Rangkaian silang kolagen kornea untuk keratitis jangkitan bakteria
Abstract
Background
Infectious keratitis is an infection of the cornea that can be caused by bacteria, viruses, fungi, protozoa, or parasites. It may be associated with ocular surgery, trauma, contact lens wear, or conditions that cause deficiency or loss of corneal sensation, or suppression of the immune system, such as diabetes, chronic use of topical steroids, or immunomodulatory therapies. Photoactivated chromophore for collagen cross‐linking (PACK‐CXL) of the cornea is a therapy that has been successful in treating eye conditions such as keratoconus and corneal ectasia. More recently, PACK‐CXL has been explored as a treatment option for infectious keratitis.
Objectives
To determine the comparative effectiveness and safety of PACK‐CXL with standard therapy versus standard therapy alone for the treatment of bacterial keratitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2019, Issue 7); Ovid MEDLINE; Embase.com; PubMed; Latin American and Caribbean Health Science Information database (LILACS); ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 8 July 2019.
Selection criteria
We included randomized controlled trials (RCTs), quasi‐RCTs, and controlled clinical trials (CCTs) of PACK‐CXL for bacterial keratitis. We included quasi‐RCTs and CCTs as we anticipated that there would not be many RCTs eligible for inclusion.
Data collection and analysis
Two review authors working independently selected studies for inclusion in the review, assessed trials for risk of bias, and extracted data. The primary outcome was proportion of participants with complete healing at four to eight weeks. Secondary outcomes included visual acuity, morphology, adverse events, and treatment failure at four to eight weeks.
Main results
We included three trials (two RCTs and one quasi‐RCT) in this review for a total of 59 participants (59 eyes) with bacterial keratitis. Trials were all single‐center and were conducted in Egypt, Iran, and Thailand between 2010 and 2014. It is very uncertain whether PACK‐CXL with standard antibiotic therapy is more effective than standard antibiotic therapy alone for re‐epithelialization and complete healing (risk ratio (RR) 1.53, 95% confidence interval (CI) 0.88 to 2.66; participants = 15). We judged the certainty of the evidence to be very low due to the small sample size and high risk of selection and performance bias. The high risk of selection bias reflects the overall review. Masking of participants was not possible for the surgical arm. No participant had a best‐corrected visual acuity of 20/100 or better at eight weeks (very low certainty evidence). There is also no evidence that use of PACK‐CXL with standard therapy results in fewer instances of treatment failure than standard therapy alone (RR 0.50, 95% CI 0.05 to 4.98; participants = 32). We judged the certainty of evidence to be low due to the small sample size and high risk of selection bias. There were no adverse events reported at 14 days (low certainty evidence). Data on other outcomes, such as visual acuity and morphological characteristics, could not be compared because of variable time points and specific metrics.
Authors' conclusions
The current evidence on the effectiveness of PACK‐CXL for bacterial keratitis is of low certainty and clinically heterogenous in regard to outcomes. There are five ongoing RCTs enrolling 1136 participants that may provide better answers in the next update of this review. Any future research should include subgroup analyses based on etiology. A core outcomes set would benefit healthcare decision‐makers in comparing and understanding study data.
PICO
Ringkasan bahasa mudah
Rangkaian silang kolagen kornea untuk keratitis berjangkit
Apakah matlamat ulasan ini?
Matlamat ulasan Cochrane ini adalah untuk mengetahui kesan kromofor yang diaktifkan cahaya bagi perangkaian silang kolagen (PACK‐CXL), rawatan yang berpotensi untuk orang dengan keratitis jangkitan bakteria. Keratitis berjangkit adalah jangkitan kornea, yang merupakan tisu jernih berbentuk kubah di bahagian depan mata. Kami mengumpul dan menganalisis semua kajian yang relevan untuk menjawab soalan ini dan menemui tiga kajian yang relevan.
Mesej‐mesej utama
Tiada bukti gabungan PACK‐CXL dengan antibiotik standard (ubat‐ubatan yang merawat jangkitan bakteria) lebih atau kurang berkesan berbanding antibiotik standard sahaja dari segi penyembuhan lengkap dan kegagalan rawatan. Lima kajian yang sedang berlangsung (1136 peserta) mungkin memberi jawapan lebih baik dalam ulasan kemas kini.
Apakah yang dikaji dalam ulasan ini?
PACK‐CXL menggunakan cahaya ultraviolet dari mesin khas untuk menguatkan kornea. Rawatan ini dipanggil ‘perangkaian silang’ kerana ia mempromosi ikatan fiber kolagen di dalam mata. Fiber kolagen berfungsi seperti rasuk sokongan untuk menstabilkan kornea.
Apakah keputusan utama ulasan ini?
Kami memasukkan tiga kajian dalam ulasan ini yang membandingkan gabungan PACK‐CXL dengan antibiotik standard berbanding antibiotik standard sahaja. Kajian ini melibatkan sejumlah 59 peserta dengan keratitis bakteria (59 mata). Susulan dibuat antara 14 hingga 120 hari selepas rawatan. Kajian dilakukan di Mesir, Iran, dan Thailand antara 2010 dan 2014.
