Rapid initiation of antiretroviral therapy for people living with HIV
Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To assess the effects of rapid initiation of ART on treatment outcomes and mortality in PLWH.
Background
Description of the condition
At the end of 2016, there were approximately 36.7 million people living with HIV worldwide, most of them in low‐ and middle‐income countries (LMICs) (WHO 2017b). Although HIV infection continues to be a leading global cause of death and disability, mortality from the virus has almost halved in the past decade due to the rapid expansion of antiretroviral therapy (ART) (UNAIDS 2016).
The latest guidelines of the World Health Organization (WHO) recognize that people living with HIV/AIDS (PLWH) need to receive a continuum (or cascade) of care in order to fully benefit from ART (WHO 2016), and highlight the importance of all elements of HIV care, including HIV diagnosis, engagement in HIV care, provision of ART, and achievement of viral suppression (Gardner 2011; MacCarthy 2015; Okeke 2014). For this reason, the Joint United Nations Programme on HIV/AIDS (UNAIDS) introduced, in 2014, the 90‐90‐90 goals, which aim to diagnose 90% of all people infected with the virus, reach 90% of ART uptake in those diagnosed with HIV, and achieve a 90% rate of viral suppression among those receiving ART by 2020 (UNAIDS 2014).
Substantial international efforts will be needed to achieve these targets, as only 53% of PLWH were receiving ART in 2016 (UNAIDS 2016). One of the main challenges is attrition in HIV services during the period of linkage from HIV diagnosis to initiating ART (Govindasamy 2014; Losina 2010; Rosen 2011). Disengagement from care while PLWH are being prepared to initiate ART is particularly relevant in sub‐Saharan Africa, where it is estimated that only 57% of those diagnosed with HIV are linked to care (Kranzer 2012). Many affected individuals who disengage from services during this period return only when they have deteriorated clinically and immunologically; this results in higher morbidity and mortality when they do start ART (Fairall 2008; Grinsztejn 2014; HMC 2015). One of the contributing factors to poor engagement in care identified in the literature is the delay in starting ART (Clouse 2013). A longitudinal study in Ethiopia conducted between 2005 and 2013 estimated that 52% of PLWH waited over a month to start ART once they were classified as eligible according to the CD4 criteria and national guidelines (Teklu 2017). Other studies carried out in sub‐Saharan African countries have found similar results (Bassett 2010; Lawn 2006; Reddy 2016). The reasons why ART initiation is frequently delayed are complex and involve structural, social, and psychological factors (Hoehn 2017; Wachira 2014). For example, lack of appropriate healthcare infrastructures or constrains involved in attending the several clinic visits that are often required before ART is offered (Govindasamy 2012).
The WHO now recommends universal ART, irrespective of CD4 cell count and clinical stage (WHO 2016). Asymptomatic PLWH with higher CD4 cell counts have been shown to adhere poorly to ART (Adakun 2013), therefore retention in care and ART adherence are critically important.
One proposed intervention for improving linkage and retention of PLWH in HIV care is rapid ART initiation (starting the therapy as soon as possible after ART eligibility, normally within seven days of HIV diagnosis) (Chan 2016; Pilcher 2017). PLWH considered eligible for ART are normally required to attend HIV services several times for counselling and medical evaluation before starting treatment. It is argued that expediting ART could potentially lead to earlier viral suppression in the medium‐ and long‐term through improved uptake and adherence to ART (Hoenigl 2016; Pilcher 2017; Wilkinson 2015).
