Exposure to the smell and taste of milk to accelerate feeding in preterm infants
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled pilot trial | |
| Participants | Inclusion criteria: tube‐fed infants with a postmenstrual age of less than 29 weeks, admitted to the Neonatal Intensive Care Unit and who had not yet received regular feeds (2‐hourly) for more than 24 hours. Exclusion criteria: any major congenital anomaly and infants with birth weight below the 10th centile measured on Fenton Growth Charts. Sample size: 51 preterm infants (treatment group (n = 28) and control group (n = 23)). Setting: neonatal intensive care unit in Melbourne, Australia. Timing: March 2014 to April 2015. | |
| Interventions | Intervention: smell and taste of human milk (own mother’s milk or pasteurised donor breast milk) before each tube feeding. Smell was provided by placing a gauze swab soaked with milk close to infants' nostrils. Taste was provided by offering a cotton wool bud soaked in milk for sucking. Control: no oral administration of milk until 32 weeks' gestation. | |
| Outcomes | Primary outcome: time from birth to full enteral feedings (in days), defined as enteral volume of 120 mL/kg/day sustained for at least 24 hours. Secondary outcomes: death; type of milk feeds at 36 weeks' postmenstrual age; postmenstrual age at removal of nasogastric tube; necrotising enterocolitis; spontaneous intestinal perforation; duration of any parenteral nutrition in days; postmenstrual age at discharge to home; weight and weight z‐scores at birth, 28 days, 36 weeks’ postmenstrual age and at discharge; time with high‐flow nasal prongs or nasal intermittent positive airway pressure and time with endotracheal ventilation in hours; any intraventricular haemorrhage and intraventricular haemorrhage higher than grade 2; any retinopathy of prematurity and retinopathy of prematurity higher than stage 2 in any zone; presence of chronic lung disease; persistent ductus arteriosus requiring treatment; bacterial sepsis diagnosed after 48 hours of life. | |
| Notes | Funding: pilot trial funded by Research Foundation for Women and Babies and Research grant from the Mercy Hospital for Women, Melbourne, Australia. Conflict of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was determined using a computer‐generated random‐number table. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was determined using sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors were not blinded but are unlikely to have influenced the outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | One participant was randomised to the control group and later excluded because they did not meet the inclusion criteria for the trial. However, analysis was performed on intention‐to‐treat and therefore exclusion is unlikely to have influenced the outcome. |
| Selective reporting (reporting bias) | Low risk | All outcomes have been reported. |
| Other bias | Low risk | No significant differences for baseline characteristics between groups and no losses to follow‐up. |
| Methods | Prospective, placebo‐controlled, partially‐blinded, single‐centre, pilot randomised trial | |
| Participants | Inclusion criteria: infants born between 28 0/7 and 33 6/7 weeks’ postmenstrual age to mothers who planned to breastfeed. Exclusion criteria: not stated. Sample size: 30 preterm infants (28 to 29 6/7 weeks’ gestation (n = 8); 30 to 31 6/7 weeks’ gestation (n = 13); and 32 to 33 6/7 weeks’ gestation (n = 9)). Setting: not stated. Timing: not stated. | |
| Interventions | Treatment group: olfactory stimulation with mother's own milk held 2 cm from the nares for 15 minutes during enteral feedings, once a day, for at least 4 days a week until transfer to a Level II nursery or attainment of full sucking feeds. Control group: olfactory stimulation with water held 2 cm from the nares for 15 minutes during enteral feedings, once a day, for at least 4 days a week until transfer to a Level II nursery or attainment of full oral feeds. | |
| Outcomes | Primary outcome: time to reach full sucking feeds. Secondary outcomes: optimal timing and sex‐specific responses to olfactory stimulation. | |
| Notes | Conflicts of interest: not stated. Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants and personnel was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Limited information was available in the abstract to assess attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available to be compared with study's final report. |
| Other bias | Unclear risk | Not possible to assess due to limited information in the abstract. |
| Methods | Prospective experimental study (quasi‐randomised) | |
