Systemic antibiotics for chronic suppurative otitis media
Abstract
Background
Chronic suppurative otitis media (CSOM) is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane. The predominant symptoms of CSOM are ear discharge and hearing loss.
Systemic antibiotics are a commonly used treatment option for CSOM, which act to kill or inhibit the growth of micro‐organisms that may be responsible for the infection. Antibiotics can be used alone or in addition to other treatments for CSOM.
Objectives
To assess the effects of systemic antibiotics for people with CSOM.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL via the Cochrane Register of Studies); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 16 March 2020.
Selection criteria
We included randomised controlled trials comparing systemic antibiotics (oral, injection) against placebo/no treatment or other systemic antibiotics with at least a one‐week follow‐up period, involving patients with chronic (at least two weeks) ear discharge of unknown cause or due to CSOM. Other treatments were allowed if both treatment and control arms also received it.
Data collection and analysis
We used the standard Cochrane methodological procedures. We used GRADE to assess the certainty of the evidence for each outcome.
Our primary outcomes were: resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not, measured at between one week and up to two weeks, two weeks to up to four weeks, and after four weeks); health‐related quality of life using a validated instrument; ear pain (otalgia) or discomfort or local irritation. Secondary outcomes included hearing, serious complications and ototoxicity measured in several ways.
Main results
We included 18 studies (2135 participants) with unclear or high risk of bias.
1. Systemic antibiotics versus no treatment/placebo
It is very uncertain if there is a difference between systemic (intravenous) antibiotics and placebo in the resolution of ear discharge at between one and two weeks (risk ratio (RR) 8.47, 95% confidence interval (CI) 1.88 to 38.21; 33 participants; 1 study; very low‐certainty evidence). The study did not report results for resolution of ear discharge after two weeks. Health‐related quality of life was not reported. The evidence is very uncertain for hearing and serious (intracranial) complications. Ear pain and suspected ototoxicity were not reported.
2. Systemic antibiotics versus no treatment/placebo (both study arms received topical antibiotics)
Six studies were included of which five presented useable data. There may be little or no difference in the resolution of ear discharge at between one to two weeks for oral ciprofloxacin compared to placebo or no treatment when ciprofloxacin ear drops were used in both intervention arms (RR 1.02, 95% CI 0.93 to 1.12; 390 participants; low‐certainty evidence). No results after two weeks were reported. Health‐related quality of life was not reported. The evidence is very uncertain for ear pain, serious complications and suspected ototoxicity.
3. Systemic antibiotics versus no treatment/placebo (both study arms received other background treatments)
Two studies used topical antibiotics plus steroids as background treatment in both arms. It is very uncertain if there is a difference in resolution of ear discharge between metronidazole and placebo at four weeks (RR 0.91, 95% CI 0.51 to 1.65; 40 participants; 1 study; very low‐certainty evidence). This study did not report other outcomes. It is also very uncertain if resolution of ear discharge at six weeks was improved with co‐trimoxazole compared to placebo (RR 1.54, 95% CI 1.09 to 2.16; 98 participants; 1 study; very low‐certainty evidence). Resolution of ear discharge was not reported at other time points. From the narrative report there was no evidence of a difference between groups for health‐related quality of life, hearing or serious complications (very low‐certainty evidence).
One study (136 participants) used topical antiseptics as background treatment in both arms and found similar resolution of ear discharge between the amoxicillin and no treatment groups at three to four months (RR 1.03, 95% CI 0.75 to 1.41; 136 participants; 1 study; very low‐certainty evidence). The narrative report indicated no evidence of differences in hearing or suspected ototoxicity (both very low‐certainty evidence). No other outcomes were reported.
4. Different types of systemic antibiotics
This is a summary of four comparisons, where different antibiotics were compared to each other. Eight studies compared different types of systemic antibiotics against each other: quinolones against beta‐lactams (four studies), lincosamides against nitroimidazoles (one study) and comparisons of different types of beta‐lactams (three studies). It was not possible to conclude if there was one class or type of systemic antibiotic that was better in terms of resolution of ear discharge. The studies did not report adverse events well.
Authors' conclusions
There was a limited amount of evidence available to examine whether systemic antibiotics are effective in achieving resolution of ear discharge for people with CSOM. When used alone (with or without aural toileting), we are very uncertain if systemic antibiotics are more effective than placebo or no treatment. When added to an effective intervention such as topical antibiotics, there seems to be little or no difference in resolution of ear discharge (low‐certainty evidence). Data were only available for certain classes of antibiotics and it is very uncertain whether one class of systemic antibiotic may be more effective than another. Adverse effects of systemic antibiotics were poorly reported in the studies included. As we found very sparse evidence for their efficacy, the possibility of adverse events may detract from their use for CSOM.
PICO
Plain language summary
Benefits and risks of antibiotics taken orally or given as an injection to treat chronic suppurative otitis media (persistent or recurring ear infection with discharge)
Why is this question important?
Chronic suppurative otitis media (CSOM), also known as chronic otitis media (COM), is an inflammation and infection of the middle ear that lasts for two weeks or more. People with CSOM usually experience recurrent or persistent discharge – fluid that leaks out from a hole or tear in the eardrum – and hearing loss.
CSOM can be treated with antibiotics (medicines that fight bacterial infections) taken orally or given as an injection (i.e. systemic treatment in which the whole body is treated). Systemic antibiotics can be used:
‐ alone;
‐ in combination with antibiotics in the form of drops, sprays, ointments or creams (topical, i.e. localised surface treatment); or
‐ in combination with other treatments such as steroids (anti‐inflammation medicines) or antiseptics (substances that stop or slow down the growth of micro‐organisms).
To find out how effective systemic antibiotics are for treating CSOM, and whether they lead to side effects, we reviewed the evidence from research studies.
How did we identify and evaluate the evidence?
First, we searched the medical literature for studies that followed people with CSOM for at least one week and compared:
‐ a systemic antibiotic used alone against a placebo (dummy) treatment, no treatment or another systemic antibiotic;
‐ a systemic antibiotic combined with another treatment, against that treatment alone.
We then compared the results, and summarised the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find?
We found 18 studies that involved a total of 2135 people with CSOM. People were treated for between five days and 12 weeks, and were followed for up to one year. Four studies provided information about how they were funded or who supplied the medicines: two were publicly funded, and medicines were provided by pharmaceutical companies in the other two studies.
Studies compared:
‐ systemic antibiotics against no treatment (one study);
‐ systemic antibiotics plus topical antibiotics against topical antibiotics alone (six studies);
‐ systemic antibiotics plus other treatments (other than topical antibiotics alone), against these same treatments without systemic antibiotics (four studies);
‐ different systemic antibiotics against one another (eight studies).
Systemic antibiotics alone against no treatment
We cannot determine from the only study we found whether systemic antibiotics alone are better or worse than no treatment. This is mainly because the study:
‐ was small;
‐ was conducted in ways that could have introduced error in the results; and
‐ reported limited information.
Systemic antibiotics plus topical antibiotics against topical antibiotics alone
Systemic antibiotics plus topical antibiotics may have little to no effect on whether discharge stops after one to two weeks, compared against topical antibiotics alone (five studies). We do not know if systemic antibiotics added to topical antibiotics have any other positive or negative effects, because:
‐ there are too few studies;
‐ available studies were small and may have been conducted in ways that introduce error in their results.
Systemic antibiotics plus other treatments (other than topical antibiotics alone), against these same treatments without systemic antibiotics
We cannot determine from the evidence available whether systemic antibiotics are effective or lead to adverse events when added to treatments other than topical antibiotics only. This is mainly because the few studies available reported limited information.
Comparisons between different systemic antibiotics
We do not know whether some systemic antibiotics are better than others. This is mainly because the way studies were conducted is likely to have introduced error in their results.
What does this mean?
There is insufficient robust evidence to determine whether systemic antibiotics are effective treatments for CSOM, and whether they lead to side effects. Evidence about side effects is particularly limited. When added to topical antibiotics, systemic antibiotics may make little to no difference to whether discharge resolves after one to two weeks. We do not know if some systemic antibiotics are better than others.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to March 2020.
Authors' conclusions
Summary of findings
| Systemic antibiotics compared to no treatment/placebo | |||||||
| Patient or population: children with CSOM | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without systemic antibiotics | With systemic antibiotics | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Assessed with: unclear if otoscopically confirmed | RR 8.47 | 33 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of systemic antibiotics (mezlocillin or ceftazidime) on the resolution of ear discharge at one to two weeks, as compared to placebo. | ||
| 8.3% | 70.6% | 62.3% more | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study reported this outcome at this time point. | — | — | ||||
| Health‐related quality of life | No study reported this outcome. | — | — | ||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | — | — | ||||
| Hearing Assessed with: air conduction and bone conduction | — | 33 | Fliss 1990 reported measuring air conduction thresholds at 0.25 kHz, 0.5 kHz, 1 kHz, 2 kHz, 4 kHz and 8 kHz, and bone conduction thresholds at 0.5 kHz, 1 kHz and 2 kHz. The time at which these were performed is not described. The authors state that "audiometric tests did not show any worsening of the hearing during or after the antimicrobial treatment". | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of systemic antibiotics (mezlocillin or ceftazidime) on hearing. | ||
| Serious complications ‐ during 6 months of follow‐up Assessed with: unclear | — | 33 | Fliss 1990 reported that no intracranial complications occurred during the study or during the follow‐up period. | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of systemic antibiotics (mezlocillin or ceftazidime) on serious complications. | ||
| Suspected ototoxicity | No study reported this outcome. | — | — | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to risk of bias (unclear methods for randomisation, allocation concealment or blinding, and placebo arm of the trial discontinued early due to lack of effect). 2Downgraded by two levels due to imprecision (very small study with only 33 participants resulting in wide confidence intervals). 3Downgraded by one level for indirectness (single study including children who were hospitalised, who did not respond to an intensive aural toileting regimen. This study involves inpatient use of mezlocillin and ceftazidime, which are broad‐spectrum antibiotics only administered parenterally (intramuscular or intravenous). It is unclear if this finding is applicable to other more commonly available antibiotics). 4Downgraded by two levels due to imprecision (very small study and numerical results were not reported for this outcome). | |||||||
| Systemic antibiotics compared to no treatment or placebo on top of topical antibiotics for CSOM | |||||||
| Patient or population: people (of any age) with CSOM | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without systemic antibiotics | With systemic antibiotics | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Assessed with: 3 RCTs otoscopically confirmed, 2 RCTs unclear | RR 1.02 | 390 | Study population | ⊕⊕⊝⊝ | The evidence suggests that adding systemic ciprofloxacin to topical antibiotics may result in little to no difference in the resolution of ear discharge at 1 to 2 weeks, when compared to topical antibiotics alone. | ||
| 76.9% | 78.5% | 1.5% more | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study reported this outcome at this time point. | — | — | ||||
| Health‐related quality of life | No study reported this outcome. | — | — | ||||
| Ear pain (otalgia) or discomfort or local irritation ‐ measured at 1 week Assessed with: self‐reported | RR 1.00 | 100 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on ear pain, discomfort or local irritation. | ||
| 6.0% | 6.0% | 0.0% fewer | |||||
| Hearing | No study reported this outcome. | — | — | ||||
| Serious complications ‐ measured at 19 to 24 days Assessed with: unclear | — | 40 | One study reported that "no side effect was recorded in any patient..." but no further information was provided. | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on serious complications. | ||
| Suspected ototoxicity ‐ measured at 10 days to 3 weeks Assessed with: unclear | RR 3.00 | 250 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on ototoxicity. | ||
| 0.8% | 2.4% | 1.6% more | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to risk of bias: four studies did not provide adequate information about randomisation or allocation concealment. Four studies did not provide any information about blinding of participants and personnel. One study was at high risk of missing outcome data, and two studies were at high risk of selective reporting. 2Downgraded by two levels due to risk of bias. Study did not provide any details on the method for random sequence generation, or allocation concealment. It was unclear if the methods for blinding were adequate, and no published study protocol was identified. 3Downgraded by one level due to imprecision (single study with small sample size and wide confidence interval). 4Downgraded to very low‐certainty evidence: downgraded by two levels due to risk of bias (study was judged to be at high risk of bias for randomisation, blinding and selective outcome reporting; study was at unclear risk of bias for allocation concealment); downgraded by one level due to imprecision (small sample size). 5Downgraded by one level due to risk of bias. Insufficient information was provided about randomisation and allocation concealment. One study was at high risk of performance and two studies were at high risk of detection bias. There was an unclear risk of bias from selective reporting as no published protocols were identified for the studies. 6Downgraded by two levels due to imprecision: wide confidence intervals in the effect estimate and a low event rate with a small sample size. | |||||||
| Systemic antibiotics compared to no treatment or placebo on top of topical antibiotics for CSOM | |||||||
| Patient or population: people with CSOM; one study included patients of unknown age; one study included children with CSOM who had not responded to initial antibiotic treatment Setting: unknown setting or tertiary care centres in the UK and the Netherlands | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without systemic antibiotics | With systemic antibiotics | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks | No study reported this outcome. | — | — | ||||
| Resolution of ear discharge ‐ measured after 4 weeks (4 weeks) (Metronidazole plus gentamicin‐steroid drops compared to gentamicin‐steroid alone) Assessed with: unclear if otoscopically confirmed | RR 0.91 | 30 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding metronidazole to gentamicin plus hydrocortisone ear drops on resolution of ear discharge after 4 weeks. | ||
| 62.5% | 56.9% | 5.6% fewer (30.6 fewer to 40.6 more) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks (6 weeks) (Oral trimethoprim/sulfamethoxazole plus topical antibiotics and steroid ear drops compared to topical antibiotic and steroid ear drops alone) Assessed with: otoscopically confirmed | RR 1.54 (1.09 to 2.16) | 98 (1 RCT) | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding oral trimethoprim/sulfamethoxazole to topical antibiotic and steroid ear drops on resolution of ear discharge after 4 weeks. | ||
| 47.1% | 72.5% (51.3 to 100%) | 25.4% more (4.2% more to 54.6% more) | |||||
| Health‐related quality of life ‐ unclear follow‐up period (Oral trimethoprim/sulfamethoxazole plus topical antibiotics and steroid ear drops compared to topical antibiotic and steroid ear drops alone) Assessed with the 6‐item otitis media questionnaire, Child Health Questionnaire and a visual analogue scale measuring ear‐related quality of life | — | 101 (1 RCT) | Results were only reported as a narrative summary. The authors stated that "during the study, the health‐related quality‐of‐life scores improved substantially in both the trimethoprim/ sulfamethoxazole and placebo groups [...]. Mean scores for the trimethoprim/ sulfamethoxazole and placebo groups for the 6‐item otitis media questionnaire, Child Health Questionnaire, and visual analog scale were the same at all visits." | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding oral trimethoprim/sulfamethoxazole to topical antibiotic and steroid ear drops on health‐related quality of life. | ||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | — | — | ||||
| Hearing ‐ unclear follow‐up period (Oral trimethoprim/sulfamethoxazole plus topical antibiotics and steroid ear drops compared to topical antibiotic and steroid ear drops alone) Assessed with pure‐tone air conduction levels at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz | — | 38 (1 RCT) | Results were only reported as a narrative summary. The authors stated "Pure‐tone air conduction levels at 500, 1000, 2000, and 4000 Hz could be determined for 20 children in the trimethoprim/ sulfamethoxazole group and 18 children in the placebo group. Although hearing levels generally improved, no differences between the groups were found" | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding oral trimethoprim/sulfamethoxazole to topical antibiotic and steroid ear drops on hearing. | ||
| Serious complications ‐ mastoid abscess measured at 12 weeks Assessed with: unclear | RR 1.02 | 101 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on ototoxicity. | ||
| 2.0% | 2.0% | 0.0% fewer (1.8 fewer to 29.1 more) | |||||
| Suspected ototoxicity | No study reported this outcome. | — | — | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to risk of bias: the study was only presented as an abstract and did not present sufficient information to assess the risk of bias across any of the study characteristics. Downgraded by two levels for imprecision: the results are from one small study (30 participants) and the confidence intervals are wide. 2Downgraded by one level due to imprecision: the results are from one small study (98 participants). 3 Downgraded by two levels for indirectness: the results are from one study conducted in children with recalcitrant CSOM of which 60% had grommets in place at the start of the study. This may not represent the target population. 4Downgraded by two levels due to imprecision: the results are only reported as a narrative and come from one small study (101 participants). 5Downgraded by two levels due to imprecision: the results are only from one small study (101 participants) with one event in each arm resulting in very wide confidence intervals. | |||||||
| Topical antiseptic compared to no treatment for chronic suppurative otitis media | |||||||
| Patient or population: schoolchildren with CSOM Setting: community setting, Malawi | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antiseptic | With topical antiseptic | Difference | |||||
| Resolution of ear discharge ‐ measured between 1 week and up to 2 weeks | No study reported this outcome. | — | |||||
| Resolution of ear discharge (4 weeks or more) ‐ measured at 3 to 4 months Assessed with: otoscopically confirmed | RR 1.03 | 136 (1 RCT) | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding systemic amoxicillin to boric acid eardrops and dry mopping on resolution of ear discharge after 4 weeks. | ||
| 54.5% | 56.2% | 1.6% more | |||||
| Health‐related quality of life | No study reported this outcome. | — | |||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | — | |||||
| Hearing ‐ measured at 3 to 4 months Assessed with: pure tone audiometry | — | 204 (1 RCT) | Results were only presented as a narrative summary. The authors state "hearing test performed before and after treatment showed that the hearing thresholds were the same and in many cases even better after the treatment" | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding systemic amoxicillin to boric acid eardrops and dry mopping on hearing. | ||
| Serious complications | No study reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity | — | 204 (1 RCT) | Results were only presented as a narrative summary. The authors state that "no signs of ototoxicity could be found." | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding systemic amoxicillin to boric acid eardrops and dry mopping on suspected ototoxicity. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to study limitations (risk of bias) because of concerns about randomisation, blinding, attrition bias and selective reporting. Downgraded by one level due to imprecision as there was one small study (136 participants) with wide confidence intervals. 2Downgraded by two levels due to study limitations (risk of bias) because of concerns about randomisation, blinding, attrition bias and selective reporting. Downgraded by two levels due to imprecision as results come from one small study (204 participants) and numeric results were not presented for this outcome. | |||||||
Background
This is one of a suite of Cochrane Reviews evaluating the comparative effectiveness of non‐surgical interventions for CSOM using topical antibiotics, topical antibiotics with corticosteroids, systemic antibiotics, topical antiseptics and aural toileting (ear cleaning) methods (Table 1).
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
|---|---|---|---|---|---|
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2020a).
CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a).
CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b).
CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2020b).
CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2020a).
CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2020b).
CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2020).