There remains, however, uncertainty about the acceptability of rapid ART initiation and its effects on morbidity and long‐term treatment outcomes (Mbonye 2016). Some concerns include: poor ART adherence with rapid ART initiation, since PLWH may not receive sufficient or adequate adherence counselling/education, or both; pill burden due to other concurrent comorbidities or conditions requiring urgent treatment (for example, tuberculosis) (Nachega 2014); and immune reconstitution inflammatory syndrome (IRIS), especially in individuals with advanced disease (CD4 counts < 200 cells/mm³) (Uthman 2015). Rapid ART initiation could be particularly relevant for PLWH with advanced disease, as they are at higher risk of mortality and morbidity (Hakim 2017). However, this cohort of individuals are also at higher risk of IRIS and, therefore, rapid ART should be considered in the context of differentiated care, in which they receive a comprehensive package of care that includes, among other factors, screening for opportunistic infections (for example, cryptococcus antigen), co‐trimoxazole prophylaxis, and more intensive adherence and follow‐up support (Mfinanga 2015; WHO 2016). It is also important to acknowledge that some of these individuals may not be eligible for rapid ART initiation if they are found to have signs and symptoms of opportunistic infections (for example, tuberculosis or cryptococcal meningitis). In these cases, ART initiation should be delayed according to the most current guidelines (WHO 2017a).
In order to evaluate the uptake, efficacy, and safety of rapid ART initiation compared with delayed ART initiation it is essential to consolidate findings from randomized controlled trials (RCTs) evaluating this intervention.
Description of the intervention
The current standard of care post‐HIV diagnosis varies across areas and settings according to the local context (MacCarthy 2015). Countries often include in their national guidelines structural and individual interventions aimed to improve linkage. These include: integration of services, point‐of‐care CD4 cell count, post‐test counselling, peer support, support with HIV disclosure, and addressing any psychosocial barriers identified (NASCOP 2016; NCASC 2009; NDOHSA 2015; Wynberg 2014). Pre‐ART care includes a comprehensive baseline assessment that often involves a clinical and a psychosocial assessment (MoH 2016). These may include several steps such as: physical examination, laboratory tests, opportunistic infection screening, nutritional status assessment, counselling, health insurance evaluation, and education sessions (NDOHSA 2015; Pilcher 2017). These interventions are often carried out over several visits to HIV services, which means that ART initiation is often delayed for several weeks after PLWH are considered eligible for ART (Lawn 2006; Teklu 2017).
High rates of disengagement from care have been reported during this period (Rosen 2011). In order to expedite this process and minimize losses to follow‐up, more rapid ART initiation has been proposed.
For the purposes of this Cochrane Review, we define rapid ART as offering the therapy within seven days after the PLWH is considered eligible for therapy. According to current guidelines, PLWH are considered eligible for ART on the same day as diagnosis unless they are found to have signs or symptoms of opportunistic infections such as tuberculosis or cryptococcal meningitis. With rapid ART, PLWH may receive usual care, but some of the interventions that form part of pre‐ART care are delivered on the same day or within a few days of HIV diagnosis. These include, among others, screening for tuberculosis, physical examination, and an initial counselling and education session (Labhardt 2016; Pilcher 2017; Rosen 2016).
How the intervention might work
Expediting the initiation of ART could improve engagement in care by simplifying and reducing the number of visits a PLWH has to make to HIV services. Delaying ART has been identified in the literature as major contributor to disengagement, particularly in LMICs (Govindasamy 2014).
The HIV continuum of care is a complex process in which every step is influenced by multiple factors, as illustrated in our conceptual model (see Figure 1). This figure shows how the feasibility of rapid ART initiation depends on various health system and provider factors, such as staffing levels, skills, infrastructure, and equipment, which vary across settings (Attawell 2003).