| Participants | Inclusion criteria: infants born after 28 and before 34 weeks' gestation, without sucking reflex (based on neonatologist evaluation), with birth weight approximately 1000 grams, "mean of Apgar scores >6," medically stable during the first 24 hours after birth, with no congenital malformation that could have caused asphyxia or otherwise affected respiration and spontaneous respiration at birth, receiving and tolerating tube feedings, receiving breast milk, mother literate in Turkish and willing to feed the baby. Exclusion criteria: intraventricular haemorrhage grade III or IV, intracranial haemorrhage, periventricular leukomalacia, necrotising enterocolitis, chromosomal anomalies, craniofacial malformation, respiratory distress syndrome, bronchopulmonary dysplasia or other chronic lung disease, need for mechanical ventilation, neonatal seizures, culture‐positive sepsis or meningitis at study screening. Sample size: 80 preterm infants: control group (n = 40) and treatment group (n = 40). Setting: neonatal intensive care unit in Turkey. Timing: September 2007 to December 2008. | |
| Interventions | Treatment group: olfactory stimulation consisting of placement of a sterile pad soaked in breast milk approximately 2 cm from the infant’s nose during three daily tube feedings in the incubator. Control group: routine tube feeding without delivery of olfactory stimulation. | |
| Outcomes | Primary outcome: time for transition to total sucking feeds. Secondary outcomes: not stated in method section but data on weight gain and duration of hospital stay were available. | |
| Notes | Funding: experimental study funded by Ataturk University Scientific Research Project Funds. Conflict of interest: none declared. Infants were sequentially allocated to treatment and control groups: the first 40 participants were allocated into control group and the next 40 participants were allocated to the treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were sequentially allocated into treatment and control groups based on date of admission (the first 40 to control, and next 40 to treatment group). |
| Allocation concealment (selection bias) | High risk | No allocation concealment was used. |
| Blinding of participants and personnel (performance bias) | Low risk | Authors state that “Although study subjects and the neonatologist were blinded to the study groups, the investigator was not blinded”. The exact method of achieving blinding was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Investigators were not blinded but are unlikely to have influenced the outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors state that when unexpected conditions emerged during the study (clinical conditions, or those induced by the mother, infant, or research conditions), those infants were excluded from the study. However, no data on excluded participants were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes have been reported. |
| Other bias | Low risk | No significant differences for baseline characteristics between groups. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Duplication not detected previously | |
| Wrong study design (cross‐over design) | |
| Wrong outcome (olfactory stimulation for pain relief) | |
| Wrong intervention (intervention given during breastfeeding trials and not related to tube feeds) |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The TASTE trial ‐ effect of smell and taste to improve nutrition in very preterm babies |
| Methods | Randomised controlled clinical trial |
| Participants | Preterm infants born < 29 weeks' gestation and/or less than 1250 grams birth weight admitted to Neonatal Intensive Care Units in Queensland and Victoria, Australia. |
| Interventions | Smell and taste of milk (mothers' breast milk, pasteurised donor breast milk or formula, whatever is fed at the time) given with every tube feeding and for the duration of the feed. For infants born before 32 weeks' gestation the intervention consists of providing a cotton bud soaked in milk, offered for sucking, and a drop of milk on a cotton pad placed close to the infants nose until infants reach 32 weeks' gestation. Once infants are 32 weeks' gestation, and until removal of nasogastric tube or discharge, the intervention will consist of 0.2 mL of milk given orally with a feeding syringe with every tube feeding. |
| Outcomes | Primary outcome: weight z‐scores at discharge home. Secondary outcomes: time (days) to full enteral feedings (120 mL/kg/day for at least 24 hours); total duration of parenteral nutrition (days); duration of parenteral nutrition (until first episode of cessation of parenteral nutrition); total duration of antibiotics (days); episodes of late onset sepsis; postmenstrual age at discharge home from hospital; |
| Starting date | 8 May 2017 |
| Contact information | Dr Friederike Beker Address: Neonatal Critical Care Unit, Mater Mothers' Hospital, Raymond Terrace, South Brisbane, QLD 4101, Australia. Email: [email protected] |
| Notes | Funding: Mater Research Institute (Australia) and Royal College of Physicians and Paediatricians ‐ Queensland Branch (Australia). Trial registration: ACTRN12617000583347. Conflict of interest: none declared. |
| Trial name or title | The DIAMOND trial ‐ DIfferent Approaches to MOderate & late preterm Nutrition: Determinants of feed tolerance, body composition and development: protocol of a randomised trial |
| Methods | Multicentre, factorial, randomised, controlled clinical trial |
| Participants | Moderate to late preterm infants (32+ 0 and 35+ 6 weeks' gestation) admitted to Neonatal Intensive Care Units in Auckland, New Zealand. |
