Paravertebral anaesthesia with or without sedation versus general anaesthesia for women undergoing breast cancer surgery
Abstract
Background
Breast cancer is one of the most common cancers among women. Surgical removal of the cancer is the mainstay of treatment; however, tumour handling during surgery can cause microscopic dissemination of tumour cells and disease recurrence. The body's hormonal response to surgery (stress response) and general anaesthesia may suppress immunity, promoting tumour dissemination. Paravertebral anaesthesia numbs the site of surgery, provides good analgesia, and blunts the stress response, minimising the need for general anaesthesia.
Objectives
To assess the effects of paravertebral anaesthesia with or without sedation compared to general anaesthesia in women undergoing breast cancer surgery, with important outcomes of quality of recovery, postoperative pain at rest, and mortality.
Search methods
On 6 April 2020, we searched the Specialised Register of the Cochrane Breast Cancer Group (CBCG); CENTRAL (latest issue), in the Cochrane Library; MEDLINE (via OvidSP); Embase (via OvidSP); the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal; and ClinicalTrials.gov for all prospectively registered and ongoing trials.
Selection criteria
We included randomised controlled trials (RCTs) conducted in adult women undergoing breast cancer surgery in which paravertebral anaesthesia with or without sedation was compared to general anaesthesia. We did not include studies in which paravertebral anaesthesia was given as an adjunct to general anaesthesia and then this was compared to use of general anaesthesia.
Data collection and analysis
Two review authors independently extracted details of trial methods and outcome data from eligible trials. When data could be pooled, analyses were performed on an intention‐to‐treat basis, and the random‐effects model was used if there was heterogeneity. When data could not be pooled, the synthesis without meta‐analysis (SWiM) approach was applied. The GRADE approach was used to assess the certainty of evidence for each outcome.
Main results
Nine studies (614 participants) were included in the review. All were RCTs of parallel design, wherein female patients aged > 18 years underwent breast cancer surgery under paravertebral anaesthesia or general anaesthesia.
None of the studies assessed quality of recovery in the first three postoperative days using a validated questionnaire; most assessed factors affecting quality of recovery such as postoperative analgesic use, postoperative nausea and vomiting (PONV), hospital stay, ambulation, and patient satisfaction.
Paravertebral anaesthesia may reduce the 24‐hour postoperative analgesic requirement (odds ratio (OR) 0.07, 95% confidence interval (CI) 0.01 to 0.34; 5 studies, 305 participants; low‐certainty evidence) compared to general anaesthesia. Heterogeneity (I² = 70%) was attributed to the fixed dose of opioids and non‐steroidal analgesics administered postoperatively in one study (70 participants), masking a difference in analgesic requirements between groups.
Paravertebral anaesthesia probably reduces the incidence of PONV (OR 0.16, 95% CI 0.08 to 0.30; 6 studies, 324 participants; moderate‐certainty evidence), probably results in a shorter hospital stay (mean difference (MD) ‐79.39 minutes, 95% CI ‐107.38 to ‐51.40; 3 studies, 174 participants; moderate‐certainty evidence), and probably reduces time to ambulation compared to general anaesthesia (SWiM analysis): percentages indicate vote counting based on direction of effect (100%, 95% CI 51.01% to 100%; P = 0.125; 4 studies, 375 participants; moderate‐certainty evidence).
Paravertebral anaesthesia probably results in higher patient satisfaction (MD 5.52 points, 95% CI 1.30 to 9.75; 3 studies, 129 participants; moderate‐certainty evidence) on a 0 to 100 scale 24 hours postoperatively compared to general anaesthesia.
Postoperative pain at rest and on movement was assessed at 2, 6, and 24 postoperative hours on a 0 to 10 visual analogue scale (VAS). Four studies (224 participants) found that paravertebral anaesthesia as compared to general anaesthesia probably reduced pain at 2 postoperative hours (MD ‐2.95, 95% CI ‐3.37 to ‐2.54; moderate‐certainty evidence). Five studies (324 participants) found that paravertebral anaesthesia may reduce pain at rest at 6 hours postoperatively (MD ‐1.54, 95% CI ‐3.20 to 0.11; low‐certainty evidence). Five studies (278 participants) found that paravertebral anaesthesia may reduce pain at rest at 24 hours postoperatively (MD ‐1.19, 95% CI ‐2.27 to ‐0.10; low‐certainty evidence). Differences in the methods of two studies (119 participants) and addition of clonidine to the local anaesthetic in two studies (109 participants), respectively, contributed to the heterogeneity (I² = 96%) observed for these two outcomes.
Two studies (130 participants) found that paravertebral anaesthesia may reduce pain on movement at 6 hours (MD‐2.57, 95% CI ‐3.97 to ‐1.17) and at 24 hours (MD ‐2.12, 95% CI ‐4.80 to 0.55; low‐certainty evidence). Heterogeneity (I² = 96%) was observed for both outcomes and could be due to methodological differences between studies.
None of the studies reported mortality related to the anaesthetic technique. Eight studies (574 participants) evaluated adverse outcomes with paravertebral anaesthesia: epidural spread (0.7%), minor bleeding (1.4%), pleural puncture not associated with pneumothorax (0.3%), and Horner's syndrome (7.1%). These complications were self‐limiting and resolved without treatment.
No data are available on disease‐free survival, chronic pain, and quality of life.
Blinding of personnel or participants was not possible in any study, as a regional anaesthetic technique was compared to general anaesthesia. Risk of bias was judged to be serious, as seven studies had concerns of selection bias and three of detection bias.
Authors' conclusions
Moderate‐certainty evidence shows that paravertebral anaesthesia probably reduces PONV, hospital stay, postoperative pain (at 2 hours), and time to ambulation and results in greater patient satisfaction on the first postoperative day compared to general anaesthesia. Paravertebral anaesthesia may also reduce postoperative analgesic use and postoperative pain at 6 and 24 hours at rest and on movement based on low‐certainty evidence. However, RCTs using validated questionnaires are needed to confirm these results. Adverse events observed with paravertebral anaesthesia are rare.
PICO
Plain language summary
Comparison of paravertebral anaesthesia to general anaesthesia for breast cancer surgery
What is the issue?
Breast cancer is one of the most common cancers in women. Surgical removal of the tumour is the main treatment; however, dissemination of cancer cells may occur due to tumour handling during surgery. The body's response to surgery (stress response or release of hormones and mediators) as well as drugs used to produce general anaesthesia (complete loss of consciousness), such as opioids and inhalational anaesthetics, can also depress immunity and promote cancer cell survival.
Paravertebral anaesthesia acts by numbing the nerves on one side of the body and can allow surgery to be performed without general anaesthesia. The lightly sedated participant can report pain, cold, thirst, or hunger during or after surgery, allowing timely treatment. This, combined with pain relief produced by paravertebral anaesthesia, has the potential to improve immunity, reduce surgical stress, and improve quality of life and cancer‐free survival.
Review question
We collected and analysed data from all relevant studies to ascertain whether paravertebral anaesthesia would provide better quality of recovery and pain relief at rest and on movement, and to determine whether paravertebral anaesthesia would have any affect on mortality, compared to general anaesthesia.
Study characteristics
We included randomised controlled trials in which participants in one group received paravertebral anaesthesia with or without sedation and participants in the other group received general anaesthesia during breast cancer surgery. We did not include studies in which paravertebral anaesthesia was administered in addition to general anaesthesia and then this was compared to the use of general anaesthesia.
Key results
Nine studies involving 614 participants were included. Four studies were conducted in Europe, three in Asia, and one each in Africa and North America. All studies took place in medical college hospitals with no external funding.
When reviewing evidence on the quality of recovery, these studies reported various outcomes. It was found that paravertebral anaesthesia:
• may reduce 24‐hour postoperative analgesic use (61 per 1000 versus 480 per 1000 in the general anaesthesia group);
• probably reduces the incidence of postoperative nausea and vomiting (72 per 1000 versus 340 per 1000 in the general anaesthesia group);
• probably reduces pain at 2 hours (by 2.95 points on a 0 to 10 point scale) and may reduce pain at 24 hours at rest (0.21 points on a 0 to 10 point scale) compared to general anaesthesia; and
• may reduce pain on movement at 6 hours and at 24 hours post surgery (at 6 hours by 2.57 points; at 24 hours by 2.12 points on a scale of 0 to 10) compared to general anaesthesia.
No study reported on death with each anaesthetic technique. None of the included studies reported on disease‐free survival, chronic pain, or quality of life after breast cancer surgery, probably because these outcomes were not envisaged or planned.
Reports of adverse events with paravertebral block were rare. Out of 290 participants who received paravertebral anaesthesia, two developed epidural spread, four had minor bleeding, and one had pleural puncture. Seventeen of 240 participants developed Horner's syndrome. All adverse events were self‐limited and resolved without treatment.
Quality of the evidence
Most evidence was judged to be of moderate certainty, meaning that we are moderately confident in the reported result. In some cases, evidence was seen to be of low certainty, meaning that our confidence in the reported result is limited.
