Quinolones | 411 | RR 1.86 | Study population | ⊕⊕⊕⊝ | Topical quinolones are likely to increase the number of people with resolution of ear discharge at 2 weeks compared with topical boric acid | ||
| 31.9% | 59.3% | 27.4% more | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study measured resolution of ear discharge at 4 weeks |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study measured quality of life |
| Ear pain, discomfort, irritation | 510 | RR 0.56 | Study population | ⊕⊕⊝⊝ | Topical quinolones may result in less ear pain, discomfort or irritation at 4 weeks compared to topical boric acid | ||
| 11.8% | 6.6% | 5.2% fewer | |||||
| Average change in hearing from baseline | 390 | — | The mean average change in hearing from baseline without topical quinolones was 2.69 dB | The mean average change in hearing from baseline with topical quinolones was 5.42 dB | MD 2.79 dB higher | ⊕⊕⊝⊝ | Topical quinolones may result in greater improvement in mean hearing from baseline compared with topical boric acid; however this effect size may not be clinically important |
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by one level to moderate‐certainty evidence due to suspected publication bias. We identified two unpublished studies comparing antibiotics and antiseptics, which indicates that there may be more unpublished studies. | |||||||
| Topical antibiotics compared to povidone‐iodine for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media | |||||||
| Outcomes | Number of participants (studies) | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge (1 to 2 weeks) | 39 | RR 1.02 | Study population | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the resolution of ear discharge at 2 weeks between topical antibiotics and topical povidone‐iodine | ||
| 88.9% | 90.7% | 1.8% more | |||||
| Resolution of ear discharge (after 4 weeks) ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Quality of life ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Ear pain, discomfort, irritation ‐ not measured | — | — | — | — | — | — | No study measured this outcome |
| Hearing | 40 (1 RCT) | "There was no deterioration of hearing as assessed by pure tone audiometry" | very low2 | — | |||
| Serious complications ‐ not measured | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity | 40 | "No patient developed allergic manifestations or ototoxic effects" | very low3 | — | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low‐certainty evidence. Downgraded by one level due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting). Downgraded by one level due to imprecision (small study size: 39 participants, confidence interval crosses the line of minimally clinical important difference). Downgraded by one level due to suspected publication bias (one study referred to long‐term results that appear to be unpublished and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). 2Downgraded to very low‐certainty evidence. Downgraded by one level due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting). Downgraded by two levels due to imprecision (no numeric results were presented and very small study size (39 participants)). Downgraded by one level due to suspected publication bias (one study referred to long‐term results, which appear to be unpublished, and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). 3Downgraded to very low‐certainty evidence. Downgraded by two levels due to risk of bias (uncertain randomisation, allocation concealment and possibility of selective reporting as it is unclear how the outcome was defined). Downgraded by two levels due to imprecision (no numeric results were presented and very small study size (39 participants). Downgraded by one level due to suspected publication bias (one study referred to long‐term results that appear to be unpublished and we identified one abstract that appeared to be relevant to this comparison but for which no paper was obtainable). | |||||||
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
|---|---|---|---|---|---|
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
| CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018a). CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a). CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b). CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018b). CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a). CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b). CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018). | |||||
| Class of antibiotics | Examples | Route of administration |
|---|---|---|
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
| Antiseptic agent used aurally | Target and mechanism of action |
|---|---|
| Rubbing alcohol (ethanol, isopropanol) | Penetrating agents that cause loss of cellular membrane function, leading to release of intracellular components, denaturing of proteins, and inhibition of DNA, RNA, protein and peptidoglycan synthesis. |
| Povidone‐iodine | Highly active oxidising agents that destroy cellular activity of proteins. Disrupts oxidative phosphorylation and membrane‐associated activities. Iodine reacts with cysteine and methionine thiol groups, nucleotides and fatty acids, resulting in cell death. |
| Chlorhexidine | Membrane‐active agents that damage cell wall and outer membrane, resulting in collapse of membrane potential and intracellular leakage. Enhanced passive diffusion mediates further uptake, causing coagulation of cytosol. |
| Hydrogen peroxide | Produces hydroxyl free radicals that function as oxidants, which react with lipids, proteins and DNA. Sulfhydryl groups and double bonds are targeted in particular, thus increasing cell permeability. |
| Boric acid | It is likely that the change in the pH media of the ear canal interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. |
| Aluminium acetate/acetic acid | Acetic acid changes the pH media of the ear canal and interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. Aluminium acetate is an astringent that helps reduce itching, stinging and inflammation. |
| Sources: Gupta 2015; McDonnell 1999; Sheldon 2005. | |
| Ref ID (no. participants) | Setting | Population | Antibiotic | Topical antiseptic | Treatment | Follow‐up | Background treatment | Notes |
|---|---|---|---|---|---|---|---|---|
| Topical antibiotics versus acetic acid | ||||||||
| (159 participants) | South Africa, city (secondary care) | Patients with otorrhoea because of active mucosal COM Age over 6 years (90% between 20 and 34 years) | Ciprofloxacin, ear drops, (no concentration), 6 drops/8 hours | 1% acetic acid 6 drops/12 hours | 4 weeks | Up to 8 weeks | Aural cleaning at 1st visit | Part of a 3‐arm trial; third arm used boric acid (see below) |
