Cannabis for the treatment of ulcerative colitis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group pilot study | |
| Participants | Male or female participants (N = 60) aged 18 years or above (18‐65 years in the Czech Republic) Inclusion criteria included: (1) diagnosed with mild to moderate ulcerative colitis and on a fixed dose of 5‐ASA treatment and have been on a stable dose for at least 2 weeks prior to screening (0 mg dose of 5‐ASA is acceptable) (2) had at screening (Visit 1) and baseline (Visit 2) with a Mayo assessment score of greater than or equal to 4 (≥ 4) but less than or equal to 10 (≤ 10) and with an endoscopy score of at least 1 (≥ 1) , following an adequate exposure to oral or topical 5‐ASA, in the opinion of the investigator (3) In the opinion of the investigator, capable of complying with the study requirements and completing the study (4) Willing and able to give informed consent (5) Willing for his or her name to be notified to the responsible authorities for participation in this study, as applicable (6) Willing to allow his or her primary care practitioner and consultant, if appropriate, to be notified of participation in the study | |
| Interventions | Patients received either cannabidiol with up to 4.7% THC (n = 29) or placebo (n = 31) gelatin capsules The cannabidiol dose ranged from 50 mg to 250 mg twice daily(GWP42003 is purified from a proprietary Cannabis sativa L. chemotype containing predominantly CBD, up to 4.7 % THC and other compounds) The placebo capsules had excipients alone The cannabidiol with 4.7% THC dose was titrated up to maximal tolerated dose over two‐weeks with maximum dose of 250 mg twice daily (i.e. intervention ranged from 1‐5 capsules taken twice daily) Treatment duration was 10 weeks. | |
| Outcomes | Note: The original outcome measures submitted in March 2012 were changed in July 2015, one year after completion of the study 2012: Primary outcome was percentage of participants achieving remission, defined as a Mayo Score of < 2 with no sub‐score > 1 Secondary outcomes included serum C‐reactive protein (CRP), serum cytokines (IL‐6, IL‐2 & TNF‐α), fecal calprotectin, body weight measurement, clinical assessments with IBDQ, SGIC, PGAS, MAYO score; and daily diary of stool frequency (0‐4 numerical rate scale), rectal bleeding (0‐4 numerical rate scale), and pain (0‐10 numerical rate scale) 2015: Primary outcome was same, but included PP analysis as well as ITT Secondary outcomes included CRP, IL‐2, IL‐6, TNF‐alpha, stool calprotectin, Inflammatory Bowel Disease Questionnaire (IBDQ) total score, Subject Global Impression of Change (SGIC), Physician's Global Assessment of Illness Severity (PGAS), pain scores using a 0‐10 numerical rate scale, stool frequency scores using a 0‐4 numerical rate scale and PP analysis, rectal bleeding scores using a 0‐4 numerical rate scale and PP analysis, plasma endocannabinoid levels (2‐arachidonoyl glycerol (2‐AG), anandamide (AEA), oleoylethanolamide (OEA), and PEA, Mayo Total Score, Mayo Partial Score, Mayo responder analysis (responder defined as participant with a decrease in their Mayo total score of ≥ 3 points, compared to baseline, with a reduction of at least 1 point in endoscopy findings sub‐score, and body weight. | |
| Notes | Industry funded by GW Research Limited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician produced a randomization schedule which was held centrally A unique number was then assigned to either the treatment or placebo group according to the randomization schedule |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding of participants and personnel (performance bias) | Low risk | Reported double blinding, but also "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" Used identical gelatin capsules for the treatment and placebo groups |
| Blinding of outcome assessment (detection bias) | Low risk | Reported double blinding, but also "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" The review of the data was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | There was a higher rate of withdrawals in the treatment group (13/29) compared to the placebo group (8/31) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the manuscript or on the clinicaltrials.gov web site |
| Other bias | Low risk | Although a higher proportion of the intervention group had previously used cannabis compared to the placebo group and the time since last cannabis use was greater in intervention group compared to the placebo group, these differences were not statistically significant The study appears to be free of other sources of bias |