Authors' conclusions
Summary of findings
| Paravertebral anaesthesia with or without sedation compared to general anaesthesia for women undergoing breast cancer surgery | ||||||
| Patient or population: women undergoing breast cancer surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | №. of participants | Certainty of evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with general anaesthesia | Risk with paravertebral anaesthesia with or without sedation | |||||
| Postoperative analgesic requirement | Study population | OR 0.07 | 305 | ⊕⊕⊝⊝ | ||
| 480 per 1000 | 61 per 1000 | |||||
| Postoperative nausea and vomiting (PONV) | Study population | OR 0.16 | 323 | ⊕⊕⊕⊝ | ||
| 340 per 1000 | 72 per 1000 | |||||
| Postoperative pain at 2 hours | Mean postoperative pain at 2 hours was 0 | MD 2.95 lower | ‐ | 224 | ⊕⊕⊕⊝ | |
| Postoperative pain at 24 hours on rest | Mean postoperative pain at 24 hours on rest was 0 | MD 0.21 lower | ‐ | 169 | ⊕⊕⊝⊝ | |
| Postoperative pain at 6 hours on movement | Mean postoperative pain at 6 hours on movement was 0 | MD 2.57 lower | ‐ | 130 | ⊕⊕⊝⊝ | |
| Postoperative pain at 24 hours on movement | Mean postoperative pain at 24 hours on movement was 0 | MD 2.12 lower | ‐ | 130 | ⊕⊕⊝⊝ | |
| Mortality | Study population | not estimable | (9 RCTs) | ‐ | No RCT has studied this outcome; therefore no data are available | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aData from five studies were analysed to assess this outcome (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015; Terheggen 2002). Blinding of participants was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in four studies (Das 2012; Naja 2003; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Pusch 1999; Sundarathiti 2015), and blinding of outcome assessors in one study led to downgrading of risk of bias to serious (Naja 2003). bHeterogeneity was observed for this outcome (I² = 70%) that included five RCTs; with the exclusion of 1 study, Sundarathiti 2015, heterogeneity decreased to 0%, probably because in this study, all patients in both groups received analgesics (Ultracet and Celebrex twice a day) irrespective of pain; only when they needed additional postoperative analgesia were morphine boluses administered. This routine administration of analgesics could have masked the difference in analgesic requirements between groups. In the other four studies, analgesics were administered only when patients expressed that they were in pain. cData from six studies were analysed to assess this outcome (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Tedore 2011; Terheggen 2002). Blinding of participants or personnel was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in five studies (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Paleczny 2005; Pusch 1999), and blinding of outcome assessors in two studies led to downgrading of risk of bias to serious (Naja 2003; Paleczny 2005). dData from four studies were analysed to assess this outcome (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002). Blinding of participants or personnel was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in all four studies (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Paleczny 2005; Pusch 1999), and blinding of outcome assessors in one study led to downgrading of risk of bias to serious (Paleczny 2005). eData from five studies were analysed to assess this outcome (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011). Blinding of participants was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in three studies (Das 2012; Naja 2003; Paleczny 2005), allocation concealment in two studies (Paleczny 2005; Sundarathiti 2015), and blinding of outcome assessors in two studies led to downgrading of risk of bias to serious (Naja 2003; Paleczny 2005). fData from five studies were used to evaluate this outcome (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011). We noted heterogeneity for this outcome (I² = 96%). On performing subgroup analysis and excluding two studies (Naja 2003; Paleczny 2005), where a potent adjunctive drug (alpha‐2 agonist clonidine) was added to the local anaesthetic solution used for paravertebral anaesthesia, we found a reduction in heterogeneity. gData from two studies were used to analyse this outcome (Naja 2003; Sundarathiti 2015). Blinding of participants or personnel was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it is acceptable. Uncertainty about random sequence generation and blinding of outcome assessors in one study ‐ Naja 2003 ‐ and allocation concealment in the other study ‐ Sundarathiti 2015 ‐ led to downgrading of evidence to serious. hSignificant heterogeneity was noted in the results and sensitivity analysis could not be performed, as only two studies could be included in assessing this outcome (Naja 2003; Sundarathiti 2015). Both studies showed reduction in pain on movement with the paravertebral block, but one study showed a greater reduction in pain probably due to methodological differences (Naja 2003). The use of clonidine as an adjunct to local anaesthetic as well as multiple injections at each paravertebral level could have contributed to this effect (Naja 2003). In the other study, only local anaesthetic was administered in the paravertebral space, injection was performed at one level, a catheter was inserted 8 cm into the space, and drug was injected through the catheter at this point, as well as on withdrawing the catheter by 2 cm and then 4 cm (single‐injection, multi‐level block) (Sundarathiti 2015). | ||||||
Background
Description of the condition
According to worldwide public health data, breast cancer is one of the most common cancers among women. More than one million women are diagnosed with the disease globally per year (Ban 2014; Coughlin 2009; Ferlay 2015; Youlden 2012). Before the global surveys of cancer, breast cancer was considered to be a disease of high‐income countries; however since then, its incidence in low‐ and middle‐income countries has increased (Ferlay 2015).
Women living in low‐ and middle‐income countries have higher mortality than those living in high‐income countries because they reach hospital at later stages of disease due to lack of facilities for screening and early diagnosis (Coughlin 2009; Ferlay 2015; Youlden 2012). Cancer that has spread to distant organs of the body (i.e. metastasis) is the main cause of death among women with breast cancer (Weigelt 2005).
Surgery is the mainstay of breast cancer treatment (Niwa 2013; Sessler 2008). However, despite the apparent complete surgical removal of a tumour, some residual disease may remain. Handling of the tumour during surgery may release cancer cells into the circulation, which results in the spread of cancer to other parts of the body (Coffey 2003). Suppression of a woman's immune system during surgery and under general anaesthesia may also contribute to cancer spread and recurrence at a later date (Coffey 2003; Niwa 2013; Tedore 2015).
Description of the intervention
Paravertebral block is a regional anaesthesia technique. It involves injecting local anaesthetic drug into the space alongside the vertebral column (i.e. paravertebral space) where the spinal nerves emerge from the intervertebral foramina (Karmakar 2001; Richardson 1998). Paravertebral block results in a sympathetic and sensory nerve block only on the side where the local anaesthetic is deposited. This technique can be performed as a single injection or as multiple injections. In either case, as the block occurs only on the side where the anaesthetic is injected (i.e. unilateral), it may produce minimal physiological changes in haemodynamic and respiratory variables and temperature changes (Karmakar 2001; Richardson 1998).
A single‐injection paravertebral block as an adjunct to general anaesthesia has been used to provide pain relief (Boughey 2009; Kairaluoma 2004; Moller 2007). The number of nerve segments blocked by a single injection can vary and depends on the volume of local anaesthetic injected. On average, eight sympathetic and five sensory nerve segments are estimated to be blocked with 15 mL of local anaesthetic (Cheema 1995). Some studies have used a single‐level paravertebral injection at the third to fourth thoracic level to provide surgical anaesthesia for breast surgery (Pusch 1999; Terheggen 2002).
In contrast, multiple (multi‐level) paravertebral block involves injecting 3 mL to 5 mL of local anaesthetic at each paravertebral space from the seventh cervical or the first thoracic vertebral level to the sixth thoracic vertebral level; this can provide reliable surgical anaesthesia (Dabbagh 2007; Greengrass 1996; Naja 2003; Weltz 1995; Wu 2015), along with excellent perioperative analgesia, following breast surgery (Abdallah 2014; Wu 2015). Multiple‐injection or single‐injection paravertebral block may minimise the perioperative use of long‐acting opioids such as morphine that promote angiogenesis (i.e. growth of new blood vessels) and survival of circulating tumour cells (Ecimovic 2011; Kairaluoma 2004; Moller 2007; Wu 2015).
Therefore, paravertebral block with or without sedation can be used as a stand‐alone technique to provide anaesthesia for breast surgery without the need for administering general anaesthesia (Dabbagh 2007; Greengrass 1996; Naja 2003; Pusch 1999; Weltz 1995).
How the intervention might work
General anaesthesia (GA) for breast cancer surgery can be provided via inhaled anaesthetic drugs or intravenous infusion of propofol (propofol‐based total intravenous anaesthesia (TIVA)) (Tedore 2015; Vaghari 2014). Regardless of whether an inhalational or intravenous technique is used, opioid medication is given to provide pain relief unless a local anaesthetic block is administered. Inhaled anaesthetic drugs and opioids such as morphine are the more common methods of providing general anaesthesia for women undergoing breast cancer surgery as compared to propofol‐based TIVA (Tedore 2015; Vaghari 2014).
Retrospective reviews and in vitro studies have shown that inhaled anaesthetic drugs and opioids suppress the body’s immune response and therefore may promote tumour spread and recurrence following surgery (Buckley 2014; Deegan 2009; Deegan 2010; Ecimovic 2011; Ecimovic 2013; Exadaktylos 2006; Jaura 2014). In contrast, retrospective evidence indicates that intravenous propofol‐based TIVA may have a protective effect against tumour spread (Gottschalk 2010; Vaghari 2014). Irrespective of the general anaesthesia technique used (inhalational or intravenous), the body responds to the pain and trauma of surgery by releasing hormones and mediators that suppress the body's immunity (surgical stress response) (Gottschalk 2010; Tedore 2015; Vaghari 2014).
Regional anaesthesia techniques produce a dense sensory block that is more effective in blunting this surgical stress response than general anaesthesia via opioids (Cata 2011; Tedore 2015). Paravertebral block has been shown to obtund the stress response to surgery (O'Riain 2005). In addition, factors such as perioperative hypothermia, hypoglycaemia, nausea and vomiting following surgery, and resulting delayed oral intake can contribute to immunosuppression. These are more likely to occur in unconscious patients under general anaesthesia than in lightly sedated patients under regional anaesthesia, who can communicate and maintain a patent airway without the need for intubation or supra‐laryngeal airway devices (Gottschalk 2010; Tedore 2015; Vaghari 2014).
Regional anaesthesia could result in better‐preserved immune function and lymphocyte (white blood cell) activity with reduced chance of tumour spread and recurrence compared to general anaesthesia (O'Riain 2005; Wu 2015). Local anaesthetic drugs used during regional anaesthesia have also been shown to cause direct inhibition of tumour cell multiplication and migration and increased tumour cell breakdown (Cassinello 2015; Ramirez 2015; Votta‐Velis 2015). Surgery under paravertebral anaesthesia (with or without sedation) could result in awake, pain‐free, comfortable patients with normal body temperature and minimal postoperative nausea and vomiting. Some studies have shown that paravertebral block even in combination with GA is likely to result in better quality recovery (Abdallah 2014; Buckenmaier 2010), improved quality of life (Karmakar 2014), and decreased chronic pain at six months (Chiu 2014).
Why it is important to do this review
Several systematic reviews have assessed the use of paravertebral block during surgery, but these reviews have included mixed populations (i.e. not specifically women with breast cancer) and non‐randomised, retrospective, and case series studies (Schnabel 2010; Tahiri 2011; Wu 2015). These reviews have included studies that compared paravertebral block and general anaesthesia to conventional general anaesthesia alone (Schnabel 2010; Tahiri 2011; Wu 2015).