Tiada bukti PACK‐CXL dengan antibiotik standard lebih berkesan berbanding antibiotik standard sahaja untuk penyembuhan lengkap. Kami berkeyakinan sangat rendah terhadap penemuan ini kerana bilangan peserta yang kecil risiko bias yang tinggi. Tiada bukti kadar kegagalan rawatan yang berbeza antara PACK‐CXL dengan antibiotik standard berbanding antibiotik standard sahaja. Kami berkeyakinan rendah terhadap penemuan ini kerana bilangan peserta yang kecil dan risiko bias yang tinggi. Satu kajian yang melibatkan 32 peserta tidak melaporkan kejadian buruk dari PACK‐CXL pada 14 hari.
Adakah ulasan ini mutakhir?
Pengarang ulasan mencari kajian‐kajian yang telah diterbitkan hingga 8 Julai 2019.
Authors' conclusions
Summary of findings
| PACK‐CXL with standard therapy compared with standard therapy alone for bacterial keratitis | ||||
| Patient or population: participants of any age with confirmed cases of bacterial keratitis Settings: outpatient or inpatient Intervention: PACK‐CXL with standard therapy Comparison: standard therapy alone | ||||
| Outcomes | Anticipated absolute effects (95% CI) | No. of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Proportion of participants with re‐epithelialization and complete healing with or without scar formation at 4 to 8 weeks Assessed by slit‐lamp biomicroscopy | (RR 1.53, 95% CI 0.88 to 2.66) | 15 (1 quasi‐RCT) | ⊕⊝⊝⊝ | Data were reported at 8 weeks. |
| Proportion of participants with BCVA of 20/100 or better at 4 to 8 weeks Assessed using a logMAR chart | Not estimable | 12 (1 quasi‐RCT) | ⊕⊝⊝⊝ | Data were reported at 8 weeks. RR is not available because there were no participants with a BCVA of 20/100 or better at 8 weeks. |
| Mean change from baseline in BCVA at 4 to 8 weeks Assessed using a logMAR chart | None | 0 | None | No data were reported. |
| Proportion of participants with a reduction in corneal infiltrate at 4 to 8 weeks Assessed by outcome assessors | None | 0 | None | No data were reported. |
| Proportion of participants with reduction in intraocular inflammation at 4 to 8 weeks Assessed by corneal keratic precipitates, anterior chamber cellular reaction, or other appropriate measure | None | 0 | None | No data were reported. |
| Proportion of participants with treatment failure at 4 to 8 weeks Assessed by outcome assessors | (RR 0.50, 95% CI 0.05 to 4.98) | 32 (1 RCT) | ⊕⊕⊝⊝ | Data were reported at 14 days. |
| Proportion of participants with adverse events at 4 to 8 weeks Assessed by outcome assessors | Not estimable | 32 (1 RCT) | ⊕⊕⊝⊝ | Data were reported at 14 days. RR is not available because there were no participants with adverse events at 14 days. |
| BCVA: best‐corrected visual acuity; CI: confidence interval; logMAR: logarithm of the minimal angle of resolution; PACK‐CXL: photoactivated chromophore for collagen cross‐linking; RCT: randomized controlled trial; RR: risk ratio | ||||
| GRADE Working Group grades of evidence | ||||
| 1Downgraded for imprecision (‐1) due to low sample size. | ||||
Background
Description of the condition
Infectious keratitis is an infection of the cornea that can be caused by bacteria, viruses, fungi, protozoa, or parasites (Bourcier 2003). It may be associated with ocular surgery, trauma, contact lens wear, or conditions that cause deficiency or loss of corneal sensation, or suppression of the immune system, such as diabetes, chronic use of topical steroids, or immunomodulatory therapies (Green 2008). The cornea is the clear, curved surface that covers the front of the eye. It is composed of five layers; from outermost, they are the epithelium, Bowman's membrane, the stroma, Descemet’s membrane, and the endothelium. Infectious keratitis first affects the outermost epithelium and can progress to damage all five layers and perforate the cornea. Untreated infectious keratitis can lead to permanent visual impairment.
Clinically, patients often present with a rapid onset of pain accompanied by conjunctival injection (red eye), photophobia (light sensitivity), and decreased vision. A corneal ulcer is an epithelial defect with surrounding and underlying inflammation caused by an influx of white blood cells. Generally, this results in necrosis of the surrounding tissue. For the purpose of this systematic review, the terms 'infectious keratitis' and 'corneal ulcer' are used interchangeably. Bacterial corneal ulcers display a sharp epithelial demarcation with underlying dense, suppurative stromal inflammation and edema (AAO 2013). Corneal ulcers are typically treated with topical antimicrobials/antibiotics; however, the widespread use of topical antibiotics may increase the incidence of multidrug‐resistant bacteria (Neu 1992). The high cost of developing new antimicrobial drugs and the rapid emergence of drug resistance make the development of alternative treatment approaches desirable (Bertino 2009; Wollensak 2006).
According to the Centers for Disease Control and Prevention (CDC), the overall burden and epidemiology of keratitis in the United States is estimated to include 930,000 doctor office and outpatient clinic visits and 58,000 emergency department visits annually; 76.5% of keratitis visits resulted in antimicrobial prescriptions with an overall cost of about USD 175 million in direct healthcare expenditures, including USD 58 million for Medicare patients and USD 12 million for Medicaid patients, each year (Collier 2014). Outpatient clinic visits resulted in over 250,000 hours of clinician time annually. The disease burden of keratitis in low‐income countries is not well established, but is speculated to be high enough to warrant neglected tropical disease classification (Ung 2019).