| (58 participants in relevant arms) | Malawi (community setting) | CSOM (no details) "Children" ‐ no age information provided | 0.3% ofloxacin 3 drops/8 hours | 2% acetic acid in spirit 25% and glycerine 30% 3 drops/8 hours | 2 weeks | 8 weeks | Suction cleaning at the start of trial, at 1‐week and 2‐week follow‐up | Part of a 3‐arm trial; third arm used topical antiseptics + steroids |
| Neomycin 0.5%/polymixin B 0.1%, 3 drops/8 hours | ||||||||
| (100 participants) | India (secondary care) | Tubotympanic (safe) type of CSOM Mean age 69 years (range: 10 to 60 years) | Gentamicin (0.3%), ear drops, 3 drops every 8 hours | Acetic acid (1.5%), ear drops, 3 drops every 8 hours | 2 weeks | 2 weeks | None listed | Resolution of ear discharge measured as symptom score |
| Topical antibiotics versus aluminium acetate (Burow's solution) | ||||||||
| (51 participants, 60 ears) | Israel (ENT outpatient clinic) | Chronic otitis media Mean: 44.4 years (range 18 to 73 years) | Ciprofloxacin (no concentration), 15 drops per day | 1% aluminium acetate solution 5 drops/8 hours | 3 weeks | 3 weeks | None mentioned | Randomisation by ear Not possible to use results 3‐arm trial |
| Tobramycin (no concentration), 15 drops per day | ||||||||
| Topical antibiotics versus boric acid | ||||||||
| (159 participants) | South Africa, city (secondary care) | Patients with otorrhoea because of active mucosal COM Age over 6 years (90% between 20 and 34 years) | Ciprofloxacin, ear drops, (no concentration), 6 drops/8 hours | Boric acid powder Single administration | 4 weeks (antibiotics) | Up to 8 weeks | Aural cleaning at 1st visit | Part of a 3‐arm trial; third arm used acetic acid (see above) |
| Macfadyen 2005 (427 participants) | Kenya, rural (community, school setting) | Children (aged over 5 years) with CSOM Mean age 11.1 ± 3.15 years | 0.3% ciprofloxacin, ear drops, no volume given every 12 hours | 2% boric acid in 45% alcohol, ear drops, no volume given every 12 hours | School days only for 2 weeks | 4 weeks | Daily dry mopping before application | — |
| Topical antibiotics versus povidone‐iodine | ||||||||
| Jaya 2003 (40 participants) | India, city (ENT outpatient clinic) | Actively discharging CSOM with moderate to large central perforation Age over 10 years (50% between 21 to 21 to 40) | Ciprofloxacin 0.3% ear drops, 3 drops 3 times daily | Povidone‐iodine 5% solution, 3 drops 3 times daily | 10 days | 4 weeks | Suction cleaning before trial and then daily dry mopping | — |
| Systemic and topical antibiotics versus acetic acid and aural toileting | ||||||||
| (100 participants) | India (secondary care) | CSOM Mean age: 36.4 years (range: 6 to 72 years) | Topical ciprofloxacin (no concentration/volume) daily for 3 months, plus oral ciprofloxacin, 500 mg twice daily for 15 days | Diluted acetic acid (2 mL) daily. Every second day this was completed at the hospital with suction ear cleaning. Continued until no further discharge | See details for each treatment arm | 3 months | Dry mopping at 1st visit | — |
| Reference | Unit of randomisation | Discharge results reported by | Definition | Otoscopically confirmed? | Time points | Notes |
|---|---|---|---|---|---|---|
| Ear | Ear | "Clinical success" defined as cessation of otorrhoea and eradication of the micro‐organisms in the post‐treatment culture | Unclear | 2 to 4 weeks: 21 days | Not possible to use these results as randomisation by ear (9/51 patients had bilateral disease) | |
| Person | Person | "Absence of discharge" | Otoscopically confirmed | 2 to 4 weeks: 15 days After 4 weeks: 1 month | — | |
| Person | Person | "Inactive" ear | Microscopic examination | 1 to 2 weeks: 2 weeks 2 to 4 weeks: 4 weeks | — | |
| Person | Person | "Inactive" ear (dry) | Otoscopically confirmed | 2 to 4 weeks: 4 weeks | Also measured patient satisfaction, which asked patients whether their ears were 'completely dry', 'better but not completely dry', 'no better, still running' | |
| Person | Both by person | Resolution of aural discharge | Otoscopically confirmed | 1 to 2 weeks: 2 weeks 2 to 4 weeks: 4 weeks | For bilateral disease results were reported for when either ear was dry and when both ears were dry. For this review we have used the 'both ears' results. | |
| Unclear | Results reported by ear | "Dry ear" | Unclear | 1 to 2 weeks: 1 week 2 to 4 weeks: 2 weeks | Results not used as it was not possible to account for correlation between ears due to bilateral disease | |
| Person | Person | "Clinical cure" defined as a score of < 3 on a symptom scale1 | Unclear | 1 to 2 weeks: 14 days | — | |
| 1Symptom scale; tinnitus: absent (0), mild (1), moderate (2), severe (3); amount of discharge: absent (0), mild (1), moderate (2), severe (3); type of discharge: absent (0), mucoid (1), mucopurulent (2), purulent (3). Sum scores in each category to give range of 0 to 9. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Aminoglycosides | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.72, 1.08] |
| 1.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Quinolone vs acetic acid | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.71, 5.04] |
| 1.3 Ear pain, discomfort, irritation Show forest plot | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.34] |
| 1.3.1 Quinolones | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.96] |
| 1.3.2 Aminoglycosides | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Ototoxicity Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Quinolones | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.48, 2.35] |
| 3.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Quinolones | 2 | 488 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.07, 1.49] |
| 3.3 Ear pain, discomfort, irritation Show forest plot | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 3.3.1 Quinolones | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 3.4 Change in hearing Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Quinolone | 1 | 390 | Mean Difference (IV, Fixed, 95% CI) | 2.79 [0.48, 5.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Quinolones | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 4.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Quinolone | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.81, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Quinolone | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 5.2 Resolution of ear discharge (after 4 weeks) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.90] |
| 5.2.1 Quinolone | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.90] |