This Cochrane Review aimed to assess whether paravertebral block as the main anaesthetic technique with or without sedation improved quality of recovery in the initial postoperative period (one to three days), reduced acute pain (at 2 hours, at 6 hours, and at 24 hours post surgery on the first postoperative day), and reduced chronic pain (3 to 12 months post surgery). It also assessed mortality associated with either anaesthetic technique and whether paravertebral anaesthesia compared to general anaesthesia is associated with improved quality of life (1 to 2 weeks, 3 to 12 months) and improved disease‐free survival in women undergoing breast cancer surgery.
Objectives
To assess the effects of paravertebral anaesthesia with or without sedation compared to general anaesthesia in women undergoing breast cancer surgery, with important outcomes of quality of recovery, postoperative pain at rest, and mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that examined paravertebral anaesthesia with or without sedation compared to general anaesthesia for breast cancer surgery.
Types of participants
We included women (over 18 years of age) with early or locally advanced breast cancer scheduled for breast cancer surgery, irrespective of whether they had received chemotherapy in the past.
Types of interventions
Intervention
Paravertebral anaesthesia with or without sedation was the intervention.
Comparator
General anaesthesia (including opioids and inhaled anaesthetic drugs or propofol‐based TIVA) was the comparator.
We planned to include studies that used truncal regional anaesthesia block in addition to paravertebral block.
We excluded studies in which paravertebral block was used as an adjunct to general anaesthesia and then this was compared to general anaesthesia alone.
Types of outcome measures
Primary outcomes
-
Quality of recovery during the initial postoperative period (1 to 3 days) assessed by outcomes such as incidence of postoperative nausea and vomiting, postoperative analgesic requirement, hospital stay, time to ambulation, and patient satisfaction
-
Postoperative pain at rest at 2 hours, at 6 hours, and at 24 hours on the first postoperative day measured on a visual analogue scale (VAS) with a range of 0 to 10, or 0 to 100, or a numerical rating scale (NRS) with a similar range
-
Postoperative pain on movement at 2 hours, at 6 hours, and at 24 hours on the first postoperative day measured on a VAS with a range of 0 to 10, or 0 to 100, or an NRS with a similar range
-
Mortality related to the anaesthetic technique, if any
Secondary outcomes
-
Adverse events due to paravertebral block (i.e. epidural block denoted by hypotension, bilateral block, pleural tap, Horner's syndrome, bleeding, or neurological deficit)
-
Disease‐free survival (often defined as time to first detection of local recurrence or distant metastasis, or progression‐free survival, time to progression, or time to treatment failure)
-
Chronic pain that persisted beyond the immediate postoperative period (i.e. at 3 to 12 months)
-
Quality of life at 1 to 2 weeks and at 3 to 12 months postoperatively assessed by a validated questionnaire
Search methods for identification of studies
Electronic searches
We searched the following databases on 6 April 2020.
-
Cochrane Breast Cancer Group (CBCG) Specialised Register. We will apply the search strategies used by the CBCG and outlined in the CBCG module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). In addition, we will extract and will consider for inclusion trials with the key words breast cancer surgery, conduction anaesthesia, paravertebral anaesthesia, paravertebral block, and nerve block.
-
Central Register of Controlled Trials (CENTRAL; latest issue), in the Cochrane Library. See Appendix 1.
-
MEDLINE (via OvidSP) from 1966 to the present (see Appendix 2).
-
Embase (via OvidSP) from 1947 to the present (see Appendix 3).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx), for all prospectively registered and ongoing trials (see Appendix 4).
-
ClinicalTrials.gov (http://clinicaltrials.gov/; see Appendix 5).
Searching other resources
Bibliographic searching
We screened the reference lists of identified relevant trials or reviews. We obtained a copy of the full article for each reference reporting a potentially eligible trial. When this was not possible, we tried to contact the study authors to request additional information.
Data collection and analysis
Selection of studies
Two review authors (AC and ARC) independently screened all titles and abstracts for eligibility. We obtained and assessed the full articles of all potentially eligible RCTs for relevance based on the pre‐specified inclusion criteria. Each review author independently documented the reason for excluding a study. We resolved any disagreement by discussion with a third review author (HP), who decided on inclusion or exclusion of the study. We compiled a list of all eligible studies and recorded the study selection process in the PRISMA flow diagram. If further information was required to make a decision about trial inclusion, AC contacted the authors of the relevant trial.
We did not impose any language restrictions and included all peer‐reviewed journals and trial registries. We obtained the articles and requested translation through Cochrane TaskExchange or through our network.
We recorded in the Characteristics of excluded studies table studies that were excluded from the review.
Data extraction and management
Two review authors (AC and ARC) independently extracted data onto a standard data extraction form. We resolved any disagreements through consultation with a third review author (HP). When additional information was required, AC contacted the corresponding author of the relevant trial.
For studies with more than one publication, data were extracted from the most recent publications and records related to the same study were combined under the overall trial ID.
Assessment of risk of bias in included studies
We minimised selection bias by including RCTs.
We used the recommended Cochrane ʻRisk of bias' tool to assess risk of bias in the following domains (Higgins 2011).
-
Random sequence generation (selection bias).
-
Concealment of the allocation sequence (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective outcome reporting (reporting bias).
-
Other potential sources of bias, which we assessed by comparing groups at baseline with respect to staging of disease and by noting any difference in treatment administered except for the intervention.
After reviewing the methods used, we decided whether risk of bias for a particular domain was ‘high’, ‘low’, or ‘unclear’ (insufficient data available to decide whether risk of bias existed). This was done only after we contacted the study authors to request additional information. Two review authors (AC and ARC) assessed risk of bias. In the case of any conflict of opinion, we resolved disagreements through consultation with a third review author (HP).
We noted financial or logistical support provided for each included study.
Measures of treatment effect
For continuous data, such as factors that determine quality of recovery (at 1 to 3 days), acute postoperative pain scales on the first postoperative day, and quality of life (at 1 to 2 weeks, and at 3 to 12 months postoperatively), we calculated the mean difference (MD) if data were on the same scale, or the standardised mean difference (SMD) if data were on different scales.
For dichotomous outcomes for which either one of two outcomes was possible, such as adverse events (epidural spread, pneumothorax, Horner's syndrome, bleeding) or no adverse events; chronic pain at 3 to 12 months, or no chronic pain, we planned to calculate the risk ratio (RR) and the 95% confidence interval (CI) from a fixed‐effect or random‐effects model analysis, depending on the presence of heterogeneity. We reported ratios of treatment effects for response so that RRs less than 1.0 favour the paravertebral group and RRs greater than 1.0 favour the general anaesthesia group.
For time‐to‐event outcomes, such as mortality related to the anaesthetic technique and disease‐free survival, we had planned to express results as hazard ratios (HRs). If we could not obtain the HR and associated variance directly from the trial publication, we had planned to obtain the data indirectly using methods described in Parmar 1998, or to extract them from published Kaplan‐Meier curves. However, none of the studies included in the review reported time‐to‐event outcomes.
In the case where studies did not report the effect measure, we cross‐checked all data presented and concluded that these data could not be converted to a suitable format for performing a meta‐analysis. Therefore we applied the synthesis without meta‐analysis (SWiM) approach.
Unit of analysis issues
We included RCTs that used a parallel group design.
Dealing with missing data
We performed quantitative analysis on an intention‐to‐treat (ITT) basis and contacted trial authors to request missing data. However, some studies excluded a small number of patients who were randomised but did not start their allocated treatment (often referred to as modified ITT (mITT) analysis). We included mITT results when ITT results were not available. We had planned to discuss the impact of missing data and imputation methods in the Discussion section of the review.
We performed sensitivity analysis to explore the consistency of effect size measures in trials with low versus high risk of bias, and we investigated the impact of any missing data by using the imputation method described above.
Assessment of heterogeneity
We used the Q statistic to test statistical heterogeneity between trials and considered P ≤ 0.05% as indicating significant heterogeneity. We used the I² statistic to assess the magnitude of heterogeneity, interpreting the I² statistic as follows (Cochran 1954; Higgins 2003).
-
0% to 40%: might not be important.
-
30% to 60%: may represent moderate heterogeneity.
-
50% to 90%: may represent substantial heterogeneity.
-
75% to 100%: considerable heterogeneity.
We used a random‐effects model analysis if the value of the I² statistic was greater than 50% (Higgins 2011).
We used unadjusted effect estimates for the meta‐analysis of RCTs.
For results synthesised by the SWiM approach, we assessed the clinical and methodological variability of all studies included in all SWiM analyses. When variability was found, data presented in the SWiM analysis were organised so as to show the variation (Campbell 2020).
Assessment of reporting biases
As more than 10 studies for any outcome were not available, we could not test for funnel plot asymmetry to ascertain reporting bias. However, in future updates, we plan to test for funnel plot asymmetry if more than 10 studies (for each outcome) are included in the meta‐analysis (Egger 1997).
Data synthesis
We quantitatively reviewed the included data and combined data by intervention, outcome, and population, if appropriate, using Review Manager 5 (RevMan 5) software (RevMan 2014).
For dichotomous outcomes, we expressed pooled estimates as RRs and used a fixed‐effect model in the absence of significant statistical heterogeneity (I² < 50%) (Mantel 1959). In the case of significant heterogeneity, we used the random‐effects analysis (DerSimonian 1986).
For continuous outcomes, we expressed pooled estimates of the MD and used a fixed‐effect analysis (i.e. inverse variance method) in the absence of significant statistical heterogeneity (I² < 50%). In the case of heterogeneity, we used a random‐effects analysis (i.e. inverse variance method with the variance including between‐study variation).
In future versions of this review, for time‐to‐event outcomes such as mortality and disease‐free survival, we plan to use the HR and generic inverse variance depending on available data (Parmar 1998).
For outcomes for which numerical data were lacking or outcomes had been measured in diverse ways and only direction of effect was available, vote counting based on direction of effect was used and results reported. When possible, this was presented alongside results of the meta‐analysis (McKenzie 2019).