Description of the intervention
Photoactivated chromophore for collagen cross‐linking (PACK‐CXL) of the cornea uses ultraviolet (UV)‐A rays in combination with riboflavin (vitamin B2, a photosensitizer) to increase the biomechanical strength of the cornea and halt exacerbation of ectatic conditions of the cornea (Wollensak 2006). The resulting reaction involves reactive oxygen species and leads to stiffening/stabilization of the corneal stroma via the formation of covalent bonds (cross‐links) between the corneal stromal fibers (Wollensak 2003). Riboflavin has previously been shown to be activated by UV light to inactivate pathogens by damaging their ribonucleic acid (RNA) and DNA by oxidation (Goodrich 2000; Tsugita 1965).
How the intervention might work
In a number of research studies PACK‐CXL has been shown to improve healing in bacterial keratitis and block the progression of corneal melting (enzymatic degradation by bacteria) (Iseli 2008; Makdoumi 2010; Makdoumi 2012; Morén 2010). When activated by UV light, riboflavin induces a change in collagen properties, and has a hardening and strengthening effect on corneal stroma. As a result, a compromised cornea treated with activated riboflavin can become more resistant to corneal melting (Schilde 2008; Spoerl 2004). However, an intact cornea is protective in that it allows less than 10% of UV light to penetrate the eye. In individuals with corneal ulcers, there is greater penetration of UV light, which could cause endothelial cell loss, consequently compromising the integrity of the cornea and corneal function and potentially resulting in decreased vision.
Why it is important to do this review
Observations have shown that corneal PACK‐CXL could be a novel treatment for infectious keratitis. PACK‐CXL has been shown to be effective in halting corneal melting, but the absence of control groups in past studies and previous reviews has meant that it has not been possible to confirm whether it is less, as, or more effective than antimicrobial treatments. Two systematic reviews of PACK‐CXL have been published, which together included two randomized controlled trials (RCTs) (Alio 2013; Papaioannou 2016). Since these systematic reviews were published, however, other RCTs of PACK‐CXL have been conducted. It is imperative to review and re‐evaluate these trials in order to provide up‐to‐date information to use in evidence‐based recommendations.
Objectives
To determine the comparative effectiveness and safety of PACK‐CXL with standard therapy versus standard therapy alone for the treatment of bacterial keratitis.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs or quasi‐RCTs that evaluated PACK‐CXL for infectious keratitis. We considered quasi‐RCTs as we anticipated that few RCTs would be available for inclusion.
Types of participants
We included studies that enrolled participants who had a diagnosis of bacterial keratitis. Where possible, we documented studies that enrolled participants with polymicrobial etiology (mixed infections) and cases involving bacterial, viral, fungal, protozoal, or parasitic infiltration, as well as cases with complications such as corneal perforations that warranted surgical intervention. However, for the purposes of this review, we focused on microbiologically proven cases of bacterial keratitis. We excluded studies in which keratitis was not clearly diagnosed as having an infectious etiology.
Types of interventions
We included studies comparing PACK‐CXL with standard therapy for the management of bacterial keratitis. Standard therapy typically includes topical antibiotic treatments. Studies that examined PACK‐CXL in combination with antimicrobial/antibiotic treatment versus standard therapy alone were eligible.
Types of outcome measures
Primary outcomes
The proportion of participants with re‐epithelization and complete healing with or without scar formation at four to eight weeks. Complete healing was defined as: absence of infiltrate, epithelial healing, and no sign of inflammation or epithelial defect.
Secondary outcomes
-
Proportion of participants with best‐corrected visual acuity (BCVA) of 20/100 or better at four to eight weeks based on measurements made on a logMAR chart. We planned to analyze BCVA data from measurements made using any type of correction of refractive error (e.g. spectacles, rigid gas‐permeable contact lens). For participants whose premorbid BCVA was worse than 20/100, we planned to consider those who regained their premorbid BCVA.
-
Mean change from baseline in BCVA at four to eight weeks, as measured on a logMAR chart.
-
Proportion of participants with a reduction in corneal infiltrate, as defined by study investigators, at four to eight weeks.
-
Proportion of participants with reduction in intraocular inflammation including, but not limited to, corneal keratic precipitates or anterior chamber cellular reaction, at four to eight weeks.
-
Proportion of participants with treatment failure, including, but not limited to, persistence or worsening of existing infectious keratitis, anterior chamber cellular reaction, corneal infiltrate, or epithelial defect, at four to eight weeks.
In the event that a trial reported an outcome for more than one time point within the range of four to eight weeks (e.g. at four weeks' and at six weeks' follow‐up), we used the longest time point for analysis. When the follow‐up time was shorter than four weeks, we used the time point at the longest follow‐up.
Adverse effects
We documented and compared the proportion of participants with adverse events at four to eight weeks in each group. Specific adverse events of interest included:
-
increased infiltrate size;
-
increased thinning or melting of the cornea, or need for corneal transplant;
-
development or worsening of corneal edema;
-
endophthalmitis; and
-
recurrence of corneal ulcer.
We summarized and reported other adverse effects related to PACK‐CXL therapy reported in all included studies; however, it is challenging to separate adverse outcomes of PACK‐CXL from worsening infectious keratitis.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. There were no restrictions on language or year of publication. The electronic databases were last searched on 8 July 2019.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 7) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 8 July 2019) (Appendix 1).
-
MEDLINE Ovid (January 1946 to 8 July 2019) (Appendix 2).
-
Embase.com (January 1947 to 8 July 2019) (Appendix 3).
-
PubMed (1948 to 8 July 2019) (Appendix 4).
-
Latin American and Caribbean Health Science Information database (LILACS) (January 1982 to 8 July 2019) (Appendix 5).
