Exposure to the smell and taste of milk to accelerate feeding in preterm infants
Abstract
Background
Preterm infants are often unable to co‐ordinate sucking, swallowing and breathing for oral feeding because of their immaturity; in such cases, initial nutrition is provided by orogastric or nasogastric tube feeding. Feed intolerance is common and can delay attainment of full enteral feeds and sucking feeds, which prolongs the need for intravenous nutrition and hospital stay. Smell and taste play an important role in the activation of physiological pre‐absorptive processes that contribute to food digestion and absorption. However, during tube feedings, milk bypasses the nasal and oral cavities, which limits exposure to the smell and taste of milk. Provision of the smell and taste of milk with tube feedings is non‐invasive and inexpensive; and if it does accelerate the transition to enteral feeds, and then to sucking feeds, it would be of considerable potential benefit to infants, their families, and the healthcare system.
Objectives
To assess whether exposure to the smell or taste (or both) of milk administered with tube feedings can accelerate progress to full sucking feeds without adverse effects in preterm infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 5), MEDLINE via PubMed (1966 to 1 June 2018), Embase (1980 to 1 June 2018), and CINAHL (1982 to 1 June 2018). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised and quasi‐randomised trials.
Selection criteria
We included randomised and quasi‐randomised studies that compared the provision of the smell or taste of milk (or both) immediately before or at the time of tube feedings, with no provision of smell or taste.
Data collection and analysis
Two review authors independently abstracted data according to Cochrane Neonatal methodology; they also assessed risk of bias, and the quality of evidence at the outcome level using the GRADE approach. We performed meta‐analyses using risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data, with their respective 95% confidence intervals (CIs).
Main results
Three trials involving a total of 161 preterm infants were included in this review, but only two trials (131 infants) contributed data for meta‐analysis. There was no evidence of a clear effect of exposure to the smell and taste of milk with tube feedings on time taken to reach full sucking feeds (MD ‐2.57 days, 95% CI ‐5.15 to 0.02; I2 = 17%; 2 trials, 131 infants; very low‐quality evidence). One trial reported no adverse effects. There was no evidence of a clear effect of exposure to the smell and taste of milk on the following outcomes: time taken to reach full enteral feeds (MD ‐1.57 days, 95% CI ‐6.25 to 3.11; 1 trial, 51 infants; very low‐quality evidence), duration of parenteral nutrition (MD ‐2.20 days, 95% CI ‐9.49 to 5.09; 1 trial, 51 infants; very low‐quality evidence), incidence of necrotising enterocolitis (RR 0.62, 95% CI 0.15 to 2.48; 1 trial, 51 infants; low‐quality evidence), and late infection (RR 2.46, 95% CI 0.27 to 22.13; 1 trial, 51 infants; low‐quality evidence). There was very low‐quality evidence demonstrating that exposure to the smell and taste of milk decreased duration of hospitalisation by almost four days (MD ‐3.89 days, 95% CI ‐7.03 to ‐0.75; I2 = 51%; 2 trials, 131 infants). In two trials, an increased growth velocity was noted in infants exposed to the intervention, but we were unable to combine data to perform meta‐analysis. No data were available to assess feed intolerance and rates of exclusive breastfeeding at discharge. Included trials were small and had methodological limitations including lack of randomisation (one trial), lack of blinding, and different inclusion criteria and administration of the interventions.
Authors' conclusions
Evidence from two trials suggests that exposure to the smell and taste of milk with tube feedings has no clear effect on time taken to reach full sucking feeds, but it may decrease length of hospitalisation. However, these results are uncertain due to the very low quality of the evidence. There is also limited evidence about the impact on other important clinical outcomes and on safety. Future research should examine the effect of exposure to the smell and taste of milk with tube feedings on clinical outcomes during hospitalisation, such as attainment of full enteral and sucking feeds, safety, feed tolerance, incidence of infection, and infant growth. Additionally, future research should be sufficiently powered to evaluate the effect of the intervention in infants of different gestational ages, on each sex separately, and on the optimal frequency and duration of exposure.
PICO
Plain language summary
Exposure to the smell and taste of milk to accelerate feeding in preterm infants
Review question
We reviewed the evidence available from clinical studies to find out about exposing infants born early (preterm) to the smell and taste of milk with feedings given by a tube which goes through the nose or mouth into the stomach. We compared this with not exposing preterm infants to the smell and taste of milk during tube feeds, to see which approach would decrease the time required to achieve full sucking feeds, without causing side effects.
Background
Preterm infants often need to be fed via a thin tube inserted through the mouth or nose into the stomach (orogastric or nasogastric) until they are able to suck all of their feeds. Initially, only small volumes of milk are given, and this is gradually increased depending on how well feeds are tolerated. Infants who are fed by tube may not experience the smell and taste of milk because the milk is placed directly into the stomach. Smell and taste have a significant role in assisting with digestion and absorption of food. Therefore, providing some milk for the infant to smell and to taste when milk is given via an orogastric or nasogastric tube could potentially help them tolerate greater volumes of milk more quickly.
Study characteristics
In a search up to 1 June 2018, we identified three completed studies involving 161 preterm infants admitted to a neonatal intensive care unit (NICU) at a tertiary hospital. One study involved 51 preterm infants, and each infant had an equal chance of being chosen to receive either treatment (a randomised controlled trial). One study involved 80 preterm infants who were sequentially assigned to control and treatment groups (a quasi‐randomised trial). One study was a prospective randomised trial involving 30 infants, but the way it was reported meant there was not enough information for us to include in our analyses.
Key results
We found that exposure to the smell and taste of milk with orogastric or nasogastric tube feedings had no clear effect on the time to reach full sucking feeds. One study reported no adverse effects. Exposure to the smell and taste of milk also had no clear effect on time to reach full tube feeding, feed tolerance, incidence of late infection and severe intestinal infection, duration of intravenous nutrition, and growth. Very low‐quality evidence from two studies suggested that exposure to the smell and taste of milk decreased the length of hospital stay by almost four days compared to no exposure to the smell and taste of milk. However, the included studies were small and had several limitations in terms of how they were done.