This method does not provide information on the magnitude of effect nor take into consideration the relative size of studies. P values were calculated via sign test and binomial probability; 95% CIs were calculated by Wilson's interval method (Brown 2001).
We prepared SWiM tables showing direction of effect based on vote counting with study ID, direction of effect as reported by study authors, and estimated risk of bias.
ʻSummary of findings' table
Two review authors (AC and ARC) used the principles of the GRADE system to assess the overall quality of evidence associated with specific outcomes (Guyatt 2011; Schünemann 2011).
We constructed a ʻSummary of findings' table using GRADEpro GDT software (GRADEpro GDT 2015). The GRADE approach appraises the quality of a body of evidence for each outcome based on five domains: (1) risk of bias of included studies (methodological quality), (2) inconsistency (i.e. heterogeneity), (3) indirectness (relevance to the review question), (4) imprecision (i.e. confidence intervals), and (5) risk of publication bias.
We listed the following main outcomes in the ʻSummary of findings' table and assessed the quality of evidence.
-
Analgesic requirement on the first postoperative day.
-
Postoperative nausea and vomiting on the first postoperative day.
-
Postoperative pain on the first postoperative day at 2 hours at rest based on a VAS with a range of 0 to 10.
-
Postoperative pain on the first postoperative day at 24 hours at rest based on a VAS with a range of 0 to 10.
-
Postoperative pain on the first postoperative day at 6 hours on movement based on a VAS with a range of 0 to 10.
-
Postoperative pain on the first postoperative day at 24 hours on movement based on a VAS with a range of 0 to 10.
-
Mortality related to either anaesthetic technique.
Other outcomes such as disease‐free survival and quality of life at 1 to 2 weeks and at 3 to 12 months postoperatively assessed by a validated questionnaire were not reported by any study and therefore are not listed in the 'Summary of findings' table.
For results summarised by vote counting when pooled estimation of data was not possible, we applied GRADE domains (methodological limitations of studies or risk of bias, indirectness, imprecision, inconsistency, and likelihood of publication bias) guided by Murad 2017.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analysis was not possible, as no study had reported these outcomes. We conducted an additional subgroup analysis on the use of local anaesthetic alone or in combination with adjuncts such as the alpha‐2 agonist drug clonidine in patients receiving paravertebral anaesthesia because adding clonidine to local anaesthetic can prolong the duration of analgesia as compared to that produced by local anaesthetic alone (Pöpping 2009).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the consistency of effect size measures in trials with low versus high risk of bias. We considered studies at high or unclear risk of bias in two or more domains to be at high risk of bias. We interpreted absence of blinding in outcome assessment, such as incidence of mortality from medical records, as showing low risk of bias. However, absence of blinding in patient‐reported outcomes was taken as showing high risk of bias; an exception was blinding of participants and personnel (performance bias). This was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review and therefore was acceptable. However, bias in any other domain, especially selection, detection, attrition, reporting, or other bias, was taken to show high risk of bias.
Results
Description of studies
Results of the search
We identified a total of 773 records through electronic database searches and one additional record by bibliographic searches. After we had removed 45 duplicate records, 729 records remained. After title and abstract screening, 683 records were found to be irrelevant and were excluded. The full text of 46 records was assessed for eligibility; 37 records did not fulfil the eligibility criteria and were excluded. Nine records fulfilled the criteria of our review, and we quantitatively analysed data from seven studies. See Figure 1.

Study flow diagram.
We contacted the lead authors of all included studies by email to request missing data. The authors of two studies confirmed that their studies did not study the quality of recovery by using a validated questionnaire, mortality related to the anaesthetic technique, disease‐free survival, chronic pain persisting 3 to 12 months postoperatively, or quality of life at 1 to 2 weeks and at 3 to 12 months with a validated questionnaire (Sundarathiti 2015; Tedore 2011).
The corresponding authors of seven studies did not respond to our request and did not provide further information (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Terheggen 2002).
See Characteristics of included studies and Characteristics of excluded studies.
Included studies
We included in our review nine studies with 614 participants (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). All were RCTs of parallel design wherein participants underwent breast cancer surgical procedures under paravertebral anaesthesia or general anaesthesia. All studies were carried out in adult female patients aged > 18 years with American Society of Anaesthesiologists physical status 1 to 3.
One study was published in the Polish language (Paleczny 2005), and another one in the Russian language (Shkol'nik 2006); both were translated to English. All other studies were published in English.
Participants
Two studies were carried out among patients undergoing ambulatory breast cancer surgery with or without sentinel lymph node biopsy or axillary lymph node dissection (Tedore 2011; Terheggen 2002). Two studies included patients undergoing mastectomy for breast cancer without axillary clearance (Das 2012; Megahed 2010). Two studies included patients undergoing only major breast cancer surgery (i.e. modified radical mastectomy (MRM) ‐ Paleczny 2005 ‐ or radical/reconstructive breast cancer surgery ‐ Shkol'nik 2006). The remaining studies included all types of breast cancer surgery ranging from lumpectomy to modified radical mastectomy (Naja 2003; Pusch 1999; Sundarathiti 2015).
One study was carried out only on patients with type 2 diabetes who were undergoing mastectomy for breast cancer to assess perioperative P selectin levels as a predictor of thrombotic events (Megahed 2010). Another study estimated serum cortisol levels in paravertebral and general anaesthesia groups (Shkol'nik 2006).
Interventions
Pre‐medication was administered to participants in the paravertebral group before block was performed in eight studies as intravenous midazolam (Naja 2003; Paleczny 2005; Pusch 1999; Sundarathiti 2015), intramuscular midazolam (Megahed 2010), or a combination of intravenous midazolam and fentanyl (Das 2012; Shkol'nik 2006; Tedore 2011). In only one study involving day case procedures, no pre‐medication was administered for the block (Terheggen 2002).
During the intraoperative period (i.e. during surgery), sedation was offered to participants in the paravertebral group in all nine studies either as propofol infusion alone (Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), propofol infusion in combination with fentanyl boluses (Das 2012; Shkol'nik 2006; Tedore 2011), or propofol infusion with a single ketamine bolus (Sundarathiti 2015). In one study, despite sedation offered to all participants, two‐thirds of participants did not opt to receive it (Naja 2003). In all studies, care was taken to maintain 'conscious' sedation (i.e. participants were sedated but could respond to verbal commands and could breathe spontaneously).
Six participants in three studies (one participant in Naja 2003, two in Paleczny 2005, three in Pusch 1999) required supplementary analgesia. This involved local anaesthetic infiltration in Naja 2003 and single‐dose opioids in Paleczny 2005 and Pusch 1999 on axillary dissection in the paravertebral group. In addition, two participants in Das 2012 required supplementary local anaesthetic infiltration in the surgical site intraoperatively due to inconsistent effect in blocked dermatomes even without axillary dissection.
Excluded studies
We excluded Sultan 2013 despite inclusion of a paravertebral anaesthesia group and a general anaesthesia group of participants undergoing breast cancer surgery because study authors assessed only cytokines using either technique and did not provide quantitative or descriptive information on any of the outcomes mentioned in our review.
Risk of bias in included studies
The characteristics of included studies for assessment of risk of bias are shown in Figure 2 and in Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Overall, one study was found to be of high methodological quality with the exception of high performance bias (Tedore 2011). Three studies had at least one more domain with high risk of bias (Das 2012; Sundarathiti 2015; Terheggen 2002). The remaining studies had unclear bias in more than two domains (Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006), as detailed below.
Allocation
We judged six studies as having unclear risk of bias for random sequence generation (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002). Five studies showed uncertainty of allocation concealment (Megahed 2010; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015). We found one study to be of high methodological quality with no risk of selection bias (Tedore 2011).
Blinding
Performance bias was high in the nine studies, as participants in one group received paravertebral anaesthesia for breast cancer surgery and those in the other group received general anaesthesia; therefore blinding of participants was not possible. As this comparison was the main question of the review, this bias was considered acceptable by all review authors.
In addition, in four studies, we detected uncertainty in blinding of outcome assessors (Megahed 2010; Naja 2003; Paleczny 2005; Shkol'nik 2006).
Incomplete outcome data
We found no evidence of attrition bias in the nine included studies.
Selective reporting
We found that all planned outcomes were reported in the studies. Study authors mentioned all outcomes in their methods and reported advantages and disadvantages of both paravertebral and general anaesthesia. However, only two studies had prospectively registered protocols on the trial registries (Sundarathiti 2015; Tedore 2011); none of the other studies had trial registration records.
Other potential sources of bias
We detected no other potential sources of bias.
Effects of interventions
See summary of findings Table 1.
Quality of recovery during the initial postoperative period (1 to 3 days)
No study assessed quality of recovery in the initial 1 to 3 days using a validated questionnaire. However, many studies compared outcomes that affect quality of recovery following breast cancer surgery. These outcomes are postoperative pain, need for postoperative analgesics, postoperative nausea and vomiting, ambulation, hospital stay, and patient satisfaction. Postoperative pain is one of the primary outcomes and is described separately.
Postoperative analgesic use
Nine studies in total reported this outcome, five of which provided data suitable for meta‐analysis (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015; Terheggen 2002); four provided data used for synthesis without meta‐analysis ((SWiM) (Megahed 2010; Paleczny 2005; Shkol'nik 2006; Tedore 2011).
Meta‐analysis
Data from five studies were used to analyse the number of participants who required postoperative analgesia in the first 24 hours post surgery in the paravertebral and general anaesthesia groups (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015; Terheggen 2002). Data regarding the number of participants requiring rescue non‐steroidal anti‐inflammatory drugs (NSAIDs) were obtained from one study (Das 2012), three studies reported the number of patients requiring both NSAIDs and opioids (Naja 2003; Pusch 1999; Terheggen 2002), and another study reported the number of participants requiring rescue opioid in the postoperative period (Sundarathiti 2015).