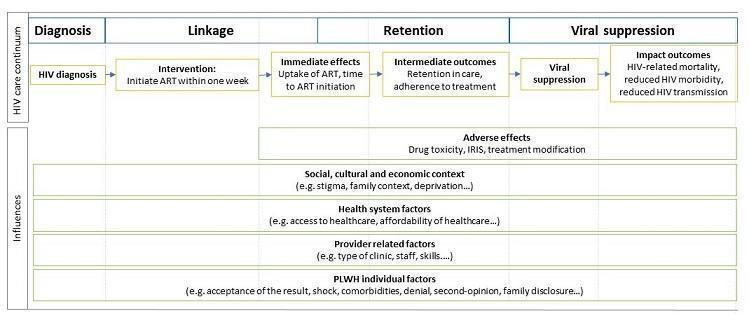
Conceptual model of factors influencing the HIV care continuum. Abbreviations: PLWH: people living with HIV, ART: antiretroviral therapy, IRIS: immune reconstitution inflammatory syndrome
Uptake of ART is also influenced by various factors (Bolsewicz 2015). Rapid ART could improve uptake of the therapy by reducing the number of PLWH lost to follow‐up in the period of linkage from HIV diagnosis to ART commencement (Pilcher 2017; Rosen 2016). However, as Figure 1 shows, uptake of ART is also influenced by social, economic, and cultural factors, such the family context, as well as individual factors, such as acceptance of the result or the desire for a second opinion. There is lack of consensus on the impact that offering rapid ART may have on these individual factors (Black 2013; Black 2014; Katirayi 2016).
By increasing the number of PLWH starting treatment, rapid ART could increase the absolute number of people retained in care and adhering to treatment. However, initiating rapid ART in PLWH before all baseline screening test results (for opportunistic infections and renal function) are available could result in a higher lost to follow‐up (LTFU) rate (Abay 2015; Chan 2016; Pilcher 2017). This could also result in a higher frequency of regimen modification in those being offered rapid ART initiation (Pilcher 2017).
Finally, rapid ART could also increase the number of PLWH achieving viral suppression through rising numbers of PLWH receiving ART, being engaged in care, and adhering to treatment (Pilcher 2017; Rosen 2016). As ART is started earlier, viral suppression could be achieved faster in those receiving rapid ART, having a positive impact on HIV transmission and HIV‐related morbidity and mortality (Eshleman 2017; Lesko 2016).
Why it is important to do this review
With universal ART being recommended and adopted worldwide, interventions aimed to address poor linkage of care are increasingly relevant. One of these proposed interventions is rapid initiation of ART.
Although it has been suggested that rapid ART initiation could improve engagement in care and increase rates of viral suppression (Rosen 2016), there is conflicting evidence regarding the acceptability and appropriateness of this intervention. Although qualitative and observational studies have found greater than 90% acceptance rates among PLWH who were offered rapid ART (Black 2013; Pilcher 2017), several other studies have found that issues, such as denial, shock, difficulty committing to life‐long treatment, or the need to consult with a partner, may, in addition to other known barriers, hamper the uptake or adherence to rapid ART initiation (Black 2014; Katirayi 2016). Most of these studies have focused on pregnant women receiving same‐day ART under Option B+ of the WHO's programme to prevent mother‐to‐child transmission of HIV. This option was introduced by the WHO in 2011 and offers pregnant women lifelong treatment as opposed to previous approaches where ART was offered for a limited time (Table 1) (UNICEF 2012). Although pregnant women constitute a distinct group with unique motivations for taking ART, the barriers identified in this group may be relevant to other settings. Another area of concern is that with expedited ART initiation, laboratory test results for opportunistic infection screening and renal function may not be available at the time of treatment initiation, which may have a negative impact on the incidence of adverse events, including drug side effects, regimen changes, and the incidence of IRIS (Abay 2015; Pilcher 2017).
| Options | Treatment for pregnant women with CD4 count < 350 cells/mm³ | Prophylaxis for pregnant women with CD4 count > 350 cells/mm³ |
| Option A | ART started as soon as HIV is diagnosed, continued for life | Antivirals started as soon as 14 weeks of gestation and continued until 7 days post‐partum |
| Option B | ART started as soon as HIV is diagnosed, continued for life | Antivirals started as soon as 14 weeks of gestation until childbirth if not breastfeeding or until one week after cessation of breastfeeding |
| Option B+ | ART initiated as soon as HIV diagnosis and continued for life | |
Source: WHO 2012.
