Parto planificado a término o cerca del término para mejorar los resultados de salud en pacientes embarazadas con diabetes preexistente y sus neonatos
Resumen
Antecedentes
Las pacientes embarazadas con diabetes preexistente (Tipo 1 o Tipo 2) tienen un aumento en las tasas de resultados maternos y neonatales adversos. Las guías clínicas actuales apoyan el parto electivo, a término o cerca del término, debido al aumento de la mortalidad perinatal durante el tercer trimestre del embarazo.
Esta revisión reemplaza una revisión previamente publicada en 2001 que incluyó "embarazadas con diabetes", que ahora se ha dividido en dos revisiones. La revisión actual se centra en las pacientes embarazadas con diabetes preexistente (tipo 1 o tipo 2), y una revisión similar se centra en las pacientes con diabetes gestacional.
Objetivos
Evaluar el efecto del parto planificado (ya sea por inducción del trabajo de parto o parto por cesárea) a término o cerca del término (37 a 40 semanas de gestación) en comparación con un enfoque expectante, para mejorar los resultados de salud de las pacientes embarazadas con diabetes preexistente y sus neonatos. Los resultados primarios se relacionan con la mortalidad y la morbilidad materna y perinatal.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth’s Trials Register), ClinicalTrials.gov, en la WHO International Clinical Trials Registry Platform (ICTRP) (15 agosto 2017), y en listas de referencias de estudios recuperados.
Criterios de selección
Se planificó incluir los ensayos aleatorios (que incluyen los que utilizaron un diseño aleatorio grupal) y los ensayos no aleatorios (p.ej. los ensayos cuasialeatorios con asignación alterna) que compararon el parto planificado, a término o cerca del término, con un enfoque expectante en pacientes embarazadas con diabetes preexistente.
Obtención y análisis de los datos
Dos de los autores de la revisión evaluaron la elegibilidad de los estudios de forma independiente. En futuras actualizaciones de esta revisión, por lo menos dos autores de la misma extraerán los datos y evaluarán el riesgo de sesgo de los estudios incluidos. También se evaluará la calidad de la evidencia mediante el enfoque GRADE.
Resultados principales
No se identificaron ensayos publicados elegibles para su inclusión en esta revisión.
Se identificó un ensayo aleatorio que examinó si la conducta expectante redujo la incidencia de parto por cesárea en embarazos sin complicaciones de pacientes con diabetes gestacional (que requerían insulina) y con diabetes preexistente. Sin embargo, los datos publicados por este ensayo no diferenciaron entre la diabetes preexistente y gestacional, por lo que se excluyó.
Conclusiones de los autores
A falta de evidencia no es posible establecer conclusiones acerca de los resultados de salud asociados con el parto planificado, a término o cerca del término, en comparación con un enfoque expectante en las pacientes embarazadas con diabetes preexistente.
Esta revisión demuestra la necesidad urgente de ensayos de alta calidad que evalúen la efectividad del parto planificado a término o cerca del término de la gestación en pacientes embarazadas con diabetes preexistente (tipo 1 o tipo 2) en comparación con un enfoque expectante.
PICO
Resumen en términos sencillos
Parto planificado a término o cerca del término para mejorar los resultados de salud en pacientes embarazadas con diabetes preexistente y sus neonatos
¿Cuál es el problema?
El objetivo de esta revisión Cochrane fue determinar si la planificación de un parto electivo a término o cerca del término del embarazo, en comparación con esperar que el trabajo de parto comience espontáneamente, tiene alguna repercusión sobre la salud de las pacientes con diabetes y la salud de los neonatos. Esta revisión se centra en las pacientes con diabetes antes del embarazo (diabetes preexistente). El parto electivo se realiza por inducción del trabajo de parto o por cesárea y "a término o cerca del término" significa a las 37 a 40 semanas de gestación.
Para responder a esta pregunta, se buscaron todos los estudios relevantes (fecha de búsqueda: 15 de agosto de 2017), con el objetivo de recopilarlos y analizarlos juntos.
¿Por qué es esto importante?
