Pruebas sin contacto para identificar a las personas con riesgo de glaucoma de cierre de ángulo primario
Resumen
Antecedentes
El glaucoma de cierre de ángulo primario (GCAP) representa el 50% de la ceguera por glaucoma en todo el mundo. Más de tres cuartos de los individuos con GCAP residen en Asia. En estas poblaciones, el GCAP a menudo se desarrolla de forma insidiosa, lo que da lugar a una presión intraocular elevada crónica y a daños en el nervio óptico, que a menudo son asintomáticos. Las pruebas sin contacto para identificar a las personas en riesgo de cierre de ángulo son relativamente rápidas y pueden ser llevadas a cabo por profesionales sanitarios o técnicos debidamente capacitados como prueba de triaje. Si la prueba es positiva, el paciente será derivado para una evaluación más especializada.
Objetivos
Determinar la precisión diagnóstica de las pruebas sin contacto (profundidad de la cámara anterior del limbo [PCAL] [prueba de van Herick]; prueba con linterna oblicua; analizador mediante escáner de la profundidad de la cámara anterior periférica [ECAP], fotografía Scheimpflug; tomografía de coherencia óptica del segmento anterior [TCO‐SA]), para identificar a las personas con un ángulo ocluible.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes tres bases de datos bibliográficas en octubre 2019: CENTRAL; MEDLINE; Embase; BIOSIS; OpenGrey; ARIF y registros de ensayos clínicos. Las búsquedas fueron limitadas para eliminar los informes de casos. No hubo restricciones de fecha ni de idioma en las búsquedas.
Criterios de selección
Se incluyeron estudios transversales prospectivos y retrospectivos, de cohortes y de casos y controles realizados en cualquier contexto que evaluaban la precisión de una o más pruebas índice para identificar a las personas con un ángulo ocluible en comparación con un estándar de referencia de gonioscopia.
Obtención y análisis de los datos
Dos autores de la revisión realizaron la extracción de datos y la evaluación de la calidad de cada estudio mediante la herramienta QUADAS‐2 de forma independiente. Para cada prueba se construyeron tablas de 2 x 2 y se calculó la sensibilidad y la especificidad. Cuando cuatro estudios o más proporcionaron datos a umbrales fijos para cada prueba, se adaptó un modelo de dos variables utilizando la macro METADAS en SAS para calcular estimaciones puntuales agrupadas para la sensibilidad y la especificidad. Para las comparaciones entre las pruebas índice y los subgrupos, se realizó una prueba de cociente de verosimilitud comparando el modelo con y sin la covariable.
Resultados principales
Se incluyeron 47 estudios con 26 151 participantes y se analizaron los datos de 23 440. La mayoría de los estudios se realizaron en Asia (36; 76,6%). Veintisiete estudios evaluaron la TCO‐SA (mediante el análisis de 15 580 participantes), 17 estudios la PCAL (7385 participantes), nueve estudios la fotografía Scheimpflug (1616 participantes), seis estudios el ECAP (5239 participantes) y cinco estudios evaluaron la prueba con linterna oblicua (998 participantes). En cuanto a la calidad de los estudios, se consideró que 36 de los estudios incluidos (76,6%) estaban en riesgo alto de sesgo en al menos un dominio. El uso de un diseño de casos y controles (13 estudios) o las exclusiones inapropiadas (6 estudios) suscitó inquietudes en cuanto a la selección de los pacientes en el 40,4% de los estudios, y las inquietudes en el dominio de la prueba índice en el 59,6% de los estudios se debieron a la falta de enmascaramiento o a la determinación post hoc de los umbrales óptimos. Entre los estudios que no utilizaron un diseño de casos y controles, se realizaron 16 estudios (20 599 participantes) en un ámbito de atención primaria/comunitario y 18 estudios (2590 participantes) en ámbitos de atención secundaria, de los cuales 15 investigaron la PCAL.
Se calcularon estimaciones resumidas de los parámetros y los umbrales comunicados comúnmente para cada prueba; PCAL ≤ 25% (16 estudios, 7540 ojos): sensibilidad 0,83 (intervalo de confianza [IC] del 95%: 0,74; 0,90), especificidad 0,88 (IC del 95%: 0,84; 0,92) (certeza moderada); linterna (grado 1) (5 estudios, 1188 ojos): sensibilidad 0,51 (IC del 95%: 0,25; 0,76), especificidad 0,92 (IC del 95%: 0,70; 0,98) (certeza baja); ECAP (≤ 5 y/o S o P) (4 estudios, 4677 ojos): sensibilidad 0,83 (IC del 95%: 0,70; 0,91), especificidad 0,78 (IC del 95%: 0,70; 0,83) (certeza moderada); fotografía Scheimpflug (PCA central) (9 estudios, 1698 ojos): sensibilidad 0,92 (IC del 95%: 0,84; 0,96), especificidad 0,86 (IC del 95%: 0,76; 0,93) (certeza moderada); TCO‐SA (opinión subjetiva de la oclusión) (13 estudios, 9242 ojos): sensibilidad 0,85 (IC del 95%: 0,76; 0,91); especificidad 0,71 (IC del 95%: 0,62; 0,78) (certeza moderada).
Para las comparaciones de la sensibilidad y la especificidad entre las pruebas índice se utilizó la PCAL (≤ 25%) como categoría de referencia. La prueba con linterna (umbral de grado 1) mostró una sensibilidad menor estadísticamente significativa que la de la PCAL (≤ 25%), mientras que la TCO‐SA (opinión subjetiva) tuvo una especificidad menor estadísticamente significativa. No hubo diferencias estadísticamente significativas para las otras comparaciones de las pruebas índice. Se realizó un análisis de subgrupos para la PCAL (≤ 25%), en el que se comparó el ámbito comunitario (7 estudios, 14,4% de prevalencia) con el de atención secundaria (7 estudios, 42% de prevalencia). No se encontró evidencia de una diferencia estadísticamente significativa en el rendimiento de la prueba según el ámbito.
Si se realiza la PCAL en 1000 personas en riesgo de cierre de ángulo con una prevalencia de ángulos ocluibles del 10%, la PCAL pasaría por alto alrededor de 17 casos de los 100 con ángulos ocluibles y clasificaría de forma incorrecta a 108 de 900 sin cierre de ángulo.
Conclusiones de los autores
El hallazgo de que la PCAL tuvo el mismo rendimiento que las pruebas índice que utilizan tecnologías de imagen sofisticadas, confirma el potencial de esta prueba para la detección de casos de ángulos ocluibles en poblaciones de alto riesgo. Sin embargo, las cuestiones metodológicas de los estudios pueden haber dado lugar a que las estimaciones de la precisión de las pruebas sean más altas de lo que se esperaría en la práctica clínica estándar. Sigue siendo necesario realizar estudios de alta calidad para evaluar el rendimiento de las pruebas no invasivas para la evaluación del ángulo, tanto en el ámbito comunitario como en el de la atención secundaria.
Resumen en términos sencillos
¿Qué tan precisas son las pruebas de detección para identificar a las personas que están en riesgo de desarrollar glaucoma de cierre de ángulo primario?
¿Por qué es importante mejorar el diagnóstico del glaucoma de cierre de ángulo primario?
El glaucoma es un grupo de enfermedades oculares que causan daño al nervio óptico en la parte posterior del ojo. Si no se trata, el glaucoma puede provocar ceguera. El glaucoma de cierre de ángulo primario es un tipo de glaucoma en el que la vía de drenaje del líquido dentro del ojo (conocido como el ángulo) presenta un estrechamiento o un bloqueo, lo que provoca un aumento de la presión ocular y la pérdida del campo de visión. El glaucoma de ángulo cerrado primario representa una cuarta parte de todos los casos de glaucoma a nivel mundial y es más probable que provoque la pérdida de la visión que la forma más común, el glaucoma de ángulo abierto primario.
Se dispone de una variedad de pruebas no invasivas para identificar a las personas en riesgo de padecer glaucoma de cierre de ángulo primario en la comunidad o en un ámbito clínico no especializado. Los pacientes con pruebas positivas son derivados para una investigación más especializada y para un posible tratamiento. El fracaso en la detección de este trastorno (un resultado negativo falso) puede dar lugar a un mayor riesgo de daño progresivo del nervio óptico y ceguera. Un diagnóstico incorrecto (un resultado positivo falso) podría dar lugar a una investigación innecesaria y costosa.
¿Cuál es el objetivo de esta revisión?
El objetivo de esta revisión fue averiguar cuán precisas son las pruebas de detección no invasivas para identificar a quienes están en riesgo de desarrollar glaucoma de cierre de ángulo primario.
¿Qué se estudió en esta revisión?
Se estudiaron cinco pruebas no invasivas. Las mismas varían desde pruebas sencillas que requieren una linterna tipo lapicera o un equipo clínico ampliamente disponible conocido como microscopio de lámpara de hendidura (prueba con linterna oblicua; profundidad de la cámara anterior del limbo [PCAL]) hasta un equipo de imágenes más sofisticado (tomografía de coherencia óptica del segmento anterior (TCO‐SA), fotografía Scheimpflug y analizador mediante escáner de la profundidad de la cámara anterior periférica [ECAP]) que pueden explorar y medir las dimensiones del ángulo de drenaje.
¿Cuáles son los principales resultados de esta revisión?
La revisión incluyó 47 estudios pertinentes, con un total de 26 151 participantes. Veintisiete estudios evaluaron la TCO‐SA, 17 estudios evaluaron la PCAL, nueve estudios la fotografía Scheimpflug, seis estudios el ECAP y cinco estudios evaluaron la prueba con linterna.
El rendimiento diagnóstico general de la PCAL fue similar a las tecnologías de imágenes más avanzadas, TCO‐SA, fotografía Scheimpflug y ECAP, sin embargo, la prueba con linterna mostró un rendimiento inferior. Utilizando la PCAL como ejemplo, si esta prueba se realizara en 1000 personas, de las cuales 100 estuvieran en riesgo de cierre del ángulo primario, se estima que 83 serían identificadas de forma correcta y 17 casos se pasarían por alto (negativos falsos). La prueba identificaría de forma correcta a 792 de los 900 que no se encuentran en riesgo de glaucoma de cierre de ángulo y clasificaría de forma incorrecta a 108 (12%), que serían derivados innecesariamente (positivos falsos).
¿Qué tan confiables son los resultados de los estudios de esta revisión?
La mayoría de los estudios fueron de calidad baja debido a la forma en que se reclutó a los participantes o a la forma en que se realizaron las pruebas. Este hecho podría haber dado lugar a que las pruebas parecieran más precisas de lo que realmente son. Por lo tanto, no es posible tener seguridad en cuanto a que las pruebas producen siempre los resultados comunicados.
¿Cuáles son las implicaciones de esta revisión?
Los estudios incluidos en esta revisión se realizaron en su mayoría en Asia, que soporta la mayor carga de glaucoma de cierre de ángulo primario. Los resultados de esta revisión han demostrado que la PCAL, que es una prueba rápida y sencilla que puede realizarse con un mínimo de capacitación, puede identificar a los pacientes en riesgo de padecer glaucoma de cierre de ángulo primario, lo que permite un tratamiento temprano y adecuado. Aunque esta prueba podría pasar por alto potencialmente a alrededor de una de cada seis personas en riesgo de padecer la afección y dar lugar a una derivación excesiva del 12%, la prueba podría ser útil para la detección selectiva en zonas con una prevalencia alta de la afección.
¿Qué grado de actualización tiene esta revisión?
La evidencia de esta revisión está actualizada hasta el 3 de octubre 2019.
Authors' conclusions
Summary of findings
| Test (measure and/or threshold) | N. studies (N. with occludable angles/total analysed) | Accuracy estimates | False positives and false negatives at prevalence 10%: 100 participants with and 900 without occludable angles | False positives and false negatives at prevalence 30%: 300 participants with and 700 without occludable angles | Certainty of evidence for sensitivity and specificity | |||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (95%CI) | Specificity (95%CI) | False positives (95% CI) | False negatives (95% CI) | False positives (95% CI) | False negatives (95% CI) | |||
| LACD (cut‐off: ≤25%) | 16 studies (1490/7540) | 0.83 (0.74 to 0.90) | 0.88 (0.84 to 0.92) | 108 (72 to 144) | 17 (10 to 26) | 51 (30 to 78) | 84 (56 to 112) | Moderatea |
| Oblique flashlight test (grade 1) | 5 studies (298/1188) | 0.51 (0.25 to 0.76) | 0.92 (0.70 to 0.98) | 72 (18 to 270) | 49 (24 to 46) | 56 (14 to 210) | 147 (75 to 228) | Lowb |
| SPAC (≤5 and/or S or P) | 4 studies (994/4677) | 0.83 (0.70 to 0.91) | 0.78 (0.70 to 0.83) | 207 (162 to 261) | 17 (10 to 26) | 161 (126 to 203) | 51 (30 to 78) | Moderatec |
| Scheimpflug photography (ACD central) | 9 studies (461/1698) | 0.92 (0.84 to 0.96) | 0.86 (0.76 to 0.93) | 126 (63 to 216) | 8 (4 to 16) | 98 (49 to 168) | 24 (12 to 48) | Moderated |
| AS‐OCT (subjective assessment) | 13 studies (1850/9239) | 0.85 (0.76 to 0.91) | 0.71 (0.62 to 0.78) | 270 (198 to 351) | 14 (8 to 24) | 210 (154 to 273) | 42 (24 to 72) | Moderatee |