Conclusion
Exposure to the smell and taste of milk with orogastric or nasogastric tube feedings may decrease length of hospitalisation for preterm infants. However, the effect of this treatment to accelerate feeding in preterm infants is uncertain due to limited and very low‐quality evidence. Future research needs to explore the effect of exposure to the smell and taste of milk with tube feedings on important clinical outcomes, such as time to full sucking feeds, adverse effects, time to reach full tube feedings, feed tolerance, incidence of infection, and growth.
Authors' conclusions
Summary of findings
| Exposure to the smell and taste of milk with tube feeds compared to no exposure in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no exposure | Risk with exposure to smell and taste of milk with tube feeds | |||||
| Time to reach full sucking feeds (days) | The mean time to reach full sucking feeds (days) ranged from 12.6 to 76.3 days | MD 2.57 days lower | ‐ | 131 | ⊕⊝⊝⊝ | |
| Adverse effects related to intervention ‐ not reported | ‐ | ‐ | ‐ | 51 | ⊕⊝⊝⊝ | No data on adverse effects were reported. One trial stated that “No adverse events or side effects, no concerns with regard to acceptability to parents and no logistical implications for the delivery of smell and taste were observed in this study”. |
| Time to reach full enteral feedings (days) | The mean time to reach full enteral feedings (days) was 17.7 days | MD 1.57 days lower | ‐ | 51 | ⊕⊝⊝⊝ | |
| Duration of parenteral nutrition (days) | The mean duration of parenteral nutrition (days) was 18.7 days | MD 2.2 days lower | ‐ | 51 | ⊕⊝⊝⊝ | |
| Necrotising enterocolitis | Study population | RR 0.62 | 51 | ⊕⊕⊝⊝ | ||
| 174 per 1,000 | 108 per 1,000 | |||||
| Late infection | Study population | RR 2.46 | 51 | ⊕⊕⊝⊝ | ||
| 43 per 1,000 | 107 per 1,000 | |||||
| Time to first discharge home (days) | The mean time to first discharge home (days) ranged from 22.8 to 85.7 days | MD 3.89 days lower | ‐ | 131 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias due to lack of blinding and lack of allocation concealment | ||||||
Background
Description of the condition
Due to immaturity of neurologic and digestive systems, preterm infants (those born before 37 weeks’ gestation) are often unable to co‐ordinate sucking, swallowing, and breathing in order to feed. Initial nutrition is usually provided intravenously and via a tube which goes through the nose (nasogastric) or mouth (orogastric) into the stomach, with a gradual transition to sucking feeds as co‐ordination improves (Toce 1987). Usually, enteral feeds (feeds provided via the gut) start at small volumes and are increased slowly until full enteral feeds are tolerated.
Feeding intolerance is defined as the inability to digest enteral feedings in association with increased gastric residuals (fluid remaining in the stomach after tube feeds), abdominal distension, vomiting, or both (Moore 2011). It often leads to a delay in attainment of full enteral feeds and prolonged intravenous nutrition (Fanaro 2013). Prolonged intravenous nutrition can increase the risk of: infection; cholestasis (impaired bile flow) (Gargasz 2012); impaired development of the gut mucosa; necrotising enterocolitis (severe intestinal inflammation) (Fanaro 2013); and morbidity and mortality (The SIFT Investigators Group 2013).
Smell and taste are important for the appreciation of food, but also have a significant role in nutrition. In response to these sensory cues, a sequence of pre‐absorptive physiological responses is triggered by the brain, collectively referred to as cephalic phase responses (Smeets 2010). The cephalic phase response plays an important role in the activation of physiological processes at multiple sites to optimise digestion, including increased salivation, increased peristaltic movements, and increased secretion of digestive enzymes and digestive‐related hormones, all of which are active in the newborn (Lipchock 2011; Mattes 1997; Zolotukhin 2013).
The pathways underlining the cephalic phase response to smell and taste stimulation are diverse and stimulate different parts of the digestive system. First, the increase in salivation starts the process of digestion as a result of the presence of salivary enzymes (such as α‐amylase and lingual lipase), salivary insulin, and the moistening of the digestive bolus to assist swallowing. Further down the gastrointestinal tract, the cephalic phase response initiates the release of gastric secretions containing gastrin, gastric acid, trypsin and gut peptides. It also initiates the release of hormones such as ghrelin, glucagon‐like peptide‐1, leptin and somatostatin, as well as increasing gut motility. Smell and taste also are known to stimulate gastric emptying by increasing contraction of segments of the gastrointestinal tract. Lastly, the release of pancreatic secretions that are rich in digestive enzymes such as lipase, amylase and cholecystokinin assist further digestion of nutrients. In addition to the pancreatic secretions released in the gut, the pancreas also releases insulin and glucagon into the bloodstream in response to sensory stimulation.
All of these responses contribute to food digestion and absorption (Mattes 1997; Zolotukhin 2013). However, little is known about the effects of smell and taste stimulation in preterm infants, despite the presence of functional taste receptors in the fetus from 18 weeks’ gestation and flavour perception from around 24 weeks’ gestation (Lipchock 2011).
Distinct olfactory reflexes have been demonstrated in neonates after 32 weeks of gestation, with infants presenting different responses to the smell of substances (such as amniotic fluid, colostrum or peppermint oil), varying from sucking response alone to a combination of sucking and arousal‐withdrawal reflex (Bingham 2003; Marlier 1998; Sarnat 1978). These findings suggest that the olfactory system is fully functional in preterm infants after 32 weeks of gestational age.
Fetal swallowing of amniotic fluid starts by the end of the first trimester and reaches up to 750 mL/day by 34 weeks' gestation (Dasgupta 2016). Thus, fetal smell and taste receptors are exposed to the components of amniotic fluid for many weeks before birth and at equivalent gestations to those of infants born preterm (Bloomfield 2017), suggesting that the first sensory experiences happenin utero.
Tube feedings bypass the oral and nasal cavities, so tube‐fed infants have limited exposure to the smell and taste of their feeds. Therefore, there is little stimulation of the cephalic phase response of digestion.
The provision of smell and taste exposure to preterm infants receiving tube feedings is currently being applied in the care of some preterm infants based on the assumption that there is biological plausibility for a possible benefit, despite lack of evidence to support this practice. More importantly, potential adverse effects have not been assessed; these could include risks such as aspiration, gagging or choking, bradycardia, desaturations or increase in oxygen requirement.
Description of the intervention
The intervention consists of placing a cotton bud or gauze soaked with a few drops of milk with which the infant is being fed close to the infant’s nostril to provide the smell of milk, and placing a few drops of milk on the infant’s lips and tongue in order to provide the taste of milk. The exposure should be done before starting the tube feeding in order to stimulate the cephalic phase response of digestion.
How the intervention might work
Preterm infants being fed via orogastric or nasogastric tube have limited exposure to smell and taste stimulation which triggers the cephalic phase response of digestion, and this might contribute to feed intolerance and the need for prolonged intravenous nutrition.
Exposure to the smell and taste of milk before tube feeding may stimulate the cephalic phase response of digestion and assist digestion by increasing salivation, triggering peristaltic movements of the gut, secretion of digestive enzymes and release of digestion‐related hormones such as ghrelin, leptin, gastrin, insulin and others (Power 2008).
Why it is important to do this review
Prolonged intravenous nutrition increases the risk of late‐onset sepsis, prolonged hospital stay, and an increase in health costs. In addition, delayed enteral feeding can result in degeneration of the gastrointestinal mucosa and increase the risk of necrotising enterocolitis once the tube feedings start, significantly impacting infant survival and hospital costs (Johnson 2014). Thus, any interventions that accelerate transition to enteral feeding, and then to sucking feeds, would be of considerable potential benefit to infants, their families, and the healthcare system.
It is increasingly common for staff in neonatal nurseries to include exposure to the smell or taste (or both) of milk in the process of tube feeding preterm infants. This is largely based on the belief that this must be beneficial, which could lead to performance bias when assessing the effects of the intervention. Furthermore, this additional intervention requires staff time (and therefore cost), and there is also the potential for adverse effects such as choking or aspiration. Reliable evidence is required on the clinical benefits and possible risks of this intervention.
Objectives
To determine whether exposure to the smell and taste (or both) of milk administered with tube feedings can accelerate progress to full sucking feeds without adverse effects in preterm infants.
We also planned to assess in subgroups the effects of different modes of administration of the intervention, gestational age, birthweight, and type of milk, but data were insufficient for these analyses.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised or quasi‐randomised trials where the unit of randomisation was the infant, or cluster‐randomised trials where the neonatal unit or hospital was the unit of randomisation. We excluded cross‐over trials and non‐randomised trials such as before‐and‐after studies.
Types of participants
We included preterm infants (born before 37 weeks’ gestation) of both sexes and all ethnicities who were receiving any orogastric or nasogastric tube feedings and had not yet reached full sucking feeds.
Types of interventions
We included studies that reported exposure to the smell and taste (or both) of breast milk or formula milk, immediately before or at the time of tube feedings.
For smell stimulation, we included in this review studies that reported delivering the smell of milk to preterm infants using a gauze with a few drops of milk placed in the cot/incubator close to the infant's nose, or using a cotton bud soaked with milk, or other forms of administration of the smell of milk (e.g. using an olfactometer adapted to a pacifier).
For taste stimulation, we included in this review studies that reported placing a few drops of milk on the infant’s lips or tongue using a syringe, or other forms of oral administration of a small amount of milk (e.g. using a pacifier or swab dipped in milk).
We planned to undertake subgroup analyses to explore the effects of different modes of administration of the intervention ( smell of milk versus no smell exposure; and taste of milk versus no taste exposure). However, there were insufficient data to perform these analyses.
Types of outcome measures
Primary outcomes
-
Time to reach full sucking feeds (defined as the removal of the feeding tube), measured in days.
-
Adverse effects related to the intervention, such as aspiration, gagging/choking, bradycardia, desaturations or increase in oxygen requirement during the intervention period.
Secondary outcomes
-
Duration of parenteral nutrition (defined as the removal of intravenous nutrition line), measured in days.
-
Time to reach full enteral feedings (150 mL/Kg/day, or as defined by the trialists), measured in days.
-
Feed intolerance (resulting in cessation or reduction in enteral feeding), during the period of hospitalisation.
-
Necrotising enterocolitis (Bell’s stage 2 or more) (Walsh 1986), during the period of hospitalisation.
-
Late infection (bacterial or fungal infection confirmed by presence of blood or cerebrospinal fluid infection with initiation of symptoms beyond 48 hours after birth) (ANZNN 2016), during the period of hospitalisation.
-
Growth from birth to discharge (weight, height/length, head circumference and z‐scores; gain in these parameters from birth to 36 weeks’ postmenstrual age or to term equivalent age; body composition).
-
Exclusive breastfeeding at time of discharge (WHO 2008).
-
Time to first discharge home, measured in days.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We searched for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed) on 1 June 2018. We did not limit the search to any particular geographical region, language or timing of publication.
Electronic searches
We searched: the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1966 to 1 June 2018); Embase (1980 to 1 June 2018); and CINAHL (1982 to 1 June 2018), using the following search terms: (("Taste"[MeSH] OR "Taste Perception"[MeSH] OR "Smell"[Mesh] OR "Olfactory Perception"[Mesh] OR "Odorants"[MeSH] OR taste*[tiab] OR tasting[tiab] OR gustat*[tiab] OR smell*[tiab] or smelt[tiab] OR olfact*[tiab] OR odor*[tiab]) AND ("Milk, Human"[MeSH] OR "Infant Formula"[MeSH] OR "Colostrum"[MeSH] OR milk*[tiab] or breastmilk*[tiab] OR formula*[tiab] OR colostrum[tiab] OR colostral[tiab])), plus database‐specific limiters for randomised controlled trials and neonates (see Appendix 1 for the full search strategies for each database).