This review compares the effectiveness of systemic antibiotics against a placebo or no treatment, or against another systemic antibiotic, for CSOM.
Description of the condition
Chronic suppurative otitis media (CSOM), which is also often referred to as chronic otitis media (COM), is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane.
The predominant symptoms of CSOM are ear discharge and hearing loss. Ear discharge can be persistent or intermittent, and many sufferers find it socially embarrassing (Orji 2013). Some patients also experience discomfort or earache. Most patients with CSOM experience temporary or permanent hearing loss with average hearing levels typically between 10 and 40 decibels (Jensen 2013). The hearing loss can be disabling, and it can have an impact on speech and language skills, employment prospects, and on children's psychosocial and cognitive development, including academic performance (Elemraid 2010; Olatoke 2008; WHO 2004). Consequently, quality of life can be affected. CSOM can also progress to serious complications in rare cases (and more often when cholesteatoma is present): both extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) have been reported (Dubey 2007; Yorgancılar 2013).
CSOM is estimated to have a global incidence of 31 million episodes per year, or 4.8 new episodes per 1000 people (all ages), with 22% of cases affecting children under five years of age (Monasta 2012; Schilder 2016). The prevalence of CSOM varies widely between countries, but it disproportionately affects people at socio‐economic disadvantage. It is rare in high‐income countries, but common in many low‐ and middle‐income countries (Mahadevan 2012; Monasta 2012; Schilder 2016; WHO 2004).
Definition of disease
There is no universally accepted definition of CSOM. Some define CSOM in patients with a duration of otorrhoea of more than two weeks but others may consider this an insufficient duration, preferring a minimum duration of six weeks or more than three months (Verhoeff 2006). Some include diseases of the tympanic membrane within the definition of CSOM, such as tympanic perforation without a history of recent ear discharge, or the disease cholesteatoma (a growth of the squamous epithelium of the tympanic membrane).
In accordance with a consensus statement, here we used CSOM only to refer to tympanic membrane perforation, with intermittent or continuous ear discharge (Gates 2002). We have used a duration of otorrhoea of two weeks as an inclusion criterion, in accordance with the definition used by the World Health Organization, but we have used subgroup analyses to explore whether this is a factor that affects observed treatment effectiveness (WHO 2004).
Many people affected by CSOM do not have good access to modern primary healthcare, let alone specialised ear and hearing care, and in such settings health workers may be unable to view the tympanic membrane to definitively diagnose CSOM. It can also be difficult to view the tympanic membrane when the ear discharge is profuse. Therefore we have also included, as a subset for analysis, studies where participants have had chronic ear discharge for at least two weeks, but where the diagnosis is unknown.
At‐risk populations
Some populations are considered to be at high risk of CSOM. There is a high prevalence of disease among Indigenous people such as the Aboriginal and Torres Strait Islander Australian, Native American and Inuit populations. This is likely due to an interplay of factors, including socio‐economic deprivation and possibly differences resulting from population genetics (Bhutta 2016). Those with primary or secondary immunodeficiency are also susceptible to CSOM. Children with craniofacial malformation (including cleft palate) or chromosomal mutations such as Down syndrome are prone to chronic non‐suppurative otitis media ('glue ear'), and by extrapolation may also be at greater risk of suppurative otitis media. The reasons for this association with craniofacial malformation are not well understood, but may include altered function of the Eustachian tube, coexistent immunodeficiency, or both. These populations may be less responsive to treatment and more likely to develop CSOM, recurrence or complications.
Children who have a grommet (ventilation tube) in the tympanic membrane to treat glue ear or recurrent acute otitis media may be more prone to develop CSOM; however, their pathway to CSOM may differ and therefore they may respond differently to treatment. Children with grommets who have chronic ear discharge meeting the CSOM criteria are therefore considered to be a separate high‐risk subgroup (van der Veen 2006).
Treatment
Treatments for CSOM may include topical antibiotics (administered into the ear) with or without steroids, systemic antibiotics (given either by mouth or by injection), topical antiseptics and ear cleaning (aural toileting), all of which can be used on their own or in various combinations. Whereas primary healthcare workers or patients themselves can deliver some treatments (for example, some aural toileting and antiseptic washouts), in most countries antibiotic therapy requires prescription by a doctor. Surgical interventions are an option in cases where complications arise or in patients who have not responded to pharmacological treatment; however, there is a range of practice in terms of the type of surgical intervention that should be considered and the timing of the intervention. In addition, access to or availability of surgical interventions is setting‐dependent. This series of Cochrane Reviews therefore focuses on non‐surgical interventions. In addition, most clinicians consider cholesteatoma to be a variant of CSOM, but acknowledge that it will not respond to non‐surgical treatment (or will only respond temporarily) (Bhutta 2011). Therefore, people with cholesteatoma are not included in these reviews.
Description of the intervention
Antibiotics are the most commonly used treatment for CSOM. They can be administered topically (as drops, ointments, sprays or creams to the affected area) or systemically (either by mouth or by injection into a vein (intravenous) or muscles (intramuscular)).
Topical application has the advantage of potentially delivering high concentrations of antibiotic to the affected area, whereas systemic antibiotics are absorbed and distributed throughout the body. However, the penetration of topical antibiotics into the middle ear may be compromised if the perforation in the tympanic membrane is small or there is copious mucopurulent discharge in the ear canal that cannot be cleaned. It may also be difficult to achieve compliance with topical dosing in young children. In these cases, systemic antibiotics may have an advantage.
How the intervention might work
CSOM is a chronic and often polymicrobial (involving more than one micro‐organism) infection of the middle ear. Broad‐spectrum antibiotics such as second‐generation quinolones and aminoglycosides, which are active against the most frequently cultured micro‐organisms (Pseudomonas aeruginosa and Staphylococcus aureus), are therefore commonly used (Mittal 2015) (Table 2). It is possible that antibiotics for CSOM that target Pseudomonas aeruginosa may have an advantage over antibiotics that do not. Dose and duration of treatment are also important factors but are less likely to affect relative effectiveness if given within the therapeutic range. Generally, treatment for at least five days is necessary and a duration of one to two weeks is sufficient to resolve uncomplicated infections. However, in some cases it may take more than two weeks for the ear to become dry and therefore longer follow‐up (more than four weeks) may be needed to monitor for recurrence of discharge.
| Class of antibiotics | Examples | Route of administration |
|---|---|---|
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
Some antibiotics (such as aminoglycosides) can be toxic to the inner ear (ototoxicity), which might be experienced as sensorineural hearing loss, dizziness or tinnitus.
Systemic antibiotics can have off‐target side effects, such as diarrhoea or nausea, and also carry a risk of systemic allergic reactions such as a skin rash. The risk or incidence of these events is not expected to be different from other common infections since the doses and duration of treatment used are similar in CSOM. A broader concern is the association of the overuse of antibiotics with increasing resistance among community‐ and hospital‐acquired pathogens.
Why it is important to do this review
Although topical antibiotics are widely recommended as the first‐line treatment for CSOM, systemic antibiotics are still used in situations where the delivery of drops to the middle ear is difficult. These include the treatment of young children and people with small perforations and/or copious ear discharge. Some antibiotics may be unsuitable for formulation as a topical ear drop so systemic antibiotics remain a viable option for the delivery of broad‐spectrum antibiotics. Evidence‐based knowledge of the effectiveness of different systemic antibiotics could help to optimise their use.
Objectives
To assess the effects of systemic antibiotics for people with chronic suppurative otitis media (CSOM).
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
-
Randomised controlled trials (including cluster‐randomised trials where the unit of randomisation is the setting or operator) and quasi‐randomised trials.
-
Patients were followed up for at least one week.
We excluded studies with the following design characteristics:
-
Cross‐over trials, because CSOM is not expected to be a stable chronic condition. Unless data from the first phase were available, we excluded such studies.
-
Studies that randomised participants by ear (within‐patient controlled) because by definition the effects of systemic interventions are not localised.
Types of participants
We included studies with patients (adults and children) who had:
-
chronic ear discharge of unknown cause; or
-
chronic suppurative otitis media.
We defined patients with chronic ear discharge as those with at least two weeks of ear discharge, where the cause of the discharge was unknown.
We defined patients with chronic suppurative otitis media (CSOM) as patients with:
-
chronic or persistent ear discharge for at least two weeks; and
-
a perforated tympanic membrane.
We did not exclude any populations based on age, risk factors (cleft palate, Down syndrome), ethnicity (e.g. Australian Aboriginal or Torres Strait Islanders) or the presence of ventilation tubes (grommets). Where available, we recorded these factors in the patient characteristics section during data extraction from the studies. If any of the included studies recruited these patients as a majority (80% or more), we analysed them in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We excluded studies where the majority (more than 50%) of participants:
-
had an alternative diagnosis to CSOM (e.g. otitis externa);
-
had underlying cholesteatoma;
-
had ear surgery within the last six weeks.
We did not include studies designed to evaluate interventions in the immediate peri‐operative period, which were focused on assessing the impact of the intervention on the surgical procedure or outcomes.
Types of interventions
Intervention
All studies with (systemic) antibiotics administered orally or parenterally (intramuscular or intravenous) were included.
We excluded studies that conduct swabs and tests for antimicrobial sensitivity and then base the choice of antibiotics for each participant on the results of the laboratory test.
Duration
At least five days of treatment with antibiotics was required, except for antibiotics where a shorter duration has been proven to be equivalent (e.g. azithromycin for systemic antibiotics).
Dose
There was no limitation on the dose or the frequency of administration.
Comparisons
The following were the comparators:
-
Placebo or no intervention (systemic antibiotic versus placebo; systemic antibiotic versus no intervention).
-
Another systemic antibiotic (systemic antibiotic A versus systemic antibiotic B).
We analysed these as three main scenarios depending on which common therapy is applied in the background:
-
Systemic antibiotics as a single treatment (main therapy): this included studies where all participants in both treatment groups either received no other treatment or only received aural toileting. This also included situations where antiseptics were applied only once (e.g. as part of microsuction at the start of treatment).
-
Systemic antibiotics as an add‐on therapy to antiseptics: this included studies where all participants in both treatment groups also used a daily antiseptic, with or without aural toileting. Topical antiseptics are agents applied locally (to the ear) that have an antimicrobial effect, helping to kill or inhibit the growth of bacteria.
-
Systemic antibiotics as an add‐on therapy to other systemic or topical antibiotics: this included studies where all participants in both treatment groups also received a systemic or topical antibiotic, with or without aural toileting or antiseptics.
Many comparison pairs were possible in this review. The main comparisons of interest that are summarised and presented in the 'Summary of findings' tables are:
-
systemic antibiotics as a single treatment (main therapy) versus placebo or no intervention;
-
systemic antibiotics versus placebo or no intervention (where other systemic or topical antibiotics were used in both arms);
-
systemic antibiotics versus placebo or no intervention (where topical antibiotics with steroids were used in both arms);
-
systemic antibiotics versus placebo or no intervention (where topical antiseptics were used in both arms).
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
We extracted and reported data from the longest available follow‐up for all outcomes.
Primary outcomes
-
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at:
-
between one week and up to two weeks;
-
two weeks to up to four weeks; and
-
after four weeks.
-
-
Health‐related quality of life using a validated instrument for CSOM (e.g. Chronic Otitis Media Questionnaire (COMQ)‐12 (Phillips 2014a; Phillips 2014b; van Dinther 2015), Chronic Otitis Media Outcome Test (COMOT)‐15 (Baumann 2011), Chronic Ear Survey (CES) (Nadol 2000)).
-
Ear pain (otalgia) or discomfort or local irritation.
Secondary outcomes
-
Hearing, measured as the pure‐tone average of air conduction thresholds across four frequencies (500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) of the affected ear. If this was not available, we reported the pure‐tone average of the thresholds measured.
-
Serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy), and death.
-
Ototoxicity; this was measured as 'suspected ototoxicity' as reported by the studies where available, and as the number of people with the following symptoms that may be suggestive of ototoxicity:
-
sensorineural hearing loss;
-
balance problems/dizziness/vertigo;
-
tinnitus.
-
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 16 March 2020.
Electronic searches
The Information Specialist searched:
-
the Cochrane ENT Register (searched via the Cochrane Register of Studies to 16 March 2020);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies Web to 16 March 2020);
-
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 16 March 2020);
-
Ovid EMBASE (1974 to 16 March 2020);
-
EBSCO CINAHL (1982 to 16 March 2020);
-
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (search to 16 March 2020);
-
Web of Knowledge, Web of Science (1945 to 16 March 2020);
-
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies to 16 March 2020);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search to 16 March 2020).
We also searched:
-
IndMed (search to 22 March 2018);
-
African Index Medicus (search to 22 March 2018).
The search strategies for major databases are detailed in Appendix 1. The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. The strategies have were designed to identify all relevant studies for a suite of reviews on various interventions for chronic suppurative otitis media (Bhutta 2020; Brennan‐Jones 2020a; Brennan‐Jones 2020b; Chong 2018a; Chong 2018b; Head 2020a; Head 2020b). Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011).
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects; we considered adverse effects described in included studies only.
We contacted original authors for clarification and further data if trial reports were unclear and we arranged translations of papers where necessary.
Data collection and analysis
Selection of studies
At least two review authors (KH/LYC/JD/KW) independently screened all titles and abstracts of the references obtained from the database searches to identify potentially relevant studies. At least two review authors (KH/LYC/JD/KW) evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
At least two review authors (KH/LYC/CBJ/JD/KWMJB) independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved any differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the included studies, such as study design, setting (including location), year of study, sample size, age and sex of participants, and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers (see Appendix 2). For this review, this included the following information whenever available:
-
duration of ear discharge at entry to the study;
-
diagnosis of ear discharge (where known);
-
number people who may have been at higher risk of CSOM, including those with cleft palate or Down syndrome;
-
ethnicity of participants including the number who were from Indigenous populations;
-
number who had previously had ventilation tubes (grommets) inserted (and, where known, the number who had tubes still in place);
-
number who had previous ear surgery;
-
number who had previous treatments for CSOM (non‐responders, recurrent versus new cases).
We recorded concurrent treatments alongside the details of the interventions used. See the 'Data extraction form' in Appendix 2 for more details.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis, i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
-
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from disease‐specific quality of life scales such as COMQ‐12, COMOT‐15 and CES as continuous data.
-
For binary data: the number of participants who experienced an event and the number of patients assessed at the time point.
-
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted it into binary data.
-
Time‐to‐event outcomes: we did not expect any outcomes to be measured as time‐to‐event data. However, if outcomes such as resolution of ear discharge were measured in this way, we reported the hazard ratios.
For resolution of ear discharge, we extracted the longest available data within the time frame of interest, defined as from one week up to (and including) two weeks (7 days to 14 days), from two weeks up to (and including) four weeks (15 to 28 days), and after four weeks (28 days or one month).
For other outcomes, we reported the results from the longest available follow‐up period.
Extracting data for pain/discomfort and adverse effects
For these outcomes, there were variations in how studies had reported the outcomes. For example, some studies reported both 'pain' and 'discomfort' separately whereas others did not. Prior to the commencement of data extraction, we agreed and specified a data extraction algorithm for how data should be extracted.
We extracted data for serious complications as a composite outcome. If a study reported more than one complication and we could not distinguish whether these occurred in one or more patients, we extracted the data with the highest incidence to prevent double counting.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper, we attempted to contact the study authors to try to obtain the raw values. When the raw values were not provided, we extracted information from the graphs using an online data extraction tool, using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
At least two review authors (KH/LYC/CBJ/JD/KW/MJB) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), using the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
-
sequence generation;
-
allocation concealment;
-
blinding of participants, personnel and outcome assessment;
-
incomplete outcome data;
-
selective reporting;
-
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with complete resolution of ear discharge) as risk ratios (RR) with confidence intervals (CIs). For the key outcomes that are presented in the 'Summary of findings' table, we have expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also planned to calculate the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk was typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies, which is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we also attempted to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD). If different scales were used to measure the same outcome, we used the standardised mean difference (SMD) and provided a clinical interpretation of the SMD values.
Unit of analysis issues
Cross‐over studies
This review did not use data from phase II of cross‐over studies.
The patient as the unit of randomisation
Some studies randomised by patient and those with bilateral CSOM received the same intervention for both ears. In some studies the results may be reported as a separate outcome for each ear (the total number of ears is used as the denominator in the analysis). The correlation of response between the left ear and right ear when given the same treatment was expected to be very high, and if both ears were counted in the analysis this was effectively a form of double counting, which may be especially problematic in smaller studies if the number of people with bilateral CSOM was unequal. We did not exclude these studies, but we only reported the results if the paper presents the data in such a way that we could include the data from each participant only once (one data point per participant) or if we had enough information to reliably estimate the effective sample size or inflated standard errors as presented in chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If this was not possible, we attempted to contact the authors for more information. If there was no response from the authors, then we did not include data from these studies in the analysis.
If we found cluster‐randomised trials by setting or operator, we analysed these according to the methods in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We attempted to contact the study authors via email whenever the outcome of interest was not reported, but the methods of the study had suggested that the outcome had been measured. We did the same if not all of the data required for the meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors, or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we did not conduct any other imputations. We extracted and analysed data for all outcomes using the available case analysis method.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included studies for potential differences in the types of participants recruited, interventions or controls used, and the outcomes measured. We did not pool studies where the clinical heterogeneity made it unreasonable to do so.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculated the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis was likely to occur. We tried to find further information from the study authors, but if no further information could be obtained, we noted this as being a high risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an unclear risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We intended to create funnel plots if sufficient studies (more than 10) were available for an outcome. If we observed asymmetry of the funnel plot, we would have conducted a more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we pooled the mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the SMD had to be used as an effect measurement, we did not pool change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies where most participants (80% or more) met the criteria stated below in order to determine whether the effect of the intervention was different compared to other patients. Due to the risks of reporting and publication bias with unplanned subgroup analyses of trials, we only analysed subgroups reported in studies if these were prespecified and stratified at randomisation.
We planned to conduct subgroup analyses regardless of whether statistical heterogeneity was observed for studies that included patients identified as high risk (i.e. thought to be less responsive to treatment and more likely to develop CSOM, recurrence or complications) and patients with ventilation tubes (grommets). 'High‐risk' patients include Indigenous populations (e.g. Australian Aboriginal and Torres Strait Islanders, Native American and Inuit populations of Alaska, Canada and Greenland), and people with craniofacial malformation (e.g. cleft palate), Down syndrome or with known immunodeficiency.
We planned to present the main analyses of this review in the form of forest plots based on this main subgroup analysis.
-
For the high‐risk group, this applies to the outcomes: resolution of ear discharge (dry ear), quality of life, pain/discomfort, development of complications and hearing loss.