| Certainty of evidence applies to both sensitivity and specificity. Explanations a Downgraded one level due to risk of bias: 75% of studies had a high risk of bias in one or more domains. Two studies (13%) used a case‐control design. Legend ACD: anterior chamber depth | ||||||||
Background
Clinical problem
Primary angle closure (PAC) is characterised by appositional or adhesional (synechial) narrowing (and eventually occlusion) of the drainage angle in the anterior chamber of the eye, resulting in elevated intraocular pressure (IOP) and subsequent glaucomatous optic neuropathy, a condition known as primary angle closure glaucoma (PACG). The occlusion of the drainage angle may occur rapidly or slowly. Rapid occlusion results in symptomatic IOP elevation that requires emergency medical treatment (known as acute angle closure). Individuals presenting with acute angle closure, characterised by eye pain, headache, corneal oedema and vascular congestion, are treated initially with topical and oral medications to lower the IOP. This is followed by laser peripheral iridotomy (LPI) as soon as possible after angle closure, usually with prophylactic treatment of the fellow eye (Emanuel 2014). An occlusion that develops insidiously results in chronically raised IOP, which is often asymptomatic. Management for chronic angle closure involves: medical therapy (topical hypotensives); LPI; filtration surgery or a combination of these to lower the IOP and open up the drainage angle. Although LPI remains the first‐line intervention for acute and chronic PAC, there is a growing evidence that clear lens extraction is associated with better clinical and patient‐reported outcomes than LPI and may therefore be a better first‐line treatment option (Azuara‐Blanco 2016; Tanner 2020).
The prevalence of PACG varies across ethnic groups; in European‐derived populations PACG has been estimated to be 0.40% of those aged 40 years and older, compared to 1.09% in Asian populations (Tham 2014). For those aged 70 years and older, the prevalence increases to 0.94% (Day 2012) and 2.32% (Cheng 2014), respectively. Although, globally, open‐angle glaucoma is more common (3%) (Tham 2014), PACG is more likely to result in bilateral blindness (Foster 2001; Quigley 1996; Quigley 2006; Resnikoff 2004).
A classification scheme for PAC designed for use in prevalence surveys and epidemiological research was published by Foster and colleagues (Foster 2002). This identifies three stages in the natural history of angle closure from initial irido‐trabecular contact (ITC) to anterior segment signs of disease (raised IOP, peripheral anterior synechiae (PAS), or both), culminating in glaucomatous optic neuropathy.
-
Primary angle closure suspect (PACS): an eye in which appositional contact between the peripheral iris and posterior trabecular meshwork is considered in two or more quadrants, in dark room conditions using static gonioscopy.
-
PAC: an eye with an occludable drainage angle and features indicating that trabecular obstruction by the peripheral iris has occurred, such as PAS, elevated IOP (> 21 mmHg), iris whorling (distortion of the radially orientated iris fibres), “glaucomfleken” lens opacities, or excessive pigment deposition on the trabecular surface. There is no evidence of glaucomatous optic neuropathy or associated glaucomatous field loss.
-
PACG: signs of PAC, as described above, and evidence of glaucomatous optic neuropathy.
There are various anatomical and demographic risk factors for PAC (Amerasinghe 2008; Lowe 1970). Anatomical risk factors include: a shallow anterior chamber depth (ACD), thickening of the crystalline lens, small corneal diameter and a short axial length (Nolan 2006; Wang 2019). The risk of PACG increases with age (Day 2012; Wang 2019), and the prevalence also varies with ethnicity, with higher rates occurring in Inuit and Asian populations (Clemmesen 1971; Drance 1973; Tham 2014).
The natural history of angle closure disease is not well documented due to the sparsity of long‐term observational data (Alsbirk 1992; Thomas 2003; Wilensky 1993; Yip 2008). A recent large randomised controlled trial, conduced in China (Zhongshan Angle‐closure Prophylaxis Study) (He 2019), carried out LPI in one randomly selected eye of participants with bilateral PACS, with the other eye acting as an untreated control. The primary outcome was incident primary angle closure disease as a composite endpoint of elevated IOP, PAS, or an acute angle‐closure episode during the 72‐month follow‐up period. The rate of developing any angle closure endpoint in this population was very low (less than 1% per year). Although eyes that underwent LPI showed a significant reduction in the risk of developing PAC or an acute attack, the authors concluded that prophylactic treatment is of limited benefit and was unlikely to be cost‐effective. However, in view of differences in the causative mechanism of angle closure between Europeans and East Asians (He 2006), the generalisability of these findings is unclear.
Target condition being diagnosed
For this review we used an occludable angle as the target condition indicative of an anatomical predisposition to angle closure as identified by gonioscopy (Weinreb 2006). In this review we defined an occludable angle as either:
-
an eye which has appositional contact between the peripheral iris and posterior trabecular meshwork in two or more quadrants (≥180°); or
-
an eye with, or at risk of, angle closure as judged by an experienced eye care professional using gonioscopy with or without indentation.
Conditions that are similar to the target condition include secondary angle closure glaucoma, such as aqueous misdirection, neovascular glaucoma and ciliary body swelling. The clinical features and management of conditions that cause secondary angle closure glaucoma have been reviewed by Parivadhini 2014 and were not investigated in this review.
Index test(s)
Targeted screening for PAC/PACG has established the effectiveness of measuring anterior chamber dimensions to identify occludable angles (Congdon 1996; Devereux 2000; Kurita 2009). A variety of non‐contact tests are available for the assessment of the ACD, anterior chamber angle (ACA), or both.
Oblique flashlight test
The flashlight test is an accessible method to detect a potentially occludable angle if no other equipment is available. The test can be carried out in a primary‐ or secondary‐care setting and involves shining a pen torch into the eye from the temporal limbus parallel to the iris to assess the ACD. Quantitative grading uses a four‐point scale, based on the proportion of the nasal iris that is in shadow (grade 4 = minimal or no shadow; grade 1 = nasal iris in complete shadow (Van Herick 1969; Vargas 1973;) grade 1 is associated with a high risk of angle closure. Alternatively, qualitative grading can be used to describe the amount of shadow falling on the iris as shallow, medium or deep, and is further described by He 2007.
Limbal anterior chamber depth assessment (van Herick technique)
The van Herick technique is used to assess the ACD at the limbus using a slit lamp biomicroscope (Van Herick 1969). The illumination system is set at 60° from the observation system. A focused vertical slit‐beam is positioned at the limbus and moved just onto the cornea until the beam separates into a corneal section and reflection of the beam onto the iris. An estimate of the thickness of the dark space between the beams (which corresponds to the limbal anterior chamber depth (LACD)) is recorded as a fraction (or percentage) of the corneal section thickness over the central portion of the beam. Van Herick 1969 originally described a four‐point grading scheme, which was extended to a seven‐point scale by Foster 2000, in an effort to improve the precision of the measurement. Van Herick 1969 considered that an eye with a LACD of grade 2 or less (≤ 25%) required gonioscopy and that a grade 1 angle was at a high risk of angle closure. Foster 2000 further subdivided grade 1 into 5% and 15% cut‐off values and also included a 0% grade, which was defined as iridocorneal contact for at least one clock hour within the observed quadrant. The augmented scale was associated with improved test accuracy.
Scanning peripheral anterior chamber depth analyser
Scanning peripheral anterior chamber depth analysis (SPAC) is an objective method for measuring the peripheral and central ACD by automatically taking 21 slit lamp images of the anterior chamber using a 1 mm‐wide slit at 0.4‐mm intervals from the optical axis towards the limbus (Kashiwagi 2006). These measurements are compared to a normative database and converted into a numerical scale ranging from 1 to 12, with 12 representing the deepest ACD. In addition, the instrument provides a categorical grading of the risk of angle closure, S (suspect angle closure), P (potential angle closure), or N (normal). The device has been shown to be reproducible and easy to operate (Kashiwagi 2004).
Scheimpflug photography
The Scheimpflug principle is used to correct perspective distortion in aerial photographs and has been adapted for ocular imaging. The Oculus Pentacam (Oculus, Wetzlar, Germany) device employs this principle using monochromatic blue light at a wavelength of 475 nm. By rotating the apparatus around the optical axis of the eye, a series of radially oriented images is generated in three dimensions around the 360° extent of the anterior segment. Between 12 and 50 real‐time sections from the anterior surface of the cornea to the posterior vertex of the lens are acquired within a two‐second acquisition frame. This generates a set of measurements that provide a detailed description of the biometric configuration of the anterior segment, which includes the ACA, ACD and the anterior chamber volume (ACV). When calculating the ACA, it should be noted that this is not a direct measurement of the ACA, but is extrapolated from the measurements taken by the Pentacam. . Currently there is no consensus on which parameter or cut‐off value to use in the determination of an occludable angle.
Anterior segment‐optical coherence tomography
Anterior segment‐optical coherence tomography (AS‐OCT) allows both qualitative and quantitative analysis of the angle. The technique is based on low‐coherence interferometry whereby the delay and intensity of light reflected from the ocular tissue structures is measured. There are currently several AS‐OCT devices available on the market; depending on the device, they use one of the following methods to obtain clinical data: time domain, spectral domain or the more recent swept source domain method. Spectral and swept source domain methods have a higher scan speed and resolution than time domain methods. A wavelength of 1310 nm is used to image the anterior segment and inbuilt software is used to quantitatively assess in detail angle parameters, which include: the trabeculo‐iris space area (TISA, measured at 500 microns and 750 microns), angle recess area (ARA) and angle opening distance (AOD) at 500 microns and 750 microns (Quek 2011). Qualitative interpretation has been typically defined by contact between the peripheral iris and any part of the angle wall anterior to the scleral spur. There is currently no consensus on which threshold values to use for any of the quantitative parameters mentioned to identify an occludable angle (Smith 2013).
Clinical pathway
A variety of non‐contact devices with varying degrees of sophistication have been developed to evaluate the risk of angle closure. The high prevalence of PAC and the burden of blindness attributable to PACG raises the possibility of using such techniques as triage tests in high‐risk populations who may not have access to eye care services (see Figure 1) (Nolan 2003; Nolan 2006). More commonly, non‐invasive assessment of the dimensions of the anterior chamber, including ACD, angle, or both are part of a standard ophthalmic examination in a primary/community or secondary care setting. If the index test is positive, such individuals are identified as being 'at risk' of PACG and are referred for further assessment, usually to a glaucoma sub‐specialist ophthalmologist. The ophthalmologist will carry out gonioscopy (the reference standard for qualitative and quantitative assessment of the ACA). If an occludable angle is diagnosed, additional tests are then performed to further diagnose the condition as PACS/PAC/PACG. Depending on the clinical presentation, the affected individual may be closely monitored or undergo prophylactic treatment with LPI or lens extraction, possibly in conjunction with IOP‐lowering eye drops.