Evidence shows that paravertebral anaesthesia may reduce the need for postoperative analgesia on the first postoperative day compared to general anaesthesia (odds ratio (OR) 0.07, 95% confidence interval (CI) 0.01 to 0.34; 305 participants; low‐certainty evidence; Analysis 1.1). Considerable heterogeneity was noted for this outcome (I² = 70%). When we excluded one study (Sundarathiti 2015), heterogeneity decreased to zero and the difference among participants requiring postoperative analgesics was OR 0.03 (95% CI 0.01 to 0.09), probably because in this study, all participants in both groups were administered fixed doses of postoperative analgesics (synthetic opioid and NSAID) twice a day, and only participants who still had pain were administered rescue analgesia with morphine boluses. This routine administration of analgesics to all participants could have masked the difference in analgesic requirements between groups, notwithstanding the fact that participants underwent more extensive breast surgery with axillary clearance in this study. In the other four included studies, analgesics were administered only when participants expressed that they were in pain.
SWiM approach
Data from four of the nine studies used this approach because numerical data were lacking (Megahed 2010), and because outcomes were measured in different ways (i.e. total doses instead of number of participants) (Paleczny 2005; Shkol'nik 2006; Tedore 2011). In addition, two studies described postoperative opioid use (Megahed 2010; Tedore 2011), one reported postoperative NSAID (ketoprofen) use (Paleczny 2005), and another analysed total perioperative opioid (fentanyl) use in the two groups (Shkol'nik 2006). Therefore, vote counting based on direction of effect was performed for data from these studies. Three of the four studies found a decrease in analgesic use in the paravertebral group (Megahed 2010; Paleczny 2005; Shkol'nik 2006), whereas one study found comparable 24‐hour analgesic consumption between the two groups (Tedore 2011), indicating that paravertebral anaesthesia may reduce the postoperative analgesic requirement compared to general anaesthesia. In the SWiM analysis, percentages indicate vote counting based on direction of effect (75%, 95% CI 30% to 95%; P = 0.625; 310 participants; low‐certainty evidence; Figure 4). Due to inconsistency and concerns of risk of bias, we downgraded the certainty of evidence to low.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis and from the SWiM analysis were concordant.
Postoperative nausea and vomiting (PONV)
Nine studies in total reported this outcome, six of which had data suitable for meta‐analysis (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Tedore 2011; Terheggen 2002); three used the SWiM approach (Megahed 2010; Shkol'nik 2006; Sundarathiti 2015).
Meta‐analysis
Data from six studies show the number of participants with vomiting and persistent nausea or retching in the first 24 hours postoperatively that required treatment with antiemetics (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Tedore 2011; Terheggen 2002). Paravertebral anaesthesia probably reduces the incidence of PONV in the first 24 hours post surgery compared to general anaesthesia (OR 0.16, 95% CI 0.08 to 0.30; 324 participants; moderate certainty of evidence; Analysis 1.2). No heterogeneity was observed for this outcome. Five studies had serious risk of bias (selection bias in five studies: Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002; detection bias in two studies: Naja 2003; Paleczny 2005). Only one study had low risk of bias (Tedore 2011). Performance bias was present in all studies, but as the meta‐analysis compared two anaesthetic techniques, this was considered acceptable.
SWiM approach
Data from three studies could not be pooled in meta‐analysis because they were presented narratively; therefore vote counting based on direction of effect was assessed (Megahed 2010; Shkol'nik 2006; Sundarathiti 2015). Data from these studies show that paravertebral anaesthesia probably decreases PONV compared to general anaesthesia (percentages indicate vote counting based on direction of effect (100%, 95% CI 44% to 100%; P = 0.25; 290 participants; moderate‐certainty evidence; Figure 5). All three studies had concerns of selection bias, and two studies also had concerns of detection bias (Megahed 2010; Shkol'nik 2006), downgrading the certainty of evidence to moderate.
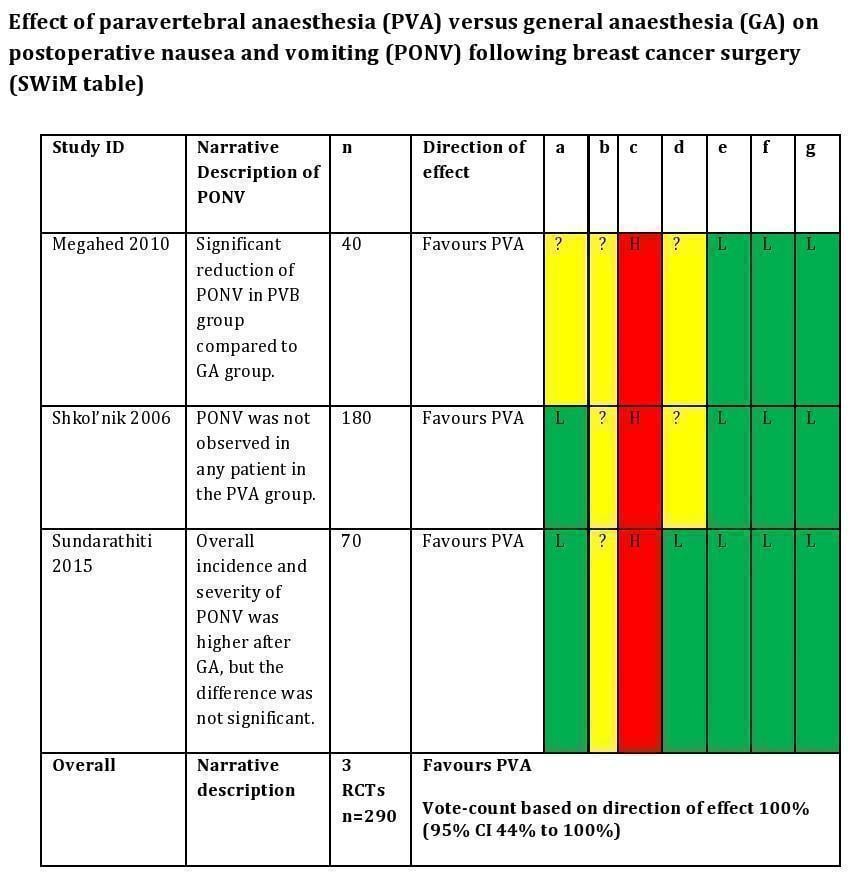
a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis was concordant with evidence from the SWiM analysis.
Hospital stay
Three studies estimated time to discharge from the hospital (Naja 2003; Sundarathiti 2015; Tedore 2011). Sundarathiti 2015 assessed time to readiness for discharge using the Post Anaesthetic Discharge Scoring System (PADSS), but all participants were discharged 2 days after surgery based on a fixed surgical protocol. Analysis of data from these studies revealed that hospital stay was probably shorter in the paravertebral group as compared to the general anaesthesia group (mean difference (MD) ‐79.39 minutes, 95% CI ‐107.38 to ‐51.40; 174 participants; moderate‐certainty evidence; Analysis 1.3). No heterogeneity was observed for this outcome. Two studies had selection bias (Naja 2003; Sundarathiti 2015), and one study had low risk of selection bias (Tedore 2011).
Ambulation
Four studies reported this outcome (Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006). Data could not be pooled for meta‐analysis for this outcome, as two studies gave a narrative description of earlier ambulation in the paravertebral group (Naja 2003; Shkol'nik 2006), and two studies described outcomes in diverse ways (Paleczny 2005; Pusch 1999). Paleczny 2005 reported reduced time to standing in the paravertebral block group compared to the general anaesthesia group, and Pusch 1999 reported that a greater number of participants in the general anaesthesia group had painful restricted movement (21/42 participants) compared to none (0/44 participants) in the paravertebral group at 24 hours post surgery. Therefore the SWiM approach with vote counting based on direction of effect was used for this outcome. It was found that paravertebral anaesthesia probably reduced time to ambulation compared to general anaesthesia (percentages indicate vote counting based on direction of effect: 100%, 95% CI 51% to 100%; P = 0.125; 375 participants; moderate‐certainty evidence; Figure 6). Four studies had concerns of selection bias (Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006) and three studies showed uncertainty in detection bias (Naja 2003; Paleczny 2005; Shkol'nik 2006), which led to downgrading of evidence certainty to moderate.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Patient satisfaction
Six studies reported this outcome; three studies had data suitable for meta‐analysis (Das 2012; Tedore 2011; Terheggen 2002), and three had data that could be used for SWiM (Paleczny 2005; Shkol'nik 2006; Sundarathiti 2015).
Meta‐analysis
Analysis of data from three studies revealed that participants receiving paravertebral anaesthesia probably had higher patient satisfaction at 24 hours post surgery compared to participants receiving general anaesthesia (MD 5.52 points, 95% CI 1.30 to 9.75 on a 0 to 100 scale; 129 participants; moderate‐certainty evidence; Analysis 1.4) (Das 2012; Tedore 2011; Terheggen 2002). No heterogeneity was observed for this outcome, and one study had low risk of bias (Tedore 2011), whereas the other two studies had selection bias (Das 2012; Terheggen 2002).
SWiM approach
Due to narrative descriptions or missing data, data from three studies were analysed using vote counting based on direction of effect. Two of the three studies reported higher satisfaction in the paravertebral anaesthesia group compared to the general anaesthesia group (percentages indicate vote counting based on direction of effect: 66.7%, 95% CI 20.8% to 94%; P = 1; 299 participants; Figure 7). There were concerns of selection bias in all three studies (Paleczny 2005; Shkol'nik 2006; Sundarathiti 2015), and two studies indicated detection bias (Paleczny 2005; Shkol'nik 2006). Therefore paravertebral anaesthesia may be associated with higher patient satisfaction compared to general anaesthesia (low‐certainty evidence).

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis was concordant with evidence from the SWiM analysis. The level of certainty of evidence for the meta‐analysis was moderate, and that for the SwiM analysis was low.