| Methods | Randomized placebo controlled trial | |
| Participants | Patients with UC who did not respond to conventional medical treatment (N = 32) | |
| Interventions | Either two cigarettes of cannabis (0.5 g of cannabis, corresponding to 11.5 mg THC, n = 17) or placebo (cannabis leaves from which THC was extracted, n = 15) daily for eight weeks | |
| Outcomes | General outcomes reported as Disease activity (DAI), Mayo endoscopic score, endoscopic findings and laboratory tests (CRP, fecal calprotectin) Abstract does not specify primary outcome | |
| Notes | Additional information was supplied by the principal investigator Timna Naftali which informed our risk of bias assessment NCT01040910 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using block method in a 1:1 ratio to receive either medical cannabis or placebo |
| Allocation concealment (selection bias) | Low risk | The investigators used sequentially numbered drug containers of identical appearance, which were given to the patients outside of the hospital by the pharmacy staff so the medical team did not see them |
| Blinding of participants and personnel (performance bias) | High risk | Although placebo cigarettes were used, blinding was likely to be broken due to the psychotropic effects of cannabis |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded Blinding was open only at the end of the study |
| Incomplete outcome data (attrition bias) | Low risk | There were no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome reported in the study protocol was for Crohn's disease ‐ a reduction in CDAI of 70 points (the study enrolled participants with Crohn's disease and ulcerative colitis) The secondary outcomes reported in the study protocol included adverse events due to cannabis smoking, change in quality of life, change in IL‐10, IL‐2, and TGF beta None of these outcomes were reported in the abstract publication but could potentially be reported in a full manuscript |
| Other bias | Low risk | The study appears to be free of other sources of bias |
5‐ASA: 5‐aminosalicylates
THC: D9‐tetrahydrocannabinol
CDAI: Crohn's disease activity index
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Unable to acquire separate data for participants with ulcerative colitis There were 10 participants with ulcerative colitis and 22 participants patients with Crohn's disease Combined results for both groups reported | |
| This randomized trial assessed the use of cannabis in participants with Crohn's disease |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission at 10 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 1 Clinical remission at 10 weeks. | ||||
| 2 Clinical response at 10 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 2 Clinical response at 10 weeks. | ||||
| 3 CRP at 10 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 3 CRP at 10 weeks. | ||||
| 4 Inflammatory Bowel Disease Questionnaire (IBDQ) ‐ at 10 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 4 Inflammatory Bowel Disease Questionnaire (IBDQ) ‐ at 10 weeks. | ||||
| 5 Symptom measure ‐ pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 5 Symptom measure ‐ pain. | ||||
| 6 Symptom measure ‐ rectal bleeding Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 6 Symptom measure ‐ rectal bleeding. | ||||
| 7 Symptom measure ‐ stool frequency Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 7 Symptom measure ‐ stool frequency. | ||||
| 8 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 8 Adverse events. | ||||
| 9 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 9 Serious adverse events. | ||||
| 10 Withdrawal due to adverse event Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 10 Withdrawal due to adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DAI Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 1 DAI. | ||||
| 2 CRP Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 2 CRP. | ||||
| 3 Fecal calprotectin Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 3 Fecal calprotectin. | ||||
| 4 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 4 Serious adverse events. | ||||
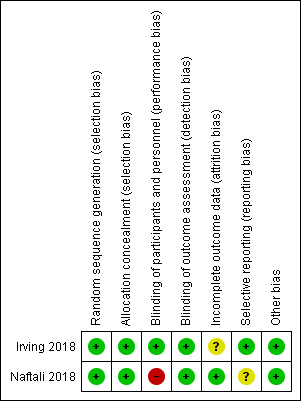
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
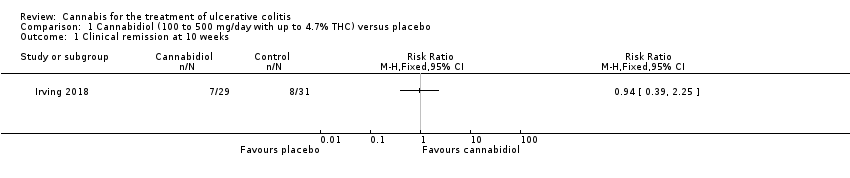
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 1 Clinical remission at 10 weeks.

Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 2 Clinical response at 10 weeks.
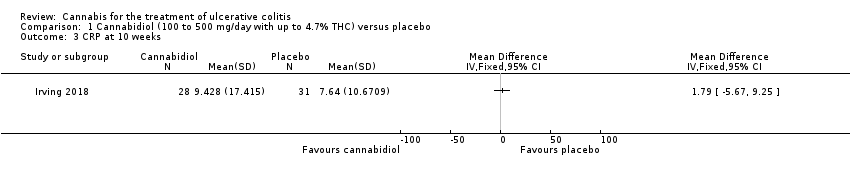
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 3 CRP at 10 weeks.
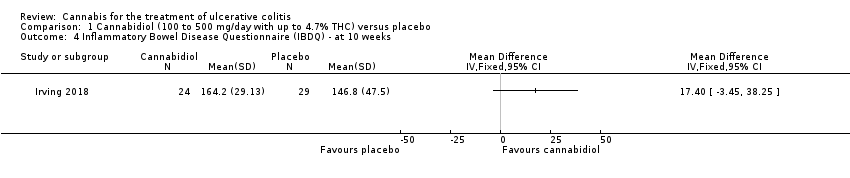
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 4 Inflammatory Bowel Disease Questionnaire (IBDQ) ‐ at 10 weeks.

Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 5 Symptom measure ‐ pain.
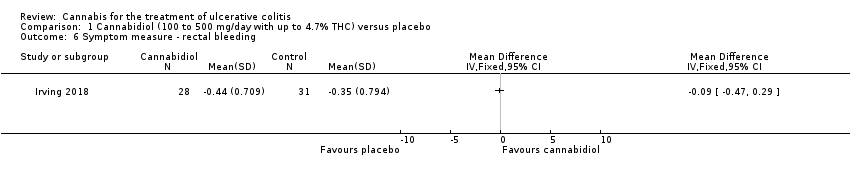
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 6 Symptom measure ‐ rectal bleeding.
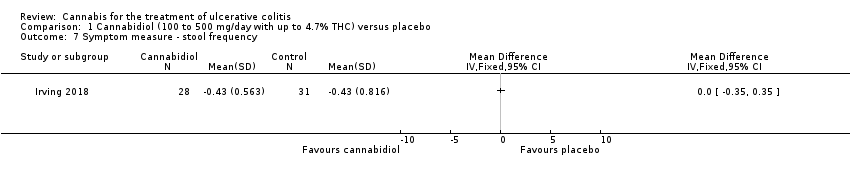
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 7 Symptom measure ‐ stool frequency.

Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 8 Adverse events.
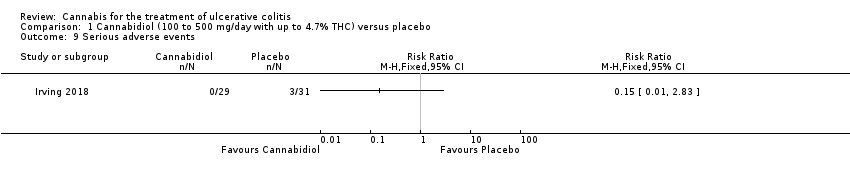
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 9 Serious adverse events.

Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 10 Withdrawal due to adverse event.

Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 1 DAI.
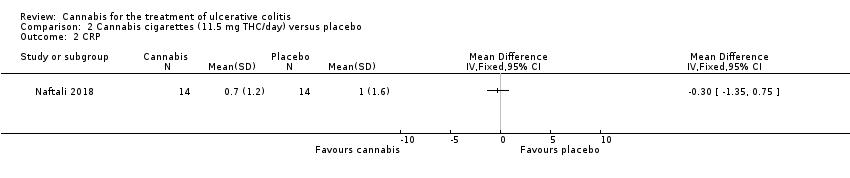
Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 2 CRP.