The WHO now recommends initiation of ART within one week of diagnosis for those who are ready (WHO 2017a). Evidence for the rapid initiation of ART was investigated in 2017 in a systematic review (Ford 2018). Since the publication of this review, however, new studies have been published that address rapid initiation of ART in the context of complex interventions. Given the emergence of this new evidence, a rigorous appraisal of the current literature, including complex interventions and using established Cochrane methods, will help to clarify the role of rapid ART initiation in HIV care. We also aim to describe the complexity of the above‐mentioned interventions, in order to help readers understand the context in which rapid initiation of ART has been evaluated.
Objectives
To assess the effects of rapid initiation of ART on treatment outcomes and mortality in PLWH.
Methods
Criteria for considering studies for this review
Types of studies
We will include all RCTs in which the unit of randomization is either the individual or a cluster. We will not include non‐randomized studies as we anticipate there to be a significant evidence base from randomized studies. Additionally, any effect seen in non‐randomized studies is likely to be confounded due to the nature of the intervention.
Types of participants
Inclusion criteria
We will include:
-
adults (aged > 19 years), adolescents (aged > 10 to 19 years) and children (aged 1 to 10 years) with a positive HIV test who are known not to have previously received ART;
-
pregnant women who are receiving life‐long ART for their own health (Option B+ of the WHO system) (WHO 2012).
Exclusion criteria
We will exclude:
-
infants (aged 0 to < 1 years);
-
pregnant women not receiving life‐long ART for their own health.
These groups will be excluded because they may receive ART as part of prevention of mother to child transmission programmes, which do not include life‐long ART. For example, under WHO's Options A and B‐, pregnant women with high CD4 cell counts and infants receive only a short course of antiretrovirals (WHO 2012); hence, linkage to care and retention in HIV services differ in comparison with other PLWH.
Types of interventions
Experimental interventions
Any intervention that aims to initiate life‐long ART within seven days of HIV diagnosis. This may be combined with several other services, including education, counselling, addressing social determinants, clinical and laboratory assessments, or treatment of comorbid conditions.
Comparator interventions
Comparison interventions should offer the standard package of HIV care. The same CD4/clinical stage thresholds for ART initiation should be used in both intervention and comparison groups.
Cointerventions
We will include studies in which rapid ART initiation is offered within a package of care alongside other cointerventions, even if these are not provided in the comparator group. We will take into account the differences between the package of care in the intervention and comparator arms in our analyses (see Sensitivity analysis).
Types of outcome measures
Primary outcomes
-
All‐cause mortality rate;
-
virological suppression 12 months after a positive HIV test. According to WHO, viral suppression refers "to a viral load below the detection threshold using viral assays" (WHO 2016). There is inconsistency in the thresholds used by different countries to define viral suppression (AIDSinfo 2015; EACS 2017; NASCOP 2016; NDOHSA 2015), which is due to the variable sensitivity and specificity of the different HIV‐viral load assays available in the market (Sollis 2014; Swenson 2014). For this reason, we will use the investigators' trial definitions of virological suppression or undetectable viral load.
Secondary outcomes
-
Retention in HIV care at 12 months after a positive HIV test. We define retention according to WHO guidelines, as PLWH "who are enrolled in HIV care and routinely attend these services in accordance to their needs" (WHO 2016). This definition excludes those PLWH who either die or are LTFU. We will consider the follow‐up period to start at the point of randomization to the intervention or comparator arm of any study. There is a lack of consensus on the period of time that a PLWH has to be disengaged with HIV services to be considered LTFU, usually ranging from 3 to 6 months after the last attendance to services (Hønge 2013; Pilcher 2017). A systematic review that analysed the sensitivity and specificity of LTFU thresholds in 41 countries concluded that the most appropriate definition would be failure to engage with services for > 180 days after the last visit (Chi 2011). Due to the variability and lack of a standard definition, we will use the investigators' trial definitions of LTFU and we will explore, if possible, different LTFU thresholds in our sensitivity analyses;
-
uptake of ART, defined as the proportion of eligible PLWH initiated on ART;
-
ART adherence, as documented by self‐report and/or pill count and/or pharmacy refills and/or real‐time electronic monitors such as MEMScaps or Wisepill (Pellowski 2014);
-
incidence of IRIS, as defined by the study authors. We will include both paradoxical and unmasking IRIS (AIDSinfo 2017);
-
incidence of treatment modification, defined as the number and proportion of PLWH on ART who experience a regimen modification in the intervention and control groups.