Postoperative pain at rest on the first postoperative day
Nine studies assessed postoperative pain on the first postoperative day (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). Data from one study could not be included because units of analysis were not clearly stated (Shkol'nik 2006). One study involving participants undergoing minor day case breast cancer surgery assessed pain scores up to 2 hours postoperatively or until discharge (Terheggen 2002), another assessed pain scores up to 12 hours postoperatively (Pusch 1999), three assessed pain up to 24 hours (Das 2012; Sundarathiti 2015; Tedore 2011), and another three studies assessed pain up to 3 to 5 days postoperatively (Naja 2003; Paleczny 2005; Shkol'nik 2006).
Pain was assessed at 2 hours, at 6 hours, and at 24 hours at rest on the first postoperative day.
Pain at 2 hours postoperatively
Pain at 2 hours postoperatively was assessed by four studies (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002). It was found that the paravertebral technique probably reduced pain compared to general anaesthesia (MD ‐2.95, 95% CI ‐3.37 to ‐2.54 on a 0 to 10 VAS; 224 participants; moderate‐certainty evidence; Analysis 1.5). No heterogeneity was observed for this outcome; all four studies had concerns of selection bias, and one study also had detection bias (Paleczny 2005).
Pain at 6 hours postoperatively
Pain at rest at 6 hours postoperatively was assessed by five studies (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Sundarathiti 2015). It was found that use of paravertebral anaesthesia may reduce pain (MD ‐1.54, 95% CI ‐3.20 to 0.11 on a 0 to 10 VAS; 324 participants; low‐certainty evidence; Analysis 1.6).
We noted heterogeneity for this outcome (I² = 96%). On performing sensitivity analysis, we found that two studies contributed to the heterogeneity (Das 2012; Naja 2003), possibly because participants in Das 2012 at 6, 12, and 24 hours postoperatively had comparable VAS scores in the paravertebral and general anaesthesia groups due to higher analgesic consumption in the general anaesthesia group. In contrast, participants in Naja 2003 had low VAS scores in the paravertebral group, perhaps owing to the admixture of clonidine with the local anaesthetic solution used for paravertebral block. Clonidine is a potent alpha‐2 agonist that prolongs the effects of a block. Contrasting VAS scores in these two studies could have contributed to the heterogeneity. On excluding these studies, we found that heterogeneity was reduced to 0 and evidence showed that use of paravertebral anaesthesia may reduce pain (MD ‐1.57, 95% CI ‐2.46 to ‐0.68 on the 0 to 10 VAS).
Four studies showed uncertainty about random sequence generation (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999), three studies about allocation concealment (Paleczny 2005; Pusch 1999; Sundarathiti 2015), and two studies about blinding of the outcome assessor (Naja 2003; Paleczny 2005), which led to downgrading of risk of bias to serious. The certainty of evidence was downgraded to low.
Pain at 24 hours postoperatively
Pain at 24 hours at rest was assessed by five studies (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011).
Meta‐analysis
It was found that use of paravertebral anaesthesia may result in reduced pain (MD ‐1.19, 95% CI ‐2.27 to ‐0.10 on a 0 to 10 VAS; 278 participants; low‐certainty evidence; Analysis 1.7). We noted heterogeneity for this outcome (I² = 96%). On performing subgroup analysis and excluding two studies in which a potent adjunctive drug (clonidine) was added to the local anaesthetic solution used for paravertebral anaesthesia (Naja 2003; Paleczny 2005), we found a reduction in heterogeneity. Analysis of data from the remaining three homogenous studies showed that paravertebral anaesthesia may reduce pain (MD ‐0.21, 95% CI ‐0.42 to 0.00; 169 participants) (Das 2012; Sundarathiti 2015; Tedore 2011).
Of studies included for this outcome, only one had low risk of bias (Tedore 2011), whereas two others showed uncertainty in selection bias (Das 2012; Sundarathiti 2015); the remaining two studies showed uncertainty in two domains (Naja 2003; Paleczny 2005).
SWiM approach
Two studies provided evidence that paravertebral block probably reduces postoperative pain at 24 hours at rest compared to general anaesthesia (percentages indicate vote counting based on direction of effect: 100%, 95% CI 34.24% to 100%; P = 0.5; 220 participants; Figure 8) (Megahed 2010; Shkol'nik 2006). Both studies had uncertainty in selection bias and in detection bias. The certainty of evidence was downgraded to moderate.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis was concordant with evidence from the SwiM analysis. The certainty of evidence for the meta‐analysis was low, and that for the SWiM analysis was moderate.
Postoperative pain on movement on the first postoperative day
We assessed pain on movement at 2, 6, and 24 hours on the first postoperative day.
Pain on movement at 2 hours
None of the included studies reported this outcome at this time point.
Pain on movement at 6 hours
Pain on movement at 6 hours was assessed by two studies (Naja 2003; Sundarathiti 2015). It was found that paravertebral anaesthesia may reduce pain on movement at 6 hours (MD‐2.57, 95% CI ‐3.97 to ‐1.17; 130 participants; low‐certainty evidence; Analysis 1.8). Considerable heterogeneity was noted for this outcome (I² = 96%). Sensitivity analysis could not be performed, but heterogeneity could be attributed to methodological differences. One study included multiple‐level paravertebral block (Naja 2003), whereas the other study involved inserting a paravertebral catheter at one level (fourth thoracic vertebral level), advancing it 8 cm into the space, injecting local anaesthetic at that depth, and withdrawing the catheter by 2 cm twice, so as to administer local anaesthetic drug at multiple levels (single injection, multi‐level block) (Sundarathiti 2015). In addition, a lower VAS score may have been observed in one study (Naja 2003), as the potent alpha‐2 agonist clonidine was added to the local anaesthetic solution. This is known to prolong the duration of analgesia produced by a regional anaesthesia block.
In both studies, there were concerns regarding selection bias, and one study had concerns of detection bias (Naja 2003); therefore the certainty of evidence was downgraded to low.
Pain on movement at 24 hours
Pain on movement at 24 hours was assessed by two studies (Naja 2003; Sundarathiti 2015), which showed that pain in the paravertebral group may be reduced compared to pain in the general anaesthesia group (MD ‐2.12, 95% CI ‐4.80 to 0.55 on a 0 to 10 VAS; 130 participants; low‐certainty evidence; Analysis 1.9). Considerable heterogeneity was noted for this outcome (I² = 96%). Sensitivity analysis could not be performed, but heterogeneity may be due to methodological differences in the two studies, as explained above.
Uncertainty about random sequence generation and blinding of outcome assessors in Naja 2003 and allocation concealment in Sundarathiti 2015 led to downgrading of risk of bias to serious, and the certainty of evidence to low.
Shkol'nik 2006 indicated that paravertebral block probably reduces pain on movement at 24 postoperative hours.
Mortality related to either anaesthetic technique
This outcome was not reported by any of the included studies.
Adverse events due to paravertebral block
Eight studies comprising 574 participants evaluated adverse outcomes due to paravertebral anaesthesia compared to general anaesthesia (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002).
Epidural spread and bilateral block
All eight studies evaluated the incidence of bilateral block indicative of epidural spread of local anaesthetic administered in the paravertebral space (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). Two of 290 participants who received the paravertebral block developed a bilateral block, indicating an incidence of 0.7% (Analysis 2.1). One of these participants developed mild breathlessness and hypotension lasting for five minutes that responded to 500 mL fluid bolus administration. This participant was subsequently administered general anaesthesia on request (Terheggen 2002). The second participant developed epidural spread with paraparesis lasting 280 minutes and Horner's syndrome lasting 170 minutes, which resolved spontaneously (Pusch 1999). In Shkol'nik 2006, two patients developed hypotension without epidural spread or bilateral block, which study authors attributed to use of two‐injection paravertebral technique with large volumes of local anaesthetics (10 to 15 mL) at each level. Study authors subsequently changed to using multi‐level paravertebral technique with 4 to 5 mL local anaesthetic at each level and found no hypotension.
A pooled estimate of this adverse event was not possible; this adverse event can be observed only with a regional anaesthesia technique involving injections in the proximity of the vertebral canal and is not seen with general anaesthesia.
As there was uncertainty about random sequence generation in five studies (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in four studies (Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015), and blinding of outcome assessors in three studies (Naja 2003; Paleczny 2005; Shkol'nik 2006), the certainty of evidence was downgraded to moderate.
Horner's syndrome
Six studies comprising 475 participants assessed the incidence of Horner's syndrome following paravertebral anaesthesia (Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Tedore 2011; Terheggen 2002). Seventeen of the total 240 participants developed this complication, indicating an incidence of 7.1%. Eleven participants had received multiple‐level paravertebral blocks from cervical seventh to thoracic sixth vertebral level (Shkol'nik 2006), and six participants received a single‐level paravertebral block at the thoracic 3/4 vertebral level (Paleczny 2005; Pusch 1999). A pooled estimate of this adverse event was not possible, as this complication is possible only with a regional anaesthesia technique involving injections in the proximity of the upper thoracic vertebrae. Therefore it occurred in the paravertebral group ‐ not in the general anaesthesia group.
As there was uncertainty about random sequence generation in four studies (Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), about allocation concealment in three studies (Paleczny 2005; Pusch 1999; Shkol'nik 2006), and about blinding of outcome assessors in three studies (Naja 2003; Paleczny 2005; Shkol'nik 2006), the certainty of evidence was downgraded to moderate.
Bleeding
Eight studies comprising 574 participants evaluated the incidence of bleeding following paravertebral block (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). Four of the 290 participants in the paravertebral group developed minor bleeding during paravertebral block, indicating an incidence of 1.4%. No participants needed interventions/transfusion for minor bleeding. A pooled estimate of this adverse event was not possible, as this complication is possible only with a regional anaesthesia technique involving injections. Therefore it is observed only in the paravertebral group ‐ not in the general anaesthesia group.
Amongst the eight included studies, seven studies had selection bias (five studies had uncertainty in random sequence generation ‐ Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002 ‐ and four studies in allocation concealment ‐ Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015). Three studies had uncertainty in blinding of outcome assessors (Naja 2003; Paleczny 2005; Shkol'nik 2006). This led to downgrading of risk of bias to serious and certainty of evidence to moderate.