-
For patients with ventilation tubes, this applied to the outcome resolution of ear discharge (dry ear) for the time point of four weeks or more (because this group was perceived to be at lower risk of treatment failure and recurrence than other patient groups). If statistical heterogeneity was observed, we conducted subgroup analysis for the effect modifiers below. If there were statistically significant subgroup effects, we presented these subgroup analysis results as forest plots.
For this review, effect modifiers included:
-
Diagnosis of CSOM: it was likely that some studies would include patients with chronic ear discharge but who had not had a diagnosis of CSOM. Therefore, we subgrouped studies where most patients (80% or more) meet the criteria for CSOM diagnosis in order to determine whether the effect of the intervention was different compared to patients where the precise diagnosis was unknown and inclusion into the study was based purely on chronic ear discharge symptoms.
-
Duration of ear discharge: there is uncertainty about whether the duration of ear discharge prior to treatment has an impact on the effectiveness of treatment and whether more established disease (i.e. discharge for more than six weeks) is more refractory to treatment compared with discharge of a shorter duration (i.e. less than six weeks).
-
Patient age: patients who were younger than two years old versus patients up to six years old, versus adults. Patients under two years are widely considered to be more difficult to treat.
We presented the results as subgroups regardless of the presence of statistical heterogeneity based on two factors:
-
Class of antibiotics. We grouped by pharmacological class, e.g. quinolone, aminoglycoside, penicillin etc. The rationale for this was that different classes may have had different effectiveness and side effect profiles.
-
Spectrum of activity against Pseudomonas aeruginosa (groups with known activity against Pseudomonas aeruginosa versus groups without activity against Pseudomonas aeruginosa). This is the most commonly found bacteria in patients with CSOM and its presence is associated with tissue damage.
When other antibiotics were also used as a treatment common to both the intervention and comparison group, we investigated the class and anti‐pseudomonal activity if statistical heterogeneity was present and could not be explained by other subgroup analyses.
No other subgroups based on the pharmacological properties of antibiotics were planned, but we considered the method and frequency of aural toileting if there was remaining unexplained heterogeneity despite conducting other subgroup analyses.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
-
Impact of model chosen: fixed‐effect versus random‐effects model.
-
Risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias: overall loss to follow‐up of 20%, differential follow‐up observed).
-
Where there was statistical heterogeneity, studies that only recruited patients who had previously not responded to one of the treatments under investigation in the randomised controlled trial (RCT). Studies that specifically recruited patients who did not respond to a treatment could potentially have reduced the relative effectiveness of an agent.
If any of these investigations found a difference in the size of the effect or heterogeneity, we mentioned this in the Effects of interventions section and/or presented the findings in a table.
Summary of findings and assessment of the certainty of the evidence
Using the GRADE approach, at least two review authors (KH/KW) independently rated the overall certainty of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The certainty of evidence reflects the extent to which we were confident that an estimate of effect was correct and we applied this in the interpretation of results. There were four possible ratings: 'high', 'moderate', 'low' and 'very low' (Handbook 2011). A rating of 'high' certainty evidence implies that we were confident in our estimate of effect and that further research was very unlikely to change our confidence in the estimate of effect. A rating of 'very low' certainty implies that any estimate of effect obtained was very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors could lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading was determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision;
-
publication bias.
The 'Summary of findings' tables present the following outcomes:
-
resolution of ear discharge or 'dry ear':
-
at between one week and up to two weeks;
-
after four weeks;
-
-
health‐related quality of life;
-
ear pain (otalgia) or discomfort or local irritation;
-
hearing;
-
serious complications;
-
suspected ototoxicity.
Results
Description of studies
Results of the search
The searches retrieved a total of 8900 references, with identification of five additional references. This reduced to a total of 3447 after removal of duplicates. We screened the titles and abstracts and subsequently removed 3218 references. We assessed 229 full texts for eligibility of which we excluded 199 references; we excluded 94 of these references (86 studies) with reasons recorded in the review (see Excluded studies). We included 18 studies (27 references) (Baba 1982c; Bajwa 2018; de Miguel 1999; Eason 1986; Esposito 1990; Fliss 1990; Ghosh 2012; Minja 2006; Nwokoye 2015; Onali 2018; Picozzi 1984; Ramos 2003; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001; Somekh 2000; Van der Veen 2007).
Of the included studies, we noticed that two had the same study design, inclusion criteria, location of study and overlap in key authors (de Miguel 1999; Ramos 2003). Ramos 2003 had 50 rather than 25 participants per treatment arm and an additional treatment arm (six versus five). The proportion of patients achieving resolution of ear discharge was identical. We contacted the authors to clarify whether these data were obtained from the same set of participants and they clarified that these are separate studies. We have therefore included both studies in the review.
There is one study (one reference) awaiting assessment because we are uncertain whether the participants were randomised in this study (Mehboob 2019).
One study (two references) was recently completed but the data were not available when this review was published, so it is included as an ongoing study (I‐HEAR‐BETA).
A flow chart of study retrieval and selection is provided in Figure 1.

Study flow diagram
Included studies
Eighteen studies were included (Baba 1982c; Bajwa 2018; Eason 1986; Esposito 1990; Fliss 1990; Ghosh 2012; Minja 2006; Nwokoye 2015; Onali 2018; Picozzi 1984; Ramos 2003; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001; Somekh 2000; Van der Veen 2007; de Miguel 1999).
Table 3 provides a summary of the included studies.
| Ref ID (no. participants) | Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background treatment | Notes |
|---|---|---|---|---|---|---|---|---|
| 1. Systemic antibiotics versus no treatment/placebo | ||||||||
| (n = 51) | Israel, tertiary hospital | Otorrhoea in children with CSOM for at least 2 months with no response to daily microsuction and debridement for 7 days Age range 11 months to 12 years | Intravenous (IV) mezlocillin, 200 mg/kg given in 3 divided doses daily; or IV ceftazidime 150 mg/kg given in 3 divided doses daily | No treatment | Until 3 days after resolution of discharge, up to maximum 3 weeks | 6 months | Daily suction and debridement | The first 19 patients in the trial did not receive additional antibiotics. The remaining 32 patients received daily prophylactic amoxicillin for at least 2 months after hospital discharge |
| 2. Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics) | ||||||||
| (n = 100) | Pakistan, ENT outpatient department | Severe episode of chronic suppurative otitis media (mucopurulent ear discharge > 4 weeks with central tympanic membrane perforation) Age range 20 to 69 years | Oral ofloxacin 500 mg, 2 times a day | No treatment | 14 days | 6 weeks | Topical ofloxacin (concentration not reported), 3 drops/3 times a day PLUS dry mopping prior to instilling ear drops | — |
| (n = 50) | Spain, general | Chronic otitis media, presenting with chronic otorrhoea as a major symptom Mean age 39.6 years (but 68% were children) | Oral ciprofloxacin 500 mg/12 hours | No treatment | 7 days | 15 days | Topical ciprofloxacin (0.2%) 3 drops/8 hours PLUS aspiration and cleaning of ear secretions before starting treatment | Part of a 5‐arm trial |
| (n = 40) | Italy, University clinic | Mild or moderate chronic OM in the acute stage Mean age 38 years | Oral ciprofloxacin 250 mg twice daily | No treatment | At least 5 days If not resolved by 5 days, interventions were continued for a maximum of 10 days | 4 weeks | Topical ciprofloxacin 250 µg/ml in saline solution, 3 drops twice daily | Part of a 3‐arm trial |
| (n = 100) | Pakistan, hospital | Tubotympanic type CSOM Mean age 33.2 years | Oral ciprofloxacin 200 mg every 12 hours | Oral placebo every 12 hours | 7 days | 14 days | Topical ciprofloxacin (concentration not reported) 3 times a day PLUS aural hygiene and water prevention | — |
| (n = 100) | Spain, ENT department of tertiary | Chronic otorrhoea (> 6 weeks), or recurrent sporadic otorrhoea (> 3 episodes in the last year) Age range 5 to 73 years; 12% of children under 14 years | Oral ciprofloxacin 500 mg 12‐hourly | No treatment | 7 days | 10 days | Topical ciprofloxacin 0.2% 0.5 ml 8‐hourly | Part of a 5‐arm trial |
| (n = 100) | India, ENT outpatient department of tertiary hospital | Active ear discharge (mucopurulent or purulent) otorrhoea of more than 3 weeks duration, with a tympanic membrane perforation Age range 20 to 69 years | Oral ciprofloxacin 500 mg twice daily | No treatment | 14 days | 8 weeks | Topical ciprofloxacin (concentration not reported), 3 drops 3 times a day PLUS dry mopping before instilling ear drops Water prevention was advised | |
| 3. Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics plus steroids) | ||||||||
| (n = 62) | Solomon Islands, hospital with community screening | CSOM with otorrhoea for more than 3 months and tympanic membrane perforation Mean age 5.4 years | Oral clindamycin (15 mg/kg/day) in 3 divided daily doses | No treatment | 6 weeks | 6 weeks | Topical Sofradex ear drops (concentration or frequency not reported) PLUS aural toilet 4 times per day | Part of a 5‐arm trial |
| (n = 40) | Unclear location ‐ researchers from United Kingdom | Active chronic otitis media Participant information not reported | Metronidazole (route, dose and frequency of administration not reported) | Placebo | 2 weeks | 4 weeks | Gentamicin‐hydrocortisone ear drops (dose and frequency not reported) for 4 weeks PLUS self‐mopping | — |
| (n = 101) | The Netherlands, | Chronic otitis media that had failed conventional therapy (topical/short‐term systemic antibiotics) Age range 1 to 12, median 4 years | Oral trimethoprim/sulfamethoxazole 18 mg/kg twice daily | Oral placebo twice daily | 6 weeks, or 12 weeks if there was still otorrhoea at 6 weeks | 1 year | Hydrocortisone/bacitracin/colistin Ear drops were given at baseline for 7 to 10 days, and repeated at 6 and 12 weeks if otorrhoea was present at these study visits | — |
| 4. Systemic antibiotics versus no treatment/placebo (both study arms had topical antiseptic plus dry mopping) | ||||||||
| (n = 204) | Tanzania, schools (community) | Children with history of ear discharge for 3 months or more Mean age 11.8 years | Amoxicillin (dose, frequency, route of administration not reported) | No treatment | 10 days | 3 to 4 months | Boric acid in alcohol (concentration and frequency not reported) ear drops for one month PLUS daily aural toilet (dry mopping) | Part of a 3‐arm cluster‐randomised trial |
| 5. Quinolones versus beta‐lactams | ||||||||
| (n = 305) | Japan, university and general hospitals | Acute suppurative otitis media or acute exacerbation of chronic otitis media Mean age not reported, study inclusion if over 15 years | Oral norfloxacin 200 mg 4 times a day | Oral aminobenzylpenicillin 500 mg 4 times a day | 7 days | 2 weeks | None | — |
| (n = 46) | India, ENT outpatient department of tertiary care teaching hospital | Tubotympanic type CSOM (acute exacerbation of longstanding chronic suppuration of middle ear and deafness in adults) Age range 18 to 60 years | Ciprofloxacin 500 mg twice daily | Cefpodoxime 200 mg twice daily | 7 days | 14 days | None | — |
| (n = 603) | Japan, university and general hospitals | Suppurative otitis media and tympanic membrane perforation Mean age not reported, study inclusion if 15 years or older | Oral pipemidic acid 500 mg, 4 times a day | Oral aminobenzyl penicillin 500 mg, 4 times a day | 14 days | 14 days | None | — |
| (n = 30) | Mexico, regional hospital | CSOM Mean age 38 (range 26 to 60) | Oral levofloxacin 500 mg once daily | Oral amoxicillin‐clavulanic acid 500/125 mg, every 8 hours | 10 days | Up to 25 days | None | — |
| 6. Different beta‐lactams | ||||||||
| (n = 36) | Israel, tertiary hospital | Otorrhoea in children with CSOM for at least 2 months, no response to daily microsuction and debridement for 7 days Mean age not reported (range 11 months to 12 years) | Intravenous (IV) mezlocillin 200 mg/kg given in 3 divided doses daily | IV ceftazidime 150 mg/kg given in 3 divided doses daily | Until 3 days after resolution of discharge, up to maximum 3 weeks | 6 months | Daily suction and debridement | The first 19 patients in the trial did not receive additional antibiotics. The remaining 32 patients received daily prophylactic amoxicillin for at least 2 months after hospital discharge. |
| (n = 30) | Israel, paediatric infectious disease unit at medical centre | Pseudomonal CSOM Mean age 4.2 years (range 1 to 12) | IV aztreonam 100 mg/kg/day, given in 3 divided doses daily | IV ceftazidime 100 mg/kg/day, given in 3 divided doses daily | At least 10 days. Treatment continued until 3 days after complete cessation of discharge. | 3 months | Daily suction and debridement | — |
| (n = 54) | Nigeria, University teaching hospital | Otorrhoea for at least 3 months Age range 5 months to 10 years; 63% under 2 years | Oral amoxicillin‐clavulanate (amoxicillin 80 mg/kg/day in 2 divided doses daily; clavulanate acid dose and frequency not reported) | Oral amoxicillin 80 mg/kg/day, given in 2 divided doses daily | 7 to 10 days | Not reported | Aural toilet with warm saline was advised 4 times daily using dry cotton wool wisps | Part of a 3‐arm trial (third arm involved treatment based on culture and antibiotic sensitivity results) |
| 7. Lincosamides versus nitroimidazoles(both study arms also received gentamicin) | ||||||||
| (n = 119) | Nigeria, University teaching hospital | Mucopurulent ear discharge, perforated tympanic membrane, and associated hyperaemic and oedematous middle ear mucosa Age range 2 weeks to > 40 years | Oral clindamycin sulfate capsules (300 mg) or oral lincomycin (300 mg), 4 times a day | Oral metronidazole 400 mg 3 times a day | 7 days | 6 weeks | Intramuscular (IM) gentamicin 1.5 mg/kg/day in divided doses for 5 days PLUS suction and cleaning of the external meatus Self‐cleaning of ears with cotton wool buds twice a day | Part of 4‐arm trial |
Study design
Ten studies were two‐arm trials (Baba 1982c; Bajwa 2018; Ghosh 2012; Onali 2018; Picozzi 1984; Renukananda 2014; Sambe 1977; Sanchez Gonzales 2001; Somekh 2000; Van der Veen 2007), and four studies were three‐arm trials (Esposito 1990; Fliss 1990; Minja 2006; Nwokoye 2015). Four studies were multi‐arm trials: four‐arm (Rotimi 1990), five‐arm (Eason 1986; de Miguel 1999) or six‐arm (Ramos 2003). Only the study arms that compared systemic antibiotics with another intervention (placebo/no intervention or an alternative systemic antibiotic) were used in this review. Details of the other study arms for each study can be found in the Characteristics of included studies table.
All studies provided an indication that they were 'randomised controlled trials' and were parallel‐group studies.
Minja 2006 indicates in the abstract that this was a randomised controlled trial but states in the methods that "all children with CSOM attending the same school were included in the same treatment group", indicating that it was probably a cluster‐randomised trial.
Sample size
The total sample size was 2135 participants. Sixteen studies reported the sample size in terms of the number of participants (not ears); these had a total of 1973 participants (Baba 1982c; de Miguel 1999; Esposito 1990; Fliss 1990; Ghosh 2012; Minja 2006; Nwokoye 2015; Onali 2018; Picozzi 1984; Ramos 2003; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001; Somekh 2000; Van der Veen 2007). Two studies reported both the number of participants and the number of ears, representing 162 participants and 214 ears (Bajwa 2018; Eason 1986).
Unit of randomisation
The individual (rather than the ear) was randomised to treatment group in 17 studies (Baba 1982c; Bajwa 2018; de Miguel 1999; Eason 1986; Esposito 1990; Fliss 1990; Ghosh 2012; Nwokoye 2015; Onali 2018; Picozzi 1984; Ramos 2003; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001; Somekh 2000; Van der Veen 2007). Of these 17 studies, only two reported the number of patients with bilateral disease (33% of participants in Bajwa 2018 and 31% in Eason 1986), but where the denominator was reported by person, it was assumed that no double counting occurred.
The remaining study is described as a randomised controlled trial, but the study description suggests that this was a cluster‐RCT (Minja 2006). Minja 2006 states that children attending the same school were in the same treatment group and that there were 24 schools included in the study (although the number of children at each school is not provided). In order to adjust the results for intra‐cluster correlation we have re‐calculated the results with an intra‐cluster correlation coefficient (ICC) of 0.015 (see Unit of analysis issues for more details). No estimates from the literature were available for this population, but in general for cluster‐randomised trials the ICC is between 0.01 and 0.02. We carried out sensitivity analyses to determine the impact of the ICC.
Location
The studies were conducted in 13 countries around the world, including Japan, Pakistan, the Solomon Islands, Italy, Israel, India, Tanzania, Nigeria, the United Kingdom, Spain, Mexico and the Netherlands (see Table 3).
Setting of trial
With regard to clinical setting, nine studies were undertaken in university clinics/teaching hospitals (Bajwa 2018; Esposito 1990; Fliss 1990; Ghosh 2012; Nwokoye 2015; Onali 2018; Rotimi 1990; Somekh 2000; Van der Veen 2007).
Two studies were conducted in the ENT departments of university and general hospitals (Baba 1982c; Sambe 1977). Three studies were based in specialist hospitals (Ramos 2003; Renukananda 2014; Sanchez Gonzales 2001) and one study was based in a general hospital (de Miguel 1999).
One study was conducted in schools (Minja 2006) and one study with participants identified through community screening and treated in a rural setting (Eason 1986).
The setting was unclear in one study (Picozzi 1984).
The years in which the studies were conducted was often not well reported. One study was published in the 1970s (Sambe 1977), while three studies were published in the 1980s (Baba 1982c; Eason 1986; Picozzi 1984). Four studies were published in the 1990s (de Miguel 1999; Esposito 1990; Fliss 1990; Rotimi 1990), while five studies were published in the 2000s (Minja 2006; Ramos 2003; Sanchez Gonzales 2001; Somekh 2000; Van der Veen 2007). There were five studies published in the last 10 years (Bajwa 2018; Ghosh 2012; Nwokoye 2015; Onali 2018; Renukananda 2014).
Funding and declarations of Interest
Four studies addressed funding sources. Three studies provided information for the research grant or company funding the study (Eason 1986; Minja 2006; Somekh 2000). Esposito 1990 stated that the "the ciprofloxacin tablets and powder used in this study were kindly provided by Bayer Italia Spa, Milan, Italy." Rotimi 1990 was funded by Wellcome Fund, Nigeria, and had clindamycin supplied by UpJohn Nigeria and metronidazole supplied by May & Baker Ltd. Minja 2006 did not specifically mention a declaration of interest but noted that one of the authors was responsible for securing the funds. The remaining 13 studies did not provide any information on funding sources or declarations of interest.