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform, the ISRCTN Registry, and ANZCTR).
Searching other resources
We searched the reference lists of articles selected for inclusion in this review, in order to identify additional relevant articles. We also approached well‐known researchers in this area to identify any unpublished or ongoing research.
Data collection and analysis
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Selection of studies
Search results from different databases were merged and duplicates were removed using reference management software. Two review authors (MM and LL) independently assessed the retrieved studies, following the steps below.
-
Screened titles and abstracts to select relevant reports and excluded studies not relevant for this review.
-
Accessed the full text of potentially relevant reports.
-
Used a reference management software (Covidence 2018) to combine search results and remove duplicate records of the same report and combine multiple reports of the same study.
-
Examined full‐text studies for compliance with the eligibility criteria for this review.
-
Where appropriate, corresponded with study authors in order to request missing results or seek additional information.
-
Made final decisions on study inclusion and proceeded to data collection.
The review authors did not encounter disagreements when selecting reports to include in the review.
Details of the selection process are shown in the PRISMA flow diagram (Moher 2009) (Figure 1).

Study flow diagram.
Data extraction and management
We extracted data from included studies using a specially developed data extraction form. Information extracted included, but was not limited to: source details; eligibility assessment; methodological details; characteristics of participants; details of intervention, and outcomes reported. Disagreements were resolved by discussion with a third assessor (JH). Data from the included studies were entered into Review Manager 5 (Review Manager 2014). When review authors were authors of an included trial, we have ensured that those authors were excluded from any decision‐making regarding inclusion of the trial in this review, and they were not involved in data extraction or quality assessment relating to that trial. We requested additional information from Beker 2017a (mean and standard deviation for the outcomes of interest) and Davidson 2015 (only an abstract was published). The authors of Beker 2017a provided the additional information requested.
Assessment of risk of bias in included studies
Two review authors (MM and LL) independently assessed the methodological quality of all included trials to determine potential risk of bias (low, high, or unclear) using the Cochrane ‘Risk of bias’ tool (Higgins 2017) for the following domains.
-
Sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants and personnel (performance bias)
-
Blinding of outcome assessment (detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective reporting (reporting bias)
-
Any other bias
Any disagreements were resolved by discussion or by a third review author (JH). See Appendix 2 for a more detailed description of risk of bias for each domain. We entered the assessed risk of bias into Review Manager 5 (Review Manager 2014). See Figure 2 and Figure 3.
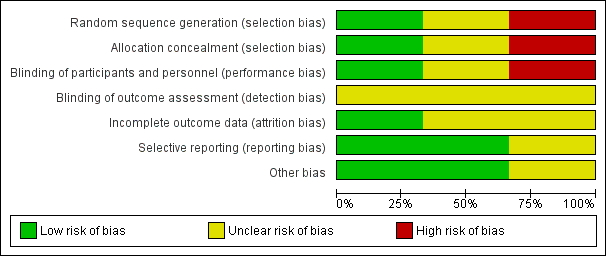
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
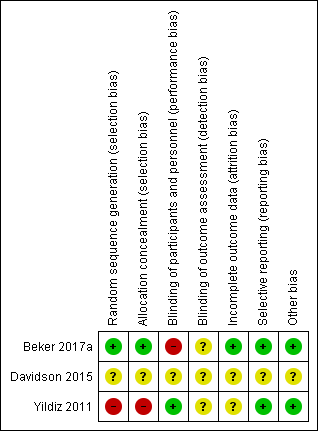
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Measures of treatment effect
We analysed treatment effects in individual trials by using Review Manager 5 (Review Manager 2014). We used the numbers of events in the control and intervention groups of each study to calculate risk ratios (RRs) for dichotomous data. We calculated mean differences (MDs) for outcomes measured on a continuous scale. Where outcomes were measured differently, we intended to report data as standardised mean differences (SMDs) and risk differences (RDs), and if a significant effect was found we planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). We reported 95% confidence intervals (CIs) for all outcomes.
The included studies reported data about infant growth differently and we were not able to combine these in a meta‐analysis. However, from the reported mean weights and length of hospitalisation we were able to estimate growth rate using the exponential model (Patel 2005), which uses difference in weights between two time points and elapsed time (in this case birth and discharge weights and duration of hospitalisation), allowing us to estimate mean growth velocity from birth to discharge in grams per kilo per day (g/kg/day).
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. We planned to include cluster‐randomised trials in analyses, along with individually randomised trials, but no cluster‐randomised trials were identified.
If cluster‐randomised trials were identified, the participating neonatal unit or section of a neonatal unit or hospital would have been the unit of analysis. We would have analysed them using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial or from a study with a similar population as described in Section 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). If we used ICCs from a similar trial or from a study with a similar population, we planned to report this and conduct a sensitivity analysis to investigate the effect of variation in the ICC.
If we identified both cluster‐randomised trials and individually randomised trials, we planned to only combine the results from both if there was little heterogeneity between the study designs, and the interaction between the effect of the intervention and the choice of randomisation unit was considered to be unlikely.
We planned to acknowledge any possible heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate possible effects of the randomisation unit.
Dealing with missing data
We contacted the investigators to request information on missing or unclear data for outcomes of interest. We analysed all participants in the treatment group to which they were randomised, regardless of the actual treatment received, where possible. We carried out analyses on an intention‐to‐treat basis for all outcomes, where feasible. If we had concerns regarding the impact of including trials with high levels of missing data in the overall assessment of treatment effect, we planned to explore this through sensitivity analysis, but no included studies had high levels of missing data.