Adverse outcomes
We will analyse the number and proportion of PLWH experiencing adverse drug reactions associated with ART in the intervention and control groups, the number of cases of IRIS in the intervention and control groups, and the number of HIV‐negative people partnered with a HIV‐positive person who became HIV infected during the study in each group.
Search methods for identification of studies
Electronic searches
Databases
We will search the following databases for relevant studies using terms listed in Appendix 1:
-
the Central Register of Controlled Trials (CENTRAL);
-
Cochrane Database of Systematic Reviews (CDSR);
-
MEDLINE (PubMed);
-
Embase (Ovid);
-
African Index Medicus (AIM);
-
Latin American and Caribbean Health Sciences Literature (LILACS);
-
Web of Science (Core Collection).
There will be no restriction on date, language, or publication status.
International trial registries
We will search the following registries for unpublished or ongoing studies:
-
ClinicalTrials.gov (www.clinicaltrials.gov);
-
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
Searching other resources
Grey literature
We will search the following sources of grey literature to identify any relevant unpublished literature, including conference abstracts:
-
International AIDS Society Online Resource Library (library.iasociety.org/GlobalSearch.aspx);
-
websites of the International AIDS Conference (IAS) on HIV science, the AIDS conference, the International Conference on AIDS and STIs in Africa (ICASA), and the Conference on Retroviruses and Opportunistic Infections (CROI) for the years 2013, 2014, 2015, and 2016;
-
the RAND publication database (www.rand.org/search.html).
Reference lists
We will handsearch the reference lists of all included studies and relevant systematic reviews to identify additional studies (for example, unpublished or in‐press citations).
Correspondence
We will contact trialists and subject experts for information on unpublished or ongoing studies, or to request additional trial data.
Data collection and analysis
Selection of studies
We will merge studies identified by the keyword searches of different databases and remove duplicate reports. Two review authors will independently evaluate all the studies by reading the abstracts to identify potentially relevant studies. We will obtain full‐text copies of those articles that are potentially eligible and we will further decide on whether the studies meet the inclusion criteria with the aid of an amended version of the study eligibility form available at Cochrane 2017b (see Appendix 2). We will resolve all disagreements by consulting a third review author. We will list all studies excluded after full‐text assessment in a ‘Characteristics of excluded studies' table. We will illustrate the study selection process in a PRISMA diagram.
Data extraction and management
Two review authors will independently extract data from included studies using a prepiloted data collection tool. We will resolve any discrepancies by discussion, consulting a third review author if necessary. If there are any missing data, we will contact the original study authors. Data points for extraction will include the following:
-
methods: study aim, design, unit of allocation, method of allocation, and duration of study. For cluster‐randomized trials we will extract the unit of analysis, the method of analysis, the average cluster size, and the intraclass correlation coefficient (ICC);
-
participants: setting, number, inclusion/exclusion criteria, participant's sociodemographic characteristics, method of recruitment, withdrawals, and losses to follow‐up;
-
intervention and control: number of participants/clusters randomized to intervention and control, description of intervention and control, including time of ART initiation, eligibility criteria, and complexity of intervention;
-
outcomes: definition of outcome, method of measurement, time points measured, person measuring, unit of measurement, statistical power, and imputation of missing data;
-
other: ethical approval, information consent, source of finding, and possible conflicts of interests.