Cuando las pacientes con diabetes (tipo 1 o tipo 2) están embarazadas tienen mayor riesgo de complicaciones que las pacientes que no presentan diabetes. Por ejemplo, los fetos pueden ser más grandes y tener un riesgo mayor de muerte en las últimas semanas del embarazo. Debido a estos riesgos, muchos médicos han recomendado que a las pacientes con diabetes se les realice un parto electivo (generalmente por inducción) a término o cerca del término (37 a 40 semanas de gestación), en lugar de esperar a que el trabajo de parto comience espontáneamente o hasta las 41 semanas de gestación si todo está bien. La inducción tiene la desventaja de aumentar la incidencia de partos con fórceps o ventosa, y las pacientes a menudo tienen dificultades para afrontar un trabajo de parto inducido. La cesárea es una operación mayor que puede provocar pérdida sanguínea, infecciones y aumenta las probabilidades de problemas con los partos posteriores. El parto temprano puede aumentar las probabilidades de problemas respiratorios en los neonatos. Es importante saber qué enfoque para el parto tiene una mejor repercusión sobre los resultados de salud de las pacientes con diabetes preexistente y sus neonatos.
¿Qué evidencia se encontró?
No se encontraron estudios que abordaran la pregunta específica.
¿Qué significa esto?
A falta de estudios aleatorios, no fue posible determinar si las pacientes con diabetes preexistente y sus neonatos presentan mejores resultados de salud si tienen un parto planificado (por inducción del trabajo de parto o cesárea a las 37 a 40 semanas de gestación) en comparación con esperar a que el trabajo de parto comience espontáneamente o hasta las 41 semanas de gestación si todo está bien. Se necesita más investigación para responder a esta cuestión.
Authors' conclusions
Background
There are several clinical situations where planned birth has been advocated with the aim of reducing adverse outcomes for both mother and baby (Dodd 2014). These include: an otherwise low‐risk singleton pregnancy after 41 weeks (Gulmezoglu 2012); for women with an uncomplicated twin pregnancy at 37 weeks' gestation (Dodd 2014); and for women at 37 to 40 weeks' gestation where a clinical suspicion of macrosomia exists (Boulvain 2016). Systematic reviews of these three scenarios reveal that induction of labour after 41 weeks is the only intervention associated with a reduction in perinatal mortality (Gulmezoglu 2012). A previously published Cochrane review concluded that elective birth at term in pregnant women with insulin‐requiring diabetes reduces the risk of macrosomia but does not impact on maternal or neonatal morbidity (Boulvain 2001).
The original review 'Elective delivery in diabetic pregnant women' (Boulvain 2001) has now been split into the following two reviews.
-
Planned birth at or near term for improving health outcomes for pregnant women with pre‐existing diabetes and their infants (this review)
-
Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants (Biesty 2018)
As gestational diabetes is typically a transient glucose abnormality occurring late in the second trimester of pregnancy, whilst pre‐existing diabetes exists throughout the entire pregnancy, it is important to clearly differentiate between these different conditions when approaching the issue of planned birth. It is acknowledged that there are similarities in the background, methods and outcomes between these two systematic reviews.
Description of the condition
Pre‐existing diabetes refers to maternal diabetes that existed prior to the pregnancy. This typically refers to Type 1 and Type 2 diabetes, however the term also encompasses rarer forms of diabetes, e.g. Maturity Onset Diabetes of the Young (MODY). Type 1 diabetes is characterised by autoimmune destruction of beta cells, usually leading to absolute insulin deficiency; Type 2 diabetes occurs due to a progressive loss of insulin secretion on a background of insulin resistance, and is likely to be the result of interactions between genetic, environmental and immunological factors (ADA 2016; Zaccardi 2016). Although estimates vary, it is believed that 0.5% to 0.9% of pregnancies are complicated by pre‐existing diabetes (Correa 2015; NICE 2015). While the prevalence of both Type 1 and Type 2 diabetes has increased in recent years (NICE 2015), the rate of Type 2 diabetes in pregnant women in the USA more than quadrupled in the period from 1994 to 2004, overtaking the rates of pre‐existing Type 1 diabetes (Albrecht 2010). This increase in prevalence of Type 2 diabetes is in line with the worldwide rise in obesity rates and advancing maternal age (ACOG 2005; Egan 2015; Zhu 2016).