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 8 July 2019) (Appendix 6).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 8 July 2019) (Appendix 7).
Searching other resources
We did not handsearch any journals or conference proceedings for this review.
Data collection and analysis
Selection of studies
Two review authors (SD and RB) independently reviewed the titles and abstracts obtained from the literature searches and classified each record according to inclusion criteria eligibility as 'yes, relevant,' 'maybe,' or 'no, not relevant.' We used the web application Covidence to manage the screening process (Covidence). We obtained the full‐text reports of records classified as 'yes' or 'maybe' relevant. Two review authors evaluated these full‐text reports and categorized them as 'include,' 'awaiting assessment,' or 'exclude.' For future studies that are categorized as awaiting assessment, we plan to contact the primary investigators to obtain more information and clarification. All disagreements regarding the inclusion of a trial were resolved through discussion at each stage of the screening process.
Data extraction and management
Two review authors (RB and GH) independently recorded data from study reports related to study methods, participants, interventions, and outcomes. Any disagreements or inconsistencies were resolved through discussion. We contacted the corresponding author of the study for information on missing data or to obtain clarification about unclear data. For all but one study, we did not receive responses after two weeks, therefore we used the data as reported in those study reports.
We used data extraction forms developed by Cochrane Eyes and Vision, and administered by Covidence (Covidence), to record data for individual studies. One review author entered data into Review Manager 5 (RevMan 5) (Review Manager 2014), and a second review author verified the data entry.
Assessment of risk of bias in included studies
Two review authors independently examined each included study for risk of bias according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We examined the studies for the following types of bias: (a) random sequence generation and allocation concealment before randomization (selection bias); (b) masking of study personnel (performance bias); (c) masking of outcome assessors (detection bias); (d) completeness of outcome data/follow‐up and intention‐to‐treat analysis (attrition bias); and (e) selective outcome reporting (reporting bias). Since masking of participants is uncommon, and often impossible, in surgical studies, we considered this a measure of risk of bias for the overall review rather than for individual studies.
Measures of treatment effect
We evaluated the data according to the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We planned to calculate mean differences with 95% confidence intervals for continuous outcomes, such as mean change in BCVA from baseline, and risk ratios with 95% confidence intervals for dichotomous outcomes, including the proportion of participants with re‐epithelialization and complete healing and the proportion of participants with BCVA of 20/100 or better.
Unit of analysis issues
The unit of analysis was the individual in all included studies (one study eye per participant).
Dealing with missing data
We contacted the corresponding author of the study for information on missing data or to obtain clarification about unclear data; however, for all but one study, we did not receive a response. We did not impute data for the purposes of this review.
Assessment of heterogeneity
We assessed methodological and clinical heterogeneity by examining the study methods, population, interventions, and outcomes among studies. We did not assess statistical heterogeneity since only one study provided outcome data in four to eight weeks.
When future RCTs become eligible for inclusion, we will assess statistical heterogeneity using an I² value of more than 50% as indicative of substantial statistical heterogeneity.
Assessment of reporting biases
We assessed selective outcome reporting for each study by comparing the outcomes specified in a protocol, research plan, or clinical trial registry with the reported results. In future updates of this review, if outcome data from 10 or more studies are included in a meta‐analysis we will assess the symmetry of the funnel plot as a method of examining small‐study effects (Deeks 2017). We assessed risk of selective outcome reporting as part of our assessment of risk of bias in the included studies.
Data synthesis
We did not combine study results since none of the prespecified review outcomes were reported by more than one study. We provided a description and summarized the findings of each study.
In future review updates, meta‐analysis may be possible if data for one or more outcomes are reported in additional included studies. If there is no clinical or methodological heterogeneity and no substantial statistical heterogeneity, we will combine the data for each outcome in a meta‐analysis using a random‐effects model; if the number of included studies is less than three, we will use a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
In future updates of this review, we will conduct a subgroup analysis to assess whether type of infection (bacterial, viral, fungal, protozoal, or parasitic) has an effect on response to PACK‐CXL.
Sensitivity analysis
In future review updates, when sufficient data are available, we will perform sensitivity analyses to determine the consistency of effect estimates when excluding studies at high risk of bias, unpublished trials, and studies funded by industry.
Summary of findings
We examined the overall certainty of evidence via the GRADE approach using the domains of risk of bias, imprecision, inconsistency, indirectness, and publication bias (Schünemann 2011). We presented our findings in a 'Summary of findings' table for the following outcomes.
-
The proportion of participants with re‐epithelization and complete healing with or without scar formation at four to eight weeks.
-
The proportion of participants with BCVA of 20/100 or better at four to eight weeks. For participants whose premorbid BCVA was worse than 20/100, we considered those who regained their premorbid BCVA.
-
The proportion of participants with a reduction in corneal infiltrate at four to eight weeks.
-
The proportion of participants with a reduction in intraocular inflammation at four to eight weeks.
-
The proportion of participants with treatment failure at four to eight weeks.
-
The proportion of participants with adverse events at four to eight weeks.
Results
Description of studies
Results of the search
The electronic search yielded 8019 records (Figure 1). After removing 10 duplicates, we screened the remaining 8009 records and excluded a further 7965 records based on information in the title and abstract. We obtained the full‐text reports of 44 records for further investigation. At full‐text screening, we included three reports of three studies (see Characteristics of included studies), three ongoing studies that potentially met our inclusion criteria (see Characteristics of ongoing studies), and three studies awaiting classification (see Characteristics of studies awaiting classification). We identified and excluded 35 reports of 35 studies (see Characteristics of excluded studies).