Population
Age and sex
One study did not report any participant characteristics for either age or gender (Picozzi 1984).
Two studies did not report participants' mean age or range, but gave the number of participants in different age categories (Baba 1982c; Sambe 1977). In Sambe 1977, participants were at least 15 years old and included a subset of participants older than 71 years.
Four studies included a mixture of adults and children. The mean age in de Miguel 1999 was reported as 39.6 years, although 17/25 participants were children. The mean age was not reported by Ramos 2003, but ages ranged from 5 to 73 years old, and 36/300 (12%) participants were children younger than 14 years. Rotimi 1990 had participant ages ranging from 2 weeks to > 40 years. Sanchez Gonzales 2001 had participants from ages 15 to 71 years (mean 38 ± 18.5 years)
Six studies included only children (Eason 1986 (mean 5.4 ± 3.1 years); Fliss 1990 (range 11 months to 148 months old); Minja 2006 (mean 11.8 years ± 2.7 years); Nwokoye 2015 (range 5 months to 10 years, with 52/82 (63%) under 2 years); Somekh 2000 (mean 4.2 years, range 1 to 12 years); Van der Veen 2007 (mean 4 years, range 1 to 12)).
Five studies included only adult participants (Bajwa 2018 (range 20 to 69); Esposito 1990 (mean 38); Ghosh 2012 (range 18 to 60); Onali 2018 (mean 33.2 ± 8.7 years, range 18 to 50); Renukananda 2014 (20 to 69 years)).
Three studies did not report the gender characteristics (Nwokoye 2015; Picozzi 1984; Somekh 2000). Fifteen studies reported the gender characteristics of individuals randomised in the study (Baba 1982c; Bajwa 2018; de Miguel 1999; Eason 1986; Esposito 1990; Fliss 1990; Ghosh 2012; Minja 2006; Onali 2018; Ramos 2003; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001; Van der Veen 2007). In total, 2305 individuals were included in these trials; 1029 (44.6%) of these were female and 1276 (55.4%) were male. The percentage of females in these studies ranged from 33% to 53%.
High‐risk populations
Nine studies did not report whether individuals in 'high‐risk' groups were included ‐ this included those who were immunocompromised, Indigenous groups, and individuals with Down syndrome or cleft palate (Baba 1982c; de Miguel 1999; Ghosh 2012; Minja 2006; Picozzi 1984; Ramos 2003; Sambe 1977; Sanchez Gonzales 2001; Somekh 2000).
Three studies reported no immunocompromised patients (Bajwa 2018; Onali 2018; Renukananda 2014). Nwokoye 2015 and Rotimi 1990 reported that no Indigenous groups participated in their study. Esposito 1990 reported that "no patients had diabetes or any other comorbidities". Fliss 1990 reported that 0% of participants had cleft palate, Down syndrome or were immunocompromised. Van der Veen 2007 did not include any individuals in high‐risk groups.
Eason 1986 recruited participants from the Solomon Islands, which we considered to be a 'high‐risk' Indigenous group. The paper stated that the incidence of CSOM in the population was 3.8% for under 15‐year olds.
Diagnosis
Thirteen studies stated that they included participants with CSOM (Bajwa 2018; Eason 1986; Esposito 1990; Fliss 1990; Ghosh 2012; Onali 2018; Picozzi 1984; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001; Somekh 2000; Van der Veen 2007). However, it was not always clear how CSOM was defined in the studies. Three studies stated that they included individuals with ear discharge (Minja 2006; Nwokoye 2015; Ramos 2003). de Miguel 1999 stated that individuals with CSOM were included, but this study also included a number of individuals with alternative diagnoses of ear pain, including cholesteatoma and post‐surgical ear discharge. One study included individuals with chronic or acute suppurative otitis media (Baba 1982c).
Seven studies reported using otoscopy to diagnose tympanic membrane perforation and/or identify mucopurulent discharge (de Miguel 1999; Eason 1986; Fliss 1990; Minja 2006; Ramos 2003; Sanchez Gonzales 2001; Van der Veen 2007). An additional study used otoscopy, but there was no stated requirement for perforated tympanic membrane (Nwokoye 2015).
Six studies reported inclusion of participants with tympanic membrane perforation and/or mucopurulent discharge, but did not report the method used to determine this (Bajwa 2018; Ghosh 2012; Renukananda 2014; Rotimi 1990; Sambe 1977; Somekh 2000).
The remaining four studies did not report on confirmation of tympanic membrane perforation or mucopurulent discharge (Baba 1982c; Esposito 1990; Onali 2018; Picozzi 1984).
Duration of ear discharge
Seven studies did not report the duration of ear discharge (Baba 1982c; de Miguel 1999; Esposito 1990; Ghosh 2012; Picozzi 1984; Sambe 1977; Sanchez Gonzales 2001).
Eleven studies reported the duration of ear discharge (Renukananda 2014 (> 3 weeks); Bajwa 2018 (> 4 weeks); Eason 1986 (> 3 months); Minja 2006 (> 3 months); Nwokoye 2015 (> 3 months); Fliss 1990 (2 to 123 months, median 20 months); Onali 2018 (mean 55.2 days ± 33.3, range 14 to 140 days); Ramos 2003 (> 6 weeks or sporadically > 3 episodes in the last year); Rotimi 1990 (range < 2 weeks (11% of completers) to 32 years, 67% at least 1 month); Somekh 2000 (mean 8 weeks, range 8 to 12 weeks); Van der Veen 2007 (median 8 months in intervention group, 5 months in placebo group)).
Other important effect modifiers
Six studies did not report on any important effect modifiers (Eason 1986; Minja 2006; Nwokoye 2015; Picozzi 1984; Sambe 1977; Sanchez Gonzales 2001).
Five studies reported that none of the patients had an alternative diagnosis (Fliss 1990; Ghosh 2012; Onali 2018; Rotimi 1990; Somekh 2000). Two studies reported on participants with alternative diagnoses for ear discharge (de Miguel 1999 (n = 17, 13.6%); Ramos 2003 (n = 42, 14%)).
Four studies reported on the number of participants who had previously had grommets (Fliss 1990 (n = 3, 6%); Ramos 2003 (n = 12, 4%); Renukananda 2014 (n = 0%); Van der Veen 2007 (n = 91, 90%)). Van der Veen 2007 also reported that 61 (60.4%) still had grommets in place at inclusion.
Six studies reported on the number of participants who had previously had ear surgery (Bajwa 2018 (n = 0% in last year); de Miguel 1999 (n = 31 (24.8%); Fliss 1990 (n = 0%); Ramos 2003 (n = 73, 24.3%); Renukananda 2014 (n = 0% within last year); Van der Veen 2007 (n = 12, 12%)). The reasons and type of surgery were not reported in the studies.
Nine studies reported on the number of participants who had received antibiotics for CSOM previously (Baba 1982c; Bajwa 2018 (n = 0% in last year); de Miguel 1999 (n = 79, 63.2%); Esposito 1990 (n = 38, 63%); Fliss 1990; Ramos 2003 (n = 197, 65.6%); Renukananda 2014 (n = 0% within last month); Somekh 2000 (n = 100%); Van der Veen 2007 (n = 91, 90%). Baba 1982c reported that 10 of the 395 randomised participants were treated with other antibiotics for acute exacerbation of chronic otitis media (AECSOM) just before enrolment into the trial. Fliss 1990 reported that all participants had at least one failed course of systemic antibiotics.
Intervention
Intervention details
Details of the intervention, background treatments and treatment durations for each of the included studies are summarised in Table 3.
Fourteen studies used oral antibiotics (Baba 1982c; Bajwa 2018; de Miguel 1999; Eason 1986; Esposito 1990; Ghosh 2012; Nwokoye 2015; Onali 2018; Ramos 2003; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001; Van der Veen 2007). Two studies used intravenous antibiotics (Fliss 1990; Somekh 2000), while two studies had unspecified routes of systemic administration (Minja 2006; Picozzi 1984). We presumed that these two studies used oral administration.
Background treatment
Six studies used aural toilet as a background treatment (Bajwa 2018; Fliss 1990; Minja 2006; Nwokoye 2015; Rotimi 1990; Somekh 2000). Five studies had topical antibiotics as a background treatment (Bajwa 2018; de Miguel 1999; Esposito 1990; Onali 2018; Ramos 2003; Renukananda 2014), and an additional three used a steroid combination (Eason 1986; Picozzi 1984; Van der Veen 2007). One study used intramuscular antibiotics (Rotimi 1990), and one study used topical antiseptics (Minja 2006). Of these studies, six had combinations of background treatment. For details, see Table 3. Four studies reported no additional, concurrent interventions (Baba 1982c; Ghosh 2012; Sambe 1977; Sanchez Gonzales 2001).
Duration of intervention
Six studies had a duration of treatment of seven days (Baba 1982c; de Miguel 1999; Ghosh 2012; Onali 2018; Ramos 2003; Rotimi 1990), two studies were for 10 days (Minja 2006; Sanchez Gonzales 2001), and five studies were for 14 days (Bajwa 2018; Eason 1986; Picozzi 1984; Renukananda 2014; Sambe 1977).
Five studies had variable duration of treatment depending on response (Esposito 1990 (5 to 10 days); Fliss 1990 (3 days after complete cessation of discharge to 3 weeks) Nwokoye 2015 (7 to 10 days); Somekh 2000 (10 to 14 days); Van der Veen 2007 (6 to 12 weeks)).
Comparison
One study compared systemic antibiotics to no treatment:
-
Fliss 1990 (51 participants) ‐ intravenous mezlocillin versus no treatment; and intravenous ceftazidime versus no treatment.
Six studies compared systemic antibiotics to no treatment, with both study arms receiving topical antibiotics:
-
Bajwa 2018 (100 participants, 133 ears) ‐ oral ofloxacin versus no treatment, background treatment of topical antibiotic drops (ofloxacin).
-
de Miguel 1999 (50 participants) ‐ oral ciprofloxacin versus no treatment, background treatment of topical antibiotic drops (ciprofloxacin).
-
Esposito 1990 (40 participants) ‐ oral ciprofloxacin versus no treatment, background treatment of topical antibiotic drops (ciprofloxacin).
-
Onali 2018 (100 participants) ‐ oral ciprofloxacin versus no treatment, background treatment of topical antibiotic drops (ciprofloxacin).
-
Ramos 2003 (100 participants) ‐ oral ciprofloxacin versus no treatment, background treatment of topical antibiotic drops (ciprofloxacin).
-
Renukananda 2014 (100 participants) ‐ oral ciprofloxacin versus no treatment, background treatment of topical antibiotic drops (ciprofloxacin).
Three studies compared systemic antibiotics versus no treatment/placebo, with both study arms receiving topical antibiotics plus steroids:
-
Eason 1986 (62 participants, 81 ears) ‐ oral clindamycin versus no treatment, background treatment of gramicidin‐framycetin‐dexamethasone ear drops.
-
Picozzi 1984 (40 participants) ‐ metronidazole versus placebo, background treatment of gentamicin plus hydrocortisone ear drops.
-
Van der Veen 2007 (101 participants) ‐ oral trimethoprim/sulfamethoxazole versus placebo, with varied antibiotic/steroid drops formulation (hydrocortisone/bacitracin/colistin eardrops were used initially, then changed to hydrocortisone/ neomycin polymyxin B).
One study compared systemic antibiotics to no treatment, with both study arms receiving topical antiseptic plus dry mopping:
-
Minja 2006 (204 participants) ‐ amoxicillin versus no treatment, background treatment of boric acid in alcohol ear drops.
Four studies compared systemic quinolones to beta‐lactams:
-
Baba 1982c (305 participants) ‐ oral aminobenzyl penicillin versus oral norfloxacin.
-
Ghosh 2012 (46 participants) ‐ oral ciprofloxacin versus oral cefpodoxime.
-
Sambe 1977 (603 participants) ‐ oral aminobenzyl penicillin versus oral pipemidic acid.
-
Sanchez Gonzales 2001 (30 participants) ‐ oral levofloxacin versus co‐amoxiclav.
Three studies compared different beta‐lactams:
-
Fliss 1990 (36 participants) ‐ intravenous mezlocillin versus intravenous ceftazidime.
-
Nwokoye 2015 (54 participants) ‐ amoxicillin alone versus amoxicillin plus clavulanic acid.
-
Somekh 2000 (30 participants) ‐ intravenous aztreonam versus intravenous ceftazidime.
One study compared lincosamides versus nitroimidazoles, with all study arms also receiving gentamicin:
-
Rotimi 1990 (119 participants) ‐ oral lincomycin versus oral metronidazole; and oral clindamycin versus oral metronidazole; background treatment of intramuscular gentamicin.
Outcomes
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not)
The definitions, methods and timing of assessment differed between studies, and these are summarised in Table 4.
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
|---|---|---|---|---|---|---|
| Person | Person | Resolution of ear discharge | Unclear | 1 to 2 weeks (7 days) | The worst affected ear was selected as the study ear. When both sides were equal, the right side was selected. | |
| Person | Person | "Cured" as non‐existence of the otorrhoea or otoscopically dormant (no discharge pooling; non‐swollen middle ear mucosa) or existence of serous mucous otorrhoea with bacteriological culture negative after therapy period | Unclear | 1 to 2 weeks (2 weeks), 2 to 4 weeks (3 weeks), after 4 weeks (8 weeks) | — | |
| Person | Person | "Global index of clinic microbiological cure" | Yes | 1 to 2 weeks (7 days) | — | |
| Person | Ear | "Dry" or "not discharging" | Yes | 2 to 4 weeks (3 weeks), after 4 weeks (6 weeks) | Bilateral ears counted separately. Results not used as it was not possible to account for correlation between ears due to bilateral disease. | |
| Person | Person | "Clinically cured" | Unclear | 1 to 2 weeks (6 to 11 days), 2 to 4 weeks (19 to 24 days) | The 1‐ to 2‐week outcome was examined but not reported | |
| Person | Person | Complete resolution of ear discharge | Yes | 1 to 2 weeks (9 days), 2 to 4 weeks (18 days) | Time points unclear | |
| Person | Person | “Clinical cure” if the otological symptom score was < 3 at day 14 visit | Unclear | 1 to 2 weeks (14 days) | — | |
| School | Person | "Dry ear" | Yes | 2 to 4 weeks (1 month), after 4 weeks (3 to 4 months) | Patients with bilateral ear disease were only counted as dry ears if both ears were dry | |
| Person | Person | "Recovery" (no further details provided) | Yes | 1 to 2 weeks (time point unclear; treatment for 7 to 10 days), 2 to 4 weeks (time point unclear) | — | |
| Person | Person | "Resolution of discharge" | Unclear | 1 to 2 weeks (7 days) | — | |
| Person | Person | "Inactive" (no further details provided) | Unclear | After 4 weeks (4 weeks) | — | |
| Person | Person | "Cured" according to "indices de curacion" | Yes | 2 to 4 weeks (10 days) | — | |
| Person | Person | "Cured" as absence of otorrhoea or otoscopically inactive, i.e. no pooling of discharge, non‐inflamed middle ear mucosa | Yes | 1 to 2 weeks (2 weeks) | 33/100 participants had bilateral disease. Unclear how these were assessed for resolution of ear discharge. | |
| Person | Person | "Clinical response" defined as cessation of discharge with no hyperaemic areas in the mucosa | Unclear | 1 to 2 weeks (1 week), 2 to 4 weeks (3 weeks), after 4 weeks (6 weeks) | Patients with bilateral ear disease were only counted as resolved if both ears were dry. | |
| Person | Person | Disappearance of otorrhoea or reduction of ear leak | Yes | 1 to 2 weeks (14 days) | — | |
| Person | Person | Not reported | Unclear | 1 to 2 weeks (10 days, 12 days), 2 to 4 weeks (15 days), after 4 weeks (4 weeks) | — | |
| Person | Person | "Complete disappearance of ear discharge within the period of treatment" | Unclear | 1 to 2 weeks (14 days) | — | |
| Person | Person | "No otomicroscopic sign of otorrhoea at either ear" | Yes | After 4 weeks (6 weeks, 12 weeks, 1 year) | Patients with bilateral ear disease were only counted as resolved if both ears were dry. |
Health‐related quality of life
One study reported on this outcome (Van der Veen 2007), but results were only reported as a narrative summary.
Ear pain (otalgia) or discomfort or local irritation
Five studies reported on this outcome (Baba 1982c; Esposito 1990; Fliss 1990; Onali 2018; Sambe 1977).
Hearing
Six studies reported on this outcome. Two studies performed hearing tests at diagnosis, at 8 days and at 15 days, but reported no results (de Miguel 1999; Ramos 2003). Minja 2006 performed audiometry (pure tone hearing thresholds) at three to four months of follow‐up. The authors state that the "hearing test performed before and after treatment showed that the hearing thresholds were the same and in many cases even better after the treatment". The authors of Sanchez Gonzales 2001 report that computerised audiometry was conducted, but report the results as categories of hearing loss (none, mild, moderate or severe). There is no description of the audiometric thresholds used for this classification.
Fliss 1990 reported measuring air‐conduction thresholds at 0.25 kHz, 0.5 kHz, 1 kHz, 2 kHz, 4 kHz and 8 kHz, and bone conduction thresholds at 0.5 kHz, 1 kHz and 2 kHz. The time at which these were performed is not described.
Van der Veen 2007 presented median values and interquartile ranges for air conduction hearing thresholds at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz.
Serious complications
Four studies reported on this outcome. Fliss 1990 reported that no intracranial or intratemporal complications occurred during the study period or the follow‐up period. Esposito 1990 reported that "no side effect was recorded in any patient...", but no further information was provided. One study reported on the occurrence of mastoid abscesses at 12 weeks follow‐up (Van der Veen 2007). Eason 1986 stated that "suppurative complications were limited to one case of mastoiditis and one of meningitis with focal encephalitis.” However, it was unclear whether this was detected pre‐randomisation or during/after treatment.
Ototoxicity
Six studies reported on this outcome. Two studies reported on the number of individuals with balance problems (vertigo) at seven days (Onali 2018; Ramos 2003). Ramos 2003 reported no ototoxicity, as diagnosed with audiometry tests, but provided no definition of limits. Onali 2018 stated that one episode of ototoxicity was identified. One additional study reported no incidence of ototoxicity, but no definition of this was provided (de Miguel 1999). One study reported on dizziness or vertigo, but no details were provided regarding the method used to assess this (Sanchez Gonzales 2001). The same study reported that no participants suffered with tinnitus in either treatment group. One study reported that "there was no deterioration of hearing in groups 2 and 3, as compared to group 1. Thus, no signs of ototoxicity could be found." (Minja 2006). One study reported that "no side effect was recorded in any patient...", but no further information was provided (Esposito 1990).