In the setting of pre‐existing diabetes, pregnancy is considered to be high risk and is associated with increased rates of adverse maternal and neonatal outcomes. Studies from the United Kingdom and Ireland reveal a congenital malformation rate twice that of the background population (24/1000), a five‐fold increased risk of stillbirth (25/1000), and a three‐fold increased risk of perinatal mortality and caesarean birth (25/1000) (Macintosh 2006; Dunne 2009; Egan 2015). During the third trimester, issues of significant concern are: late fetal death; complications necessitating premature birth; and the potential for birth trauma associated with fetal macrosomia (Cousins 1987; Hanson 1993; Dunne 2009). Although recent research has noted a higher risk of adverse neonatal outcomes including stillbirths and congenital abnormalities in offspring of women with Type 1 compared to Type 2 diabetes (Owens 2015), an earlier systematic review found perinatal mortality to be higher for women with Type 2 compared with Type 1 diabetes (Balsells 2009). It should be noted that women with Type 2 diabetes are more commonly from ethnic minorities and are often cared for in community settings with minimal access to specialist care (Murphy 2010). This makes them an especially vulnerable group.
The hyperglycaemia‐hyperinsulinaemia hypothesis, also known as the Pederson Hypothesis, aims to explain the underlying pathology that leads to the disordered fetal growth associated with the diabetic pregnancy. It states that "maternal hyperglycaemia results in fetal hyperglycaemia and, hence, in hypertrophy of fetal islet tissue with insulin‐hypersecretion. This again means a greater fetal utilisation of glucose" (Pedersen 1952; Pedersen 1967). More recently, it has been suggested that additional factors such as alterations in lipid metabolism and inflammatory change may contribute to the abnormal metabolic environment associated with diabetic pregnancies, particularly when obesity co‐exists (Catalano 2011). Such metabolic disruptions can affect organogenesis in early pregnancy, and cardiac malformations in particular are more common in infants of women with diabetes (Inkster 2006). As the pregnancy progresses, this abnormal intrauterine environment may result in the aforementioned neonatal morbidity, including being large‐for‐gestational age; having neonatal hypoglycaemia, hyperbilirubinaemia, hypocalcaemia; and increased need for admission to an intensive care unit (Macintosh 2006; Dunne 2009; Middleton 2016). It is becoming increasingly evident that exposure to maternal diabetes in utero may also have a longer‐term negative impact on the offspring, with one recent study noting that adolescent offspring of women with Type 1 diabetes have lower cognitive function compared with a control group even after adjusting for confounders (Bytoft 2016). In addition, long‐term follow up of offspring of women with diabetes reveal that they have elevated rates of obesity and Type 2 diabetes later in life (Dabelea 2000).
Due to the association between hyperglycaemia and poor pregnancy outcomes, pregnant women with diabetes are advised to keep blood glucose levels as close to normal as possible. Frequent capillary blood‐glucose monitoring and tight targets such as fasting glucose of less than 5.3 mmol/L (96 mg/dL) and one‐hour post prandial of less than 7.8 mmol/L (140 mg/dL) are typically recommended (NICE 2015). While HbA1c is not entirely reliable in pregnancy, higher levels (HbA1c more than 6.0% to 6.5% or more than 42 to 48 mmol/mol) may still be used as a marker of poor glycaemic control and a higher risk pregnancy (Egan 2015; Maresh 2015). In early pregnancy, there is increased insulin sensitivity, lower glucose levels and lower insulin requirements in women with Type 1 diabetes (ADA 2016). During the second and early third trimesters, physiological insulin resistance increases to facilitate the transfer of glucose across the placenta to the fetus and ensure adequate growth and development (Farrar 2016). This creates a significant challenge for women with diabetes who must adjust their treatments regularly to match these increasing insulin requirements and achieve their therapeutic goals. Typically, care of these women involves significant input from a multidisciplinary team of specialists, with intensive monitoring throughout the pregnancy, including frequent ultrasound surveillance of fetal growth (NICE 2015). Unfortunately, the prevalence of large‐for‐gestational age or macrosomia (or both) remains high in infants of women with diabetes, even in pregnancies that are considered 'well‐controlled' (Evers 2002). In the past, some authors have proposed to perform birth before full term in women with pre‐existing diabetes, because of increased perinatal mortality during the third trimester (Hunter 1989). This viewpoint is also reflected in current clinical guidelines (NICE 2015).