Study flow diagram.
We conducted a top‐up search in July 2019 that yielded 766 records (Figure 1). We removed one duplicate and performed title and abstract screening of the remaining 765 records. We excluded 749 records, and assessed the full texts of 16 records. We excluded 14 full‐text records (see Characteristics of excluded studies) and identified two ongoing studies that will be assessed when data become available (see Characteristics of ongoing studies).
We identified a total of three included studies, five ongoing studies, and three studies awaiting classification.
Included studies
For further details on included studies, see Characteristics of included studies.
Type of studies
We included two RCTs, Bamdad 2015; Kasetsuwan 2016, and one quasi‐RCT, Said 2014. Two studies were conducted in the Middle East: Said 2014 (Egypt) and Bamdad 2015 (Iran), while the third study was conducted in Thailand (Kasetsuwan 2016). All studies were single‐center. Study participants were recruited between 2010 to 2014. One study reported participant‐level outcomes data (Said 2014), whereas the other two studies reported summary outcomes data (Bamdad 2015; Kasetsuwan 2016).
Type of participants
The three studies randomized a total of 82 participants (82 eyes, range per study: 21 to 32), 59 of whom were diagnosed with bacterial keratitis. The studies were of comparable sizes, although the number of participants with bacterial keratitis varied from 12 to 32 per study. One study included only participants with bacterial keratitis (Bamdad 2015), while the remaining two studies included participants who were heterogenous with regard to causative organism (Kasetsuwan 2016; Said 2014).
Demographic and baseline characteristics of participants with bacterial keratitis were inconsistently reported in the three studies. While Bamdad 2015 reported all characteristics, Said 2014 reported only baseline characteristics of participants with bacterial keratitis, and Kasetsuwan 2016 did not report any characteristics. Communication with study investigators yielded no additional information.
The average age of randomized participants was comparable across the three studies despite different inclusion criteria: Kasetsuwan 2016 included children older than six years of age, and Said 2014 included adults 18 years of age or older. Randomized participants were on average middle‐aged (mean age of 43 years), which is consistent with findings from retrospective analyses (Bourcier 2003; Mun 2019). Participants also had variable baseline levels of corneal ulcer severity, which was determined based on size and infiltration: Kasetsuwan 2016 included participants with moderate (defined as being 2 to 6 mm in size and involving the superficial two‐thirds of the cornea) to severe ulcers (defined as being over 6 mm in size and involving the posterior one‐third of the cornea, or presence of hypopyon), and Bamdad 2015 only included participants with moderate ulcers (defined as being 2 to 6 mm in size and involving the superficial two‐thirds of the cornea). Said 2014 did not use standardized ulcer grading in their inclusion criteria. All participants in Said 2014 had advanced keratitis due to presence of corneal melting and received treatment for infectious keratitis prior to enrollment.
Type of interventions
The three studies compared photoactivated chromophore for collagen cross‐linking (PACK‐CXL) with standard therapy versus standard therapy alone for the treatment of infectious keratitis.
PACK‐CXL with standard therapy versus standard therapy alone
Sixty participants were assigned to one of two intervention arms: PACK‐CXL with standard therapy or standard therapy alone. Participants who were assigned to the PACK‐CXL arm received riboflavin (MedioCross 0.1% riboflavin/20% dextran solution; Peschke Meditrade) before and during 30‐minute UVA illumination (365 nm with 3.0 mW/cm²) with a UV‐X lamp (Peschke Meditrade). Riboflavin administration took place for an initial 30 minutes and then another 30 minutes while irradiating with UVA. The respective frequency of riboflavin administration varied across studies: every 2 minutes and then every 5 minutes (Kasetsuwan 2016); every 2 minutes and then every 2 minutes (Said 2014); and every 3 minutes and then every 5 minutes (Bamdad 2015). After PACK‐CXL, participants received standard antibiotic therapy.
Standard therapy for cases of bacterial keratitis consisted of antibiotic therapy; studies varied in the type and number of antibiotics given, but all antibiotics were given hourly. Kasetsuwan 2016 used fortified cefazolin (50 mg/mL) and fortified amikacin (20 mg/mL); Said 2014 used vancomycin eye drops (50 mg/mL), fortified ceftazidime eye drops (50 mg/mL hourly), and itraconazole (100 mg orally twice daily); and Bamdad 2015 used fortified cefazolin (50 mg/mL hourly), gentamicin (15 mg/mL hourly), and systemic doxycycline (every 12 hours). These antibiotics are typical of usual practice (AAO 2019). Loading doses, which are a high initial dose to rapidly achieve desired levels in a patient, were used in Bamdad 2015 (fortified cefazolin and gentamicin every 5 minutes for 30 minutes).
Type of outcomes
Follow‐up
Bamdad 2015 conducted follow‐up at 14 days, and Kasetsuwan 2016 conducted follow‐up at 30 days. Said 2014 conducted follow‐up examinations until healing as defined by study authors; the longest time until healing was 120 days.
Morphology
One study reported information with which we could assess our primary outcome (proportion of participants with re‐epithelialization and complete healing with or without scar formation) (Said 2014). Although both Bamdad 2015 and Said 2014 reported participants' time to complete healing, only Said 2014 reported sufficient data to determine the proportion of participants with complete healing. Bamdad 2015 and Kasetsuwan 2016 reported mean area of epithelial defects at day 14 and median area of epithelial defects at day 30, respectively.