Excluded studies
We excluded 94 papers (86 studies) after reviewing the full text. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table. The following are the main reasons for exclusion.
We excluded five studies (six references) as the comparisons were not appropriate for this review but were relevant to another review in this suite of Cochrane CSOM reviews (Esposito 1992; Gupta 2015; Mira 1993; Povedano 1995; Yuen 1994).
We excluded 36 studies (37 references) on the basis of their study design (Arguedas 1993; Baba 1986; Baba 2008; Bakir 2013; Brook 1979; Brook 1980; Browning 1984; Chowdhury 2002; Deitmer 2002; Esposito 2000; Gehanno 1997; Hwang 2015; Jahn 1984; Jang 2004; Kadar 2003; Kenna 1986; Kothari 1969; Kovacic 1999; Kurilin 1976; Lancaster 1999; Lancaster 2003; Lang 1992; Lautala 1983; Legent 1994; Merifield 1993; Morgon 1976; Poliakova 1991; Singhal 1992; Sugiyama 1981; Sultan 2017; Sumitsawan 1995; Supiyaphun 1995; Tachibana 1986; Thomsen 1976; Van de Heyning 1986; Wintermeyer 1997).
We excluded 23 studies (26 references) due to the population characteristics included in their study (Abbott 2016; Adler 2000; Baba 1982b; Baba 1983a; Baba 1983b; Baba 1987; Berman 1990; Block 2000; Bogomil'skii 1999; Bross Soriano 1996; Granath 2007; Gyde 1981; Gyde 1982; IRCT20130427013136N6; IRCT2016082313136N4; Mendelman 1992; Mesure 1973; Principi 1995; Quick 1973; Quick 1975; Saez‐Llorens 2005; Stenstrom 1991; van Dongen 2014).
We excluded 15 studies (17 references) as the interventions were outside of our protocol (Baba 1983c; Browning 1983; Browning 1983b; CTRI/2019/09/021197; Dellamonica 1995; Fraysse 1988; ISRCTN86106121; Jiang 2016; Khanna 2000; Li 2004; NCT02592096; NCT02817347; Roydhouse 1981; Shkil' 1964; van Hasselt 1998b).
Seven studies (eight references) had multiple reasons for exclusion (Baba 1980; Fombeur 1994; Hemlin 1997; Kantawala 1976; Kashiwamura 2004; Khon 2012; Lorentzen 1978).
Risk of bias in included studies
See Figure 2 for the 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for the 'Risk of bias' summary (our judgements about each risk of bias item for each included study).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We assessed only three studies to have adequate sequence generation, which were therefore at low risk of bias (Bajwa 2018; Ghosh 2012; Van der Veen 2007). We assessed three studies to be at high risk of bias (Eason 1986; Esposito 1990; Rotimi 1990). Eason 1986 had an uneven participant distribution between groups, with the larger number of patients belonging to the more effective treatment groups. In Esposito 1990, patients had a differing prevalence of Pseudomonas between the treatment groups, and the distribution of unsuccessful antibiotic treatment was unclear between groups. In Rotimi 1990, patient characteristics were only available for people who completed follow‐up and showed imbalance in the unilateral:bilateral ratio across groups; it was unclear if this was an effect of selection or attrition bias. The remaining 12 studies did not describe the method of sequence generation and we considered them to have unclear risk of bias.
Allocation concealment
We assessed only one study to be at low risk of bias for adequate allocation concealment (Van der Veen 2007). Two studies were assessed as a high risk of bias (Ghosh 2012; Minja 2006). Ghosh 2012 used a method that could not conceal the allocation, and there was uncertainty as to whether the people completing the allocation in the study by Minja 2006 knew which group each school was going to be allocated to. The remaining 15 studies did not describe the methods used for allocation concealment, and we assessed them as at unclear risk of bias.
Blinding
Performance bias
We assessed three studies as having adequate methods for blinding (through the use of a placebo that effectively masked the interventions) and therefore at low risk of bias (Baba 1982c; Sambe 1977; Van der Veen 2007). We assessed nine studies as being at high risk of bias for being unblinded (Bajwa 2018; Eason 1986; Ghosh 2012; Minja 2006; Ramos 2003; Renukananda 2014; Rotimi 1990; Sanchez Gonzales 2001; Somekh 2000). Bajwa 2018, Eason 1986, Ramos 2003, Renukananda 2014 and Rotimi 1990 were unblinded due to the absence of an oral placebo, while Ghosh 2012 could not be conducted as a double‐blinded study due to "financial constraints and logistic problems". For Minja 2006, this was because it was not clear if participants and teachers were aware of all treatment options and how this might affect compliance of antibiotics etc. We assessed the remaining six studies as being at unclear risk of bias.
Detection bias
We assessed three studies as being at low risk of bias because they described how they had attempted to blind the outcome assessors (Baba 1982c; Sambe 1977; Van der Veen 2007). We assessed 10 studies as being at high risk of bias (de Miguel 1999; Eason 1986; Esposito 1990; Fliss 1990; Minja 2006; Nwokoye 2015; Ramos 2003; Rotimi 1990; Sanchez Gonzales 2001; Somekh 2000). For de Miguel 1999, Eason 1986, Esposito 1990, Nwokoye 2015, Ramos 2003, Rotimi 1990 and Somekh 2000, this was because no information was provided regarding who assessed the subjective outcomes. Sanchez Gonzales 2001 was a non‐blinded study and clinicians provided a subjective assessment. In Fliss 1990, there was no mention of blinding, a high number of bilateral ear participants and adaption to method due to discontinuation of a study arm resulting from a lack of efficacy. For Minja 2006, it was unclear whether assessors were aware of the treatments received. We assessed the remaining five studies as being at unclear risk of bias.
Incomplete outcome data
We assessed five studies to be at low risk of bias (Baba 1982c; Esposito 1990; Ghosh 2012; Ramos 2003; Van der Veen 2007). We assessed the risk of attrition bias to be high for eight studies (Bajwa 2018; Minja 2006; Nwokoye 2015; Picozzi 1984; Renukananda 2014; Rotimi 1990; Sambe 1977; Sanchez Gonzales 2001). For Bajwa 2018 and Renukananda 2014, participants with moderate/poor compliance were removed from the study, which made participant numbers unclear, and the authors did not provide any numerical data to describe the number of participants "cured". Minja 2006 had an uneven distribution of loss to follow‐up between groups, and this was higher for groups with more treatment interventions. The authors of Nwokoye 2015 suggested that patients could be excluded from the analysis and loss to follow‐up was not reported. Picozzi 1984 had a high rate of exclusion from analysis (25%); only those who complied were analysed and there were low compliance rates (57% and 63%). Rotimi 1990 had high loss to follow‐up with unclear distribution, difference in the proportion with bilateral condition in follow‐up, and participant numbers that were not balanced between groups. Sambe 1977 and Sanchez Gonzales 2001 had a high proportion of missing outcome data, which may have a significant impact on results. The remaining five studies were at unclear risk of bias.
Selective reporting
We assessed one study to be at low risk of bias (Ghosh 2012). We assessed three studies as being at high risk of bias as they did not fully report all the outcomes planned and measured (Bajwa 2018; Esposito 1990; Van der Veen 2007). For the remaining 14 studies, we rated the risk as unclear, either because the protocol could not be found, or because there was information suggesting certain outcomes (e.g. hearing loss) were probably measured in the trials but not reported completely or at all.
Other potential sources of bias
Only three studies described how outcomes were measured and defined for patients with bilateral ear disease (Baba 1982c; Eason 1986; Rotimi 1990). All other studies either stated that no bilateral cases were included or we assumed that the studies had randomised by patient and the number of events corresponds to patients rather than ears based on the information reported in the study.
Effects of interventions
See: Summary of findings 1 Systemic antibiotics compared to no treatment/placebo; Summary of findings 2 Systemic antibiotics compared to no treatment or placebo on top of topical antibiotics for CSOM; Summary of findings 3 Systemic antibiotics compared to no treatment or placebo on top of topical antibiotics for CSOM; Summary of findings 4 Systemic antibiotics compared to no treatment or placebo on top of topical antiseptics for CSOM
Comparison 1: Systemic antibiotics versus no treatment/placebo
A single study provided data for this comparison (Fliss 1990; 51 participants). This three‐arm trial compared treatment with mezlocillin (200 mg per kg, administered intravenously, three times daily), ceftazidime (150 mg per kg administered intravenously, three times daily) or no treatment. All patients received daily suction and debridement for seven days before entry to the trial, and only those in whom otorrhoea persisted were recruited to the study. Participants were hospitalised for the duration of the trial. Both of the antibiotics used are active against Pseudomonas.
Although the trial initially enrolled participants to three arms (mezlocillin, ceftazidime or placebo), after enrolling 33 participants the authors discontinued the placebo arm, due to lack of effect. The results presented here are the data obtained from the first 33 trial participants, according to their randomised group.
See summary of findings Table 1.
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not)
Between one week and up to two weeks
In Fliss 1990 (51 participants) the risk ratio (RR) for resolution of ear discharge at one to two weeks was higher in the antibiotic group compared to the placebo/no treatment group, but the evidence was very uncertain (RR 8.47, 95% confidence interval (CI) 1.88 to 38.21; 33 participants; 1 study; very low‐certainty evidence; Analysis 1.1).
Between two weeks and up to four weeks
The study did not report results for this time point.
After four weeks
The study did not report results for this time point.
Health‐related quality of life
The study did not report this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not report this outcome.
Hearing
Fliss 1990 (51 participants) reported measuring air conduction thresholds at 0.25 kHz, 0.5 kHz, 1 kHz, 2 kHz, 4 kHz and 8 kHz, and bone conduction thresholds at 0.5 kHz, 1 kHz and 2 kHz. The time at which these were performed is not described. The authors state that "audiometric tests did not show any worsening of the hearing during or after the antimicrobial treatment" (very low‐certainty evidence).
Serious complications
Fliss 1990 (51 participants) reported that no intracranial complications occurred during the study or the follow‐up period (very low‐certainty evidence).
Suspected ototoxicity
The study did not report this outcome.
Subgroup analysis
Although we had planned to complete subgroup analyses, as only one study was included in this comparison this was not possible.
Comparison 2: Systemic antibiotics versus no treatment/placebo (both study arms received topical antibiotics)
Six studies (490 participants) reported on this comparison (Bajwa 2018; de Miguel 1999; Esposito 1990; Onali 2018; Ramos 2003; Renukananda 2014), but only five studies (390 participants) contributed data as it was not possible to interpret the results from Bajwa 2018 (see below). All of the included studies compared oral quinolone antibiotics (five used ciprofloxacin, one used ofloxacin) to no treatment, and background treatment for all participants included topical antibiotic drops (five used ciprofloxacin, one used ofloxacin) (see Table 3). All antibiotics were active against Pseudomonas.
Both de Miguel 1999 (50 participants) and Ramos 2003 (100 participants) assigned patients to treatment with oral ciprofloxacin (500 mg every 12 hours for seven days) or no treatment. Background treatment in these studies was topical ciprofloxacin 0.2%, three ear drops every eight hours.
Esposito 1990 (40 participants) compared treatment with oral ciprofloxacin (250 mg twice daily for 5 to 10 days) to no treatment. Background treatment in this study was three drops of topical ciprofloxacin (250 μg/ml) twice a day.
Onali 2018 (100 participants) assigned participants to oral ciprofloxacin (200 mg every 12 hours for seven days) or placebo, with a background treatment of topical ciprofloxacin ear drops (three to four drops eight‐hourly, concentration was not reported).
Renukananda 2014 (100 participants) compared oral ciprofloxacin (500 mg twice a day for 14 days) to no treatment, with a background treatment of ciprofloxacin ear drops (three drops, three times a day, concentration not reported).
Bajwa 2018 reportedly included 100 participants (133 ears), however there was great uncertainty regarding the study methodology, including the number of participants, interventions used, "cure" rates and the assessment time points. Therefore the results are not currently included in the review. We have attempted to contact the study authors but are yet to receive a response.
See summary of findings Table 2.
Resolution of ear discharge at one to two weeks (whether otoscopically confirmed or not)
Between one week and up to two weeks
Five studies (390 participants) found no evidence of a difference in the resolution of ear discharge at one to two weeks for oral ciprofloxacin compared to placebo or no treatment (RR 1.02, 95% CI 0.93 to 1.12; 390 participants; 5 studies; I2 = 0%; Analysis 2.1; low‐certainty evidence).
Two of the studies had reported exactly the same percentages of "cure" across all but one of the intervention arms of the studies (de Miguel 1999; Ramos 2003). We had concerns that these could have been the same set of patients, but the authors clarified that these were different patients (and studies).
Between two weeks and up to four weeks
There were no useable data from the studies at this time point.
After four weeks
There were no useable data from the studies at this time point.
Health‐related quality of life
No study reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
Onali 2018 (100 participants) found no evidence of a difference in "mild earache" at seven days follow‐up between those allocated to ciprofloxacin or placebo (RR 1.00, 95% CI 0.21 to 4.72; 100 participants; 1 study; Analysis 2.2; very low‐certainty evidence).
One further study (Esposito 1990; 40 participants) reported that "no side effect was recorded in any patient..." but no additional information was provided, therefore it is not clear whether ear pain was specifically assessed.
Hearing
Two studies performed hearing tests at diagnosis, at eight days and at 15 days, but reported no results (de Miguel 1999; Ramos 2003).
Serious complications
One study (Esposito 1990; 40 participants) reported that "no side effect was recorded in any patient..." but no further information was provided.
Suspected ototoxicity
Three studies (de Miguel 1999; Onali 2018; Ramos 2003; 250 participants) reported on suspected ototoxicity, which showed no evidence of a difference between oral ciprofloxacin and placebo, although the confidence interval was extremely wide as only one episode of vertigo was identified (RR 3.00, 95% CI 0.13 to 71.92; 250 participants; 3 studies; Analysis 2.4; very low‐certainty evidence).
Subgroup analysis
No subgroup analysis was completed as there were no studies that were relevant for the identified subgroups:
-
High‐risk population ‐ none of the studies reported high‐risk populations as defined in our methods.
-
Patients with ventilation tubes ‐ none of the studies reported the inclusion of patients with ventilation tubes.
-
Diagnosis of CSOM ‐ although de Miguel 1999 and Ramos 2003 included mixed populations of CSOM along with ear discharge from other causes, there were no studies that included only patients with 'ear discharge' as an inclusion criterion.
-
Duration of ear discharge ‐ studies either did not report the duration of discharge, or included individuals with a wide range of discharge duration.
-
Patient age ‐ none of the studies included participants younger than two years of age, or exclusively aged two to six years.
Comparison 3: Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics plus steroids)
Three studies (203 participants) were included in this comparison, but all used different combinations of systemic antibiotics and topical antibiotic‐steroid drops.
Picozzi 1984 (40 participants) compared metronidazole (route of administration and dose not stated) to placebo for two weeks, with background treatment for all participants of gentamicin plus hydrocortisone ear drops (for four weeks; dose/frequency not reported).
Eason 1986 (62 participants) included two arms of a five‐arm trial that compared oral clindamycin (15 mg/kg per day in three divided doses) to no treatment for two weeks, with background treatment of gramicidin‐framycetin‐dexamethasone ear drops (dose/frequency not reported).
Van der Veen 2007 (101 participants) compared oral trimethoprim/sulfamethoxazole (18 mg/kg twice daily for 6 to 12 weeks, depending on response) to placebo. All participants received antibiotic‐steroid drops but the formulation varied: hydrocortisone/bacitracin/colistin eardrops were used initially, then changed to hydrocortisone/neomycin polymyxin B.
Metronidazole and clindamycin are not active against Pseudomonas, but these studies did not present results for the same outcomes therefore no meta‐analysis was possible. Trimethoprim/sulfamethoxazole does have anti‐pseudomonal activity.
See summary of findings Table 3.
Resolution of ear discharge (whether otoscopically confirmed or not)
Between one week and up to two weeks
No studies reported the outcome at this time point.
Between two weeks and up to four weeks
One study (Eason 1986; 62 participants) investigated the addition of oral clindamycin to gramicidin‐framycetin‐dexamethasone ear drops at three weeks. Although this study reported the number of participants with bilateral disease, the results were only reported according to the number of ears cured. We were unable to determine the number of participants who were cured with each intervention, therefore these data have not been included in the review.
After four weeks
Three studies (203 participants) reported at this time point, but investigated different comparisons of systemic antibiotics against placebo or no treatment when added to topical antibiotic plus steroid drops and so we have not combined the data.
One study (Picozzi 1984; 40 participants) investigated metronidazole added to gentamicin plus steroids at four weeks (RR 0.91, 95% CI 0.51 to 1.65; 30 participants; Analysis 3.1).
The second study (Van der Veen 2007; 101 participants) investigated co‐trimoxazole compared to placebo. In contrast to the other studies in this comparison, topical antibiotics and steroid eardrops were only prescribed when patients had discharge (at baseline or the 6‐ and 12‐week follow‐up appointments). It is uncertain if there is a difference between the groups at six weeks follow‐up (RR 1.54, 95% CI 1.09 to 2.16; 98 participants; Analysis 3.1; very low‐certainty evidence).
The final study (Eason 1986; 62 participants) investigated the addition of clindamycin to gramicidin‐framycetin‐dexamethasone ear drops at six weeks. Again, we were unable to present the results by participant and so the data have not been included.
Health‐related quality of life
One study (Van der Veen 2007; 101 participants) used the six‐item otitis media questionnaire and Child Health Questionnaire and a visual analogue scale measuring ear‐related quality of life, but results were only reported as a narrative summary. The authors stated that "during the study, the health‐related quality‐of‐life scores improved substantially in both the trimethoprim/sulfamethoxazole and placebo groups [...]. Mean scores for the trimethoprim/sulfamethoxazole and placebo groups for the 6‐item otitis media questionnaire, Child Health Questionnaire, and visual analog scale were the same at all visits" (very low‐certainty evidence).
Ear pain (otalgia) or discomfort or local irritation
No study reported this outcome.
Hearing
One study (Van der Veen 2007; 101 participants) reported on this outcome. Data were presented as median values and interquartile ranges. The authors state that "pure‐tone air conduction levels at 500, 1000, 2000, and 4000 Hz could be determined for 20 children in the trimethoprim/sulfamethoxazole group and 18 children in the placebo group. Although hearing levels generally improved, no differences between the groups were found" (very low‐certainty evidence).
Serious complications
Mastoid abscess
One study (Van der Veen 2007; 101 participants) reported on the occurrence of mastoid abscesses at 12 weeks follow‐up. There was one event in each treatment arm and no evidence of a difference in this outcome between those who received systemic antibiotics and those who did not (RR 1.02, 95% CI 0.07 to 15.86; 101 participants; Analysis 3.2; very low‐certainty evidence).