| Interventions | (i) Parenteral nutrition: intravenous amino acid solution versus intravenous dextrose solution until full milk feeds established. (ii) Enteral nutrition: milk supplement versus exclusive breast milk. (iii) Sensory stimulation: taste and smell given or not given before gastric tube feeds. |
| Outcomes | For parenteral nutrition (i) and milk supplement interventions (ii), body composition at 4 months' corrected age. For taste/smell intervention (iii), time to full enteral feeds (defined as 150 mL/kg/day) or exclusive breastfeeding. |
| Starting date | 29 March 2017 |
| Contact information | Professor Frank H. Bloomfield Address: Liggins Institute, University of Auckland. Private Bag: 92019. Auckland, 1142. New Zealand. Email: [email protected]. |
| Notes | Funding: Health Research Council of New Zealand and Counties Manukau Health. Trial registration: ACTRN12616001199404. Conflict of interest: none declared. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to reach full sucking feeds (days) Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐5.15, 0.02] |
| Analysis 1.1  Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 1 Time to reach full sucking feeds (days). | ||||
| 2 Time to reach full enteral feedings (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐6.25, 3.11] |
| Analysis 1.2  Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 2 Time to reach full enteral feedings (days). | ||||
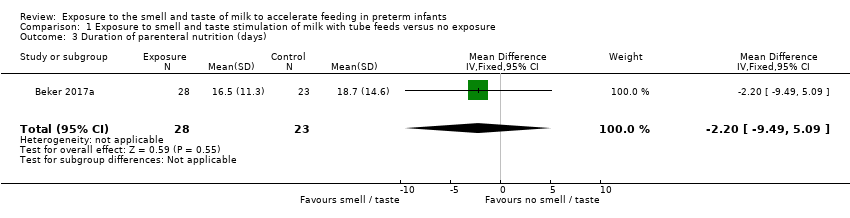
| 3 Duration of parenteral nutrition (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐9.49, 5.09] |
| Analysis 1.3  Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 3 Duration of parenteral nutrition (days). | ||||
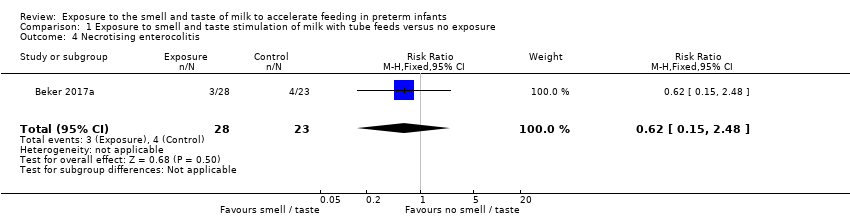
| 4 Necrotising enterocolitis Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.48] |
| Analysis 1.4  Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 4 Necrotising enterocolitis. | ||||
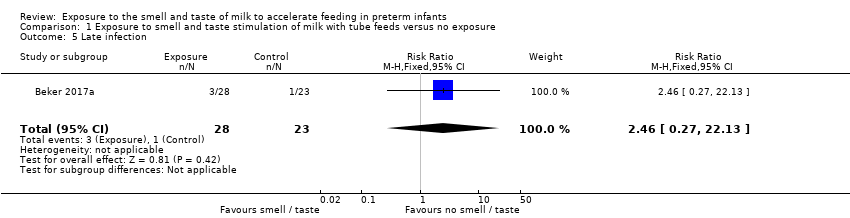
| 5 Late infection Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.27, 22.13] |
| Analysis 1.5  Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 5 Late infection. | ||||
| 6 Time to first discharge home (days) Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐3.89 [‐7.03, ‐0.75] |
| Analysis 1.6  Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 6 Time to first discharge home (days). | ||||

Study flow diagram.
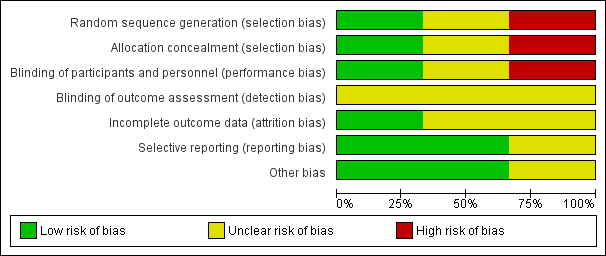
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 1 Time to reach full sucking feeds (days).

Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 2 Time to reach full enteral feedings (days).
Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 3 Duration of parenteral nutrition (days).
Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 4 Necrotising enterocolitis.
Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 5 Late infection.

Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 6 Time to first discharge home (days).
| Exposure to the smell and taste of milk with tube feeds compared to no exposure in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no exposure | Risk with exposure to smell and taste of milk with tube feeds | |||||
| Time to reach full sucking feeds (days) | The mean time to reach full sucking feeds (days) ranged from 12.6 to 76.3 days | MD 2.57 days lower | ‐ | 131 | ⊕⊝⊝⊝ | |
| Adverse effects related to intervention ‐ not reported | ‐ | ‐ | ‐ | 51 | ⊕⊝⊝⊝ | No data on adverse effects were reported. One trial stated that “No adverse events or side effects, no concerns with regard to acceptability to parents and no logistical implications for the delivery of smell and taste were observed in this study”. |
| Time to reach full enteral feedings (days) | The mean time to reach full enteral feedings (days) was 17.7 days | MD 1.57 days lower | ‐ | 51 | ⊕⊝⊝⊝ | |
| Duration of parenteral nutrition (days) | The mean duration of parenteral nutrition (days) was 18.7 days | MD 2.2 days lower | ‐ | 51 | ⊕⊝⊝⊝ | |
| Necrotising enterocolitis | Study population | RR 0.62 | 51 | ⊕⊕⊝⊝ | ||
| 174 per 1,000 | 108 per 1,000 | |||||
| Late infection | Study population | RR 2.46 | 51 | ⊕⊕⊝⊝ | ||
| 43 per 1,000 | 107 per 1,000 | |||||
| Time to first discharge home (days) | The mean time to first discharge home (days) ranged from 22.8 to 85.7 days | MD 3.89 days lower | ‐ | 131 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias due to lack of blinding and lack of allocation concealment | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to reach full sucking feeds (days) Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐5.15, 0.02] |
| 2 Time to reach full enteral feedings (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐6.25, 3.11] |
| 3 Duration of parenteral nutrition (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐9.49, 5.09] |
| 4 Necrotising enterocolitis Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.48] |
| 5 Late infection Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.27, 22.13] |
| 6 Time to first discharge home (days) Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐3.89 [‐7.03, ‐0.75] |