We were also interested in the proportion of participants with a reduction in corneal infiltrate. All included studies measured the final corneal infiltration using slit‐lamp biomicroscopy, but reported infiltrate morphology in various ways.
Bamdad 2015 graded the ulcer at day 14 (grading considered the severity of anterior segment infiltrates). Kasetsuwan 2016 reported the median infiltrate area at day 30; however, the study authors did not report sufficient participant‐level data to determine the proportion of participants with a reduction in corneal infiltrate. Said 2014 considered clearing of stromal infiltrate as part of their definition of complete healing.
No studies reported sufficient information to assess the proportion of participants with reduction in intraocular inflammation (e.g. hypopyon). The authors of Bamdad 2015 and Said 2014 reported the number of participants who reached complete healing, which was defined as the disappearance of hypopyon, among other requirements (e.g. no anterior chamber activity, re‐epithelialization). However, data were insufficient to assess only the proportion of participants with disappearance of hypopyon.
Bamdad 2015 reported the proportion with treatment failure at day 14.
Visual acuity
Two studies reported visual acuity outcomes using measurements made on a logMAR chart (Kasetsuwan 2016; Said 2014). The two studies reported baseline and final visual acuity: Said 2014 reported BCVA, and Kasetsuwan 2016 reported BPVA (best pinhole‐corrected visual acuity). Measuring BPVA eliminates the effect of ocular opacity that corneal ulcers would have on visual acuity, potentially equalizing the visual acuity results of PACK‐CXL and control groups. Said 2014 reported individual‐level baseline and final BCVA measurements at time of healing, which ranged from 14 to 120 days, from which we calculated the proportion of participants with BCVA of 20/100 or better and mean change from baseline in BCVA. We were unable to extract visual acuity data on bacterial keratitis cases in Kasetsuwan 2016 because only summary‐level visual acuity data (i.e. proportion with improved BPVA, mean BPVA) were reported.
Bamdad 2015 reported that an examiner assessed baseline visual acuity, but the authors did not report the measurements nor specify whether visual acuity measured as BCVA or BPVA. Moreover, authors did not measure final visual acuity.
Adverse events
Bamdad 2015 reported adverse events at day 14. Although Kasetsuwan 2016 and Said 2014 reported adverse events, we were not able to determine whether participants with bacterial keratitis were affected. Kasetsuwan 2016 reported two cases of uncontrolled infection in the PACK‐CXL with standard therapy group at day 30, and three cases of uncontrolled infection and one case of endophthalmitis in the standard therapy‐alone group. Said 2014 reported no complications in the PACK‐CXL with standard therapy group at time of healing (range: 14 to 120 days), whereas there were three cases of corneal perforation and one case of recurrent infection in the standard therapy‐alone group.
Ongoing studies
We identified five ongoing studies (n = 1136). All studies are described as RCTs. They are geographically diverse, taking place or proposing to take place in Australia, India, the United States, and Switzerland. One study compares PACK‐CXL with standard therapy versus placebo surgery with standard therapy (CTRI/2019/02/017601). Four studies compare PACK‐CXL with standard therapy versus standard therapy (ACTRN12611000189921; CTRI/2019/01/017136; NCT02570321; NCT02717871).
Studies awaiting classification
We identified three RCTs (n = 380) awaiting classification. One study compares PACK‐CXL with standard therapy versus placebo surgery with standard therapy (NCT02865876), and two studies compare PACK‐CXL with standard therapy versus standard therapy (NCT02088970; PACTR201701001843366). NCT02865876 was discontinued due to difficulty recruiting participants. We contacted the authors of NCT02865876 and NCT02865876 with a data request; however, we did not receive a response. PACTR201701001843366 (n = 78) was published recently and will be included in a future update of this review.
Excluded studies
We excluded 20 studies at full‐text assessment because they were not RCTs or quasi‐RCTs. We additionally excluded eight studies with ineligible patient population and seven studies with ineligible interventions. There were a total of 35 excluded studies.
For further details on excluded studies, see Characteristics of excluded studies.
Risk of bias in included studies
We summarized the risk of bias in all included studies in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All studies reported methods of randomization: two RCTs used simple randomization (Bamdad 2015; Kasetsuwan 2016), and one quasi‐RCT used alternate allocation (Said 2014). Randomization in Said 2014 was inadequately generated, and may have introduced selection bias.
Allocation concealment
One study reported allocation concealment using sealed envelopes (Kasetsuwan 2016). The quasi‐RCT used alternate allocation and was therefore at high risk of bias (Said 2014). One study did not report method of allocation concealment (Bamdad 2015). Overall, allocation concealment was poorly conducted or reported.
Blinding
Two studies reported masking outcome assessors (Bamdad 2015; Kasetsuwan 2016). However, masking of outcome assessors failed in Bamdad 2015 due to participants informing the assessor of their treatment group.
As reported in the review protocol, the nature of surgical studies means that masking of participants is impossible. We therefore considered the overall review as at high risk of bias for this domain (see summary of findings Table 1).
Incomplete outcome data
None of the studies reported missing data from attrition. We considered studies to have complete outcome data.
Selective reporting
Two studies reported prespecified outcomes in the protocols (Bamdad 2015; Kasetsuwan 2016). Insufficient information was available to assess reporting bias for Said 2014.