Eason 1986 reports one case of mastoiditis and one case of meningitis with focal encephalitis. It is not clear which group these patients were from, or whether the complications occurred pre‐ or post‐treatment.
Suspected ototoxicity
No study reported this outcome.
Subgroup analysis
Although we had planned to complete subgroup analyses, this was not possible as no meta‐analysis was performed for this comparison.
Comparison 4: Systemic antibiotics versus no treatment/placebo (both study arms had topical antiseptic plus dry mopping)
A single study (Minja 2006; 204 participants), cluster‐randomised by school, contributed data to this outcome. Individuals were allocated to treatment with amoxicillin for 10 days (treatment was according to body weight; no further details provided) or no treatment. All participants received background treatment of daily boric acid in alcohol ear drops for one month (no further information provided about concentration or dose). Amoxicillin does not have activity against Pseudomonas.
See summary of findings Table 4.
Resolution of ear discharge (whether otoscopically confirmed or not)
Between one week and up to two weeks
The study did not report the outcome at this time point.
Between two weeks and up to four weeks
It is unclear whether adding a systemic antibiotic (amoxicillin, dose unspecified) to boric acid in alcohol plus daily dry mopping changes the resolution rates at two to four weeks of follow‐up (RR 1.07, 95% CI 0.71 to 1.61; 136 participants; 1 study; Analysis 4.1). No difference was observed, but the confidence intervals were wide, indicating uncertainty in the finding.
After four weeks
It is unclear whether there is a difference in resolution of ear discharge at three to four months of follow‐up (RR 1.03, 95% CI 0.75 to 1.41; 136 participants; 1 study; Analysis 4.2; very low‐certainty evidence).
Sensitivity analysis
As Minja 2006 appeared to be a cluster‐randomised controlled trial we adjusted the results using an intra‐cluster correlation coefficient (ICC) of 0.015 in the primary analysis (136 participants), and compared this against a high ICC of 0.03 (117 participants) and no correlation (162 participants), to account for the possible correlation of results within groups. We conducted a sensitivity analysis based on the ICC used and the results are available for two to four weeks in Analysis 4.3 and after four weeks in Analysis 4.4. The sensitivity analysis indicates that the choice of ICC does not influence the overall results greatly.
Health‐related quality of life
The study did not report this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not report this outcome.
Hearing
Minja 2006 (204 participants) performed audiometry (pure tone hearing thresholds) at three to four months of follow‐up. The authors state that "hearing test performed before and after treatment showed that the hearing thresholds were the same and in many cases even better after the treatment" (very low‐certainty evidence).
Serious complications
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Minja 2006 (204 participants) stated that there were no signs or symptoms of suspected ototoxicity amongst participants in the trial (very low‐certainty evidence).
Subgroup analysis
Although we had planned to complete subgroup analyses, this was not possible as only one study was included in this comparison.
Comparison 5: Systemic quinolones versus beta‐lactams
Four studies (984 participants) reported on this comparison (Baba 1982c; Ghosh 2012; Sambe 1977; Sanchez Gonzales 2001), but only three studies (938 participants) contributed data. All compared different combinations of quinolones and beta‐lactam antibiotics.
Sambe 1977 (603 participants) compared oral pipemidic acid (500 mg four times daily) to oral aminobenzylpenicillin (500 mg four times daily) for 14 days.
Baba 1982c (305 participants) compared oral norfloxacin (200 mg four times daily) to oral aminobenzylpenicillin (200 mg four times daily) for 7 days.
Ghosh 2012 (46 participants) compared oral ciprofloxacin (500 mg twice daily) to oral cefpodoxime (200 mg twice daily) for 7 days.
Sanchez Gonzales 2001 (30 participants) compared oral levofloxacin (500 mg once daily) to co‐amoxiclav (625 mg three times daily) for 10 days.
No additional, concurrent interventions were reported for any of these studies.
The study by Baba and colleagues included a majority of individuals with CSOM, but some participants with acute suppurative otitis media (56/252 participants) (Baba 1982c). Although results were presented separately for the two groups, it was not clear that randomisation had been stratified according to the underlying diagnosis. Therefore, we have presented the overall result for the whole population, rather than the results specifically for those with CSOM.
All quinolones used in these studies are active against Pseudomonas, whilst none of the beta‐lactam antibiotics have anti‐pseudomonal activity.
Resolution of ear discharge (whether otoscopically confirmed or not)
Between one week and up to two weeks
Three studies (Baba 1982c; Sambe 1977; Sanchez Gonzales 2001; 938 participants) compared different quinolones to beta‐lactams. There was no evidence of a difference in resolution rates at one to two weeks (RR 1.14, 95% CI 0.97 to 1.35; 702 participants; 3 studies; I2 = 0%; Analysis 5.1).
Ghosh 2012 (46 participants) assessed "clinical cure" using an otological scoring system, where a score of 3 or less was reported as "cure". It is not clear whether this required complete resolution of ear discharge, therefore this result was not included in the meta‐analysis. The authors reported that 16/22 participants receiving ciprofloxacin were "cured", compared to 20/23 participants receiving cefpodoxime.
Between two weeks and up to four weeks
No studies reported results for this time point.
After four weeks
No studies reported results for this time point.
Health‐related quality of life
No study reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
Two studies reported on the number of participants in whom ear pain resolved (Baba 1982c; Sambe 1977). However, this outcome was only reported for the subgroup of participants who had ear pain at the start of the trial, therefore it could not be included in the analysis.
Hearing
The authors of Sanchez Gonzales 2001 (30 participants) report that computerised audiometry was conducted, but report the results as categories of hearing loss (none, mild, moderate or severe). There is no description of the audiometric thresholds used for this classification.
Serious complications
No study reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
One study (Sanchez Gonzales 2001; 30 participants) reported on dizziness or vertigo, but no details were provided regarding the method used to assess this. It is unclear if there was a difference in symptoms of dizziness or vertigo between the two groups due to the small number of participants and wide confidence intervals (RR 0.28, 95% CI 0.01 to 6.25; 22 participants, 1 study; Analysis 5.2). The same study reported that no participants suffered with tinnitus in either treatment group.
Subgroup analysis
We completed no subgroup analysis as there were no studies that were relevant for the identified subgroups:
-
High‐risk population ‐ none of the studies reported high‐risk populations as defined in our methods.
-
Patients with ventilation tubes ‐ none of the studies reported the inclusion of patients with ventilation tubes.
-
Diagnosis of CSOM ‐ none of the studies included only patients with 'ear discharge' as inclusion criteria.
-
Duration of ear discharge ‐ the studies did not report the duration of discharge.
-
Patient age ‐ none of the studies included participants younger than two years of age, or exclusively aged 2 to 6 years.
Comparison 6: Different beta‐lactams
Three studies (120 participants) contributed data for this comparison (Fliss 1990; Nwokoye 2015; Somekh 2000). All three trials included children only, with no adult participants.
Fliss 1990 (36 participants) was a three‐arm trial that compared treatment with mezlocillin (200 mg per kg, administered intravenously, three times daily), ceftazidime (150 mg per kg administered intravenously, three times daily) or no treatment. All patients received daily suction and debridement for seven days before entry to the trial, and only those in whom otorrhoea persisted were recruited to the study. Participants were hospitalised for the duration of the trial. Although the trial initially enrolled participants to three arms (mezlocillin, ceftazidime or placebo), after enrolling 33 participants the authors discontinued the placebo arm, due to lack of effect. The results presented here are the data obtained from the first 33 trial participants, according to their randomised group.
Somekh 2000 (30 participants) compared intravenous aztreonam (100 mg/kg/day in three divided doses) to intravenous ceftazidime (100 mg/kg/day in three divided doses) for participants with pseudomonal CSOM. All participants had concurrent daily suction and debridement until complete cessation of discharge.
Nwokoye 2015 (54 participants) compared amoxicillin alone (80 mg/kg/day) to amoxicillin plus clavulanic acid (80 mg/kg/day amoxicillin, dose of clavulanic acid not specified) for 7 to 10 days. Participants were also advised to carry out aural toileting with dry cotton wool wisps four times per day. This trial had a third study arm that was not relevant to this review, as it included treatment based on culture and antibiotic sensitivity.
Mezlocillin, ceftazidime and aztreonam all have anti‐pseudomonal activity. Amoxicillin and clavulanic acid are not active against Pseudomonas.
Resolution of ear discharge (whether otoscopically confirmed or not)
Between one week and up to two weeks
Two studies (66 participants) compared a beta‐lactam antibiotic to ceftazidime (Fliss 1990; Somekh 2000). There was no evidence of a difference in the resolution of ear discharge at one to two weeks (RR 0.83, 95% CI 0.63 to 1.10; 66 participants; 2 studies; I2 = 0%; Analysis 6.1).
One study compared amoxicillin and clavulanic acid to amoxicillin alone (Nwokoye 2015; 54 participants). Again, there was no evidence of a difference between these two interventions at one to two weeks follow‐up (RR 1.26, 95% CI 0.96 to 1.67; 54 participants; 1 study; Analysis 6.1).
Between two weeks and up to four weeks
One study (Nwokoye 2015; 54 participants) compared amoxicillin with clavulanic acid to amoxicillin alone. There is uncertainty due to the small sample size, but the resolution of ear discharge at two to four weeks was higher in the group receiving amoxicillin plus clavulanic acid (RR 1.57, 95% CI 1.05 to 2.36; 54 participants; 1 study; Analysis 6.2).
After four weeks
No study reported results for this time point.
Health‐related quality of life
No study reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
No study reported this outcome.
Hearing
No study reported this outcome.
Serious complications
Fliss 1990 (36 participants) reported that no intracranial complications occurred during the study period or the follow‐up period for the study.
Suspected ototoxicity
No study reported this outcome.
Subgroup analysis
No subgroup analysis was completed as there were no studies that were relevant for the identified subgroups:
-
High‐risk population ‐ none of the studies reported high‐risk populations as defined in our methods.
-
Patients with ventilation tubes ‐ none of the studies reported the inclusion of patients with ventilation tubes.
-
Diagnosis of CSOM ‐ although one study included individuals with ear discharge (Nwokoye 2015), these results were not pooled with other studies, therefore we could not conduct any subgroup analysis.
-
Duration of ear discharge ‐ the studies did not report the duration of discharge.
-
Patient age ‐ none of the studies included participants exclusively younger than two years of age, or aged two to six years.
Comparison 7: Lincosamides versus nitroimidazoles (both study arms also received systemic gentamicin)
A single study contributed data to this comparison. Rotimi 1990 (119 participants) randomised individuals with chronic ear discharge to one of three groups: oral clindamycin (300 mg four times daily for seven days), oral lincomycin (300 mg four times daily for seven days) (both lincosamides) or oral metronidazole (a nitroimidazole, 400 mg three times daily for seven days). All participants also received intramuscular gentamicin, 1.5 mg/kg per day in divided doses for five days. Clindamycin, lincomycin and metronidazole are not active against Pseudomonas. However, the systemic gentamicin given to all participants does have anti‐pseudomonal activity.
Resolution of ear discharge (whether otoscopically confirmed or not)
Between one week and up to two weeks
One study (119 participants) investigated the effectiveness of lincosamides compared to metronidazole, when added to gentamicin. Metronidazole was found to be more effective at resolving ear discharge at between one to two weeks (RR 0.74, 95% CI 0.55 to 1.00; 119 participants; 1 study; Analysis 7.1). The study had three intervention arms (lincomycin, clindamycin and metronidazole).
Between two weeks and up to four weeks
One study (119 participants) also found metronidazole to be more effective at two to four weeks compared to lincosamides (RR 0.72, 95% CI 0.53 to 0.98; 119 participants; 1 study; Analysis 7.2).
After four weeks
At a longer follow‐up (six weeks), one study (119 participants) found the relative effectiveness of metronidazole was maintained (RR 0.68, 95% CI 0.48 to 0.96; 119 participants; 1 study; Analysis 7.3).
Health‐related quality of life
No study reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not report this outcome.
Hearing
The study did not report this outcome.
Serious complications
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
The study did not report this outcome.
Subgroup analysis
Although we had planned to complete subgroup analyses, as only one study was included in this comparison this was not possible.
Discussion
Summary of main results
We included 18 studies (2135 participants) reporting on seven different comparisons. Due to the choice of outcome measures used in these studies and the incomplete reporting of results, for many of the proposed comparisons we were not able to find a substantial amount of evidence.
Systemic antibiotics versus no treatment/placebo (Comparison 1)
We included one study (51 hospitalised children), which was a three‐armed trial comparing treatment with mezlocillin (administered intravenously), ceftazidime (administered intravenously) or no treatment, and found that it is very uncertain whether systemic (intravenous) antibiotics increase the resolution of ear discharge at one to two weeks compared with no treatment (risk ratio (RR) 8.47, 95% confidence interval (CI) 1.88 to 38.21; 33 participants; 1 study; very low‐certainty evidence). The study did not report resolution of ear discharge beyond four weeks. The study authors reported that they did not observe any worsening of hearing as measured by air or bone audiometry during or after antimicrobial treatment, but this evidence is of very low certainty and it was not clear at which time points hearing was measured. The authors reported that no intracranial complications occurred during the study period or the follow‐up period for the study. There were no data for other outcomes: health‐related quality of life, ear pain/discomfort/irritation or suspected ototoxicity.
Systemic antibiotics versus no treatment/placebo where both study arms received topical antibiotics (Comparison 2)
We included six studies (490 participants), which compared oral quinolones to no treatment, and background treatment for all participants included topical antibiotic drops (ciprofloxacin).
The evidence suggests that oral ciprofloxacin may result in little to no difference in the resolution of ear discharge at one to two weeks compared to placebo or no treatment when a background of topical antibiotics is used (RR 1.02, 95% CI 0.93 to 1.12; 390 participants; 5 studies; I2 = 0%; low‐certainty evidence). One study reported outcomes beyond four weeks, but the results were not useable.
Three studies reported on suspected ototoxicity, with two of those specifically addressing balance problems (vertigo). These studies showed no evidence of suspected ototoxicity at seven days between oral ciprofloxacin and placebo. It is very unclear if there is a difference in suspected ototoxicity because the confidence interval was extremely wide as only one episode of vertigo was identified (RR 3.00, 95% CI 0.13 to 71.92; 250 participants; 3 studies; very low‐certainty evidence).
One study examined "mild ear ache" at seven days follow‐up for participants receiving oral ciprofloxacin compared to placebo. Due to the limited number of events, we are uncertain if there is a difference in ear pain between groups. One study reported that no side effects were reported for any participants, but no further details were provided. There were no data for health‐related quality of life or hearing.
Systemic antibiotics versus no treatment/placebo where both study arms received topical antibiotics with steroids (Comparison 3)
We included three studies that compared the effects of different systemic antibiotics against placebo or no treatment when added to topical antibiotics plus steroid drops.
One study demonstrated that the evidence is unclear as to whether the resolution of discharge at four weeks was different between the group receiving metronidazole added to gentamicin plus steroids compared to those receiving placebo with gentamicin plus steroids (RR 0.91, 95% CI 0.51 to 1.65; 30 participants; very low‐certainty evidence). Another study investigated co‐trimoxazole added to topical antibiotics plus steroids prescribed whenever patients had discharge, and found that the evidence for the resolution rate between the groups is unclear at six weeks follow‐up (RR 1.54, 95% CI 1.09 to 2.16; 98 participants; very low‐certainty evidence).
One study narratively reported substantial improvement in health‐related quality of life scores in both the intervention and placebo groups (very low‐certainty evidence). This study also reported on hearing and occurrence of mastoid abscesses and average hearing level, with no significant differences reported between intervention and placebo groups (very low‐certainty evidence). Another study reported that "suppurative complications were limited to one case of mastoiditis and one of meningitis with focal encephalitis", but it was unclear whether these were detected before randomisation, or during/after treatment. There were no data for ear pain/discomfort/irritation or suspected ototoxicity.
Systemic antibiotics versus no treatment/placebo where both study arms had topical antiseptic plus dry mopping (Comparison 4)
We included a single study that reported the effects of treatment with amoxicillin for 10 days or no treatment in children. All participants received background treatment of daily boric acid in alcohol ear drops for one month (no further information was provided about concentration or dose). The study was a cluster‐randomised study by school where all participants in the same school were allocated to the same treatment, which was accounted for in the analysis.
Adding a systemic antibiotic (amoxicillin, dose unspecified) to a topical antiseptic (boric acid) plus daily dry mopping did not provide a difference in the resolution rates at two to four weeks follow‐up (RR 1.07, 95% CI 0.71 to 1.61; 136 participants; 1 study) or at three to four months after treatment (RR 1.03, 95% CI 0.75 to 1.41; 136 participants; 1 study; very low‐certainty evidence). The study did not report resolution of ear discharge between one to two weeks.
The authors reported no difference in hearing thresholds between groups (very low‐certainty evidence), and no signs or symptoms of suspected ototoxicity (very low‐certainty evidence) were reported amongst the participants in the trial. There were no data for other outcomes: health‐related quality of life, ear pain/discomfort/irritation or serious complications.
Systemic quinolones versus beta‐lactams (Comparison 5)
We included four studies that compared different combinations of quinolones and beta‐lactam antibiotics. Three studies compared different quinolones to beta‐lactams but there was no evidence of a difference in resolution of ear discharge at one to two weeks (RR 1.14, 95% CI 0.97 to 1.35; 702 participants; 3 studies; I2 = 0%). No additional, concurrent interventions were reported for any of these studies. No studies reported resolution of ear discharge beyond four weeks.
One study reported that audiometry was conducted but did not report hearing thresholds. The same study reported no difference in symptoms of dizziness or vertigo between groups and that no participants reported tinnitus. There were no data for health‐related quality of life or serious complications.
Different beta‐lactams (Comparison 6)
We included three studies that compared treatment with different beta‐lactams or no treatment.
Two studies compared a beta‐lactam antibiotic to ceftazidime. There was no evidence of a difference in the resolution of ear discharge at one to two weeks (RR 0.83, 95% CI 0.63 to 1.10; 66 participants; 2 studies; I2 = 0%). One study compared amoxicillin and clavulanic acid to amoxicillin alone. Again, there was no evidence for a difference between these two interventions at one to two weeks follow‐up (RR 1.26, 95% CI 0.96 to 1.67; 54 participants; 1 study). One study compared amoxicillin with clavulanic acid to amoxicillin alone and demonstrated that resolution of ear discharge at two to four weeks was higher in the group receiving amoxicillin plus clavulanic acid (RR 1.57, 95% CI 1.05 to 2.36).
One study compared mezlocillin to ceftazidime (Fliss 1990), but did not demonstrate a difference in resolution of ear discharge at two to four weeks (RR 1.00, 95% CI 0.90 to 1.11). No studies reported resolution of ear discharge beyond four weeks.