Assessment of risk of bias in included studies
Two review authors will examine the components of each included study for risk of bias using the Cochrane ‘Risk of bias' tool (Cochrane 2017a). This includes detailed information on sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessor), incomplete outcome data, selective outcome reporting, and other sources of bias (Higgins 2011). If any cluster‐randomized trial is included in the review, we will also assess the risk of bias by including the five additional criteria specified in Section 16.3.2 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We will assess the methodological components of the studies and classify these as adequate (low risk of bias), inadequate (high risk of bias), or unclear, as explained in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
We will also assess and report the likely magnitude and direction of biases and their likely impact on the findings. We will resolve any discrepancies by discussion or by consulting a third review author. Where our judgement is uncertain we will attempted to contact the study authors.
Measures of treatment effect
Dichotomous data
We will report outcome measures for dichotomous data (for example, viral suppression yes/no) as risk ratios with 95% confidence intervals (CIs).
Continuous data
If studies report any outcome measures as continuous data (for example, viral load in copies per mL) we will report them using mean differences with standard deviations.
Time‐to‐event data
If possible, we will analyse outcomes using hazard ratios with 95% CIs. If trialists report the status of all the participants at a fixed time‐point (for example, 10 or 12 months) we will analyse the results as dichotomous data by generating a 2 x 2 table, with the estimate effects being risk ratios.
Timing of outcome assessment
For the outcomes of virological suppression and retention in care at 12 months, we will accept the result closest to 12 months within a range of six to 14 months.
For the secondary outcomes of incidence of treatment modification and of number of people experiencing adverse drug events we will accept the result closest to 12 months within a range of 6 to 14 months.
Unit of analysis issues
Cluster‐randomized trials
Where cluster‐randomized trials have appropriately adjusted for the effects of clustering in their analysis we will use these adjusted effect estimates and standard errors in our meta‐analysis using the generic inverse‐variance method in Review Manager 5 (RevMan 5) (RevMan 2014).
If the included study does not perform any adjustment for clustering, we will adjust the raw data ourselves using the ICC. If the study authors do not report an ICC value in the published article, we will either obtain this value from similar studies or we will estimate the ICC value. We will not present results from cluster‐randomized trials that are not adjusted for clustering. If we estimate the ICC value, we will perform sensitivity analyses to investigate the robustness of our analyses.
Cross‐over trials
We do not anticipate any cross‐over trials addressing this review question.
Repeated observations on participants
In some studies, results from more than one timepoint may be reported. In those cases we will conduct separate analyses according to the different outcomes defined (see Primary outcomes; Secondary outcomes). For example, if viral suppression is reported at six and 12 months, we will conduct two separate analyses, one for the secondary outcome of viral suppression at six months and another for the primary outcome of viral suppression at 12 months.
Studies with multiple treatment groups
Depending on the study, we will address multiple treatment groups in the analysis in one of three ways:
-
combining groups to create a single pair‐wise comparison;
-
selecting one pair of interventions and excluding the others;
-
splitting the ‘shared’ group into two or more groups with a smaller sample size and including two or more (reasonably independent) comparisons.
Dealing with missing data
Where possible, we will contact the original authors to request missing data. If, after contacting the authors, there are still missing data and we consider the data to be missing at random, we will include only the data available in the analysis. If we do not consider the data to be missing at random, we will impute the missing data and account for the fact that the data were imputed with uncertainty, following the advice in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We will then conduct a sensitivity analysis to analyse how sensitive the results are to the assumptions we made when imputing missing data.
We will analyse all data as intention‐to‐treat.
Assessment of heterogeneity
We will assess the statistical heterogeneity in each meta‐analysis by inspecting forest plots and calculating Chi2 test values and I2 statistics. We will consider significant heterogeneity to be present if the P value of the Chi2 test is < 0.10. We will interpret the I2 statistic according to the thresholds recommended in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011):
-
0% to 40%: low heterogeneity;
-
30% to 60%: moderate heterogeneity;
-
50% to 90%: substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
We will explore the causes of statistical heterogeneity by conducting a subgroup analysis or meta‐regression. We will also explore clinical and methodological heterogeneity by assessing trial populations, methods used, and interventions delivered.