Other potential sources of bias
A study author in Said 2014 co‐invented an ultraviolet light source; an ultraviolet light source is used for PACK‐CXL, but it is unclear if the invention was used in the study. This was the only reported conflict of interest in the included studies.
Effects of interventions
See summary of findings Table 1.
Comparison 1: Collagen cross‐linking with standard therapy versus standard therapy alone
We did not conduct a meta‐analysis for any outcome since none of the prespecified review outcomes were reported by more than one study.
Proportion of participants with re‐epithelialization and complete healing with or without scar formation at 8 weeks (1 study; 15 participants)
In Said 2014, participants were followed until complete healing was achieved. We analyzed outcome data at our time point of interest (longest time point between four and eight weeks) using the participant‐level data provided. There were seven participants in the PACK‐CXL with standard therapy arm and eight participants in the standard therapy‐alone arm. Participants in the PACK‐CXL group fared slightly better than those in the only standard therapy‐alone arm: at 8 weeks, 7/7 participants in the PACK‐CXL with standard therapy arm achieved complete healing, compared to 5/8 participants in the standard therapy‐alone arm (risk ratio (RR) 1.53, 95% confidence interval (CI) 0.88 to 2.66).
We judged the evidence for complete healing to be of very low certainty due to the small sample size and high risk of selection and performance bias. The high risk of selection bias reflects the overall review, since masking of participants was not possible for the surgical arm.
Proportion of participants with BCVA of 20/100 or better at 8 weeks (1 study; 12 participants)
In Said 2014, no participants in either arm achieved a BCVA of 20/100 or better. Only 12 participants were included because BCVA was assessed at the time of complete healing, which occurred after 8 weeks for 3 participants.
We judged the evidence for visual acuity to be of very low certainty due to the small sample size and high risk of selection and performance bias. The high risk of selection bias reflects the overall review, since masking of participants was not possible for the surgical arm.
Mean change from baseline in BCVA
Not reported.
Proportion of participants with a reduction in corneal infiltrate
Not reported.
Proportion of participants with reduction in intraocular inflammation
Not reported.
Proportion of participants with treatment failure at day 14 (1 study, 32 participants)
Bamdad 2015 reported treatment failure in 1/16 participants in the PACK‐CXL with standard therapy arm, compared to 2/16 participants in the standard therapy‐alone arm (RR 0.50, 95% CI 0.05 to 4.98). Participants with treatment failure were treated with amniotic membrane transplantation; if this failed, a conjunctival flap was performed.
We judged the evidence for treatment failure to be of low certainty due to the small sample size and high risk of selection bias. The high risk of selection bias reflects the overall review, since masking of participants was not possible for the surgical arm.
Proportion of participants with adverse events at day 14 (1 study, 32 participants)
Bamdad 2015 reported no adverse events in either arm.
We judged the evidence for adverse events to be of low certainty due to the small sample size and high risk of selection bias. The high risk of selection bias reflects the overall review, since masking of participants was not possible for the surgical arm.
Discussion
Summary of main results
This systematic review included three studies that compared PACK‐CXL with standard antibiotic therapy to standard antibiotic therapy alone for the treatment of bacterial keratitis (59 participants). Two studies were RCTs, and one study used a quasi‐randomization method based on alternate allocation. The primary outcome for our review was proportion of participants with complete healing. Secondary outcomes included visual acuity and morphology measures, as well as adverse events and treatment failure. All three included studies had comparable intervention groups and protocols for PACK‐CXL with standard therapy.
The included studies were highly variable with regard to outcome reporting and follow‐up times. The clinical heterogeneity in reported outcomes made it difficult to compare data across RCTs. Moreover, there was a wide range of follow‐up times: 14 days (prespecified) to 120 days (time until healing). The methodological rigor of the studies varied. Although there were no missing data reported in the included studies, one study was quasi‐randomized, thereby introducing selection bias. There was high risk of bias overall due to performance bias, since it is impossible to mask participants to surgical interventions.
The most significant limitation we faced was that studies provided insufficient information to assess cases of bacterial keratitis. Two of the three included studies included keratitis of any etiology, but did not report outcomes based on etiology completely or at all. In addition, there were low numbers of participants with bacterial keratitis enrolled in the included studies (range 12 to 32); while one study conducted a sample size calculation, the calculation was designed to detect significance in participants with keratitis of any etiology.
It is very uncertain whether PACK‐CXL with standard antibiotic therapy is safer and more effective than standard antibiotic therapy alone for re‐epithelialization and complete healing. We have very low confidence in this evidence due to the small sample size and high risk of selection and performance bias.
Overall completeness and applicability of evidence
The overall certainty of the evidence is very low. We identified three small studies for inclusion in the review, and only one study reported sufficient information to assess outcomes for cases of bacterial keratitis. We judged all three studies as at high or unclear risk of bias for most domains.
Quality of the evidence
The evidence in this review is very limited because we were unable to assess the data on cases of bacterial keratitis for two of the three included studies. Moreover, the number of bacterial keratitis cases per study was very low (range: 12 to 32). Given these small sample sizes, we are not confident in the available evidence.
Overall, this review was at high risk of performance bias because the intervention arm involved a surgical procedure which thus made masking participants and personnel impossible. Other 'Risk of bias' domains were generally low or unclear (see Characteristics of included studies).
Potential biases in the review process
No changes were made to the review protocol published in 2018. Two review authors independently completed all steps in the Methods section to minimize errors and conferred upon disagreement.
