Rangkaian silang kolagen kornea untuk keratitis jangkitan bakteria
Información
- DOI:
- https://doi.org/10.1002/14651858.CD013001.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 junio 2020see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Conception and design of study (SAD, JK, RB)
-
Drafting the review or commenting on it critically for intellectual content (SAD, RB, JK, GH)
-
Final approval of the document to be published (SAD, JK, GH, RB)
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Cochrane Eyes and Vision US Project, supported by grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA
-
National Institute for Health Research (NIHR), UK
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
-
Declarations of interest
RB: Dr Bovelle has within the past three years received speaker fees from Allergan, which are unrelated to this work.
SAD: none
GH: none
JK: none
Acknowledgements
Lori Rosman, Information Specialist for Cochrane Eyes and Vision, created and executed the electronic search strategies. We thank Tianjing Li and Henry Jampel for providing comments on the review. We also thank the following peer reviewers for their comments: Lindsay Sicks, OD, FAAO (Illinois College of Optometry) and Vishal Jhanji, MD (University of Pittsburgh School of Medicine).
We are grateful to Dr Robert A Copeland Jr, who passed in April 2016. Dr Copeland conceived, designed, and drafted the first version of the protocol. The authors have made edits to the protocol beyond his contribution, although the main components (population, interventions, comparisons, and outcomes) are consistent with his initial draft.
This review was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jun 17 | Corneal collagen cross‐linking for bacterial infectious keratitis | Review | Shadi A Davis, Renee Bovelle, Genie Han, John Kwagyan | |
| 2018 May 30 | Corneal collagen cross‐linking for infectious keratitis | Protocol | Robert A Copeland Jr, Shadi A Davis, Young‐Joo Lee, John Kwagyan, Renee Bovelle | |
Differences between protocol and review
We planned to search for additional eligible studies that had cited the trials included in this review using the Science Citation Index database. We also planned to search the reference lists of included trials and previous review articles to find any other pertinent studies. We chose not to conduct either of these searches because we believed the electronic search to be sufficient.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti-Bacterial Agents [therapeutic use];
- Collagen [*radiation effects];
- Eye Infections, Bacterial [drug therapy, *radiotherapy];
- Keratitis [drug therapy, microbiology, *radiotherapy];
- Photosensitizing Agents [*therapeutic use];
- Randomized Controlled Trials as Topic;
- Riboflavin [*therapeutic use];
- Ultraviolet Therapy [adverse effects, *methods];
- Visual Acuity;
Medical Subject Headings Check Words
Humans;
PICO
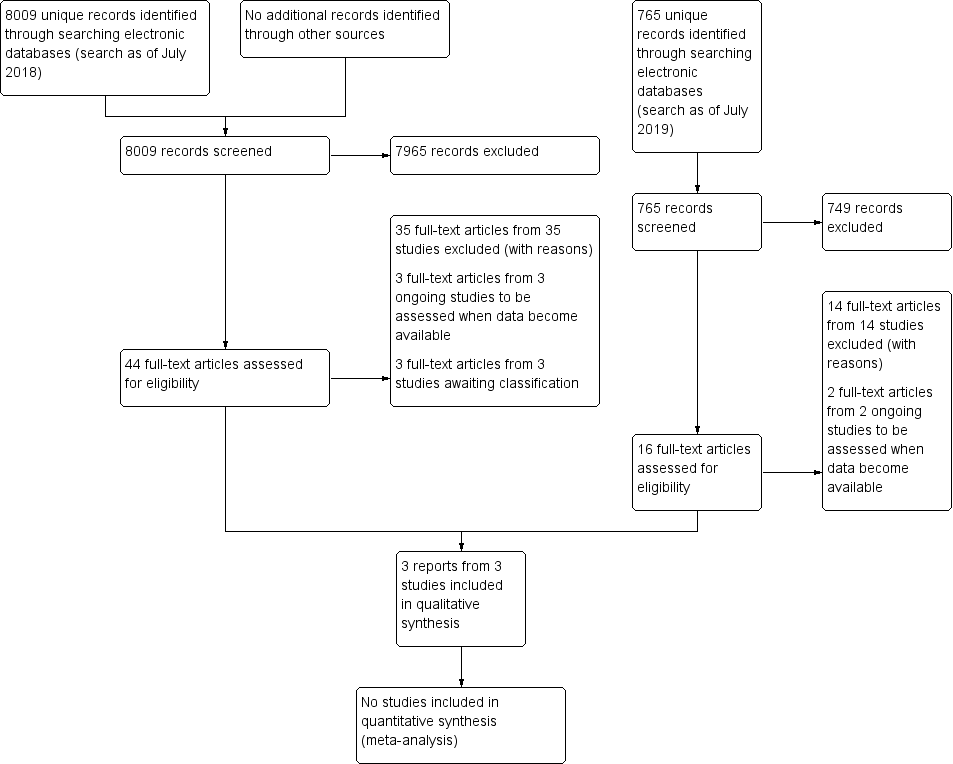
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| PACK‐CXL with standard therapy compared with standard therapy alone for bacterial keratitis | ||||
| Patient or population: participants of any age with confirmed cases of bacterial keratitis Settings: outpatient or inpatient Intervention: PACK‐CXL with standard therapy Comparison: standard therapy alone | ||||
| Outcomes | Anticipated absolute effects (95% CI) | No. of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Proportion of participants with re‐epithelialization and complete healing with or without scar formation at 4 to 8 weeks Assessed by slit‐lamp biomicroscopy | (RR 1.53, 95% CI 0.88 to 2.66) | 15 (1 quasi‐RCT) | ⊕⊝⊝⊝ | Data were reported at 8 weeks. |
| Proportion of participants with BCVA of 20/100 or better at 4 to 8 weeks Assessed using a logMAR chart | Not estimable | 12 (1 quasi‐RCT) | ⊕⊝⊝⊝ | Data were reported at 8 weeks. RR is not available because there were no participants with a BCVA of 20/100 or better at 8 weeks. |
| Mean change from baseline in BCVA at 4 to 8 weeks Assessed using a logMAR chart | None | 0 | None | No data were reported. |
| Proportion of participants with a reduction in corneal infiltrate at 4 to 8 weeks Assessed by outcome assessors | None | 0 | None | No data were reported. |
| Proportion of participants with reduction in intraocular inflammation at 4 to 8 weeks Assessed by corneal keratic precipitates, anterior chamber cellular reaction, or other appropriate measure | None | 0 | None | No data were reported. |
| Proportion of participants with treatment failure at 4 to 8 weeks Assessed by outcome assessors | (RR 0.50, 95% CI 0.05 to 4.98) | 32 (1 RCT) | ⊕⊕⊝⊝ | Data were reported at 14 days. |
| Proportion of participants with adverse events at 4 to 8 weeks Assessed by outcome assessors | Not estimable | 32 (1 RCT) | ⊕⊕⊝⊝ | Data were reported at 14 days. RR is not available because there were no participants with adverse events at 14 days. |
| BCVA: best‐corrected visual acuity; CI: confidence interval; logMAR: logarithm of the minimal angle of resolution; PACK‐CXL: photoactivated chromophore for collagen cross‐linking; RCT: randomized controlled trial; RR: risk ratio | ||||
| GRADE Working Group grades of evidence | ||||
| 1Downgraded for imprecision (‐1) due to low sample size. | ||||