Assessment of heterogeneity
We planned to consider whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary by assessing statistical heterogeneity using the Chi2 test and the I2 statistic. We used the guidelines recommended by Cochrane Neonatal for interpretation of results. We considered an I2 value of less than 25% to represent no heterogeneity; 25% to 49% to represent low heterogeneity; 50% to 74% to represent moderate heterogeneity, and more than 75% to represent high heterogeneity.
We considered an I2 value greater than 50% and a low P value (less than 0.10) in the Chi2 test for heterogeneity to indicate substantial heterogeneity (Deeks 2017). Where substantial heterogeneity was detected, we planned to explore this through sensitivity/subgroup analyses, but there were insufficient data to perform these analyses. We took statistical heterogeneity into account when interpreting the results, especially when variation in the direction of effect was detected and data were insufficient to carry out further assessment of heterogeneity.
Assessment of reporting biases
For the included trials, two reviewers (MM and LL) examined the methods of each study for the prespecified outcomes. When all prespecified outcomes were reported in the results, the studies were considered to have a low risk of bias. When prespecified outcomes were not reported in the results, the study was considered to carry high risk of bias. If we identified that a trial carried reporting bias, with the potential to introduce serious bias, we planned to conduct a sensitivity analysis to determine the effects of including and excluding such a study in the analysis. However, due to the limited number of studies included in this review, and the small sample sizes, we did not perform sensitivity analyses.
Data synthesis
We conducted meta‐analyses using Review Manager 2014, as supplied by Cochrane. We used a fixed‐effect model to combine data when it was reasonable to assume that studies were estimating the same underlying treatment effect. Where moderate or high heterogeneity existed, we planned to examine the potential causes in subgroup and sensitivity analyses.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following outcomes.
-
Time to reach full sucking feeds (defined as the removal of the feeding tube), measured in days.
-
Adverse effects related to intervention such as aspiration, gagging/choking, bradycardia, desaturations or increase in oxygen requirement.
-
Time to reach full enteral feedings (150 mL/Kg/day, or as defined by the trialists), measured in days.
-
Feed intolerance (resulting in cessation or reduction in enteral feeding).
-
Duration of parenteral nutrition (defined as the removal of the intravenous nutrition line), measured in days.
-
Necrotising enterocolitis (Bell’s stage 2 or more) (Walsh 1986).
-
Late infection (bacterial or fungal infection confirmed by presence of blood or cerebrospinal fluid infection with initiation of symptoms beyond 48 hours after birth) (ANZNN 2016).
Two review authors independently used GRADEpro GDT to assess the quality of evidence for all the outcomes above, except for adverse effects and feed intolerance, for which no data were reported. We considered evidence from randomised trials as high quality, but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias); consistency across studies; directness of the evidence; precision of estimates, and presence of publication bias. We also used GRADEpro GDT to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of the following four grades.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We had planned to perform the following subgroup analyses using a fixed‐effect model. However, insufficient data were available to conduct any subgroup analyses.
-
Type of administration of smell exposure (cotton swab or similar soaked with milk placed close to infants’ nostril versus placed by the infant’s side).
-
Type of administration of taste (cotton swab or similar soaked with milk placed on infant’s lips and tongue versus syringe administration of milk directly onto the infant’s lips and tongue versus use of pacifier to deliver taste of milk).
-
Type of exposure (provision of smell and taste versus provision of taste only versus provision of smell only).
-
Gestational age (less than 28 weeks' versus 28 to less than 32 weeks' versus 32 to less than 37 weeks' postmenstrual age).
-
Type of diet (exclusively human milk versus formula versus human milk plus formula).
-
Intrauterine growth restricted or small for gestational age (less than 10th centile or as defined by the trialists) versus appropriately grown at birth.
Sensitivity analysis
We had planned to conduct sensitivity analyses by examining only those trials considered to have a low risk of bias for allocation concealment and randomisation. We were unable to do this as only one of the included studies was judged to be of low risk of bias for both allocation concealment and randomisation.
Results
Description of studies
Results of the search
In total, 373 publications were identified by the search strategy for possible inclusion in this review. Of these, 197 were duplicates and were removed, and 176 studies were screened for eligibility. After title and abstract screening, 167 studies were considered irrelevant and were excluded. Nine studies underwent full‐text screening, of which three met the inclusion criteria for this review. We identified two ongoing registered clinical trials. For a full description of our selection process, please refer to Figure 1.
Included studies
Three studies met the inclusion criteria for this review (Beker 2017a; Davidson 2015; Yildiz 2011). All included studies were published in English between 2011 and 2017. All were single‐centre trials. One was a randomised controlled pilot trial with 51 preterm infants (Beker 2017a) and one was a quasi‐randomised study in which 80 preterm infants were sequentially allocated to treatment and control groups (the first 40 infants comprised the control group and the next 40 infants comprised the intervention group) (Yildiz 2011). One was a conference abstract and had limited information available (Davidson 2015). It provided an overall sample size, without specifying the number of participants allocated to each group, so these data were not included in meta‐analysis. In total, data on 161 preterm infants were included in this review (three trials) but only 131 infants (two trials) were included in meta‐analyses. Refer to Characteristics of included studies for a summary of the included trials.
Participants
All trials included preterm infants admitted to a neonatal intensive care unit (NICU) at a tertiary hospital. One trial included infants born between 28 and 34 weeks' gestation born in a Turkish hospital between September 2007 and December 2008 (Yildiz 2011), and one included extremely preterm infants (less than 29 weeks' gestation) born in a hospital in Melbourne, Australia between March 2014 and April 2015 (Beker 2017a). The conference abstract reported that preterm infants born between 28 and 34 weeks' gestation were included but no information was provided on setting and trial duration (Davidson 2015). All infants were being tube‐fed at the time of intervention and receiving either mother's milk, donor milk, or infant formula.
Intervention
In all three studies, the provision of the smell of milk was done at the time of tube feedings. Only one trial provided taste of milk as well as smell (Beker 2017a). Exposure to the smell of milk was achieved by placing a gauze or pad with drops of milk close to the infant's nostrils in all three trials. Exposure to the taste of milk was achieved by offering a cotton wool bud soaked in milk for sucking. While in the study of Beker 2017a the intervention was performed with all tube feeds, in the study of Yildiz 2011 smell stimulation was provided during three tube feedings each day, and in Davidson 2015 the smell stimulation was performed once a day for 15 minutes for at least four days each week. No information regarding duration (in minutes) of the intervention was provided in Beker 2017a or Yildiz 2011.
Comparators
All included studies reported a control group. In the study of Beker et al, the control group consisted of infants who were not given any milk in the mouth until 32 weeks' gestation (Beker 2017a). In the study of Yildiz et al, the comparison group consisted of infants receiving routine orogastric or nasogastric tube feedings without provision of olfactory stimulation (Yildiz 2011). In the study of Davidson et al, the comparison group received olfactory stimulation with water placed close to infants' nostrils (Davidson 2015).
Outcomes
All included trials reported at least one of the pre‐specified outcomes of this review. All three trials reported time to reach full sucking feeds. However, the abstract of Davidson 2015 provided no information about the number of participants allocated to treatment and control groups and results were descriptive without numerical values for outcomes of interest. Beker 2017a reported that no adverse effects related to smell and taste stimulation were observed. The other two trials did not provide any comments on adverse effects related to the intervention (Davidson 2015; Yildiz 2011). Growth from birth to discharge was reported in different ways by two studies (Beker 2017a; Yildiz 2011), and therefore we were not able to combine data to perform meta‐analysis, but we were able to estimate mean growth velocity by applying the exponential model of Patel 2005. Duration of hospitalisation was reported by two studies (Beker 2017a; Yildiz 2011). Only one trial, Beker 2017a, reported duration of parenteral nutrition, time to reach full enteral feeds, incidence of necrotising enterocolitis and sepsis, and type of milk feeds at 36 weeks' postmenstrual age. Only one trial reported information on time to reach full sucking feeds, stratified by gestational age and gender (Davidson 2015). However, we were not able to identify the groups to which participants were allocated to due to limited information available in the abstract (Davidson 2015).
Excluded studies
See Characteristics of excluded studies for details. We excluded four publications from this review: one because it was a cross‐over trial (Bingham 2003); one because the intervention was delivered during the first breastfeeding attempt and was not related to orogastric or nasogastric tube feeding (Raimbault 2007); one because the trial was olfactory stimulation for pain relief and was not related to nutrition (Neshat 2016); and one because it was an undetected duplication of a study already included in this review (Beker 2016).
Ongoing studies
We identified two ongoing trials (see Characteristics of ongoing studies).
Risk of bias in included studies
Details of methodological quality of each trial are provided in the Characteristics of included studies and Figure 2 and Figure 3.
Allocation
Risk of bias for allocation was low in one of the included studies (Beker 2017a). In one trial, the method of allocating participants was not clearly described and so we classified this as an unclear risk of bias (Davidson 2015). In another trial, participants were sequentially allocated to treatment and control groups (the first 40 to control, and the next 40 to intervention) and we therefore classified the trial as having high risk of selection bias (Yildiz 2011).
Blinding
Risk of performance bias was low in one trial as participants and clinicians were blinded to study group allocation (Yildiz 2011), but high in one trial as blinding of participants and clinicians was not feasible (Beker 2017a). Despite lack of blinding in Beker 2017a and Yildiz 2011, we judged that the outcome assessors were unlikely to have influenced some of the outcomes reported; and in Davidson 2015 the blinding of outcome assessors was not clearly stated. Therefore, we considered all three trials to have an unclear risk of detection bias.
Incomplete outcome data
We considered two trials to have unclear risk of bias (Davidson 2015; Yildiz 2011). In Davidson 2015, there was limited information available in the abstract, meaning we were unable to determine if all outcomes were adequately addressed. In Yildiz 2011, the authors stated that infants were excluded when unexpected conditions emerged, but no data were provided on excluded participants. Only one study was considered to be at low risk of attrition bias as all prespecified outcomes were reported (Beker 2017a).
Selective reporting
We considered two trials to be free of reporting bias as all prespecified outcomes were reported (Beker 2017a; Yildiz 2011). We deemed one trial to have unclear risk of reporting bias as no protocol was available for comparison and the abstract did not address this issue (Davidson 2015).
Other potential sources of bias
Other potential sources of bias were considered unclear for one trial as the abstract provided limited information (Davidson 2015), and low for two trials as there were no significant differences in baseline characteristics between groups (Beker 2017a; Yildiz 2011).
Effects of interventions
See summary of findings Table for the main comparison for the main comparison.
Exposure to smell and taste stimulation of milk with tube feeds versus no exposure
Time to reach full sucking feeds
Two studies contributed data for meta‐analysis on time to reach full sucking feeds (Beker 2017a; Yildiz 2011). There was no evidence of a clear effect of exposure to the smell and taste of milk with tube feedings on time to reach full sucking feeds (MD ‐2.57 days, 95% CI ‐5.15 to 0.02; I2 = 17%; 2 trials, 131 infants; very low‐quality evidence; Analysis 1.1). We downgraded the quality of evidence three levels for risk of bias (lack of blinding and lack of allocation concealment), imprecision (small sample sizes and large confidence intervals), and indirectness (trials had different inclusion criteria (less than 29 weeks' gestation versus 29 to 34 weeks' gestation) and different interventions (smell and taste of milk with all tube feeds versus smell of milk three times per day with tube feeds).
In Davidson 2015, it was reported that infants allocated to the control group attained full sucking feeds at an earlier postmenstrual age compared to the intervention group (35+2 versus 36 weeks', respectively; P = 0.05). They also reported that infants in the intervention group born at earlier gestational age, and females, demonstrated a trend towards reaching full oral feeds in a shorter time, but associations were not statistically significant. No data on sample size per group were available to allow this trial to be included in the meta‐analysis.