Agreements and disagreements with other studies or reviews
Papaioannou 2016 analyzed two of the three studies included in our systematic review, assessing a total of 25 studies (13 case series, 10 case reports). The review authors found that PACK‐CXL was most effective in cases of bacterial keratitis (87.2% healed, total of 167 eyes), but the certainty of evidence was low due to the number of non‐randomized studies that were included. Another systematic review supported PACK‐CXL for infectious keratitis (Alio 2013); however, that review did not identify any RCTs for inclusion.
We are in agreement with published systematic reviews that recommend further rigorous examination of the effectiveness of PACK‐CXL in individuals with infectious keratitis, and more specifically infectious keratitis by etiology.
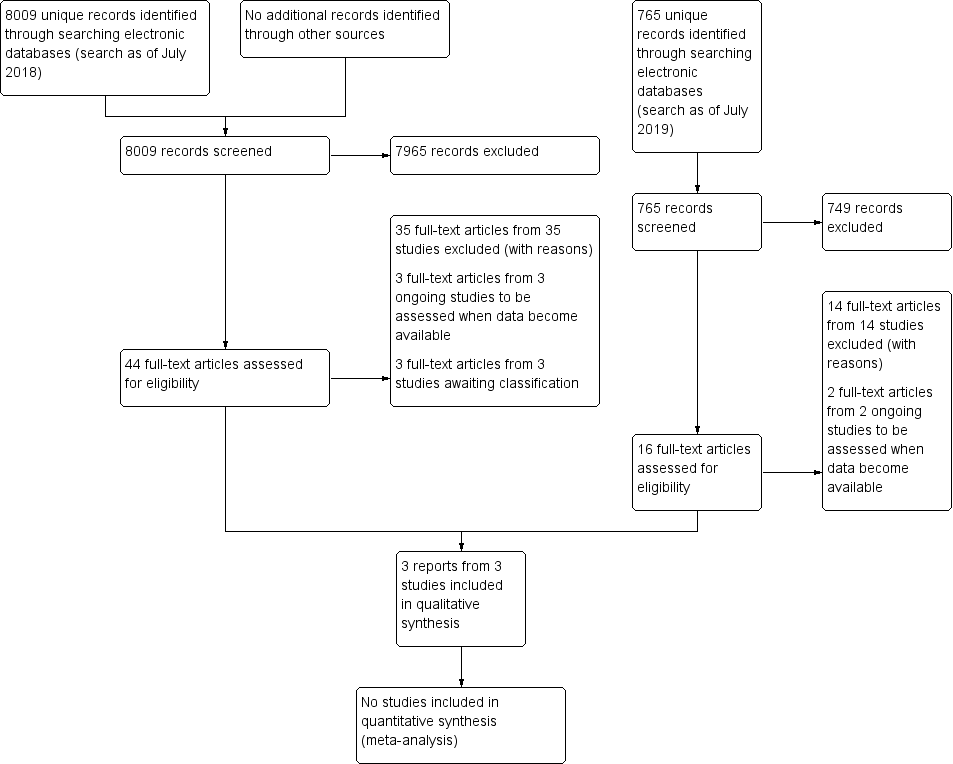
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| PACK‐CXL with standard therapy compared with standard therapy alone for bacterial keratitis | ||||
| Patient or population: participants of any age with confirmed cases of bacterial keratitis Settings: outpatient or inpatient Intervention: PACK‐CXL with standard therapy Comparison: standard therapy alone | ||||
| Outcomes | Anticipated absolute effects (95% CI) | No. of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Proportion of participants with re‐epithelialization and complete healing with or without scar formation at 4 to 8 weeks Assessed by slit‐lamp biomicroscopy | (RR 1.53, 95% CI 0.88 to 2.66) | 15 (1 quasi‐RCT) | ⊕⊝⊝⊝ | Data were reported at 8 weeks. |
| Proportion of participants with BCVA of 20/100 or better at 4 to 8 weeks Assessed using a logMAR chart | Not estimable | 12 (1 quasi‐RCT) | ⊕⊝⊝⊝ | Data were reported at 8 weeks. RR is not available because there were no participants with a BCVA of 20/100 or better at 8 weeks. |
| Mean change from baseline in BCVA at 4 to 8 weeks Assessed using a logMAR chart | None | 0 | None | No data were reported. |
| Proportion of participants with a reduction in corneal infiltrate at 4 to 8 weeks Assessed by outcome assessors | None | 0 | None | No data were reported. |
| Proportion of participants with reduction in intraocular inflammation at 4 to 8 weeks Assessed by corneal keratic precipitates, anterior chamber cellular reaction, or other appropriate measure | None | 0 | None | No data were reported. |
| Proportion of participants with treatment failure at 4 to 8 weeks Assessed by outcome assessors | (RR 0.50, 95% CI 0.05 to 4.98) | 32 (1 RCT) | ⊕⊕⊝⊝ | Data were reported at 14 days. |
| Proportion of participants with adverse events at 4 to 8 weeks Assessed by outcome assessors | Not estimable | 32 (1 RCT) | ⊕⊕⊝⊝ | Data were reported at 14 days. RR is not available because there were no participants with adverse events at 14 days. |
| BCVA: best‐corrected visual acuity; CI: confidence interval; logMAR: logarithm of the minimal angle of resolution; PACK‐CXL: photoactivated chromophore for collagen cross‐linking; RCT: randomized controlled trial; RR: risk ratio | ||||
| GRADE Working Group grades of evidence | ||||
| 1Downgraded for imprecision (‐1) due to low sample size. | ||||