Clinical Pathway
Role of index test(s)
The reference standard test to detect an occludable angle is gonioscopy; however, this is not routinely performed outside the specialist setting since it is invasive and requires a high level of skill, which may lead to missed diagnoses. Non‐contact tests are relatively quick and can be carried out by appropriately trained healthcare professionals or technicians as a triage test to identify people at risk of angle closure. A systematic review published in 2013 concluded that there was insufficient evidence for non‐contact tests to replace gonioscopy, as they do not provide sufficient information on the ACA anatomy (Smith 2013). It should be noted that in some cases, when gonioscopy fails to visualise the anterior chamber configuration and depth, typically in secondary causes of angle closure, AS‐OCT and Scheimpflug photography can be used to provide objective measurements (Kang 2013). In addition, these techniques can be used to supplement existing clinical documentation by providing objective measurements (Smith 2013).
Alternative test(s)
Tests that use contact methods, such as ultrasound biomicroscopy, have been reviewed by Smith 2013, and were not included in the current review.
Rationale
A systematic review published in 2013 evaluated whether anterior segment imaging (using ultrasound biomicroscopy, optical coherence tomography (OCT), Scheimpflug photography or SPAC aided the diagnosis of PAC (Smith 2013). This review included 79 studies and concluded that although anterior segment imaging provided useful information, none of the tests provided sufficient information about the anatomy of ACA to be considered a substitute for gonioscopy. However, no meta‐analysis of accuracy data was conducted. The current review updates and extends this review by considering the following non‐contact tests of anterior chamber assessment (flashlight test, slit‐lamp techniques for LACD assessment, AS‐OCT, Scheimpflug photography and SPAC).
Objectives
To determine the diagnostic accuracy of non‐contact tests for identifying people with an occludable anterior chamber angle of the eye.
Secondary objectives
-
To investigate the accuracy of each non‐contact test for detecting the most severe referable condition or PACG (versus PACS or PAC)
-
To explore potential causes of heterogeneity in diagnostic performance
Methods
Criteria for considering studies for this review
Types of studies
We included all prospective and retrospective cohort studies ('single‐gate' design) and case‐control studies ('two‐gate' design) that evaluated the accuracy of non‐contact tests for diagnosing occludable angles compared to a gonioscopic reference standard. We included studies comparing each method separately, and studies comparing more than one method, to the reference standard in the same population. This included studies in which participants received all the tests or were randomised to receive different tests. We included only studies that provided sufficient data to allow the calculation of sensitivity and specificity.
Non‐contact tests for the detection of occludable angles are mainly of interest in primary‐care settings as a triage test aiming to guide referrals to glaucoma specialists. The tests are also used in non‐specialist secondary care settings. Since the relative accuracy of these tests in these settings is not well known, we included studies investigating these tests in any setting, and planned to assess the effect of this on accuracy in subgroup analyses.
Participants
We included all participants who met the inclusion criteria for studies conducted in any setting, which evaluated any of the index tests against the reference standard. We considered data from both untested asymptomatic populations and other pre‐tested predominantly asymptomatic populations recruited in secondary care.
Index tests
We assessed non‐contact tests including: the oblique flashlight test, LACD using the van Herick technique, SPAC, Scheimpflug photography and AS‐OCT.
Target conditions
An occludable angle, as a referable condition that can include PACS, PAC or PACG, as described above, was the target condition of interest.
As a secondary objective, we also planned to extract data to investigate the accuracy of the test for detecting the most severe referable condition or PACG (versus PAC or PACS).
Reference standards
Gonioscopy was the reference standard for the diagnosis of an occludable angle. We included studies using any of the standard gonioscopic classification schemes and used the authors' definition of an occludable angle, based on the number of quadrants of ITC. When the information was available, we further classified an occludable angle into one of three subgroups PACS, PAC, PACG.
Gonioscopy
Gonioscopy is the acknowledged reference standard for the evaluation of eyes with or at risk of angle closure, and should be performed on both eyes in any individual with suspected angle closure. The technique should be performed under dark‐room conditions and used in the primary position to visualise angle structures, the presence of ITC, PAS, or both (Bhargava 1973). Dynamic assessment is helpful in distinguishing ITC from PAS using a four‐mirror lens, which is applied to the cornea creating pressure with the goniolens. The Shaffer grading system, which records the ACA width in four quadrants, from grade 0 (closed) to grade 4 (wide open), is the most widely adopted ACA classification scheme (Shaffer 1960). Angle morphology can be further described using the Scheie grading system (Scheie 1957). This scheme describes the angle according to the anatomical structures observed (grade IV: Schwalbe’s line not visible; grade III: Schwalbe’s line visible; grade II: anterior trabecular meshwork visible; grade I: visible scleral spur; and grade 0: ciliary body band visible). The Spaeth classification is the most detailed of the three grading systems that allows grading of the geometric angle, iris profile and level of iris insertion (Spaeth 1971).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases. We imposed no restrictions on language or year of publication.The date of the search was 3 October 2019.
-
Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 3 October 2019) (Appendix 1).
-
Health Technology Assessment Database (HTAD) in the Cochrane Library (searched 3 October 2019) (Appendix 1).
-
MEDLINE Ovid (January 1946 to 3 October 2019) (Appendix 2).
-
Embase Ovid (January 1980 to 3 October 2019) (Appendix 3).
-
BIOSIS (January 1969 to 3 October 2019) (Appendix 4).
-
System for Information on Grey Literature in Europe (OpenGrey) (1995 to 3 October 2019) (Appendix 5).
-
Aggressive Research Intelligence Facility database (ARIF) (www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx; searched 3 October 2019. ARIF database last updated June 2018) (Appendix 6).
-
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 3 October 2019) (Appendix 7).
-
US National Institutes of Health Ongoing Trials Register ‐ ClinicalTrials.gov (www.clinicaltrials.gov; searched 3 October 2019) (Appendix 8).
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched 3 October 2019) (Appendix 9).
Searching other resources
We searched the references of included studies for information about further studies. We did not handsearch journals and conference proceedings.
Data collection and analysis
Selection of studies
Two review authors (AJ and IC) independently assessed the titles and abstracts of all studies identified by the electronic searches. We labelled each record at this stage as "definitely relevant", "possibly relevant" or "definitely not relevant". We excluded records labelled as "definitely not relevant" by both review authors. We retrieved full‐text reports of records labelled as "definitely relevant" or "possibly relevant" and the two review authors independently assessed whether these met the inclusion criteria. We resolved any disagreement when present at any stage through discussion. When necessary, we consulted a third review author or contacted the study investigators for more information to determine eligibility.
Data extraction and management
Two review authors (AJ and JL) independently extracted the following data, where possible, from the included studies: the number of true positives (TP), false positives (FP), true negatives (TN) and false negatives (FN) using 2 x 2 contingency tables. From the 2 X 2 tables we calculated sensitivity (the proportion of diseased people correctly diagnosed) and specificity (the proportion of non‐diseased people correctly diagnosed) with 95% confidence intervals (CIs).
One review author entered data into Review Manager 5 (RevMan 5) (Review Manager 2014) and a second review author verified the entered data. We resolved any disagreement when present at any stage through discussion. We contacted study investigators to provide missing information or to clarify data, and we allowed two weeks for a response. If we did not receive a response during this time, we proceeded to use the information available in the published reports. We summarised the characteristics of included studies in a 'Characteristics of included studies' table. The characteristics extracted from each study are shown in Appendix 10. See Appendix 11 for abbreviations.
Assessment of methodological quality
Two review authors (AJ and JL) independently assessed each included study for risk of bias using the QUADAS 2 tool to assess the susceptibility to bias of the included studies, based on guidance presented in Appendix 12 (Whiting 2011). We assessed each study and judged each bias criterion to be at 'high', 'low' or 'unclear' risk of bias (lack of information or uncertainty over the potential for bias). Concerns regarding applicability were rated as 'high', 'low' or 'unclear' concerns.
Statistical analysis and data synthesis
We extracted and analysed the data available at fixed thresholds for each index test, in order to ease the interpretability of our summary measures of accuracy. Our preferred thresholds were:
-
oblique flashlight test: grades 1 and 2;
-
LACD using the van Herick technique: grades 1 and 2 (≤ 25%);
-
SPAC: categorical grading of suspect angle closure or potential angle closure, as provided by the device.
As there is no current consensus regarding thresholds for Scheimpflug photography and AS‐OCT, we extracted these data, when available, from the included studies.
We generated estimates of sensitivity and specificity in forest plots for each index test and also plotted them in receiver operating characteristics (ROC) space in RevMan. When four or more studies provided data at fixed thresholds for each test, we fitted a bivariate model using the METANDI function in STATA to calculate pooled point estimates for sensitivity and specificity. For comparisons between index tests and between subgroups, we performed a likelihood ratio test comparing the model with and without the covariate and assumed that the variances for the random effects for the logit sensitivities and logit specificities were similar. For the investigation of heterogeneity we used the melogit command in STATA to fit models that included particular covariates.
Takwoingi 2013 has showed that direct comparisons conducted within each study are more reliable than indirect comparisons. When direct comparisons were available, we plotted data points and joined the two estimates (one for each test) from each study by a line to show the difference in accuracy between tests. If a sufficient number of such paired studies had been available, we planned to pool them in bivariate meta‐analyses and tested their relative accuracy with a covariate coding for each test using the methods described above.
Since occludable angles are often bilateral, this complication may result in unit of analysis issues. We included studies that evaluated only one eye of each participant or, in participants with two affected eyes, studies that randomly selected only one eye. We also included studies that included both eyes in our review, but we acknowledged the unit of analysis issue when formulating our conclusions (i.e. acknowledging the overestimate of the precision in accuracy).
Investigations of heterogeneity
We investigated any heterogeneity in sensitivity and specificity through visual inspection of forest plots and the degree to which individual study results lie close together on the summary ROC curve. For diagnostic tests with a sufficient number of eligible studies, we planned to formally explore heterogeneity using the following study‐level covariates:
-
study design (e.g. single‐gate and two‐gate designs);
-
diagnostic reference thresholds (gonioscopy grading (e.g. number of quadrants occluded));
-
characteristics of the study population (e.g. high versus low prevalence, ethnicity). The comparison of low versus high prevalence level was based on the study setting. Studies undertaken in secondary care included populations with a higher prevalence, whilst studies conduced in a primary care/community setting included participants with a low prevalence of the target condition.
Sensitivity analyses
We planned to perform a sensitivity analysis to assess the impact of risk of bias on test accuracy by repeating the analysis after removing studies at high risk of bias.
Results
Results of the search
The electronic searches yielded a total of 6719 records (Figure 2). After 2460 duplicate records were removed we screened the remaining 4259 records. We excluded 4094 records at the title and abstract stage and obtained full‐text reports of 165 references for further assessment. We excluded 108 reports of 108 studies (see Characteristics of excluded studies for reasons). We identified 57 reports of 47 studies (see Characteristics of included studies) that met the inclusion criteria, recruiting 26,151 participants and providing data from 23,440 participants for quantitative analysis. Nineteen of the included studies were cohort studies, 15 were cross‐sectional and 13 used a case‐control design. Most studies were conducted in Asia (36, 76.6%), followed by Europe (5, 10.6%), North America (3, 6.4%), South America (2, 4.3%) and Africa (1, 2.1%), and over half the studies (30 studies, 4950 participants) were conducted in a secondary care setting, with the remainder (17 studies, 21,201 participants) in a primary care or community setting. The sample size ranged from 24 to 2052 participants (median 200) with most studies enrolling one eye per person (34, 72.3%).