Adverse effects related to the intervention
There were no data available on potential adverse effects related to exposure to the smell and taste of milk with tube feedings. However, Beker 2017a reported that no adverse effects related to the intervention were observed.
Time to reach full enteral feeds
One trial contributed data on time required to reach full enteral feeds, defined as 120 mL/kg/day by the trialists (Beker 2017a). There was no evidence of a clear effect of exposure to the smell and taste of milk with tube feedings on time required to reach full enteral feeds (MD ‐1.57 days, 95% CI ‐6.25 to 3.11; 1 RCT, 51 infants; very low‐quality evidence; Analysis 1.2). We downgraded the quality of evidence one level for risk of bias (lack of blinding) and two levels for imprecision (data derived from a single trial with small sample size and wide confidence intervals).
Duration of parenteral nutrition
One trial (Beker 2017a) reported duration of parenteral nutrition. There was no evidence of a clear effect of exposure to the smell and taste of milk with tube feedings on the duration of parenteral nutrition (MD ‐2.20 days, 95% CI ‐9.49 to 5.09; 1 RCT, 51 infants; very low‐quality evidence; Analysis 1.3). We downgraded the quality of evidence one level for risk of bias (lack of blinding) and two levels for imprecision (data derived from a single trial with small sample size and wide confidence intervals).
Incidence of necrotising enterocolitis
One trial reported the incidence of necrotising enterocolitis (Beker 2017a). There was no evidence of a clear effect of exposure to the smell and taste of milk with tube feedings on the incidence of necrotising enterocolitis (RR 0.62, 95% CI 0.15 to 2.48; 1 RCT, 51 infants; low‐quality evidence; Analysis 1.4). We downgraded the quality of evidence two levels for imprecision (data derived from a single trial with small sample size, and wide confidence intervals). We judged that the lack of blinding was unlikely to have influenced the assessment of this outcome.
Incidence of late infection
One trial reported the incidence of late infection (Beker 2017a). There was no evidence of a clear effect of exposure to the smell and taste of milk with tube feedings on the incidence of late infection (RR 2.46, 95% CI 0.27 to 22.13; 1 RCT, 51 infants; low‐quality evidence; Analysis 1.5). We downgraded the quality of evidence two levels for imprecision (data derived from a single trial with small sample size, and wide confidence intervals). We judged that the lack of blinding was unlikely to have influenced the assessment of this outcome.
Growth
Data on growth during hospitalisation were assessed differently in each of the included studies and we were not able to combine data to perform meta‐analysis. However, we were able to estimate mean growth rates using exponential model estimates (Patel 2005), and found that infants exposed to the smell and taste of milk with tube feedings had faster mean growth rates than infants in the control group (14.2 g/kg/day versus 12.8 g/kg/day in Beker 2017a; and 14.0 g/kg/day versus 7.9 g/kg/day in the study of Yildiz 2011).
Exclusive breastfeeding at time of discharge
No data were available to assess the effect of exposure to the smell and taste of milk with tube feedings on rates of exclusive breastfeeding at time of hospital discharge.
Episodes of feed intolerance
No data were available to assess the effect of exposure to the smell and taste of milk with tube feedings on episodes of feed intolerance.
Time to first discharge home
Two trials reported data on duration of hospitalisation (Beker 2017a; Yildiz 2011). Infants exposed to the smell and taste of milk with tube feedings had a shorter hospital stay than infants not exposed to the intervention (MD ‐3.89 days, 95% CI ‐7.03 to ‐0.75; I2 = 51%; 2 trials, 131 infants; very low‐quality evidence; Analysis 1.6). We downgraded the quality of evidence one level for risk of bias (lack of blinding and lack of allocation concealment), one level for imprecision (small sample sizes and wide confidence intervals), and one level for indirectness of evidence (trials had different inclusion criteria (less than 29 weeks' gestation versus 29 to 34 weeks' gestation) and differences in the provision of intervention (smell and taste of milk with all tube feeds versus smell of milk three times per day with tube feeds)).
Discussion
Summary of main results
The evidence from three trials, involving 161 preterm infants, was judged to be of very low quality and the overall effect of provision of the smell and taste of milk to accelerate feeding in preterm infants is uncertain. There was no evidence of a clear effect of exposure to the smell and taste of milk during tube feedings on time to reach full sucking feeds, and there were no data available to assess potential adverse effects related to the intervention. There was no evidence of a clear effect on time to reach full enteral feeds, duration of parenteral nutrition, incidence of necrotising enterocolitis, late infection and growth. No data were available for the assessment of the effects of exposure to the smell and taste of milk on episodes of feed intolerance and prevalence of exclusive breastfeeding at discharge. However, very low‐quality evidence demonstrated that exposure to the smell and taste of milk with tube feedings decreased duration of hospital stay by almost four days.
Overall completeness and applicability of evidence
The trials included in this review had small sample sizes and did not provide data for all of the outcomes of interest. We were able to include data from two trials for two outcomes only: time to reach full sucking feeds (Analysis 1.1), and time to first discharge home (Analysis 1.6). Only one trial (Beker 2017a) contributed data for the other planned outcomes: time to reach full enteral feeds (Analysis 1.2); duration of parenteral nutrition (Analysis 1.3); incidence of necrotising enterocolitis (Analysis 1.4); and late infection (Analysis 1.5). In addition, the two trials that contributed data for the meta‐analyses included different populations of preterm infants, provided the intervention differently, and used different methods of allocation to the intervention groups. Thus, caution is needed when interpreting the results of this review.