Description of the intervention
A woman’s pregnancy is considered to be 'at term' when her pregnancy duration reaches 37 weeks (Gulmezoglu 2012). Planned birth involves the early birth of the infant either by induction of labour or caesarean section. This typically takes place between 37 and 40 weeks’ gestation. Methods of induction vary according to local protocols and typically depend on cervical status. The process generally involves cervical ripening with misoprostol or prostaglandin E2 (PGE2) followed by amniotomy and oxytocin infusion if labour has not started (Boulvain 2016). An alternative is the expectant approach to the management of birth, which refers to waiting for the spontaneous onset of labour in the absence of any maternal or fetal issues that may necessitate birth (Bond 2017).
How the intervention might work
In pregnant women with pre‐existing diabetes, the rationale for performing an elective birth includes a possible reduction in perinatal morbidity and mortality, particularly in relation to complications associated with macrosomia (Brudenell 1989). Macrosomia is typically defined as a birthweight of more than 4000 g (Feig 2015). It is associated with an increased chance of prolonged labour, maternal trauma, emergency caesarean birth, and birth injuries for the infant, including clavicle fracture and brachial plexus injury (Perlow 1996; Ju 2009). This has resulted in certain clinical practice guidelines recommending that pregnant women with diabetes be offered planned birth through induction of labour or by elective caesarean section (if indicated) between 37 weeks plus 0 days, and 38 weeks plus 6 days, of pregnancy (NICE 2015).
A recent Cochrane review of induction of labour at or near term for suspected fetal macrosomia (Boulvain 2016) concluded that further trials are necessary to clarify if the benefits — including lower mean birthweight and fewer instances of birth fracture and shoulder dystocia — outweigh the risks, which include increased perineal damage.
Why it is important to do this review
In 1989, the St Vincent declaration called on governments and healthcare services to implement effective measures to achieve pregnancy outcomes in women with diabetes that approximate those of women without diabetes within five years (St Vincent Declaration 1990). While this goal was not achieved, it is important that we strive to identify any measures that may assist in meeting this target in our care for women with diabetes. Planned birth may have potential benefits, possibly reducing the risks of prolonged labour and elevated rates of caesarean section following induction of labour (Macer 1992). Birth by caesarean section, including elective caesarean, may increase the risk of maternal morbidity including postpartum infections, haemorrhage or uterine rupture during subsequent labour (Irion 1998). Induction of labour may lead to increased interventions during labour and birth and an increase in maternal morbidity (Khireddine 2013). Furthermore, early‐term birth is associated with an increased risk of multiple neonatal morbidities including respiratory distress syndrome and the need for mechanical ventilation and admission to a neonatal intensive care unit (ACOG 2013). Women's views on elective birth versus continued antenatal surveillance should also be considered (Dodd 2014). The existing Cochrane review on this topic, 'Elective delivery in diabetic pregnant women'(Boulvain 2001), does not make the distinction between women with established, pre‐existing diabetes and those with gestational diabetes (a condition associated with carbohydrate intolerance resulting in hyperglycaemia of variable severity with onset or first recognition during pregnancy) (WHO 2014). Finally, this review was published in 2001 and it is possible that additional evidence on this subject is now available for analysis.
Based on the above, it is now important to assess the effect of planned birth compared with an expectant approach for pregnant women with pre‐existing diabetes on maternal and perinatal mortality and morbidity. Women and healthcare professionals need unbiased information on this subject and this is best provided by meta‐analysis of high‐quality randomised controlled trials.