Study flow diagram.
Twenty‐seven studies assessed AS‐OCT (analysing 15,580 participants), 17 studies LACD (7385 participants), nine studies Scheimpflug photography (1616 participants), six studies SPAC (5239 participants) and five studies evaluated the flashlight (oblique handlight) test (998 participants). Thirty‐three of the studies evaluated a single index test and the remainder evaluated two or more tests on the same population. For the gonioscopic reference standard, 42 studies reported either the number of quadrants or degrees occluded. Thirty‐six (76.6%) studies (analysing 21,840 (93.2%) of participants) used a diagnostic definition 2 or more quadrants occluded, six studies used one or more quadrants occluded, three studies reported on occlusion of the nasal or temporal quadrant only, and one study used the clinicians subjective opinion of occludability. The gonioscopic reference criterion was not reported in one study.
Methodological quality of included studies
A summary of the methodological quality assessment is shown in the risk of bias and applicability graph and summary for each test (Figure 3 and Figure 4). Thirty‐six of the included studies (76.6%) were judged to have a high risk of bias in at least one domain.The risk of bias and applicability concerns are detailed below.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Patient Selection domain
Nineteen of the included studies (40.4%) were judged to have a high risk of patient selection bias. Thirteen studies adopted a case‐control design that recruited participants with the target condition (cases), and a group of control participants without the target condition. Six studies used inappropriate exclusions e.g. excluding eyes with PAS, high myopia, optic neuropathy, or used age restrictions. Five studies (10.6%) were categorised as having an unclear risk of bias due to poor reporting of recruitment strategy e.g. failure to report exclusion criteria or method of sampling,
The purpose of the index tests is to triage at‐risk populations or in opportunistic case‐detection to identify people at risk of angle closure. The inclusion of participants with a previous diagnosis of the target condition therefore raised applicability concerns, as the spectrum of participants in these studies was not representative of those who would receive the test in practice.
Index test domain
There was a high risk of bias in studies where index test thresholds were not pre‐defined. Optimal cut‐offs were determined post hoc in eight of the nine studies that evaluated Scheimpflug photography, approximately half for AS‐OCT (14 studies, 52%) and two of the six studies that evaluated SPAC. In the majority of studies, the index test was interpreted without knowledge of the results of the reference standard. However, for LACD, eight studies (47.1%) were judged at high risk of bias since either the same observer performed the index and reference test (6 studies) or the threshold was not pre‐defined (2 studies).
Applicability of the test was generally of low concern across all the index tests, as the tests and testing procedures were clearly described and executed by personal who were sufficiently trained.
Reference standard domain
For the reference standard domain, 33 studies (70.2%) were judged to be at a low risk of bias, seven studies (14.9%) were classified as high risk as gonioscopy was not masked to the index test result, and in seven studies (14.9%) masking was unclear. Concerns regarding applicability were not applicable for this review, since gonioscopy was used as the reference standard for the diagnosis of an occludable angle in all of the included studies.
Flow and Timing domain
For the flow and timing domain, the majority of studies (41, 87.2%) were classified as having a low risk of bias. In these studies, all participants receiving the index test were verified with the reference standard, the number of participants included in the study matched the number in the analysis and there was less than a three‐month interval between the execution of the index and reference tests. There were five studies (10.6%) where the time interval where the time interval between the index and reference test was not reported, and in one study it was unclear whether all participants were included in the analysis.
The overall number of participants/eyes excluded from all the studies due to gonioscopy was negligible (< 0.3%), for LACD, flashlight, SPAC and Scheimpflug photography it was small (0% to 1.9%). The number of eyes/participants excluded from final analysis using AS‐OCT was relatively high (13.9%), due to the non‐interpretation of the data owing to either the clinician or the internal software inability to identify the scleral spur.
Conflict of Interest
Conflict of interest was of high concern in 15 studies, of unclear concern in 10 studies, and of no concern in 22 studies. Conflicts of interest were reported for 13 studies that evaluated AS‐OCT (56.5%), where the authors described receiving financial support from the manufacturer and/or loan of the device. For SPAC, four studies (66%) involved the patent holder of the device who was also a co‐author.
Unit of analysis concerns
Thirteen studies analysed data from both eyes, however seven of these studies corrected for clustering of data (Congdon 1996; Foster 2000; Lavanya 2008; Narayanaswamy 2010; Nolan 2007; Li 2019; Rossi 2012).
Findings
Forty‐seven studies reported sensitivity and specificity values for one or more index tests. Table 1 presents the pooled diagnostic accuracy estimates for index test parameters with four or more studies providing data at fixed thresholds for each test.
| Test | No. of studies (Number of eyes analysed) | Se (95% CI) | Sp (95% CI) | P values for differences between [test] and LACD ≤ 25% | |
| Se | Sp | ||||
| LACD | |||||
| 25% or < 25% | 16 (7540) | 0.83 (0.74 to 0.90) | 0.88 (0.84 to 0.92) | Reference | |
| 15% | 5 (3345) | 0.61 (0.36 to 0.81) | 0.93 (0.83 to 0.97) | ||
| 5% | 4 (2920) | 0.42 (0.31 to 0.55) | 0.97 (0.96 to 0.98) | ||
| 0% | 4 (2920) | 0.08 (0.03 to 0.18) | 1.00 (0.99 to 1.00) | ||
| Flashlight | |||||
| Grade One | 5 (1188) | 0.51 (0.25 to 0.76) | 0.92 (0.70 to 0.98) | 0.0181 | 0.5508 |
| SPAC | |||||
| ≤ 5 and/or S or P | 4 (4677) | 0.83 (0.70 to 0.91) | 0.78 (0.70 to 0.83) | 0.9707 | 0.5947 |
| Scheimpflug photography | |||||
| ACD central | 9 (1698) | 0.92 (0.84 to 0.96) | 0.86 (0.76 to 0.93) | 0.0828 | 0.5325 |
| ACA | 6 (1330) | 0.79 (0.64 to 0.89) | 0.88 (0.68 to 0.96) | ||
| ACV | 6 (1474) | 0.87 (0.82 to 0.91) | 0.79 (0.70 to 0.86) | ||
| AS‐OCT | |||||
| Subjective | 13 (9239) | 0.85 (0.76 to 0.91) | 0.71 (0.62 to 0.78) | 0.6632 | 0.0003 |
| ACA | 4 (517) | 0.81 (0.72 to 0.88) | 0.91 (0.84 to 0.95) | ||
| ACD | 4 (530) | 0.89 (0.78 to 0.95) | 0.88 (0.75 to 0.95) | ||
| AOD 500 nasal | 4 (1976) | 0.95 (0.76 to 0.99) | 0.82 (0.72 to 0.89) | ||
| AOD 500 temp. | 4 (1976) | 0.94 (0.75 to 0.99) | 0.81 (0.73 to 0.87) | ||
| AOD 750 temp. | 4 (3758) | 0.93 (0.84 to 0.97) | 0.85 (0.77 to 0.90) | ||
| TISA 500 nasal | 4 (1976) | 0.79 (0.71 to 0.85) | 0.81 (0.73 to 0.86) | ||
| TISA 500 temp. | 4 (1976) | 0.84 (0.77 to 0.89) | 0.81 (0.61 to 0.92) | ||
For the comparison between index tests, we considered LACD < 25% as the reference category and used bivariate models to investigate whether sensitivity and/or specificity differs between the most commonly used test parameters for each index test.
Limbal anterior chamber depth (LACD)
Seventeen studies (recruiting 7385 participants) assessed LACD, with nine studies evaluating a single threshold and the remainder providing data on two or more thresholds. With an increasing LACD cut‐off criterion (0%, ≤ 5%, ≤ 15%, ≤ 25%), there was an increase in sensitivity (0.08 to 0.83) with a corresponding reduction in specificity (1.00 to 0.88). (Table 1). The most commonly used threshold was ≤ 25% (used in 16 studies, 7011 participants (7540 eyes)), which produced pooled sensitivity and specificity estimates of 0.83 (95% CI 0.74 to 0.90) and 0.88 (95% CI 0.84 to 0.92), respectively Figure 5. The certainty of this evidence was moderate due to risk of bias concerns, since the same observer performed the index and the reference test in many studies (see summary of findings Table 1).