Quality of the evidence
We judged the overall quality of evidence for all reported outcomes to be of low to very low quality, due to a combination of factors that might influence the overall effect of the intervention. This means that we are very uncertain about the effect estimates and their precision. Firstly, we downgraded the quality of evidence for risk of bias, taking into account the lack of allocation concealment in the quasi‐randomised trial (Yildiz 2011) and lack of blinding in one trial (Beker 2017a). Secondly, we downgraded the quality of evidence for indirectness as trials used different inclusion criteria, provided different exposures to the intervention, and had differing estimate of effects, which could have influenced the overall effect of the intervention. Lastly, we downgraded our assessment of the quality of evidence for imprecision because of the small sample size, small number of trials included, and wide confidence intervals, which can also impact the estimation of effect. Only for two outcomes (incidence of necrotising enterocolitis and late infection) did we judge that the lack of blinding in Beker 2017a was unlikely to have influenced the assessment of these outcomes.
Potential biases in the review process
Due to the small number of included studies, we were unable to create funnel plots to assess the potential risk of publication or reporting bias. We minimised bias by conducting a systematic search of the literature, and data extraction was undertaken independently by two reviewers.
Agreements and disagreements with other studies or reviews
Provision of smell and taste stimulation for preterm infants receiving tube feedings is a relatively new topic. Thus, we are not aware of any previous systematic reviews on this topic, nor of other trials not included in this review.

Study flow diagram.
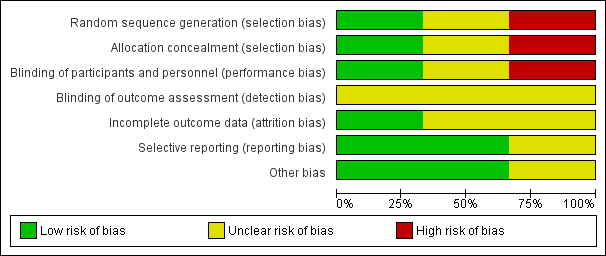
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 1 Time to reach full sucking feeds (days).

Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 2 Time to reach full enteral feedings (days).
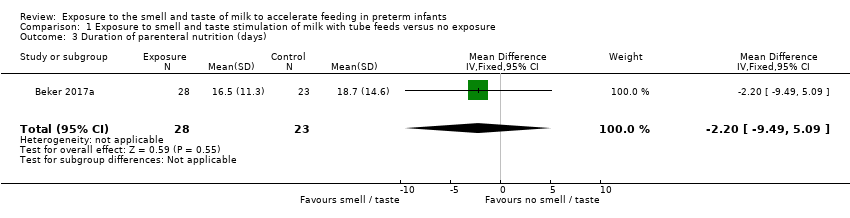
Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 3 Duration of parenteral nutrition (days).
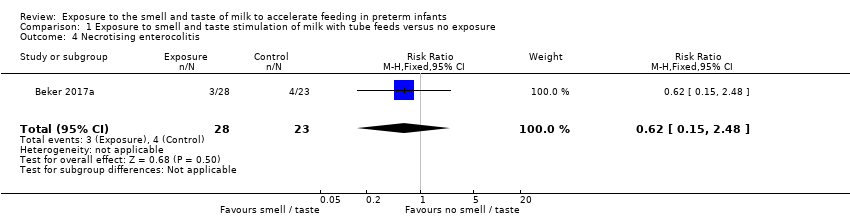
Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 4 Necrotising enterocolitis.
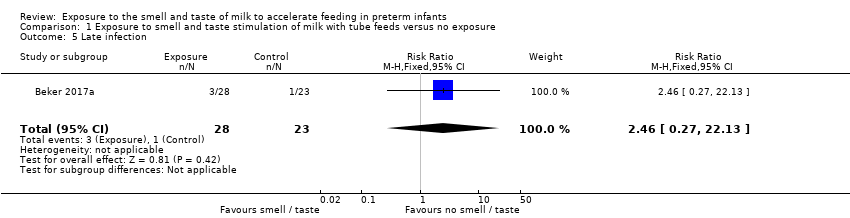
Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 5 Late infection.

Comparison 1 Exposure to smell and taste stimulation of milk with tube feeds versus no exposure, Outcome 6 Time to first discharge home (days).
| Exposure to the smell and taste of milk with tube feeds compared to no exposure in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no exposure | Risk with exposure to smell and taste of milk with tube feeds | |||||
| Time to reach full sucking feeds (days) | The mean time to reach full sucking feeds (days) ranged from 12.6 to 76.3 days | MD 2.57 days lower | ‐ | 131 | ⊕⊝⊝⊝ | |
| Adverse effects related to intervention ‐ not reported | ‐ | ‐ | ‐ | 51 | ⊕⊝⊝⊝ | No data on adverse effects were reported. One trial stated that “No adverse events or side effects, no concerns with regard to acceptability to parents and no logistical implications for the delivery of smell and taste were observed in this study”. |
| Time to reach full enteral feedings (days) | The mean time to reach full enteral feedings (days) was 17.7 days | MD 1.57 days lower | ‐ | 51 | ⊕⊝⊝⊝ | |
| Duration of parenteral nutrition (days) | The mean duration of parenteral nutrition (days) was 18.7 days | MD 2.2 days lower | ‐ | 51 | ⊕⊝⊝⊝ | |
| Necrotising enterocolitis | Study population | RR 0.62 | 51 | ⊕⊕⊝⊝ | ||
| 174 per 1,000 | 108 per 1,000 | |||||
| Late infection | Study population | RR 2.46 | 51 | ⊕⊕⊝⊝ | ||
| 43 per 1,000 | 107 per 1,000 | |||||
| Time to first discharge home (days) | The mean time to first discharge home (days) ranged from 22.8 to 85.7 days | MD 3.89 days lower | ‐ | 131 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias due to lack of blinding and lack of allocation concealment | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to reach full sucking feeds (days) Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐5.15, 0.02] |
| 2 Time to reach full enteral feedings (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐6.25, 3.11] |
| 3 Duration of parenteral nutrition (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐9.49, 5.09] |
| 4 Necrotising enterocolitis Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.48] |
| 5 Late infection Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.27, 22.13] |
| 6 Time to first discharge home (days) Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐3.89 [‐7.03, ‐0.75] |