Objectives
To assess the effect of planned birth (either by induction of labour or caesarean birth) at or near term gestation (37 to 40 weeks’ gestation) compared with an expectant approach, for improving health outcomes for pregnant women with pre‐existing diabetes and their infants. The primary outcomes relate to maternal and perinatal mortality and morbidity.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all published randomised trials (including those using a cluster‐randomised design) and non‐randomised trials which compared planned birth at or near term gestation, with an expectant approach for pregnant women with pre‐existing diabetes. Non‐randomised trials are trials in which participants are allocated to treatment groups using non‐random methods (e.g. alternate) (EPOC 2016).
Cross‐over studies were not eligible for inclusion as this design is not appropriate for this intervention.
Studies published in abstract form only were eligible for inclusion where information on risk of bias and primary or secondary outcomes could be obtained.
Types of participants
Pregnant women, at or near term gestation (37 to 40 weeks’ gestation), with pre‐existing diabetes (Type 1 or Type 2) as diagnosed according to each included study.
Women with gestational diabetes are included in a different Cochrane review, 'Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants' (Biesty 2018)
We planned to exclude trials that included women both with gestational diabetes and pre‐existing diabetes, where data could not be separated.
Types of interventions
Planned birth (induction of labour or caesarean section) at or near term gestation (37 to 40 weeks’ gestation).
Induction of labour was defined by trial authors and may include the use of prostaglandins, misoprostol, oxytocin, amniotomy or a combination of these.
Comparisons
-
Planned birth (induction of labour/caesarean section), at or near term gestation versus an expectant approach
An expectant approach to the management of birth refers to waiting for the spontaneous onset of labour in the absence of any maternal of fetal issues that may necessitate birth (Bond 2017) (or until 41 weeks' gestation or more, when induction of labour may be offered).
Types of outcome measures
For this review, we adapted the core outcome set agreed by consensus between review authors of the Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes mellitus and pre‐existing diabetes. The core outcome set was adapted to ensure that the outcome measures included were appropriate for this research question.
Primary outcomes
Maternal
-
Maternal mortality or serious maternal morbidity (e.g. cardiac arrest, respiratory arrest, admission to intensive care unit (ICU))
-
Caesarean section
-
Instrumental vaginal birth (forceps or vacuum)
Neonatal
-
Perinatal mortality rate (corrected, i.e. stillbirth and early neonatal deaths excluding lethal congenital anomalies)
-
Shoulder dystocia
-
Large‐for‐gestational age (birthweight greater than the 90th centile or as defined by the trial authors)
-
Acidaemia (as evident by a pH of less than 7.0 or a base deficit greater than 12 mmol/L in umbilical arterial cord blood or neonatal blood sample within the first hour of life, or both)
We planned to include all primary outcomes in a 'Summary of findings' table.
Secondary outcomes
Maternal
-
Maternal death
-
Cardiac arrest
-
Respiratory arrest
-
Admission to ICU
-
Intact perineum
-
Uterine rupture
-
Postpartum haemorrhage (defined as 1000 mL or more)
-
Postnatal depression (as measured by either the Edinburgh Postnatal Depression Scale, the Postpartum Depression Screening Scale, the Beck Depression Inventory or other validated scales)
-
Maternal satisfaction (as measured by trial authors)
-
Intact perineum
Neonatal
-
Brachial plexus injury
-
Bone fracture at birth
-
Intracranial haemorrhage (all grades)
-
Hypoxic ischaemic encephalopathy
-
Respiratory distress syndrome
-
Neonatal hypoglycaemia (blood glucose concentrations below the normal range, investigator defined)
-
Neonatal hyperbilirubinaemia (blood bilirubin concentrations above the normal range, investigator defined)
-
Small‐for‐gestational age (birthweight below the third centile or as defined by the trial authors)
-
Admission to neonatal ICU
-
Neurosensory disability (defined by a standardised assessment tool at approximately two years of age)
Health service outcomes
-
Length of postnatal stay (mother)
-
Length of postnatal stay (baby)
-
Cost
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (15 August 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the 'Specialized Register' section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Excluded studies; Studies awaiting classification).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (LB, DD) independently assessed for inclusion all potential eligible studies identified by our search strategy. We planned to resolve any disagreement through discussion or, if required, we planned to consult a third person.