Summary ROC Plot of LACD with thresholds of 0%, ≤5%, ≤15%, ≤ 25% or <25%, ≤40%, >25% to ≤50%. Summary point estimate and confidence region shown for LACD ≤ 25%.
Flashlight Test
Five studies (998 participants) evaluated the flashlight test, three studies evaluated grades 1 and 2, and two studies evaluated only grade 1. Visual inspection of the forest plot at this threshold revealed significant heterogeneity with respect to sensitivity, which ranged from 0.20 to 0.89. A meta‐analysis was conducted for grade 1 (1188 eyes), including all studies, with an estimated pooled sensitivity of 0.51 (95% CI 0.25 to 0.76) and specificity of 0.92 (95% CI 0.70 to 0.98) (Figure 6). The certainty of this evidence was low due to heterogeneity in accuracy estimates among studies and a high risk of bias (summary of findings Table 1). There were insufficient studies to generate a summary estimate for grade 2.

Summary ROC Plot of the flashlight test with thresholds of grade 1 and grade 2. Summary point estimate and confidence region shown for flashlight grade 1.
SPAC
Six studies (5239 participants) examined SPAC, three studies reported both categorical and numerical grades, two studies presented only the numerical grading and one study described only categorical thresholds. Four studies used both categorical and numerical thresholds Figure 7. The most common numerical grading was a threshold of ≤ 5. For the meta‐analysis, this numerical grade was amalgamated with the combined S and P categorical grade (4 studies, 4677 eyes), to produce a summary estimate of diagnostic performance for ≤ 5 and/or S or P (sensitivity 0.83 (95% CI 0.70 to 0.91); specificity 0.78 (95% CI 0.70 to 0.83)). The certainty of this evidence was moderate (summary of findings Table 1).
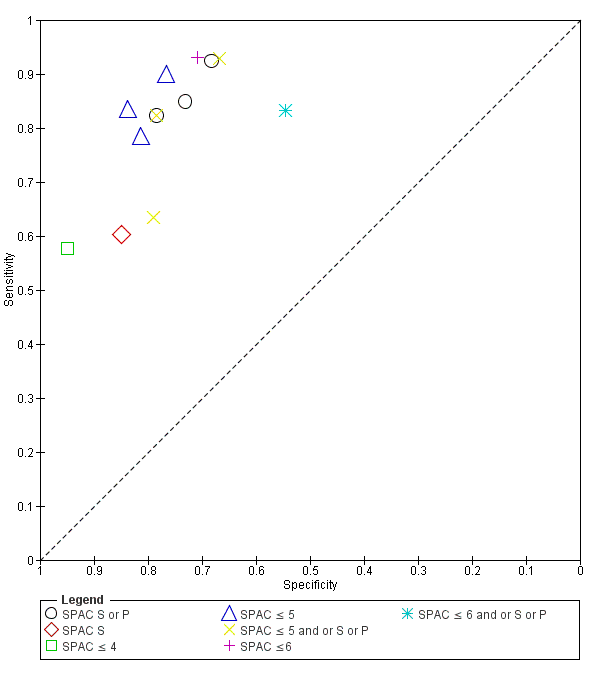
Summary ROC Plot of SPAC with thresholds of S or P, S, ≤ 4, ≤ 5, ≤ 5 and or S or P, ≤ 6, ≤ 6 and or S or P.
Scheimpflug photography
Nine studies (1616 participants) evaluated the diagnostic performance of Scheimpflug photography. Four studies reported all three anterior segment parameters (ACA, ACD and ACV), four studies evaluated two parameters and one study evaluated only ACD Figure 8. Point estimates of summary sensitivity varied between 0.79 and 0.92 across the parameters. Central ACD was the most commonly reported threshold (used in all nine studies, 1698 eyes), which produced a pooled sensitivity estimate of 0.92 (95% CI 0.84 to 0.96) and specificity 0.86 (95% CI 0.76 to 0.93). The certainty of this evidence was moderate due to the case‐control design used in over half of the studies (summary of findings Table 1).
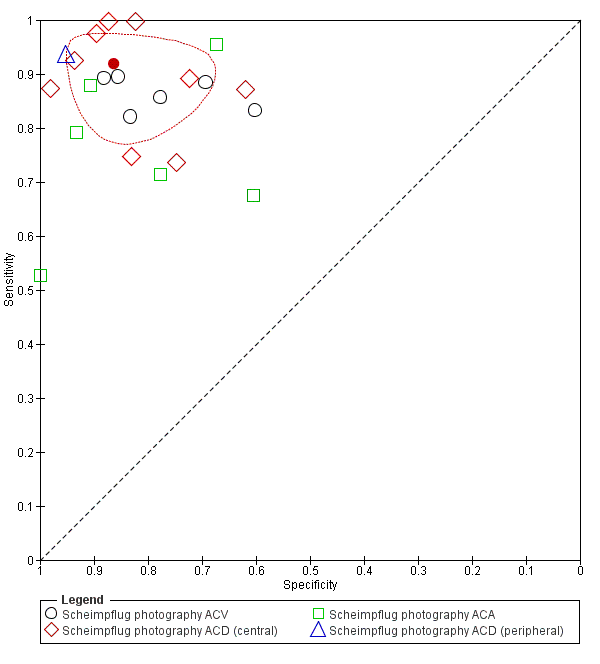
Summary ROC Plot of Scheimpflug photography with thresholds of ACV, ACD (central), ACA. and ACD (peripheral). Summary point estimate and confidence region shown for ACD (central).
Quantitative parameters reported unique cut‐off values that were derived from the data post‐hoc in eight of the nine studies.
Anterior segment optical coherence tomography (AS‐OCT)
Twenty‐seven studies (15,580 participants) assessed AS‐OCT, 17 studies used the Visante time domain AS‐OCT; four studies used a slit lamp OCT; two studies spectral domain OCT with a lens adapter and four studies utilised swept source OCT. Thirteen unique AS‐OCT parameters were reported; using either quantitative or qualitative thresholds or both. Pooled point estimates of sensitivity and specificity could only be calculated for eight parameters (subjective opinion of occludability, AOD 500 (nasal), AOD 500 (temporal), AOD 750 (temporal), TISA 500 (nasal), TISA 500 (temporal), ACA, ACD) with pooled sensitivities ranging from 0.79 to 0.95 and specificities from 0.71 to 0.88 (Table 1). Subjective judgement of occludability was the most commonly used threshold (used in 13 studies (48.1%), 9,239 eyes), which produced a pooled sensitivity estimate of 0.85 (95% CI 0.76 to 0.91); specificity 0.71 (95% CI 0.62 to 0.78) (Figure 9). The certainly of this evidence was moderate (summary of findings Table 1).