Assessment of reporting biases
If we include more than 10 studies we will perform a funnel plot to explore reporting bias. We will assess reporting bias a visual exploration of the funnel plot and by conducting an appropriate statistical test for asymmetry (for example, Begg or Egger tests) following the advice given in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We will analyse data using RevMan 5 (RevMan 2014). We will assess the clinical, methodological, and statistical heterogeneity of the trials. If we identify considerable heterogeneity, we will explore the causes by carrying out subgroup analyses or meta‐regression. If, after exploring heterogeneity, we identify considerable unexplained heterogeneity, we will consider not combining the results in a meta‐analysis. If we identify low or moderate heterogeneity, we will combine the results using a random‐effects model. If we find no heterogeneity, we will combine the results using a fixed‐effects model.
Subgroup analysis and investigation of heterogeneity
If we include a sufficient number of trials, we plan to carry out the following subgroup analyses to investigate potential sources of heterogeneity.
-
Severity of HIV infection at baseline, defined by CD4 cell count:
-
CD4 cell count > 200 copies/mL;
-
CD4 cell count < 200 copies/mL.
-
-
Time of ART initiation in the intervention group:
-
same‐day ART (< 24 hours post‐ART eligibility);
-
ART initiated between > 24 hours and < 7 days post‐ART eligibility.
-
-
Age group:
-
children (aged 1 to 10 years);
-
adolescents (aged > 10 to 19 years);
-
adults (aged > 19 years).
-
-
Geographical location, defined by the World Bank’s income classification of countries (World Bank 2017):
-
low and low‐middle income countries;
-
high and upper‐middle income countries.
-
Sensitivity analysis
We plan to conduct sensitivity analyses to investigate the effect on the outcomes of:
-
including and excluding trials we consider to be at high risk of bias for random sequence generation according to Cochrane's ‘Risk of bias' assessment;
-
analysing the different assumptions made when imputing missing data;
-
analysing retention in care using different LTFU thresholds.
If we identify, during the review process, any issues that are suitable for sensitivity analysis, we will include them in this section, as recommended in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
In studies in which rapid ART initiation is delivered as a package of care alongside other cointerventions that are not present in the comparator arm, we will consider not pooling the results if such cointerventions may confound the effect estimate.
‘Summary of findings' table
We will create a ‘Summary of findings' table using GRADEpro software (GRADEpro 2015). In the table, we will display the primary and secondary outcomes of the review (see Types of outcome measures), the comparative risks between intervention and control groups, the relative effects with 95% CIs, the number of participants in the studies, and the quality of evidence. We will classify the strength of the evidence for each of the outcomes as high, moderate, low, or very low, according to a quality assessment using the five criteria (limitations, inconsistency, indirectness, imprecision, and publication bias) of the GRADE system (GRADE 2004).

Conceptual model of factors influencing the HIV care continuum. Abbreviations: PLWH: people living with HIV, ART: antiretroviral therapy, IRIS: immune reconstitution inflammatory syndrome
| Options | Treatment for pregnant women with CD4 count < 350 cells/mm³ | Prophylaxis for pregnant women with CD4 count > 350 cells/mm³ |
| Option A | ART started as soon as HIV is diagnosed, continued for life | Antivirals started as soon as 14 weeks of gestation and continued until 7 days post‐partum |
| Option B | ART started as soon as HIV is diagnosed, continued for life | Antivirals started as soon as 14 weeks of gestation until childbirth if not breastfeeding or until one week after cessation of breastfeeding |
| Option B+ | ART initiated as soon as HIV diagnosis and continued for life | |
| Source: WHO 2012. | ||