One study reported that no intracranial complications occurred during the study period or the follow‐up period for the study. There were no data for other outcomes: health‐related quality of life, ear pain/discomfort/irritation, suspected ototoxicity or hearing outcomes.
Lincosamides versus nitroimidazoles where study arms also received systemic gentamicin (Comparison 7)
We included a single study that randomised individuals to one of three groups: oral clindamycin (300 mg four times daily for seven days), oral lincomycin (300 mg four times daily for seven days) or oral metronidazole (400 mg three times daily for seven days). All participants also received intramuscular gentamicin. Metronidazole was found to be more effective at resolving ear discharge between one to two weeks (RR 0.74, 95% CI 0.55 to 1.00; 119 participants) and at two to four weeks (RR 0.72, 95% CI 0.53 to 0.98; 119 participants), and after four weeks (at six weeks) the relative effectiveness of metronidazole was maintained (RR 0.68, 95% CI 0.48 to 0.96; 119 participants). However, these results are very uncertain as they are based on a single study with high risk of bias (see Figure 3).
There were no data for other outcomes: health‐related quality of life, serious complications, ear pain/discomfort/irritation, suspected ototoxicity or hearing outcomes.
Overall completeness and applicability of evidence
The overall completeness of the evidence base was poor. Three comparisons included only a single study. All studies were conducted at tertiary or secondary care centres including hospital departments and specialist clinics. The majority of studies were published at least 15 years ago (and up to 30 years ago). The diagnostic criteria used to identify CSOM were often unclear or not reported. Three studies reported including a mixed population of participants having ear discharge, which may not be due to CSOM. One study stated that individuals with CSOM were included, but this study also included a number of individuals with alternative diagnoses of ear pain, including cholesteatoma and post‐surgical ear discharge. Another study included individuals with chronic or acute suppurative otitis media. Seven studies reported otoscopic confirmation of CSOM, whilst in nine studies this was unclear or not reported. Six studies reported the inclusion of children under two years of age, but we were unable to extract data specific to this age group. This leaves us with limited information on this important patient group. Only one study included participants classed as 'high‐risk' in our protocol. Patients in these high‐risk groups can be a challenge for clinicians to treat effectively and evidence to support best‐practice interventions for these people is needed. Studies were conducted in every WHO region (Africa, 1; Americas, 1; South‐East Asia, 2; Europe, 7; Eastern Mediterranean, 2; Western Pacific, 3). However, the majority of studies were conducted in high‐resource settings in countries in Western Europe, East Asia and the Western Pacific, which have a below average estimated incidence of CSOM: fewer than four cases per thousand people (Monasta 2012).
There is very little evidence by which to ascertain the relative benefits of different antibiotics, or one antibiotic compared to placebo. We identified a single study that compared systemic antibiotics to placebo (with no concurrent, background treatment). The antibiotics in this study were administered intravenously, and only 33 participants were included. The evidence for any benefit of systemic antibiotics compared to placebo with no additional treatment is therefore of very low certainty.
We identified low‐certainty evidence to suggest that the addition of systemic (oral) antibiotics to topical antibiotics may lead to little or no improvement in the resolution of ear discharge at one to two weeks. Therefore, we are not certain whether systemic antibiotics provide any additional benefit when used with topical antibiotics.
Whilst there were no significant differences reported in mean hearing levels or suspected worsening of audiological measurements, this outcome was poorly reported across all studies. The effectiveness of systemic antibiotics is likely to be influenced by the sensitivity of the pathogenic micro‐organisms to that antibiotic. We were unable to carry out a subgroup analysis of the spectrum of antibiotic activity as the data were either not in the included studies or heterogeneity was not observed, which leaves us with no information on this aspect of antibiotic treatment. Aural toileting prior to application of topical antibiotics may also influence the effectiveness of background topical antibiotic treatment. All the studies included had limited use of aural toileting, either only mentioning microsuction in the initial assessment (two studies), or not mentioning any form of aural toileting (four studies).
There were very few data for outcomes other than resolution of ear discharge. One study reported health‐related quality of life, but results were only reported as a narrative summary. Adverse events, suspected ototoxicity and serious complications were all poorly reported. The length of follow‐up in all studies was between 10 days and one year, meaning that there was limited evidence regarding the long‐term effectiveness of systemic antibiotics for the resolution of discharge for people with CSOM.
This review did not assess more generic adverse effects of systemic antibiotics, such as gastrointestinal disturbance. This was in part due to the well‐recognised existence of these adverse events, which are specified in the product information sheets available for individual antibiotics. It was also because we considered that they would be poorly reported in the studies, which focused on ear complications. However, we are aware that the adverse effects associated with systemic antibiotics are numerous, and sometimes severe. Therefore this should be taken into account when making a decision regarding treatment, particularly when the evidence of efficacy for that treatment is sparse or negligible.
Quality of the evidence
The certainty of the evidence for all outcomes in these comparisons was low or very low (GRADE assessment). This was mainly due to two factors: the risk of bias in the studies and imprecision. In many cases the results were imprecise due to the small number of participants available for analysis (resulting in large confidence intervals). There were important limitations in the methods of study conduct and reporting in nearly all of the studies. Fifteen of the studies had unclear or high risk of bias in the randomisation process and allocation concealment, and none of the studies blinded participants or study personnel to treatment group.
Accuracy of the diagnosis was also a potential issue throughout the studies included in this review. Of the 18 included studies, only seven described the use of otoscopic confirmation of resolution of discharge. This may have impacted on the accuracy of the diagnostic outcome and therefore the response to treatment.
Potential biases in the review process
Within this series of CSOM Cochrane Reviews the potential for publication bias has been identified as an issue. In some reviews, unpublished studies were found and included in the review and there is a suspicion that further unpublished trials may have been completed (Brennan‐Jones 2020b; Head 2020a; Head 2020b). It is unknown whether this is a risk with this review.
Most studies in the field of CSOM have not addressed the issue of how patients with bilateral ear disease have be counted when outcomes are reported or defined, or whether the correlation of results between the ears of an individual has been accounted for. Given the little information in the included studies, we have had to make an assumption that all the numbers reported represent number of patients rather than ears, for both the number of people randomised and the event (outcome).
By only analysing the results from studies that provided the results by person, there was one study (reporting results by ear) that we could not use for the primary outcome. This reduced the amount of data that we could analyse. However, as we know that the correlation of results between ears is likely to be high, we felt that the inclusion of the results of both ears in the analysis was likely to lead to double counting and results that could lead to spurious conclusions.
Agreements and disagreements with other studies or reviews
This review is part of a series of Cochrane Reviews on CSOM (Bhutta 2020; Brennan‐Jones 2020a; Brennan‐Jones 2020b; Chong 2018a; Chong 2018b; Head 2020a; Head 2020b).
Two other reviews evaluated whether antibiotics are effective and provide information that is complementary to the findings of this review. In one review, topical antibiotics were compared against placebo and other types of topical antibiotics. Despite the serious limitation of evidence, the review suggested that topical antibiotics (aminoglycosides and quinolones) may be effective (compared against placebo) as a single treatment, and when used on top of another potentially effective treatment (such as systemic antibiotics) (CSOM‐1, Brennan‐Jones 2020a). Given the very low certainty of the evidence included in this review, it was not clear whether systemic antibiotics add any benefit to topical antibiotics alone, in terms of resolution of ear discharge.
The addition of topical steroids to topical antibiotics did not seem to enhance the effectiveness of topical antibiotics and there was little information on the adverse effects (Brennan‐Jones 2020b). The other reviews in this series did not find any good evidence that aural toileting and antiseptics are effective on their own; they are likely to be less effective than topical antibiotics.
There are few previous reviews or guidelines for CSOM. The WHO in 2004 suggested that first‐line treatment of CSOM should comprise aural toilet and topical antibiotic drops, with second‐line treatment comprising an alternative topical antibiotic (guided by the results of microbiological culture) or parenteral antibiotics (WHO 2004). The Australian government recommendations from 2010 for the treatment of Aboriginal and Torres Strait islanders gave similar recommendations, with first‐line treatment comprising aural toilet (or antiseptic washout) followed by topical antibiotics, and second‐line treatment with parenteral antibiotics (Morris 2010). An expert panel of the American Academy of Otolaryngologists in 2000 came to a similar conclusion (Hannley 2000).
Although we planned subgroup analyses for different participant characteristics (age, high‐risk, ventilation tubes), treatment duration and spectrum of antibiotic activity, these were not carried out either because the data were not available or heterogeneity was not observed.
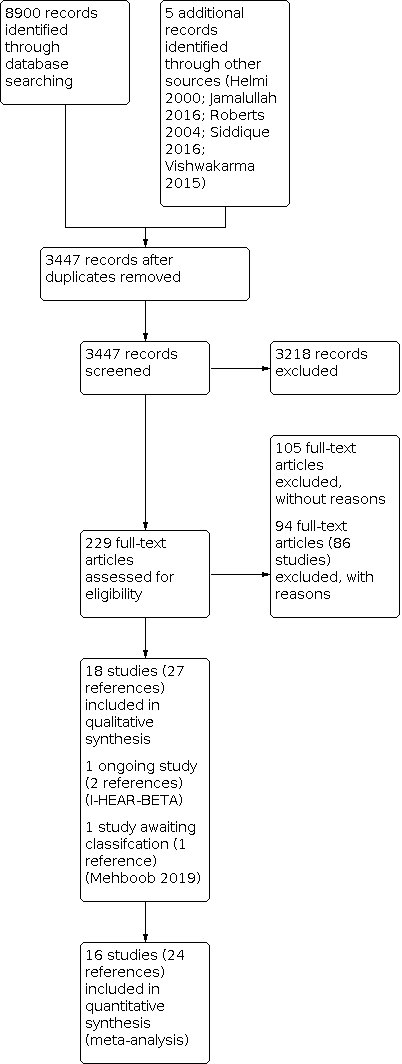
Study flow diagram

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Systemic antibiotics versus no treatment/placebo, Outcome 1: Resolution of ear discharge at 1 to 2 weeks

Comparison 2: Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics), Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 2: Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics), Outcome 2: Ear pain

Comparison 2: Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics), Outcome 3: Serious complications

Comparison 2: Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics), Outcome 4: Ototoxicity

Comparison 3: Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics plus steroids), Outcome 1: Resolution of ear discharge (after 4 weeks)

Comparison 3: Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics plus steroids), Outcome 2: Serious complications

Comparison 4: Systemic antibiotics versus no treatment/placebo (both study arms had topical antiseptic plus dry mopping), Outcome 1: Resolution of ear discharge at 2 to 4 weeks

Comparison 4: Systemic antibiotics versus no treatment/placebo (both study arms had topical antiseptic plus dry mopping), Outcome 2: Resolution of ear discharge after 4 weeks

Comparison 4: Systemic antibiotics versus no treatment/placebo (both study arms had topical antiseptic plus dry mopping), Outcome 3: Sensitivity analysis: Resolution of ear discharge at 2 to 4 weeks

Comparison 4: Systemic antibiotics versus no treatment/placebo (both study arms had topical antiseptic plus dry mopping), Outcome 4: Sensitivity analysis: Resolution of ear discharge after 4 weeks

Comparison 5: Quinolones versus beta‐lactams, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 5: Quinolones versus beta‐lactams, Outcome 2: Suspected ototoxicity

Comparison 6: Different beta‐lactams, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 6: Different beta‐lactams, Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 7: Lincosamides versus nitroimidazoles (both study arms also received gentamicin), Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 7: Lincosamides versus nitroimidazoles (both study arms also received gentamicin), Outcome 2: Resolution of ear discharge (2 to 4 weeks)

Comparison 7: Lincosamides versus nitroimidazoles (both study arms also received gentamicin), Outcome 3: Resolution of ear discharge (after 4 weeks)
| Systemic antibiotics compared to no treatment/placebo | |||||||
| Patient or population: children with CSOM | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without systemic antibiotics | With systemic antibiotics | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Assessed with: unclear if otoscopically confirmed | RR 8.47 | 33 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of systemic antibiotics (mezlocillin or ceftazidime) on the resolution of ear discharge at one to two weeks, as compared to placebo. | ||
| 8.3% | 70.6% | 62.3% more | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study reported this outcome at this time point. | — | — | ||||
| Health‐related quality of life | No study reported this outcome. | — | — | ||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | — | — | ||||
| Hearing Assessed with: air conduction and bone conduction | — | 33 | Fliss 1990 reported measuring air conduction thresholds at 0.25 kHz, 0.5 kHz, 1 kHz, 2 kHz, 4 kHz and 8 kHz, and bone conduction thresholds at 0.5 kHz, 1 kHz and 2 kHz. The time at which these were performed is not described. The authors state that "audiometric tests did not show any worsening of the hearing during or after the antimicrobial treatment". | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of systemic antibiotics (mezlocillin or ceftazidime) on hearing. | ||
| Serious complications ‐ during 6 months of follow‐up Assessed with: unclear | — | 33 | Fliss 1990 reported that no intracranial complications occurred during the study or during the follow‐up period. | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of systemic antibiotics (mezlocillin or ceftazidime) on serious complications. | ||
| Suspected ototoxicity | No study reported this outcome. | — | — | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to risk of bias (unclear methods for randomisation, allocation concealment or blinding, and placebo arm of the trial discontinued early due to lack of effect). 2Downgraded by two levels due to imprecision (very small study with only 33 participants resulting in wide confidence intervals). 3Downgraded by one level for indirectness (single study including children who were hospitalised, who did not respond to an intensive aural toileting regimen. This study involves inpatient use of mezlocillin and ceftazidime, which are broad‐spectrum antibiotics only administered parenterally (intramuscular or intravenous). It is unclear if this finding is applicable to other more commonly available antibiotics). 4Downgraded by two levels due to imprecision (very small study and numerical results were not reported for this outcome). | |||||||
| Systemic antibiotics compared to no treatment or placebo on top of topical antibiotics for CSOM | |||||||
| Patient or population: people (of any age) with CSOM | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without systemic antibiotics | With systemic antibiotics | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Assessed with: 3 RCTs otoscopically confirmed, 2 RCTs unclear | RR 1.02 | 390 | Study population | ⊕⊕⊝⊝ | The evidence suggests that adding systemic ciprofloxacin to topical antibiotics may result in little to no difference in the resolution of ear discharge at 1 to 2 weeks, when compared to topical antibiotics alone. | ||
| 76.9% | 78.5% | 1.5% more | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study reported this outcome at this time point. | — | — | ||||
| Health‐related quality of life | No study reported this outcome. | — | — | ||||
| Ear pain (otalgia) or discomfort or local irritation ‐ measured at 1 week Assessed with: self‐reported | RR 1.00 | 100 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on ear pain, discomfort or local irritation. | ||
| 6.0% | 6.0% | 0.0% fewer | |||||
| Hearing | No study reported this outcome. | — | — | ||||
| Serious complications ‐ measured at 19 to 24 days Assessed with: unclear | — | 40 | One study reported that "no side effect was recorded in any patient..." but no further information was provided. | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on serious complications. | ||
| Suspected ototoxicity ‐ measured at 10 days to 3 weeks Assessed with: unclear | RR 3.00 | 250 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on ototoxicity. | ||
| 0.8% | 2.4% | 1.6% more | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to risk of bias: four studies did not provide adequate information about randomisation or allocation concealment. Four studies did not provide any information about blinding of participants and personnel. One study was at high risk of missing outcome data, and two studies were at high risk of selective reporting. 2Downgraded by two levels due to risk of bias. Study did not provide any details on the method for random sequence generation, or allocation concealment. It was unclear if the methods for blinding were adequate, and no published study protocol was identified. 3Downgraded by one level due to imprecision (single study with small sample size and wide confidence interval). 4Downgraded to very low‐certainty evidence: downgraded by two levels due to risk of bias (study was judged to be at high risk of bias for randomisation, blinding and selective outcome reporting; study was at unclear risk of bias for allocation concealment); downgraded by one level due to imprecision (small sample size). 5Downgraded by one level due to risk of bias. Insufficient information was provided about randomisation and allocation concealment. One study was at high risk of performance and two studies were at high risk of detection bias. There was an unclear risk of bias from selective reporting as no published protocols were identified for the studies. 6Downgraded by two levels due to imprecision: wide confidence intervals in the effect estimate and a low event rate with a small sample size. | |||||||
| Systemic antibiotics compared to no treatment or placebo on top of topical antibiotics for CSOM | |||||||
| Patient or population: people with CSOM; one study included patients of unknown age; one study included children with CSOM who had not responded to initial antibiotic treatment Setting: unknown setting or tertiary care centres in the UK and the Netherlands | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without systemic antibiotics | With systemic antibiotics | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks | No study reported this outcome. | — | — | ||||
| Resolution of ear discharge ‐ measured after 4 weeks (4 weeks) (Metronidazole plus gentamicin‐steroid drops compared to gentamicin‐steroid alone) Assessed with: unclear if otoscopically confirmed | RR 0.91 | 30 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding metronidazole to gentamicin plus hydrocortisone ear drops on resolution of ear discharge after 4 weeks. | ||
| 62.5% | 56.9% | 5.6% fewer (30.6 fewer to 40.6 more) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks (6 weeks) (Oral trimethoprim/sulfamethoxazole plus topical antibiotics and steroid ear drops compared to topical antibiotic and steroid ear drops alone) Assessed with: otoscopically confirmed | RR 1.54 (1.09 to 2.16) | 98 (1 RCT) | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding oral trimethoprim/sulfamethoxazole to topical antibiotic and steroid ear drops on resolution of ear discharge after 4 weeks. | ||
| 47.1% | 72.5% (51.3 to 100%) | 25.4% more (4.2% more to 54.6% more) | |||||
| Health‐related quality of life ‐ unclear follow‐up period (Oral trimethoprim/sulfamethoxazole plus topical antibiotics and steroid ear drops compared to topical antibiotic and steroid ear drops alone) Assessed with the 6‐item otitis media questionnaire, Child Health Questionnaire and a visual analogue scale measuring ear‐related quality of life | — | 101 (1 RCT) | Results were only reported as a narrative summary. The authors stated that "during the study, the health‐related quality‐of‐life scores improved substantially in both the trimethoprim/ sulfamethoxazole and placebo groups [...]. Mean scores for the trimethoprim/ sulfamethoxazole and placebo groups for the 6‐item otitis media questionnaire, Child Health Questionnaire, and visual analog scale were the same at all visits." | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding oral trimethoprim/sulfamethoxazole to topical antibiotic and steroid ear drops on health‐related quality of life. | ||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | — | — | ||||
| Hearing ‐ unclear follow‐up period (Oral trimethoprim/sulfamethoxazole plus topical antibiotics and steroid ear drops compared to topical antibiotic and steroid ear drops alone) Assessed with pure‐tone air conduction levels at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz | — | 38 (1 RCT) | Results were only reported as a narrative summary. The authors stated "Pure‐tone air conduction levels at 500, 1000, 2000, and 4000 Hz could be determined for 20 children in the trimethoprim/ sulfamethoxazole group and 18 children in the placebo group. Although hearing levels generally improved, no differences between the groups were found" | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding oral trimethoprim/sulfamethoxazole to topical antibiotic and steroid ear drops on hearing. | ||
| Serious complications ‐ mastoid abscess measured at 12 weeks Assessed with: unclear | RR 1.02 | 101 | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of adding systemic ciprofloxacin to topical antibiotics on ototoxicity. | ||
| 2.0% | 2.0% | 0.0% fewer (1.8 fewer to 29.1 more) | |||||
| Suspected ototoxicity | No study reported this outcome. | — | — | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to risk of bias: the study was only presented as an abstract and did not present sufficient information to assess the risk of bias across any of the study characteristics. Downgraded by two levels for imprecision: the results are from one small study (30 participants) and the confidence intervals are wide. 2Downgraded by one level due to imprecision: the results are from one small study (98 participants). 3 Downgraded by two levels for indirectness: the results are from one study conducted in children with recalcitrant CSOM of which 60% had grommets in place at the start of the study. This may not represent the target population. 4Downgraded by two levels due to imprecision: the results are only reported as a narrative and come from one small study (101 participants). 5Downgraded by two levels due to imprecision: the results are only from one small study (101 participants) with one event in each arm resulting in very wide confidence intervals. | |||||||
| Topical antiseptic compared to no treatment for chronic suppurative otitis media | |||||||
| Patient or population: schoolchildren with CSOM Setting: community setting, Malawi | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without topical antiseptic | With topical antiseptic | Difference | |||||
| Resolution of ear discharge ‐ measured between 1 week and up to 2 weeks | No study reported this outcome. | — | |||||
| Resolution of ear discharge (4 weeks or more) ‐ measured at 3 to 4 months Assessed with: otoscopically confirmed | RR 1.03 | 136 (1 RCT) | Study population | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding systemic amoxicillin to boric acid eardrops and dry mopping on resolution of ear discharge after 4 weeks. | ||
| 54.5% | 56.2% | 1.6% more | |||||
| Health‐related quality of life | No study reported this outcome. | — | |||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | — | |||||
| Hearing ‐ measured at 3 to 4 months Assessed with: pure tone audiometry | — | 204 (1 RCT) | Results were only presented as a narrative summary. The authors state "hearing test performed before and after treatment showed that the hearing thresholds were the same and in many cases even better after the treatment" | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding systemic amoxicillin to boric acid eardrops and dry mopping on hearing. | ||
| Serious complications | No study reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity | — | 204 (1 RCT) | Results were only presented as a narrative summary. The authors state that "no signs of ototoxicity could be found." | ⊕⊝⊝⊝ | The evidence is very uncertain about the effects of adding systemic amoxicillin to boric acid eardrops and dry mopping on suspected ototoxicity. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded by two levels due to study limitations (risk of bias) because of concerns about randomisation, blinding, attrition bias and selective reporting. Downgraded by one level due to imprecision as there was one small study (136 participants) with wide confidence intervals. 2Downgraded by two levels due to study limitations (risk of bias) because of concerns about randomisation, blinding, attrition bias and selective reporting. Downgraded by two levels due to imprecision as results come from one small study (204 participants) and numeric results were not presented for this outcome. | |||||||
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
|---|---|---|---|---|---|
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
| CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2020a). CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a). CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b). CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2020b). CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2020a). CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2020b). CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2020). | |||||
| Class of antibiotics | Examples | Route of administration |
|---|---|---|
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
| Ref ID (no. participants) | Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background treatment | Notes |
|---|---|---|---|---|---|---|---|---|
| 1. Systemic antibiotics versus no treatment/placebo | ||||||||
| (n = 51) | Israel, tertiary hospital | Otorrhoea in children with CSOM for at least 2 months with no response to daily microsuction and debridement for 7 days Age range 11 months to 12 years | Intravenous (IV) mezlocillin, 200 mg/kg given in 3 divided doses daily; or IV ceftazidime 150 mg/kg given in 3 divided doses daily | No treatment | Until 3 days after resolution of discharge, up to maximum 3 weeks | 6 months | Daily suction and debridement | The first 19 patients in the trial did not receive additional antibiotics. The remaining 32 patients received daily prophylactic amoxicillin for at least 2 months after hospital discharge |
| 2. Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics) | ||||||||
| (n = 100) | Pakistan, ENT outpatient department | Severe episode of chronic suppurative otitis media (mucopurulent ear discharge > 4 weeks with central tympanic membrane perforation) Age range 20 to 69 years | Oral ofloxacin 500 mg, 2 times a day | No treatment | 14 days | 6 weeks | Topical ofloxacin (concentration not reported), 3 drops/3 times a day PLUS dry mopping prior to instilling ear drops | — |
| (n = 50) | Spain, general | Chronic otitis media, presenting with chronic otorrhoea as a major symptom Mean age 39.6 years (but 68% were children) | Oral ciprofloxacin 500 mg/12 hours | No treatment | 7 days | 15 days | Topical ciprofloxacin (0.2%) 3 drops/8 hours PLUS aspiration and cleaning of ear secretions before starting treatment | Part of a 5‐arm trial |
| (n = 40) | Italy, University clinic | Mild or moderate chronic OM in the acute stage Mean age 38 years | Oral ciprofloxacin 250 mg twice daily | No treatment | At least 5 days If not resolved by 5 days, interventions were continued for a maximum of 10 days | 4 weeks | Topical ciprofloxacin 250 µg/ml in saline solution, 3 drops twice daily | Part of a 3‐arm trial |
| (n = 100) | Pakistan, hospital | Tubotympanic type CSOM Mean age 33.2 years | Oral ciprofloxacin 200 mg every 12 hours | Oral placebo every 12 hours | 7 days | 14 days | Topical ciprofloxacin (concentration not reported) 3 times a day PLUS aural hygiene and water prevention | — |
| (n = 100) | Spain, ENT department of tertiary | Chronic otorrhoea (> 6 weeks), or recurrent sporadic otorrhoea (> 3 episodes in the last year) Age range 5 to 73 years; 12% of children under 14 years | Oral ciprofloxacin 500 mg 12‐hourly | No treatment | 7 days | 10 days | Topical ciprofloxacin 0.2% 0.5 ml 8‐hourly | Part of a 5‐arm trial |
| (n = 100) | India, ENT outpatient department of tertiary hospital | Active ear discharge (mucopurulent or purulent) otorrhoea of more than 3 weeks duration, with a tympanic membrane perforation Age range 20 to 69 years | Oral ciprofloxacin 500 mg twice daily | No treatment | 14 days | 8 weeks | Topical ciprofloxacin (concentration not reported), 3 drops 3 times a day PLUS dry mopping before instilling ear drops Water prevention was advised | |
| 3. Systemic antibiotics versus no treatment/placebo (both study arms had topical antibiotics plus steroids) | ||||||||
| (n = 62) | Solomon Islands, hospital with community screening | CSOM with otorrhoea for more than 3 months and tympanic membrane perforation Mean age 5.4 years | Oral clindamycin (15 mg/kg/day) in 3 divided daily doses | No treatment | 6 weeks | 6 weeks | Topical Sofradex ear drops (concentration or frequency not reported) PLUS aural toilet 4 times per day | Part of a 5‐arm trial |
| (n = 40) | Unclear location ‐ researchers from United Kingdom | Active chronic otitis media Participant information not reported | Metronidazole (route, dose and frequency of administration not reported) | Placebo | 2 weeks | 4 weeks | Gentamicin‐hydrocortisone ear drops (dose and frequency not reported) for 4 weeks PLUS self‐mopping | — |
| (n = 101) | The Netherlands, | Chronic otitis media that had failed conventional therapy (topical/short‐term systemic antibiotics) Age range 1 to 12, median 4 years | Oral trimethoprim/sulfamethoxazole 18 mg/kg twice daily | Oral placebo twice daily | 6 weeks, or 12 weeks if there was still otorrhoea at 6 weeks | 1 year | Hydrocortisone/bacitracin/colistin Ear drops were given at baseline for 7 to 10 days, and repeated at 6 and 12 weeks if otorrhoea was present at these study visits | — |
| 4. Systemic antibiotics versus no treatment/placebo (both study arms had topical antiseptic plus dry mopping) | ||||||||
| (n = 204) | Tanzania, schools (community) | Children with history of ear discharge for 3 months or more Mean age 11.8 years | Amoxicillin (dose, frequency, route of administration not reported) | No treatment | 10 days | 3 to 4 months | Boric acid in alcohol (concentration and frequency not reported) ear drops for one month PLUS daily aural toilet (dry mopping) | Part of a 3‐arm cluster‐randomised trial |
| 5. Quinolones versus beta‐lactams | ||||||||
| (n = 305) | Japan, university and general hospitals | Acute suppurative otitis media or acute exacerbation of chronic otitis media Mean age not reported, study inclusion if over 15 years | Oral norfloxacin 200 mg 4 times a day | Oral aminobenzylpenicillin 500 mg 4 times a day | 7 days | 2 weeks | None | — |
| (n = 46) | India, ENT outpatient department of tertiary care teaching hospital | Tubotympanic type CSOM (acute exacerbation of longstanding chronic suppuration of middle ear and deafness in adults) Age range 18 to 60 years | Ciprofloxacin 500 mg twice daily | Cefpodoxime 200 mg twice daily | 7 days | 14 days | None | — |
| (n = 603) | Japan, university and general hospitals | Suppurative otitis media and tympanic membrane perforation Mean age not reported, study inclusion if 15 years or older | Oral pipemidic acid 500 mg, 4 times a day | Oral aminobenzyl penicillin 500 mg, 4 times a day | 14 days | 14 days | None | — |
| (n = 30) | Mexico, regional hospital | CSOM Mean age 38 (range 26 to 60) | Oral levofloxacin 500 mg once daily | Oral amoxicillin‐clavulanic acid 500/125 mg, every 8 hours | 10 days | Up to 25 days | None | — |
| 6. Different beta‐lactams | ||||||||
| (n = 36) | Israel, tertiary hospital | Otorrhoea in children with CSOM for at least 2 months, no response to daily microsuction and debridement for 7 days Mean age not reported (range 11 months to 12 years) | Intravenous (IV) mezlocillin 200 mg/kg given in 3 divided doses daily | IV ceftazidime 150 mg/kg given in 3 divided doses daily | Until 3 days after resolution of discharge, up to maximum 3 weeks | 6 months | Daily suction and debridement | The first 19 patients in the trial did not receive additional antibiotics. The remaining 32 patients received daily prophylactic amoxicillin for at least 2 months after hospital discharge. |
| (n = 30) | Israel, paediatric infectious disease unit at medical centre | Pseudomonal CSOM Mean age 4.2 years (range 1 to 12) | IV aztreonam 100 mg/kg/day, given in 3 divided doses daily | IV ceftazidime 100 mg/kg/day, given in 3 divided doses daily | At least 10 days. Treatment continued until 3 days after complete cessation of discharge. | 3 months | Daily suction and debridement | — |
| (n = 54) | Nigeria, University teaching hospital | Otorrhoea for at least 3 months Age range 5 months to 10 years; 63% under 2 years | Oral amoxicillin‐clavulanate (amoxicillin 80 mg/kg/day in 2 divided doses daily; clavulanate acid dose and frequency not reported) | Oral amoxicillin 80 mg/kg/day, given in 2 divided doses daily | 7 to 10 days | Not reported | Aural toilet with warm saline was advised 4 times daily using dry cotton wool wisps | Part of a 3‐arm trial (third arm involved treatment based on culture and antibiotic sensitivity results) |
| 7. Lincosamides versus nitroimidazoles(both study arms also received gentamicin) | ||||||||
| (n = 119) | Nigeria, University teaching hospital | Mucopurulent ear discharge, perforated tympanic membrane, and associated hyperaemic and oedematous middle ear mucosa Age range 2 weeks to > 40 years | Oral clindamycin sulfate capsules (300 mg) or oral lincomycin (300 mg), 4 times a day | Oral metronidazole 400 mg 3 times a day | 7 days | 6 weeks | Intramuscular (IM) gentamicin 1.5 mg/kg/day in divided doses for 5 days PLUS suction and cleaning of the external meatus Self‐cleaning of ears with cotton wool buds twice a day | Part of 4‐arm trial |
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
|---|---|---|---|---|---|---|
| Person | Person | Resolution of ear discharge | Unclear | 1 to 2 weeks (7 days) | The worst affected ear was selected as the study ear. When both sides were equal, the right side was selected. | |
| Person | Person | "Cured" as non‐existence of the otorrhoea or otoscopically dormant (no discharge pooling; non‐swollen middle ear mucosa) or existence of serous mucous otorrhoea with bacteriological culture negative after therapy period | Unclear | 1 to 2 weeks (2 weeks), 2 to 4 weeks (3 weeks), after 4 weeks (8 weeks) | — | |
| Person | Person | "Global index of clinic microbiological cure" | Yes | 1 to 2 weeks (7 days) | — | |
| Person | Ear | "Dry" or "not discharging" | Yes | 2 to 4 weeks (3 weeks), after 4 weeks (6 weeks) | Bilateral ears counted separately. Results not used as it was not possible to account for correlation between ears due to bilateral disease. | |
| Person | Person | "Clinically cured" | Unclear | 1 to 2 weeks (6 to 11 days), 2 to 4 weeks (19 to 24 days) | The 1‐ to 2‐week outcome was examined but not reported | |
| Person | Person | Complete resolution of ear discharge | Yes | 1 to 2 weeks (9 days), 2 to 4 weeks (18 days) | Time points unclear | |
| Person | Person | “Clinical cure” if the otological symptom score was < 3 at day 14 visit | Unclear | 1 to 2 weeks (14 days) | — | |
| School | Person | "Dry ear" | Yes | 2 to 4 weeks (1 month), after 4 weeks (3 to 4 months) | Patients with bilateral ear disease were only counted as dry ears if both ears were dry | |
| Person | Person | "Recovery" (no further details provided) | Yes | 1 to 2 weeks (time point unclear; treatment for 7 to 10 days), 2 to 4 weeks (time point unclear) | — | |
| Person | Person | "Resolution of discharge" | Unclear | 1 to 2 weeks (7 days) | — | |
| Person | Person | "Inactive" (no further details provided) | Unclear | After 4 weeks (4 weeks) | — | |
| Person | Person | "Cured" according to "indices de curacion" | Yes | 2 to 4 weeks (10 days) | — | |
| Person | Person | "Cured" as absence of otorrhoea or otoscopically inactive, i.e. no pooling of discharge, non‐inflamed middle ear mucosa | Yes | 1 to 2 weeks (2 weeks) | 33/100 participants had bilateral disease. Unclear how these were assessed for resolution of ear discharge. | |
| Person | Person | "Clinical response" defined as cessation of discharge with no hyperaemic areas in the mucosa | Unclear | 1 to 2 weeks (1 week), 2 to 4 weeks (3 weeks), after 4 weeks (6 weeks) | Patients with bilateral ear disease were only counted as resolved if both ears were dry. | |
| Person | Person | Disappearance of otorrhoea or reduction of ear leak | Yes | 1 to 2 weeks (14 days) | — | |
| Person | Person | Not reported | Unclear | 1 to 2 weeks (10 days, 12 days), 2 to 4 weeks (15 days), after 4 weeks (4 weeks) | — | |
| Person | Person | "Complete disappearance of ear discharge within the period of treatment" | Unclear | 1 to 2 weeks (14 days) | — | |
| Person | Person | "No otomicroscopic sign of otorrhoea at either ear" | Yes | After 4 weeks (6 weeks, 12 weeks, 1 year) | Patients with bilateral ear disease were only counted as resolved if both ears were dry. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Resolution of ear discharge at 1 to 2 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Any antibiotic (mezlocillin or ceftazidime) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.47 [1.88, 38.21] |
| 1.1.2 Mezlocillin | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.27 [1.83, 37.47] |
| 1.1.3 Ceftazidime | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.31 [1.84, 37.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 5 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 2.2 Ear pain Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.21, 4.72] |
| 2.3 Serious complications Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Ototoxicity Show forest plot | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 71.92] |
| 2.4.1 Ototoxicity | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4.2 Dizziness/Vertigo/Balance | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 71.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Resolution of ear discharge (after 4 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Metronidazole plus gentamicin‐steroid drops compared to gentamicin‐steroid alone | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.65] |
| 3.1.2 Cotrimoxazole for 6 weeks: 6 week follow‐up | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.09, 2.16] |
| 3.2 Serious complications Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.86] |
| 3.2.1 Mastoid abscess (follow‐up: 12 weeks) | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Resolution of ear discharge at 2 to 4 weeks Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.61] |
| 4.2 Resolution of ear discharge after 4 weeks Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.75, 1.41] |
| 4.3 Sensitivity analysis: Resolution of ear discharge at 2 to 4 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 No correction | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.73, 1.56] |
| 4.3.2 ICC used in primary analysis (0.015) | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.61] |
| 4.3.3 High ICC (0.03) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.67] |
| 4.4 Sensitivity analysis: Resolution of ear discharge after 4 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.4.1 No correction | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.77, 1.38] |
| 4.4.2 ICC used in primary analysis (0.015) | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.75, 1.41] |
| 4.4.3 High ICC (0.03) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.74, 1.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 3 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.35] |
| 5.1.1 Levofloxacin versus co‐amoxiclav | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.68, 2.28] |
| 5.1.2 Pipemidic acid versus aminobenzylpenicillin | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.42] |
| 5.1.3 Norfloxacin versus aminobenzylpenicillin | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.85, 1.48] |
| 5.2 Suspected ototoxicity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Dizziness or vertigo | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.25] |
| 5.2.2 Tinnitus | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 Beta‐lactam versus ceftazidime | 2 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 6.1.2 Amoxicillin‐clavulanate versus amoxicillin | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.96, 1.67] |
| 6.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.05, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.55, 1.00] |
| 7.1.1 Lincomycin versus metronidazole | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.14] |
| 7.1.2 Clindamycin versus metronidazole | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.12] |
| 7.2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 7.2.1 Lincomycin versus metronidazole | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.09] |
| 7.2.2 Clindamycin versus metronidazole | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.12] |
| 7.3 Resolution of ear discharge (after 4 weeks) Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.48, 0.96] |
| 7.3.1 Lincomycin versus metronidazole | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.11] |
| 7.3.2 Clindamycin versus metronidazole | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.10] |