Summary ROC Plot of AS‐OCT with thresholds of subjective judgement), AOD 500 temporal, AOD 500 nasal, AOD 750 temporal, TISA 500 temporal, TISA 500 nasal, TISA 750 temporal, TISA 750 nasal, ACA angle, ACA area, ACD, ACV, ARA 500 average, ARA 750 average, ARA 750 nasal and LV. Summary point estimate and confidence region shown for AS‐OCT (subjective judgement).
Quantitative parameters reported unique cut‐off values that were derived from the data post‐hoc, which could have led to an overestimation of test performance.
Comparison between tests
The most commonly reported parameter for each index test was compared using LACD (≤ 25%) as the reference category Table 1. Comparisons of sensitivity and specificity were not shown to differ across all the index tests, except for the flashlight test, where a grade 1 threshold had a statistically significant lower sensitivity than LACD, and AS‐OCT (subjective judgement), which had a statistically significant lower specificity.
Direct comparisons between tests in the same studies were available for LACD ≤ 25% versus AS‐OCT (subjective opinion of occludability) in three studies, Figure 10) and for LACD ≤ 25% versus Scheimpflug photography (central ACD) in two studies (Figure 11), with no clear pattern seen. LACD ≤ 25% seemed more accurate than flashlight (grade1) in two studies (Figure 12).

Summary ROC of tests: 4 Direct comparison: LACD ≤ 25% or <25%, 20 Direct comparison: AS‐OCT (subjective judgement).

Summary ROC Plot of tests: 4 Direct comparison: LACD ≤ 25% or < 25%, 17 Direct comparison: Scheimpflug photography ACD (central).

Summary ROC Plot of tests: 4 Direct comparison: LACD ≤ 25% or < 25%, 7 Direct comparison: Flashlight grade 1.
Investigation of heterogeneity and subgroup analysis
Among pre‐planned study level covariates, we investigated the effect of the setting by comparing accuracy in 16 studies reporting LACD at a common threshold (≤ 25%) conducted in a primary care/ community setting (seven studies, mean prevalence 14.4%) with that in studies conducted in secondary care (seven studies, mean prevalence 42%) and in case‐control studies (two studies, 520 participants, mean prevalence 79.2%). Case‐control studies were analysed in a different category since prevalence is determined by study design. The sensitivity and specificity were 0.90 (95% CI: 0.75 to 0.97) and 0.82 (95%CI: 0.66 to 0.91) for primary care/community studies, 0.82 (95%CI: 0.67 to 0.91) and 0.91 (95% CI: 0.85 to 0.94) in secondary care studies, and 0.89 (95% CI: 0.64 to 0.97) and 0.86 (95% CI: 0.78 to 0.91) in case‐control studies. We found no evidence of a statistically significant difference in test performance according to setting.
There were insufficient studies to conduct the effect of other pre‐specified covariates including gonioscopic diagnostic reference thresholds. We also planned to perform a sensitivity analysis to assess the impact of risk of bias on test accuracy by repeating the analysis after removing studies at high risk of bias, however nearly all the studies were judged to have at least one domain that was labelled as high/unclear risk of bias or had applicability concern.
Discussion
This systematic review evaluated the diagnostic accuracy of non‐contact tests including: LACD (van Herick test), flashlight, SPAC, Scheimpflug photography and AS‐OCT for detecting individuals at risk of PACG. These tests were evaluated as stand‐alone triage methods that could be used by specialist or non‐specialist healthcare professionals in a primary care or secondary care setting for case‐detection. In the proposed clinical pathway, screen positive cases would be referred for gonioscopic assessment by a glaucoma specialist.
Summary of main results
We analysed data from a total of 23,440 participants in 47 studies reporting the diagnostic accuracy of one or more index tests for the detection of an occludable angle.
The LACD test (van Herick test) was investigated in 17 studies. This test is quick, relatively inexpensive and can be used by non‐glaucoma specialists with a minimal amount of training, Using a cut‐off 25% or less, our pooled estimates found that at 10% prevalence as in a community setting, 17 out of 100 people at risk of angle closure would be missed and not sent to ophthalmic assessment and would potentially be exposed to acute or chronic angle‐closure glaucoma and 108 out of 900 people who are not at risk would be sent to ophthalmic assessment unnecessarily, increasing costs with little or no benefit. These estimates of accuracy, although they are sub‐optimal, can still be suitable for case‐detection in areas where most people do not receive basic ophthalmic care, There were only two case‐control studies in the main analysis on LACD and the certainty of the evidence was moderate, meaning that we are relatively confident in our estimates. A subgroup analysis comparing community (low prevalence) studies versus secondary care (high prevalence) settings found no evidence of a statistically significant difference in diagnostic performance according to setting.
Fifty‐seven per cent of included studies evaluated AS‐OCT. This technology has a number of theoretical advantages, including the rapid and non‐invasive acquisition of high‐resolution images of the complete 360 degrees of the ACA. These images can be interpreted qualitatively or quantitatively. Although the included studies provided data on 13 separate AS‐OCT parameters, the lack of consistency in the thresholds used meant that summary estimates could only be calculated for a minority of parameters (n = 8). The largest number of studies used subjective AS‐OCT assessment of angle width (n = 13) and yielded a similar sensitivity to LACD but a statistically significant lower specificity. This evidence was moderate‐certainty. Only four studies each used objective AS‐OCT test measures and ACD and AOD 500 obtained good sensitivity and specificity. However, given the small number of studies available for each measure, we conclude that more research is needed on AS‐OCT both in community and secondary care settings. This is particularly important as OCT technology continues to develop with ongoing improvements in image resolution. It is likely that the superior resolution of newer devices e.g. swept‐source OCT, may overcome the current problem of scleral spur visualisation, which is an important anatomical landmark for ACA evaluation. Furthermore, machine learning has recently shown potential for automated detection of angle‐closure in AS‐OCT images (Fu 2019).
Scheimpflug photography, which requires a costly device that is rarely used for anterior chamber angle assessment was evaluated in nine studies. The best performing parameter was ACD (central), which had similar sensitivity and specificity estimates to LACD (moderate‐certainty evidence).
Other tests, including the oblique flashlight test and SPAC, were investigated in only five studies each and showed either a low sensitivity or unacceptable specificity, with low‐ and moderate‐certainty evidence, respectively.
Although no firm conclusions can be drawn from indirect comparisons of diagnostic tests, we find no evidence of statistically significant differences between LACD and other objective tests that use costly devices. However, based on this analysis, the flashlight test showed a statistically significant lower sensitivity than LACD. This was also shown in the small number of studies that compared the tests directly.
The majority of studies were conducted in Asia. Pre‐specified thresholds were reported for LACD, flashlight, SPAC and the subjective judgement of occludability using AS‐OCT. However, all the reported thresholds for eight out of the nine studies for Scheimpflug photography and all quantitative AS‐OCT thresholds were calculated post‐hoc and were based on the best performing cut‐points derived from each study population. The heterogeneity of sensitivity and specificity estimates for each test was large and could not be adequately explained. Furthermore, 36 of the 47 included studies (76.6%) were judged to have a high risk of bias in at least one domain, most commonly due to patient selection bias and/or not pre‐defining the index test threshold. However, 38% of included studies recruited participants with a previous diagnosis of an occludable angle, which was mainly attributed to the use of a case‐control design. These designs are known to over‐estimate the performance of diagnostic tests and therefore our estimates of test accuracy could be higher than would be expected in unscreened populations. It is therefore possible that the reported estimates of test performance may differ from what may be expected in a standard clinical setting.
Strengths and weaknesses of the review
Strengths of this systematic review included its methodological rigour, which included the following.
-
A comprehensive search strategy to identify as many potential studies for inclusion as possible, with no language, clinical setting, study design or publication year restrictions
-
All titles and abstracts were independently screened by two review authors
-
Two review authors independently extracted data and conducted a quality assessment of studies (using QUADAS‐2).
-
We obtained translations of two non‐English studies that met the inclusion criteria and undertook data extraction and conducted 'Risk of bias' assessments
-
Sufficient studies were available to conduct a meta‐analysis and produce summary estimates of sensitivity and specificity for all five index tests
There were a number of limitations of the review. Comparisons between index tests are best conducted using direct (within study) comparisons, as direct comparisons are considered to be more reliable than indirect comparisons (between studies) (Takwoingi 2013). Since there were insufficient studies that reported more than one test or parameter, comparisons of test accuracy were mostly based on indirect comparisons and therefore subject to between‐study differences in characteristics of participants, diagnostic standards and study design. The majority of studies had a high or unclear risk of bias in at least one domain and substantial heterogeneity was observed between studies. This should be taken into consideration when interpreting the review findings. Finally, there were insufficient studies to compare test performance in populations of different ethnicity or angle closure disease severity, in addition we were unable to conduct the planned sensitivity analysis on the risk of bias, as this may have impacted the applicability of such tests.
Applicability of findings to the review question
Given that the tests could be applied in either primary or secondary care, we did not place any restriction on setting, although in both pathways consecutive undiagnosed participants would be evaluated or triaged. Several included studies recruited participants with a previous diagnosis of an occludable angle, which was mainly attributed to the use of a case‐control design, which may not only overestimate accuracy, but also cause concerns regarding applicability.
Although proportionately more studies were conducted in a secondary care setting and participants were typically recruited from specialist or general ophthalmology clinics, over 80% of participants included in the quantitative analysis were recruited from a primary care or community setting. Participants in these studies included those recruited in large cross‐sectional epidemiological studies, or from community polyclinics that provide primary care services to local populations. In the context of angle closure, patients with suspected occludable angles in such settings would be referred to secondary care for specialist evaluation.
Three‐quarters of the included studies were performed in Asia, which carries the greatest burden of PACG and its associated blindness (Tham 2014). The prevalence of PACG in Asian populations is up to three times higher than in European‐derived groups (Cheng 2014; Day 2012). Consequently, case‐detection of angle closure disease in these populations is more likely to be cost‐effective (Tang 2019).
Non‐contact tests for identifying occludable angles include both subjective (flashlight, LACD) and objective tests (SPAC and Scheimpflug photography). AS‐OCT imaging can be interpreted subjectively or objectively. Subjective tests in the included studies were generally interpreted by ophthalmologists, which could have potentially led to an improved test performance. However, previous studies evaluating LACD have found no difference in performance within and between ophthalmologists and non‐medical healthcare professionals, with moderate inter‐observer agreement for each group (Jindal 2015; Johnson 2018). Similarly, a small study assessing AS‐OCT qualitative judgements by glaucoma specialists also found moderate agreement (Tay 2015).
Angle closure disease represents a spectrum of disorders from angle closure suspect to PACG. Angle closure is defined by the degree of appositional contact between the peripheral iris and trabecular meshwork and the presence or absence of trabecular damage (PAS). Although all studies used gonioscopy as the reference standard, a variety of diagnostic definitions were used. The review allowed for this flexibility in clinical definition and accepted the classification of an occludable angle adopted by the investigators. The term 'occludable angle' could encompass varying degrees of risk of angle closure, clinically it is most important to ascertain whether the angle is potentially occludable and therefore at risk of developing glaucomatous optic neuropathy. The widely accepted classification of occludability is that proposed by the International Society for Geographical and Epidemiological Ophthalmology ISGEO group (Foster 2002) (two more quadrants occluded). Thirty‐six (76.6%) of the included studies (recruiting 24,347 (93.1%) of participants) used a diagnostic definition two or more quadrants occluded, one study used a sub‐specialist ophthalmologist opinion that the angle was occludable and nine studies used a threshold of one or less quadrants occluded. We therefore feel that the majority of angles included represented a referable condition and would be classified as at risk of occludability.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Summary ROC Plot of LACD with thresholds of 0%, ≤5%, ≤15%, ≤ 25% or <25%, ≤40%, >25% to ≤50%. Summary point estimate and confidence region shown for LACD ≤ 25%.

Summary ROC Plot of the flashlight test with thresholds of grade 1 and grade 2. Summary point estimate and confidence region shown for flashlight grade 1.

Summary ROC Plot of SPAC with thresholds of S or P, S, ≤ 4, ≤ 5, ≤ 5 and or S or P, ≤ 6, ≤ 6 and or S or P.

Summary ROC Plot of Scheimpflug photography with thresholds of ACV, ACD (central), ACA. and ACD (peripheral). Summary point estimate and confidence region shown for ACD (central).

Summary ROC Plot of AS‐OCT with thresholds of subjective judgement), AOD 500 temporal, AOD 500 nasal, AOD 750 temporal, TISA 500 temporal, TISA 500 nasal, TISA 750 temporal, TISA 750 nasal, ACA angle, ACA area, ACD, ACV, ARA 500 average, ARA 750 average, ARA 750 nasal and LV. Summary point estimate and confidence region shown for AS‐OCT (subjective judgement).

Summary ROC of tests: 4 Direct comparison: LACD ≤ 25% or <25%, 20 Direct comparison: AS‐OCT (subjective judgement).

Summary ROC Plot of tests: 4 Direct comparison: LACD ≤ 25% or < 25%, 17 Direct comparison: Scheimpflug photography ACD (central).

Summary ROC Plot of tests: 4 Direct comparison: LACD ≤ 25% or < 25%, 7 Direct comparison: Flashlight grade 1.

Scheimpflug photography ACV

Scheimpflug photography ACD (central)

Scheimpflug photography ACA

Scheimpflug photography ACD (peripheral)

AS‐OCT (subjective judgement)
| Test (measure and/or threshold) | N. studies (N. with occludable angles/total analysed) | Accuracy estimates | False positives and false negatives at prevalence 10%: 100 participants with and 900 without occludable angles | False positives and false negatives at prevalence 30%: 300 participants with and 700 without occludable angles | Certainty of evidence for sensitivity and specificity | |||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (95%CI) | Specificity (95%CI) | False positives (95% CI) | False negatives (95% CI) | False positives (95% CI) | False negatives (95% CI) | |||
| LACD (cut‐off: ≤25%) | 16 studies (1490/7540) | 0.83 (0.74 to 0.90) | 0.88 (0.84 to 0.92) | 108 (72 to 144) | 17 (10 to 26) | 51 (30 to 78) | 84 (56 to 112) | Moderatea |
| Oblique flashlight test (grade 1) | 5 studies (298/1188) | 0.51 (0.25 to 0.76) | 0.92 (0.70 to 0.98) | 72 (18 to 270) | 49 (24 to 46) | 56 (14 to 210) | 147 (75 to 228) | Lowb |
| SPAC (≤5 and/or S or P) | 4 studies (994/4677) | 0.83 (0.70 to 0.91) | 0.78 (0.70 to 0.83) | 207 (162 to 261) | 17 (10 to 26) | 161 (126 to 203) | 51 (30 to 78) | Moderatec |
| Scheimpflug photography (ACD central) | 9 studies (461/1698) | 0.92 (0.84 to 0.96) | 0.86 (0.76 to 0.93) | 126 (63 to 216) | 8 (4 to 16) | 98 (49 to 168) | 24 (12 to 48) | Moderated |
| AS‐OCT (subjective assessment) | 13 studies (1850/9239) | 0.85 (0.76 to 0.91) | 0.71 (0.62 to 0.78) | 270 (198 to 351) | 14 (8 to 24) | 210 (154 to 273) | 42 (24 to 72) | Moderatee |
| Certainty of evidence applies to both sensitivity and specificity. Explanations a Downgraded one level due to risk of bias: 75% of studies had a high risk of bias in one or more domains. Two studies (13%) used a case‐control design. Legend ACD: anterior chamber depth | ||||||||
| Test | No. of studies (Number of eyes analysed) | Se (95% CI) | Sp (95% CI) | P values for differences between [test] and LACD ≤ 25% | |
| Se | Sp | ||||
| LACD | |||||
| 25% or < 25% | 16 (7540) | 0.83 (0.74 to 0.90) | 0.88 (0.84 to 0.92) | Reference | |
| 15% | 5 (3345) | 0.61 (0.36 to 0.81) | 0.93 (0.83 to 0.97) | ||
| 5% | 4 (2920) | 0.42 (0.31 to 0.55) | 0.97 (0.96 to 0.98) | ||
| 0% | 4 (2920) | 0.08 (0.03 to 0.18) | 1.00 (0.99 to 1.00) | ||
| Flashlight | |||||
| Grade One | 5 (1188) | 0.51 (0.25 to 0.76) | 0.92 (0.70 to 0.98) | 0.0181 | 0.5508 |
| SPAC | |||||
| ≤ 5 and/or S or P | 4 (4677) | 0.83 (0.70 to 0.91) | 0.78 (0.70 to 0.83) | 0.9707 | 0.5947 |
| Scheimpflug photography | |||||
| ACD central | 9 (1698) | 0.92 (0.84 to 0.96) | 0.86 (0.76 to 0.93) | 0.0828 | 0.5325 |
| ACA | 6 (1330) | 0.79 (0.64 to 0.89) | 0.88 (0.68 to 0.96) | ||
| ACV | 6 (1474) | 0.87 (0.82 to 0.91) | 0.79 (0.70 to 0.86) | ||
| AS‐OCT | |||||
| Subjective | 13 (9239) | 0.85 (0.76 to 0.91) | 0.71 (0.62 to 0.78) | 0.6632 | 0.0003 |
| ACA | 4 (517) | 0.81 (0.72 to 0.88) | 0.91 (0.84 to 0.95) | ||
| ACD | 4 (530) | 0.89 (0.78 to 0.95) | 0.88 (0.75 to 0.95) | ||
| AOD 500 nasal | 4 (1976) | 0.95 (0.76 to 0.99) | 0.82 (0.72 to 0.89) | ||
| AOD 500 temp. | 4 (1976) | 0.94 (0.75 to 0.99) | 0.81 (0.73 to 0.87) | ||
| AOD 750 temp. | 4 (3758) | 0.93 (0.84 to 0.97) | 0.85 (0.77 to 0.90) | ||
| TISA 500 nasal | 4 (1976) | 0.79 (0.71 to 0.85) | 0.81 (0.73 to 0.86) | ||
| TISA 500 temp. | 4 (1976) | 0.84 (0.77 to 0.89) | 0.81 (0.61 to 0.92) | ||
| For the comparison between index tests, we considered LACD < 25% as the reference category and used bivariate models to investigate whether sensitivity and/or specificity differs between the most commonly used test parameters for each index test. | |||||
| Test | No. of studies | No. of participants |
| 1 LACD 0% Show forest plot | 4 | 2920 |
| 2 LACD ≤ 5% Show forest plot | 4 | 2920 |
| 3 LACD ≤ 15% Show forest plot | 5 | 3345 |
| 4 LACD ≤ 25% or <25% Show forest plot | 16 | 7540 |
| 5 LACD ≤ 40% Show forest plot | 3 | 2177 |
| 6 LACD > 25% to ≤ 50% Show forest plot | 3 | 988 |
| 7 Flashlight grade 1 Show forest plot | 5 | 1188 |
| 8 Flashlight grade 2 Show forest plot | 3 | 848 |
| 9 SPAC S or P Show forest plot | 3 | 2325 |
| 10 SPAC S Show forest plot | 1 | 120 |
| 11 SPAC ≤ 4 Show forest plot | 1 | 2047 |
| 12 SPAC ≤ 5 Show forest plot | 3 | 4252 |
| 13 SPAC ≤ 5 and or S or P Show forest plot | 3 | 2630 |
| 14 SPAC ≤6 Show forest plot | 1 | 442 |
| 15 SPAC ≤ 6 and or S or P Show forest plot | 1 | 425 |
| 16 Scheimpflug photography ACV Show forest plot | 6 | 1474 |
| 17 Scheimpflug photography ACD (central) Show forest plot | 9 | 1698 |
| 18 Scheimpflug photography ACA Show forest plot | 6 | 1330 |
| 19 Scheimpflug photography ACD (peripheral) Show forest plot | 1 | 506 |
| 20 AS‐OCT (subjective judgement) Show forest plot | 13 | 9239 |
| 21 AS‐OCT AOD 500 temporal Show forest plot | 4 | 1976 |
| 22 AS‐OCT AOD 500 nasal Show forest plot | 4 | 1976 |
| 23 AS‐OCT AOD 750 temporal Show forest plot | 4 | 3758 |
| 24 AS‐OCT AOD 750 nasal Show forest plot | 3 | 1711 |
| 25 AS‐OCT AOD 500 average Show forest plot | 2 | 307 |
| 26 AS‐OCT TISA 500 temporal Show forest plot | 4 | 1976 |
| 27 AS‐OCT TISA 500 nasal Show forest plot | 4 | 1976 |
| 28 AS‐OCT TISA 500 average Show forest plot | 1 | 31 |
| 29 AS‐OCT TISA 750 temporal Show forest plot | 3 | 1711 |
| 30 AS‐OCT TISA 750 nasal Show forest plot | 3 | 1711 |
| 31 AS‐OCT TISA 750 average Show forest plot | 1 | 31 |
| 32 AS‐OCT ACA Show forest plot | 4 | 517 |
| 33 AS‐OCT ACA area Show forest plot | 2 | 3702 |
| 34 AS‐OCT ACD Show forest plot | 4 | 530 |
| 35 AS‐OCT ACV Show forest plot | 3 | 3879 |
| 36 AS‐OCT ARA 500 average Show forest plot | 1 | 31 |
| 37 AS‐OCT ARA 750 nasal Show forest plot | 1 | 1465 |
| 38 AS‐OCT ARA750 temporal Show forest plot | 1 | 1465 |
| 39 AS‐OCT ARA 750 average Show forest plot | 1 | 31 |
| 40 AS‐OCT ITC index ≥35% Show forest plot | 2 | 1997 |
| 41 AS‐OCT ITC index ≥50% Show forest plot | 2 | 1997 |
| 42 AS‐OCT ITC index ≥70% Show forest plot | 1 | 140 |
| 43 AS‐OCT ITC index ≥75% Show forest plot | 1 | 1857 |
| 44 AS‐OCT LV Show forest plot | 3 | 2260 |








