We created a study flow diagram to map out the number of records identified, included and excluded.
There were no studies identified as eligible for inclusion in this review. In future updates, if there are any eligible studies identified for inclusion, we will use the following methods.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will extract the data independently using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third person. We will pilot test the data extraction tool on two papers prior to the conduct of the full review and amend as necessary.
One review author will enter all data into Review Manager 5 (RevMan 2014) software which will be checked for accuracy against the data extraction sheets by a second review author. Where additional information is needed, we will try to contact authors of the original reports to provide further details. When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details and will note this contact in the 'Characteristics of included studies' tables.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor. The following sections refer to individually randomised trials. If cluster‐randomised trials are included we will use appropriate methods for assessing bias in these designs, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where information on risk of bias relates to unpublished data or correspondence with trialists, this will be noted in the 'Risk of bias' table.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as being at:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. alternate, odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias (insufficient information to allow a judgement).
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as being at:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess risk of detection bias for self‐reported and objective outcome measurement. We will assess the methods as being at:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will consider blinding separately for different outcomes where appropriate (for example, blinding may have the potential to differently affect subjective versus objective outcome measures).
We will assess methods used to blind outcome assessment as being at low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised women), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake. We will assess risk of attrition bias for self‐reported and objective outcome measurement.
We will assess methods as being at:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups, the proportion of missing data were less than the effect size, i.e. unlikely to overturn the results);
-
high risk of bias (For self‐reporting outcomes of maternal depression and satisfaction we will judge attrition of > 20% as high risk of bias. For other outcomes, we will explore if numbers or reasons for missing data imbalance across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation and judge based on these findings);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
· We will assess the methods as being at:
-
low risk of bias (where all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We will assess the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the comparisons of planned birth (induction of labour or caesarean section), at or near term gestation versus an expectation approach.
-
Maternal mortality or serious maternal morbidity (e.g. cardiac arrest, respiratory arrest, admission to ICU)
-
Caesarean section
-
Instrumental vaginal birth (forceps or vacuum)
-
Perinatal mortality rate (corrected, i.e. stillbirth and early neonatal deaths excluding lethal congenital anomalies)
-
Shoulder dystocia
-
Large‐for‐gestational age (birthweight greater than the 90th centile or as defined by the trial authors)
-
Acidaemia (as evident by a pH of less than 7.0 or a base deficit greater than 12 mmol/L in umbilical arterial cord blood or neonatal blood sample within the first hour of life, or both)
We will use the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) to create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the above outcomes will be produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious limitations (or by two levels for very serious limitations), depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods. We will report mean and standardised mean differences with 95% confidence intervals.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (section 16.3.4 or 16.3.6) (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Studies with multiple arms
For studies with multiple treatment arms, we will combine all relevant experimental intervention groups in the study (e.g. groups with different methods for induction of labour) into a single group and all comparable relevant control intervention groups into a single control group and perform a single pair‐wise comparison, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (section 16.5.4) (Higgins 2011).
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data (more than 20%) in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We will regard inconsistency as important if I2 is greater than 30% and either Tau2 is greater than zero, or there is a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using Review Manager 5 (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged to be sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
-
Women with Type I diabetes versus women with Type 2 diabetes
-
Parity: primiparous women versus multiparous women
-
Birth by planned caesarean section versus planned elective induction of labour
The following outcomes will be used in subgroup analysis.
Maternal
-
Maternal mortality or serious maternal morbidity (e.g. cardiac arrest, respiratory arrest, admission to ICU)
-
Caesarean section
-
Instrumental vaginal birth (forceps or vacuum)
Neonatal
-
Corrected perinatal mortality rate (stillbirths and early neonatal deaths, excluding lethal congenital anomalies)
-
Shoulder dystocia
-
Large‐for‐gestational age (birthweight greater than the 90th centile or as defined by the trial authors)
-
Acidaemia (as evident by a pH of less than 7.0 or a base deficit greater than 12 mmol/L in umbilical arterial cord blood or neonatal blood sample within the first hour of life, or both
We will assess subgroup differences using interaction tests available within Review Manager 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value.
Sensitivity analysis
We will conduct a sensitivity analysis based on risk of bias in trials. We will exclude all studies at high or unclear risk of bias for either sequence generation or allocation concealment to see if this makes any difference to the overall results. This is based on growing empirical evidence that these factors are a particularly important potential source of bias (Higgins 2011). We will limit sensitivity analyses to primary outcomes.
Results
Description of studies
No studies were eligible for inclusion in this review (see Characteristics of excluded studies and Characteristics of studies awaiting classification).
Results of the search
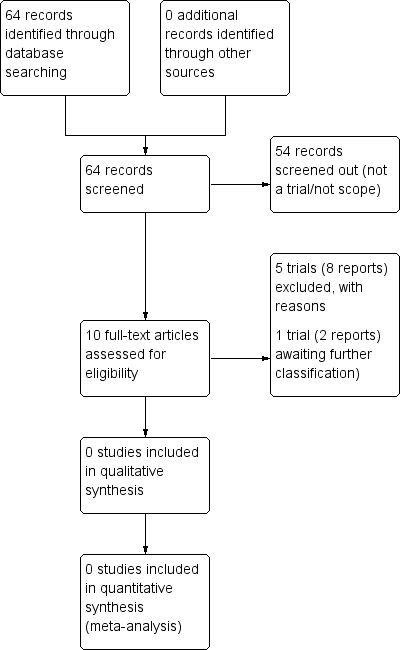
See: Figure 1.

Study flow diagram.
We identified 64 citations through our searches. After screening, we retrieved 10 reports of six trials for potential inclusion in the review.
Included studies
No studies were eligible for inclusion.
Excluded studies
We excluded five studies (Alberico 2017; Dhaneshwor 2011; Ghosh 1979; Khojandi 1974; Worda 2017) (see Characteristics of excluded studies). We excluded four studies ( Alberico 2017; Dhaneshwor 2011; Khojandi 1974; Worda 2017) because the participants were women with gestational diabetes. We excluded the remaining study (Ghosh 1979) as no further details or publications have been made available in relation to this study since the publication of the conference abstract 38 years ago.
We sent communications to the named contact author of one trial (Henry 1992) to obtain data that related specifically to the women with Type 1 or Type 2 diabetes in their trial. We are awaiting a reply (See Characteristics of studies awaiting classification).
Risk of bias in included studies
As no studies were included in this review, we could not assess risk of bias.
Allocation
No studies were included in this review, therefore we could not assess the risk of selection bias.
Blinding
No studies were included in this review, therefore we could not assess the risk of performance bias and detection bias.
Incomplete outcome data
No studies were included in this review, therefore we could not assess the risk of attrition bias.
Selective reporting
No studies were included in this review, therefore we could not assess the risk of reporting bias.
Other potential sources of bias
No studies were included in this review, therefore we could not assess the risk of other potential sources of bias.
Effects of interventions
As there were no studies included in this review, we could not assess the effects of interventions.
Discussion
We identified one completed trial (Henry 1992) which is awaiting assessment. This trial was designed to compare elective induction of labour with expectant management for reducing the incidence of caesarean birth in pregnant women with insulin‐requiring gestational diabetes or pre‐existing diabetes. However, to date, published results do not separate the data of pregnant women with pre‐existing diabetes and women with gestational diabetes.
The risks for women with pre‐existing diabetes and their neonates during pregnancy have been explored in the earlier sections of this review. The risks of having a baby diagnosed with macrosomia or large‐for‐gestational age (or both) remains high for these women, even for those considered to have "well controlled diabetes" (Evers 2002). The association between macrosomia and late fetal death, and the potential for birth trauma, remain as concerns (Dunne 2009). These concerns are reflected in clinical guidelines (e.g. NICE 2015) which propose planned birth before term for pregnant women with pre‐existing diabetes. Yet, to date, no results from randomised trials have been published relating to the effect of planned birth at or near term gestation, compared with an expectant approach, for improving health outcomes for pregnant women with pre‐existing diabetes and their babies. This review demonstrates the urgent need for such trials.
Study flow diagram.