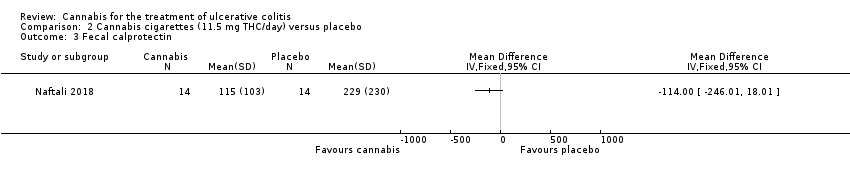
Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 3 Fecal calprotectin.
Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 4 Serious adverse events.
| Cannabidiol compared to placebo for the treatment of ulcerative colitis | ||||||
| Patient or population: participants with active ulcerative colitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Cannabidiol with up to 4.7% THC | |||||
| Clinical remission at 10 weeks | 258 per 1,000 | 243 per 1,000 | RR 0.94 | 60 | ⊕⊕⊝⊝ | Remisison was defined as a Mayo score of < 2 (with no sub‐score > 1) |
| Clinical response at 10 weeks | 226 per 1,000 | 309 per 1,000 | RR 1.37 | 60 | ⊕⊕⊝⊝ | Response defined as decrease in Mayo score of ≥3 points compared to baseline, with a reduction of at least 1 point in endoscopy findings sub‐score |
| CRP at 10 weeks | The mean CRP at 10 weeks was 9.4 mg/L | MD 1.79 mg/L higher | ‐ | 59 | ⊕⊕⊕⊝ | |
| Quality of life Inflammatory Bowel Disease Questionnaire (IBDQ) at 10 weeks | The mean IBDQ score at 10 weeks was 146.8 | MD 17.4 higher | ‐ | 53 | ⊕⊕⊕⊝ | IBDQ scores range from 32 to 224 with a higher score indicating better quality of life |
| Adverse events | 774 per 1,000 | 991 per 1,000 | RR 1.28 | 60 | ⊕⊕⊕⊝ | Common adverse events included dizziness, disturbance in attention, headache, nausea and fatigue |
| Serious adverse events | 97 per 1,000 | 15 per 1,000 | RR 0.15 | 60 | ⊕⊕⊝⊝ | There were no serious adverse events in the cannabidiol group Serious adverse events in the placebo group included worsening of ulcerative colitis and one complicated pregnancy |
| Withdrawal due to adverse event | 161 per 1,000 | 345 per 1,000 | RR 2.14 | 60 | ⊕⊕⊝⊝ | Withdrawls in the cannabidiol group were mostly due to dizziness Withdrawals in the placebo group were due to worsening ulcerative colitis |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very sparse data (15 events). 2 Downgraded two levels due to very sparse data (16 events). 3 Downgraded one level due to sparse data (59 participants). 4 Downgraded one level due to sparse data (53 participants). 5 Downgraded one level due to sparse data (53 events). 6 Downgraded two levels due to very sparse data (4 events). | ||||||
| Cannabis cigarettes (23 mg THC/day) compared to placebo for the treatment of ulcerative colitis | ||||||
| Patient or population: participants with active ulcerative colitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with cannabis cigarettes (11.5 mg THC) | |||||
| Clinical remission | Not reported | This outcome was not reported | ||||
| Clinical response | Not reported | This outcome was not reported | ||||
| CRP at 8 weeks | The mean CRP at 8 weeks was 1.0 mg/L | MD 0.3 mg/L lower | ‐ | 28 | ⊕⊕⊝⊝ | |
| Quality of life Inflammatory Bowel Disease Questionnaire (IBDQ) | Not reported | This outcome was not reported | ||||
| Adverse events | Not reported | This outcome was not reported | ||||
| Serious adverse events | 0 per 1,000 | 0 per 1,000 | not estimable | No serious adverse events were observed | ||
| Withdrawal due to adverse events | Not reported | This outcome was not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very sparse data (28 participants). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission at 10 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical response at 10 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 CRP at 10 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Inflammatory Bowel Disease Questionnaire (IBDQ) ‐ at 10 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Symptom measure ‐ pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Symptom measure ‐ rectal bleeding Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Symptom measure ‐ stool frequency Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Withdrawal due to adverse event Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DAI Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 CRP Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Fecal calprotectin Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |


