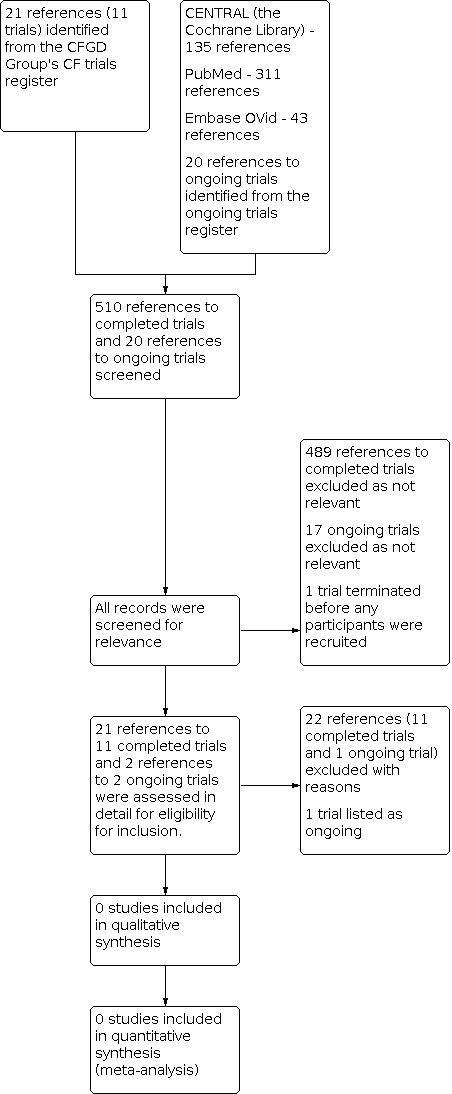
Intervenciones médicas para la rinosinusitis crónica en la fibrosis quística
Referencias
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Intervention undertaken was not considered in our protocol. In this trial, study group underwent therapeutic ultrasound insonation over each maxillary sinus for five minutes. This intervention has not been considered in our protocol. Hence, we excluded this trial. | |
| Follow‐up period was not adequate for analysis. In this trial, included participants were treated with nasal spray formulation consisting of a 10 mL aqueous solution of 0.2% sodium hyaluronate and 3% tobramycin sulphate for 14 days. They were assessed on the first and 14th day. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. | |
| Intervention undertaken was not considered in our protocol. In this trial, participants were treated with compound honey syrup. This intervention has not been considered in our protocol. Hence, we excluded this trial. | |
| Intervention undertaken was not considered in our protocol In this trial, the study group was treated with 10% (v/v) manuka honey (MEDIHONEY®; Derma Sciences; Princeton, NJ) . This intervention has not been considered in our protocol. Hence, we excluded this trial. | |
| Follow‐up period was not adequate for analysis. In this trial, included participants were treated with inhaled dornase alfa or normal saline for 28 days. They were assessed on 28th day. After a washout period of another 28 days, they were crossed over to the alternative treatment. The minimum period of follow‐up needed to include a trial in this review in 3 months. Hence, we excluded this trial. | |
| Follow‐up period was not adequate for analysis. In this trial, included participants were treated with inhaled dornase alfa or normal saline for 28 days. They were assessed on the 28th day. After a washout period of another 28 days, they were crossed over to the alternative treatment. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. | |
| Follow‐up period was not adequate for analysis. In this trial, included participants were treated with inhaled hypertonic saline (6%) or normal saline for 28 days. They were assessed on the 28th day. After a washout period of another 28 days, they were crossed over to the alternative treatment. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. | |
| Trial evaluated the effect of medical interventions on surgical outcomes. In this trial, participants were randomized to receive dornase alfa or placebo via nasal inhalation one week following a nasal surgery, to assess improvement in post‐operative sinusitis symptoms. We have planned to exclude trials evaluating effect of medical interventions on surgical outcomes. Hence, we excluded this trial. | |
| Follow‐up period was not adequate for analysis. In this trial, included participants were treated with nasal irrigation with Respimer® mineral salts solution for 8 weeks. They were assessed at begining of trial, 4 weeks into treatment and at the end of 8 weeks. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. | |
| Follow‐up period was not adequate for analysis. In the protocol of this ongoing trial, participants were planned to be treated with ivacaftor along with standard of care treatment (topical nasal steroid spray and culture‐directed antibiotics) or standard of care alone for 14 days. Outcomes are planned to be assessed on day 1, day 14 and day 30. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. | |
| Methodology of randomisation was not acceptable as per our protocol. Investigators preformed a prospective, randomized, phase I clinical trial by instilling adeno‐associated virus vector ‐ CF transmembrane conductance regulator (AAV‐CFTR) into a maxillary sinus with a surgically‐created antrostomy. The contralateral sinus served as a control. According to our protocol, we planned to exclude trials randomized by the side of the nose. Hence, we excluded this trial. | |
| Methodology of randomisation was not acceptable as per our protocol. Investigators performed a phase II double‐blind randomized placebo‐controlled trial by instilling 100,000 replication units of targeted genetics adeno‐associated virus vector – CF (tgAAVCF) into one of the maxillary sinuses. The contralateral maxillary sinus acted as a control. According to our protocol, we planned to exclude trials randomized by the side of the nose. Hence we excluded this trial. |
CF: cystic fibrosis
Characteristics of ongoing studies [ordered by study ID]
Ir a:
| Study name | Efficacy of Antibiotic (Tobramycin) Delivered by Nebulized Sonic Aerosol for Chronic Rhinosinusitis Treatment of Cystic Fibrosis Patients: A Multicenter Double‐blind Randomized Controlled Trial |
| Methods | RCT Parallel assignment Triple blinded Duration: 15 days Multicenter: 6 centers (Créteil, Marseille, Nantes, Toulouse, Clermont‐Ferrand and Nice) |
| Participants | Estimated enrolment: 86 participants aged 7 years or older Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment arm 1 ampoule tobramycin (5 mL containing 300 mg of tobramycin and 11.25 mg of sodium chloride) nebulized nasally 2x per day (morning and evening with a maximum of 12 hours but not less than 6 hours between the 2 doses) each sonic nebulization lasting approximately 15 minutes; dosage will not be adjusted to body weight. Active intervention is manufactured by the "Pharmacie à Usage Interne" of the Henri Mondor Hospital prepared with Base Tobramycin and excipients in accordance with TOBI's composition Control arm 1 ampoule placebo (5 mL containing sodium chloride 0.9%) nebulized nasally 2x per day (morning and evening with a maximum of 12 hours but not less than 6 hours between the 2 doses) each sonic nebulization lasting approximately 15 minutes; dosage will not be adjusted to body weight. Placebo is manufactured by the "Pharmacie à Usage Interne" of the Henri Mondor Hospital so that it has the same colour (light yellow transparent) as tobramycin |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | February 2017 |
| Contact information | Virginie ESCABASSE, MD Tel: 1 45 17 55 97 ext 33 |
| Notes |
CF: cystic fibrosis
CFU: colony forming units
CRS: chronic rhinosinusitis
CT: computer tomography
FEV: forced expiratory volume
FVC: forced vital capacity
RCT: randomized controlled trial
SNOT: sino‐nasal outcomes test