Pleural tap
Eight studies comprising 574 participants evaluated the incidence of pleural tap with the paravertebral anaesthetic technique (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). One of the 290 participants in the paravertebral group developed this complication, indicating an incidence of 0.3% for this complication with the paravertebral technique. The participant was kept in the post‐anaesthesia care unit (PACU) for 195 minutes before discharge to home. No radiological features of pneumothorax or respiratory difficulty developed during 2 days of follow‐up. No patient in the general anaesthesia group developed this complication, which is possible only with a regional anaesthesia technique involving injections in the proximity of the pleura.
There were concerns of selection bias in seven studies ‐ Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Terheggen 2002 ‐ and detection bias in three studies (Naja 2003; Paleczny 2005; Shkol'nik 2006). This led to downgrading of risk of bias to serious and certainty of evidence to moderate.
Disease‐free survival (often defined as time to first detection of local recurrence or distant metastasis, or progression‐free survival, time to progression, or time to treatment failure)
This outcome was not reported by any of the included studies.
Chronic pain that persists beyond the immediate postoperative period (i.e. at 3 to 12 months)
This outcome was not reported by any of the included studies.
Quality of life at 1 to 2 weeks and at 3 to 12 months postoperatively assessed by a validated questionnaire
This outcome was not reported by any of the included studies.
Postoperative surgical considerations
Technical difficulties with paravertebral block included multiple needle passes after single skin pricks in six patients; another patient described moderate pain at the time of the block, requiring supplementary doses of fentanyl and local anaesthetic (Das 2012). Sundarathiti 2015 described difficulty in catheter insertion in 20% of included participants.
In two cases of paravertebral block failure ‐ one case each in Das 2012 and Paleczny 2005 ‐ both cases needed conversion to general anaesthesia for surgery.
Haemodynamic parameters were found to be comparable between groups in four studies (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015). Three studies reported lower, more stable haemodynamic parameters in the paravertebral group than in the general anaesthesia group (Megahed 2010; Paleczny 2005; Shkol'nik 2006). Shkol'nik 2006 reported a decrease in ventricular extrasystoles and an improved ejection fraction in the paravertebral group. In addition, one of these studies ‐ Shkol'nik 2006 ‐ found reduced plasma cortisol levels, and another ‐ Megahed 2010 ‐ found reduced soluble P‐selectin levels in the paravertebral group compared to the general anaesthesia group.
Paleczny 2005 reported one participant developing laryngeal spasm after extubation in the general anaesthesia group. Sundarathiti 2015 found that 54.3% of participants in the general anaesthesia group developed sore throat after tracheal intubation.
Discussion
Summary of main results
Paravertebral anaesthesia probably reduces postoperative nausea and vomiting (PONV), hospital stay, and time to ambulation, resulting in greater patient satisfaction on the first postoperative day compared to general anaesthesia. Paravertebral anaesthesia may also reduce postoperative analgesic use. However, randomised controlled trials (RCTs) using validated questionnaires are needed to confirm these results.
Paravertebral anaesthesia probably reduces postoperative pain at 2 hours, and it may reduce pain at rest and on movement at 6 and 24 hours, postoperatively. No comment can be made regarding mortality associated with the anaesthetic technique.
Adverse events observed with paravertebral anaesthesia are rare. None of the other secondary outcomes, such as disease‐free survival, chronic pain persisting from 3 to 12 months, or quality of life described on a validated questionnaire, were assessed by any of the included studies.
Overall completeness and applicability of evidence
The study population included adult participants from Europe, Asia, Africa, and North America who underwent different types of breast cancer surgery under all techniques of paravertebral block compared to general anaesthesia.
Participants in eight RCTs were administered sedation while paravertebral block was performed. Skin infiltration with local anaesthetic and pre‐medication before any block are standard practices to decrease patient discomfort during the block procedure; they are usually administered after monitoring of vitals and when oxygen supplementation has commenced. Usually multiple‐level blocks are thought to provide more reliable surgical anaesthesia, but in two studies (Paleczny 2005; Pusch 1999), the study authors administered a greater volume of local anaesthetic (0.3 mL/kg) as a single injection at the thoracic fourth vertebral level.
In this current review, it was not possible to ascertain which technique of paravertebral block was more effective (i.e. single‐injection, multiple‐injection, or catheter‐based techniques), as only two block failures occurred (two studies each reported one block failure ‐ Das 2012; Paleczny 2005). One RCT included a single‐level paravertebral injection (Paleczny 2005), and the other a multiple‐injection technique (Das 2012). Both cases required conversion to general anaesthesia. None of the catheter‐related paravertebral blocks resulted in block failure. A failure rate ranging from 10.7% to 23.7% has been reported in the literature (Lonnqvist 1995; Najarian 2003). In this current review, failure rate and need for supplementation were low.
None of the included studies reported mortality following either anaesthetic technique, possibly because most of the included RCTs were small and mortality related to general anaesthesia for surgical patients is rare (estimated as 8.2 deaths per million hospital surgical discharges, 95% confidence interval (CI) 7.4 to 9.0). Specifically for women, it has been estimated as 6.2 deaths per million hospital surgical discharges (95% CI 5.5 to 7.0; Li 2009). In the medical literature, we found no report of mortality attributed to paravertebral anaesthesia.
As none of the studies assessed important long‐term outcomes, such as mortality, disease‐free survival, chronic pain, or quality of life following breast cancer surgery, additional large multi‐centre trials are needed.
Quality of the evidence
We selected RCTs including participants undergoing paravertebral or general anaesthesia for breast cancer surgery. As this included comparison of two techniques of providing anaesthesia (i.e. regional anaesthesia compared to general anaesthesia), blinding of participants or personnel was not possible; therefore all studies had high risk of performance bias. However as this was the main question of the review, it was considered acceptable after discussion with all review authors. Tedore 2011 had low risk of bias in all other domains. Among the remaining eight studies (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Terheggen 2002), all had uncertainty in selection bias. Three of the nine studies reported on the method of randomisation (Shkol'nik 2006; Sundarathiti 2015; Tedore 2011), and four studies clearly stated the method of allocation concealment (Das 2012; Naja 2003; Tedore 2011;Terheggen 2002). In addition, low detection bias or blinding of outcome assessors was clearly stated in five studies (Das 2012; Pusch 1999; Sundarathiti 2015; Tedore 2011; Terheggen 2002).
We used the GRADE approach to estimate the certainty of evidence for all outcomes studied. We found the certainty of evidence for outcomes of PONV and pain at rest at 2 hours to be of moderate certainty, primarily due to risk of bias (selection and/or detection bias). We found the certainty of evidence for postoperative analgesic use and pain at rest at 24 hours and pain on movement at 6 and 24 hours to be of low certainty. This was due primarily to risk of bias (selection, detection) and heterogeneity between studies due to methodological differences.
Data used for synthesis without meta‐analysis (SWiM) for quality of recovery outcomes of postoperative analgesic use and patient satisfaction were also downgraded to low certainty because of serious risk of bias and inconsistent results (Murad 2017). However, for other quality of recovery outcomes, namely, PONV and ambulation, descriptive data were downgraded by one level to moderate certainty because of risk of bias, as included studies consistently favoured the intervention group on vote counting and results showed no inconsistency.
Similarly, unpooled data on adverse events (epidural spread, Horner's syndrome, pleural tap, and bleeding) were downgraded to moderate certainty because of risk of bias. Therefore, we are uncertain about the estimates.
Potential biases in the review process
In an attempt to minimise bias, we followed the guidelines recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Eligibility for inclusion and exclusion and risk of bias of studies were assessed by two review authors independently.
Selection bias was observed in six studies due to uncertainty in random sequence generation (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), and in five studies due to allocation concealment (Megahed 2010; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015). Therefore except for Tedore 2011, all studies had uncertain selection bias. Performance bias was high in all studies, as participants in one group received paravertebral anaesthesia for breast cancer surgery, and those in the other group received general anaesthesia; therefore blinding of participants or personnel was not possible. As this comparison was the main question of the review, this bias was considered acceptable by all review authors. In addition, in four studies, uncertainty in blinding of outcome assessors was detected (Megahed 2010; Naja 2003; Paleczny 2005; Shkol'nik 2006). No attrition, reporting, or other bias was noted in any of the studies.
Agreements and disagreements with other studies or reviews
Several systematic reviews have assessed the use of paravertebral block during surgery, but these reviews included mixed populations (i.e. not specifically women with breast cancer) and non‐randomised, retrospective, and case series studies (Schnabel 2010; Tahiri 2011; Wu 2015). These reviews included studies that compared paravertebral block and general anaesthesia to conventional general anaesthesia alone (Schnabel 2010; Tahiri 2011; Wu 2015). Similar to our findings, these two other meta‐analyses found improvement in parameters that determined the quality of recovery (Schnabel 2010; Tahiri 2011). Schnabel 2010 reported reduced requirement for postoperative analgesia and reduced incidence of PONV in the paravertebral group compared to the general anaesthesia group. Tahiri 2011 found lesser incidence of PONV, decreased hospital length of stay, and greater patient satisfaction with paravertebral anaesthesia.
Two other systematic reviews studied all modalities of analgesia for breast cancer surgery, including different agents and regional anaesthesia techniques (paravertebral block included) (Cheng 2017; Jacobs 2020). They included RCTs and series (Cheng 2017), or RCTs and meta‐analyses (Jacobs 2020), as well as participants who received general anaesthesia with paravertebral block in the intervention arm. They too found that paravertebral block as a single injection or as a continuous infusion was associated with reduced acute postoperative pain, need for opioids, and occurrence of postoperative nausea and vomiting when compared with opioid‐based analgesics alone. Another systematic review found a marginal decrease in hospital stay in the paravertebral anaesthesia group, which review authors stated was not clinically relevant (standardised mean difference ‐0.60 hour, 95% CI ‐1.13 to ‐0.06; P = 0.028) and with significant heterogeneity (I² = 94.37%) (Terkawi 2015). These findings are consistent with the findings of our review, but we found no heterogeneity. In general, hospital stay is affected by the extent of surgery and hospital policy following breast cancer surgery. In one of the included studies in this review (Sundarathiti 2015), participants were kept in hospital for 2 days as per hospital policy despite fulfilling criteria for discharge earlier (this time was taken into account in the analysis). Two other studies had shorter hospital stay in the paravertebral group, which they surmised could contribute to considerable cost savings (Naja 2003; Tedore 2011).
Adverse events due to paravertebral block were reported in two meta‐analyses (Schnabel 2010; Tahiri 2011). Similar to our findings, Schnabel 2010 and Tahiri 2011 reported a very low incidence of pleural tap: Schnabel 2010 reported a risk ratio (RR) of 0.01 (95% CI 0.02 to 0.03) for pleural tap; Tahiri 2011 reported two cases of pneumothorax in 11 included studies comprising 618 mixed population participants and retrospective data. A low incidence of Horner's syndrome was reported by Schnabel 2010 (RR 0.05; 95% CI 0.02 to 0.09) in contrast to 7.1% (17/240 participants) incidence estimated in this meta‐analysis. We estimated the incidence of epidural spread to be 2 of 290 (0.7%), whereas Tahiri 2011 reported the most common adverse events to be hypotension/bradycardia (n = 12) followed by epidural spread (n = 5) among the 618 mixed participant data analysed. However in both meta‐analyses, there were no long‐term sequelae of reported adverse events.
The overall complication rate reported with paravertebral block has been reported as hypotension 4.6%, vascular puncture 3.8%, pleural puncture 1.1%, pneumothorax 0.5%, and a block failure rate of 10.1% (Lonnqvist 1995). The complication rate in the present review was lower than that reported in the study for vascular puncture, hypotension, and pleural tap, probably because the study included participants of all age groups (adults and children) undergoing all types of surgery (thoracic, abdominal, lower limb, and chronic pain procedures), whereas the present review included RCTs administering paravertebral block for breast cancer surgery to adult women alone. As breast cancer surgery requiring axillary clearance requires a higher‐level paravertebral block (C7/T1), this is more likely to block the sympathetic ganglia at the T1 level, resulting in Horner's syndrome, compared to the lower thoracic or lumbar level for other surgeries.
A recent large multi‐centre RCT including 2132 participants (women aged < 85 years undergoing breast cancer surgery) assessed the incidence of breast cancer recurrence following surgery under paravertebral analgesia. This study did not fulfil our inclusion criteria, as the intervention group received general anaesthesia (total intravenous anaesthesia with propofol) in addition to paravertebral block. This study found that the incidence of cancer recurrence was comparable between the paravertebral combined with general anaesthesia group and the general anaesthesia group at 36 months' follow‐up (10% of participants in either group). The incidence of chronic pain at 6 months and at 12 months (52%; 27% to 28% of participants, respectively) and the incidence of neuropathic pain at the same time points (10% and 7% of participants, respectively) were also comparable between groups (Sessler 2019). This study had several limitations: in 17% of participants in the paravertebral group, block was performed under sevoflurane anaesthesia. Sevoflurane has been shown in an vitro study to be associated with depressed natural killer cell activity (Buckley 2014), as well as higher mortality in cancer patients (Wigmore 2016). In addition, morphine, a drug known to promote angiogenesis and tumour cell recurrence in rats, was used as the primary postoperative analgesic (Sessler 2019). Sixty per cent of participants in this RCT were from the same country. Therefore, results of this RCT cannot be used to ascertain effects of paravertebral anaesthesia on breast cancer recurrence and other long‐term outcomes, which was the goal of this review.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.

Comparison 1: Postoperative outcomes, Outcome 1: Postoperative analgesic requirement

Comparison 1: Postoperative outcomes, Outcome 2: Postoperative nausea and vomiting

Comparison 1: Postoperative outcomes, Outcome 3: Hospital stay

Comparison 1: Postoperative outcomes, Outcome 4: Patient satisfaction score

Comparison 1: Postoperative outcomes, Outcome 5: Postoperative pain (VAS) at 2 hours

Comparison 1: Postoperative outcomes, Outcome 6: Postoperative pain (VAS) at 6 hours at rest

Comparison 1: Postoperative outcomes, Outcome 7: Postoperative pain (VAS) at 24 hours at rest

Comparison 1: Postoperative outcomes, Outcome 8: Postoperative pain (VAS) at 6 hours on movement

Comparison 1: Postoperative outcomes, Outcome 9: Postoperative pain (VAS) at 24 hours on movement

Comparison 2: Adverse events, Outcome 1: Epidural spread
| Paravertebral anaesthesia with or without sedation compared to general anaesthesia for women undergoing breast cancer surgery | ||||||
| Patient or population: women undergoing breast cancer surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | №. of participants | Certainty of evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with general anaesthesia | Risk with paravertebral anaesthesia with or without sedation | |||||
| Postoperative analgesic requirement | Study population | OR 0.07 | 305 | ⊕⊕⊝⊝ | ||
| 480 per 1000 | 61 per 1000 | |||||
| Postoperative nausea and vomiting (PONV) | Study population | OR 0.16 | 323 | ⊕⊕⊕⊝ | ||
| 340 per 1000 | 72 per 1000 | |||||
| Postoperative pain at 2 hours | Mean postoperative pain at 2 hours was 0 | MD 2.95 lower | ‐ | 224 | ⊕⊕⊕⊝ | |
| Postoperative pain at 24 hours on rest | Mean postoperative pain at 24 hours on rest was 0 | MD 0.21 lower | ‐ | 169 | ⊕⊕⊝⊝ | |
| Postoperative pain at 6 hours on movement | Mean postoperative pain at 6 hours on movement was 0 | MD 2.57 lower | ‐ | 130 | ⊕⊕⊝⊝ | |
| Postoperative pain at 24 hours on movement | Mean postoperative pain at 24 hours on movement was 0 | MD 2.12 lower | ‐ | 130 | ⊕⊕⊝⊝ | |
| Mortality | Study population | not estimable | (9 RCTs) | ‐ | No RCT has studied this outcome; therefore no data are available | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aData from five studies were analysed to assess this outcome (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015; Terheggen 2002). Blinding of participants was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in four studies (Das 2012; Naja 2003; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Pusch 1999; Sundarathiti 2015), and blinding of outcome assessors in one study led to downgrading of risk of bias to serious (Naja 2003). bHeterogeneity was observed for this outcome (I² = 70%) that included five RCTs; with the exclusion of 1 study, Sundarathiti 2015, heterogeneity decreased to 0%, probably because in this study, all patients in both groups received analgesics (Ultracet and Celebrex twice a day) irrespective of pain; only when they needed additional postoperative analgesia were morphine boluses administered. This routine administration of analgesics could have masked the difference in analgesic requirements between groups. In the other four studies, analgesics were administered only when patients expressed that they were in pain. cData from six studies were analysed to assess this outcome (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Tedore 2011; Terheggen 2002). Blinding of participants or personnel was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in five studies (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Paleczny 2005; Pusch 1999), and blinding of outcome assessors in two studies led to downgrading of risk of bias to serious (Naja 2003; Paleczny 2005). dData from four studies were analysed to assess this outcome (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002). Blinding of participants or personnel was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in all four studies (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Paleczny 2005; Pusch 1999), and blinding of outcome assessors in one study led to downgrading of risk of bias to serious (Paleczny 2005). eData from five studies were analysed to assess this outcome (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011). Blinding of participants was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in three studies (Das 2012; Naja 2003; Paleczny 2005), allocation concealment in two studies (Paleczny 2005; Sundarathiti 2015), and blinding of outcome assessors in two studies led to downgrading of risk of bias to serious (Naja 2003; Paleczny 2005). fData from five studies were used to evaluate this outcome (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011). We noted heterogeneity for this outcome (I² = 96%). On performing subgroup analysis and excluding two studies (Naja 2003; Paleczny 2005), where a potent adjunctive drug (alpha‐2 agonist clonidine) was added to the local anaesthetic solution used for paravertebral anaesthesia, we found a reduction in heterogeneity. gData from two studies were used to analyse this outcome (Naja 2003; Sundarathiti 2015). Blinding of participants or personnel was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it is acceptable. Uncertainty about random sequence generation and blinding of outcome assessors in one study ‐ Naja 2003 ‐ and allocation concealment in the other study ‐ Sundarathiti 2015 ‐ led to downgrading of evidence to serious. hSignificant heterogeneity was noted in the results and sensitivity analysis could not be performed, as only two studies could be included in assessing this outcome (Naja 2003; Sundarathiti 2015). Both studies showed reduction in pain on movement with the paravertebral block, but one study showed a greater reduction in pain probably due to methodological differences (Naja 2003). The use of clonidine as an adjunct to local anaesthetic as well as multiple injections at each paravertebral level could have contributed to this effect (Naja 2003). In the other study, only local anaesthetic was administered in the paravertebral space, injection was performed at one level, a catheter was inserted 8 cm into the space, and drug was injected through the catheter at this point, as well as on withdrawing the catheter by 2 cm and then 4 cm (single‐injection, multi‐level block) (Sundarathiti 2015). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Postoperative analgesic requirement Show forest plot | 5 | 305 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.34] |
| 1.2 Postoperative nausea and vomiting Show forest plot | 6 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.08, 0.31] |
| 1.3 Hospital stay Show forest plot | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐79.39 [‐107.38, ‐51.40] |
| 1.4 Patient satisfaction score Show forest plot | 3 | 129 | Mean Difference (IV, Fixed, 95% CI) | 5.52 [1.30, 9.75] |
| 1.5 Postoperative pain (VAS) at 2 hours Show forest plot | 4 | 224 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐3.37, ‐2.54] |
| 1.6 Postoperative pain (VAS) at 6 hours at rest Show forest plot | 5 | 324 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐3.20, 0.11] |
| 1.7 Postoperative pain (VAS) at 24 hours at rest Show forest plot | 5 | 278 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.27, ‐0.10] |
| 1.8 Postoperative pain (VAS) at 6 hours on movement Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐3.97, ‐1.17] |
| 1.9 Postoperative pain (VAS) at 24 hours on movement Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐4.80, 0.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Epidural spread Show forest plot | 8 | 574 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |

