نقش (شیمیدرمانی) پرتودرمانی کمکی شدتزدایی شده در برابر شیمیدرمانی پرتودرمانی کمکی استاندارد پس از جراحی ترانساورال با حداقل تهاجم برای کارسینوم اوروفارنژیال HPV مثبت قابل رزکسیون
چکیده
پیشینه
هر ساله در سراسر جهان، بیش از 400,000 مورد سرطان سلول سنگفرشی اوروفارنژیال (oropharyngeal squamous cell cancer; OPSCC) تشخیص داده میشود و این تعداد در حال افزایش است. بیشترین افزایش به ویروس پاپیلومای انسانی (human papillomavirus; HPV) نسبت داده شده است. بیماران مبتلا به OPSCC با HPV مثبت، اغلب جوانتر هستند و بهطور قابل توجهی بقای بهتری نسبت به بیماران با HPV منفی دارند. مدیریت مرسوم OPSCC با پرتودرمانی با یا بدون شیمیدرمانی بوده، زیرا نشان داده شده که این کار دارای بقای مشابه با جراحی باز است اما موربیدیتی آن بهطور قابل توجهی کمتر است. با این حال، با توسعه برنامهریزی کامپیوتری شده و پرتودرمانی با شدت تعدیل یافته و تکنیکهای جراحی با حداقل تهاجم، تکنیکهایی تکامل یافتهاند. سمیتهای حاد و دیرهنگام مرتبط با شیمیدرمانی پرتودرمانی، بار (burden) قابل توجهی برای بیماران مبتلا به OPSCC دارد و با یک مطالعه کوهورت از افراد جوانتر، هرگونه استراتژی که بتواند باعث کاهش موربیدیتی مرتبط با درمان شود، باید مورد بررسی قرار بگیرد.
اهداف
ارزیابی تاثیرات (شیمیدرمانی) پرتودرمانی کمکی شدتزدایی شده در مقایسه با (شیمیدرمانی) پرتودرمانی کمکی استاندارد در بیماران تحت درمان با جراحی ترانساورال با حداقل تهاجم (جراحی روباتیک ترانساورال (transoral robotic surgery) یا میکروسرجری لیزری ترانساورال (transoral laser microsurgery)) برای کارسینوم سلول سنگفرشی اوروفارنژیال قابل رزکسیون با HPV مثبت (resectable HPV‐positive oropharyngeal squamous cell carcinoma).
روشهای جستوجو
متخصص اطلاعات گروه گوش و حلق و بینی (ENT) در کاکرین برای یافتن کارآزماییهای منتشر شده و منتشر نشده به جستوجو در پایگاه ثبت کارآزماییهای گروه گوش و حلق و بینی (ENT) در کاکرین؛ پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ Ovid MEDLINE؛ Ovid Embase؛ CINAHL؛ Web of Science؛ ClinicalTrials.gov؛ ICTRP و منابع اضافی پرداخت. تاریخ جستوجو 26 اپریل 2018 بود.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) در بیماران مبتلا به کارسینوم اوروفارینکس (که بر اساس طبقهبندی C09؛ C10 سازمان جهانی بهداشت تعریف شد). سرطانها شامل تومورهای سلول سنگفرشی HPV مثبت اولیه بودند که از مخاط اوروفارنژیال برخاسته بودند. تومورها بهصورت T1‐4a با یا بدون انتشار به غدد لنفاوی و بدون شواهدی از انتشار متاستاتیک دور طبقهبندی شدند. مداخله شامل جراحی ترانساورال با حداقل تهاجم و به دنبال آن درمان کمکی شدتزدایی شده (یا حذف شیمیدرمانی یا کاهش دوز پرتودرمانی) بود. مقایسه کننده، جراحی ترانساورال با حداقل تهاجم و به دنبال آن شیمیدرمانی پرتودرمانی همزمان استاندارد یا پرتودرمانی با دوز استاندارد بود. درمانهای دریافت شده با قصد درمان قطعی بوده و بیماران به غیر از بیوپسی تشخیصی، تحت هیچ مداخله قبلی قرار نگرفته بودند.
گردآوری و تجزیهوتحلیل دادهها
ما از روشهای استاندارد روششناسی مورد انتظار کاکرین استفاده کردیم. پیامدهای اولیه ما بقای کلی (بقای مرتبط با بیماری، در جایی که امکانپذیر بود، مورد مطالعه قرار گرفت) و بقای بدون بیماری بود، که در یک، دو، سه و پنج سال اندازهگیری شدند. پیامدهای ثانویه ما عبارت بودند از ارزیابی توانایی بلعیدن و تولید صدا، که در یک، شش، 12 و 24 ماه اندازهگیری شدند. ما برای استفاده از سیستم درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) برای ارزیابی کیفیت شواهد مربوط به هر پیامد برنامهریزی کردیم.
نتایج اصلی
ما هیچ RCT تکمیل شدهای را شناسایی نکردیم که معیارهای ورود ما را داشته باشد. با این حال، سه مطالعه واجد شرایط در حال انجام هستند:
ADEPT یک کارآزمایی فاز III است که به مقایسه پرتودرمانی پس از جراحی با یا بدون سیسپلاتین (cisplatin) در بیماران مبتلا به T1‐4a OPSCC با HPV مثبت پرداخته است. بیماران وارد شده باید جراحی با حداقل تهاجم را دریافت کرده باشند و گسترش اکسترا‐کپسولار (extra‐capsular) را از بیماری در گردن نشان داده باشند.
ECOG‐E3311 یک کارآزمایی فاز II درمان برای OPSCC پیشرفته موضعی با HPV مثبت است (مراحل III‐IVa + Ivb بدون متاستاز دور). بیماران پس از جراحی با حداقل تهاجم طبقهبندی میشوند. بیماران با خطر متوسط برای پرتودرمانی استاندارد یا دوز کاهش یافته، تصادفیسازی میشوند.
PATHOS یک کارآزمایی فاز III درمان برای OPSCC با HPV مثبت (T1‐3، N0‐2b) است. بیماران پس از جراحی با حداقل تهاجم طبقهبندی میشوند. بیماران با خطر متوسط برای پرتودرمانی استاندارد یا دوز کاهش یافته، تصادفیسازی میشوند. بیماران پرخطر برای پرتودرمانی با یا بدون سیسپلاتین همزمان تصادفیسازی میشوند.
نتیجهگیریهای نویسندگان
این مرور به فقدان فعلی کارآزماییهای تصادفیسازی و کنترل شده با کیفیت بالا اشاره میکند که به مطالعه درمان با شدت کمتر پس از جراحی با حداقل تهاجم در بیماران مبتلا به OPSCC با HPV مثبت میپردازند. با این حال، کارآزماییهایی که معیارهای ورود را به این مرور با نتایج مورد انتظار داشته باشند، بین سالهای 2021 و 2023 در حال انجام هستند.
PICOs
خلاصه به زبان ساده
نقش پرتودرمانی/شیمیدرمانی با دوز کاهش یافته در مقایسه با درمان با دوز استاندارد بعد از جراحی سوراخ کلید (keyhole surgery) برای سرطان گلو ناشی از ویروس پاپیلومای انسانی(human papillomavirus)
سوال مطالعه مروری
تاثیرات درمان پرتودرمانی/شیمیدرمانی با دوز کاهش یافته در مقایسه با درمان با دوز استاندارد بعد از جراحی سوراخ کلید برای سرطان گلو ناشی از ویروس پاپیلومای انسانی (human papillomavirus; HPV) چه هستند؟
پیشینه
هر سال بیش از 400,000 مورد سرطان گلو تشخیص داده میشود و این تعداد در حال افزایش بوده و HPV عامل مهمی برای آن است. سرطان گلو ناشی از این ویروس اغلب بیماران جوانتر را تحت تاثیر قرار میدهد اما پیشآگهی بهتری نسبت به سرطان گلو غیر‐ویروسی دارد. درمان مرسوم سرطان گلو، پرتودرمانی و شیمیدرمانی است؛ زیرا نشان داده شده که پیامدهای بقای مشابه، اما عوارض جانبی کمتری نسبت به جراحی دارند. با این حال، درمانهایی مانند برنامهریزی کامپیوتری شده و بهبودهایی در پرتودرمانی، و پیشرفت جراحی سوراخ کلید تکامل یافتهاند، که عوارض جانبی بالقوه کمتری دارند. شیمیدرمانی و پرتودرمانی تاثیرات منفی طولانیمدتی بر کیفیت زندگی دارند. با توجه به آنکه بیماران جوانتر تحت تاثیر قرار میگیرند، هرگونه راه کاهش این عوارض جانبی باید مورد بررسی قرار بگیرد.
ویژگیهای مطالعه
در اپریل 2018، کارآزماییهای تصادفیسازی و کنترل شدهای (randomised controlled trials; RCTs) را جستوجو کردیم که به مقایسه پرتودرمانی/شیمیدرمانی با دوز کاهش یافته با درمان با دوز استاندارد پرداختند. به پیامدهای مربوط به بقای کلی و بقای بدون بیماری، و همچنین تاثیرات بر توانایی بلع و تولید صدا علاقهمند بودیم. جستوجوهای ما هیچ RCT تکمیل شدهای را شناسایی نکردند، با این حال سه مطالعه مرتبط در حال انجام هستند و اولین نتایج بین سالهای 2021 تا 2023 مورد انتظار هستند.
نتایج کلیدی
در حال حاضر شواهد با کیفیت بالا که به مقایسه این دو درمان بپردازند وجود ندارد، اما چنین کارآزماییهایی در حال انجام هستند.
Authors' conclusions
Summary of findings
| Low‐dose adjuvant radiotherapy compared with standard‐dose adjuvant radiotherapy for patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery | ||||||
| Patient or population: patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery Settings: post minimally invasive transoral surgery Intervention: low‐dose adjuvant radiotherapy Comparison: standard‐dose adjuvant radiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard‐dose adjuvant radiotherapy | Low‐dose adjuvant radiotherapy | |||||
| Overall survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Disease‐free survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Swallowing ability Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| Voice (Voice Handicap Index) Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Standard‐dose adjuvant radiotherapy alone compared with adjuvant chemoradiotherapy for patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery | ||||||
| Patient or population: patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery Settings: post minimally invasive transoral surgery Intervention: standard‐dose adjuvant radiotherapy alone Comparison: adjuvant chemoradiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Adjuvant chemoradiotherapy | Standard‐dose adjuvant radiotherapy alone | |||||
| Overall survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Disease‐free survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Swallowing ability Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| Voice (Voice Handicap Index) Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
More than 400,000 cases of oropharyngeal squamous cell carcinoma (OPSCC) are diagnosed each year worldwide (Chaturvedi 2013). According to the US Centers for Disease Control and Prevention there were approximately 18,917 new cases of human papillomavirus‐associated OPSCC in the United States in 2015 (Van Dyne 2018). Worldwide, the incidence of OPSCC ranges from 7 to 17 cases per 100,000 persons and is steadily rising (Chaturvedi 2013), particularly in developed countries and young males (Chaturvedi 2011; Gillison 2015; Van Dyne 2018).
Human papillomavirus (HPV) is a major carcinogen, with an estimated 4.8% of total worldwide cancers in 2008 linked to the virus (de Martel 2012). HPV now meets the epidemiological criteria for OPSCC causality, especially in non‐smokers (Gillison 2015; Sudhoff 2011). Meta‐analysis of the world literature has demonstrated that the proportion of HPV‐associated oropharyngeal cancer has increased from 40.5% in studies recruiting before the year 2000 to 72.2% in studies reporting after 2005 (Mehanna 2013), although this is known to vary by individual population (Schache 2016). In contrast to this apparent overall trend, recent published work has shown that in the UK over the period 2002 to 2011, whilst the overall incidence of OPSCC doubled, the proportion that were HPV‐positive stayed the same, demonstrating a concomitant increase in non‐HPV associated OPSCC (Schache 2016). In the UK population, therefore, the increase cannot be attributed to HPV alone.
It is worth noting that HPV‐positive OPSCC patients have significantly improved rates of both overall and disease‐free survival compared to HPV‐negative tumour groups. HPV‐positive OPSCC is associated with a 58% reduction in the risk of death compared to HPV‐negative disease (Ang 2010; Fakhry 2008). Indeed the presence or absence of HPV with regard to the tumour may have a greater impact on five‐year survival than T stage or nodal status alone (Haughey 2011). This is illustrated by its inclusion as a significant factor in the progression from the TNM Classification of Malignant Tumours (TNM) 7th to 8th edition (TNM 2009; TNM 2017; O'Sullivan 2016).
Risk factors for oral HPV infection include a history of orogenital sexual practice, a large number of sexual partners and first intercourse at an early age. The same factors also reflect changes in modern society and combine to increase the cumulative effect of HPV infection in OPSCC (Chung 2009). HPV‐negative OPSCC tends to affect an older age group and is normally associated with smoking and alcohol. HPV‐positive OPSCC behaves differently, often presenting with a small primary in the oropharynx combined with a metastatic cystic deposit in the neck. In the TNM 7th edition this previously entailed a higher stage at presentation for the majority of patients (Ang 2010; Dwivedi 2013; Evans 2010), however this has been adjusted in the TNM 8th edition.
Description of the intervention
Over the last 20 years the management of oropharyngeal cancer has changed dramatically. In 2002, Parsons et al published a review of 51 studies of patients with OPSCC who were treated with surgery with or without radiotherapy or primary radiotherapy without neck dissection (Parsons 2002). The cumulative five‐year survival was 47% for patients undergoing primary surgical resection with or without neck dissection, and 43% for those undergoing primary radiotherapy with or without neck dissection. However, the severe complication rate was 23% in the primary surgical group and only 6% in the primary radiotherapy group. This led to the conclusion that non‐operative therapy was superior to operative therapy for OPSCC of all stages. More recently a large meta‐analysis comparing primary radiotherapy with chemoradiotherapy in 16,192 head and neck squamous cell carcinoma (HNSCC) patients provided updated results. The study concluded an absolute survival benefit of 8.1% after five years in OPSCC patients treated with concurrent chemoradiotherapy (Blanchard 2011).
Acute and late toxicities associated with chemoradiotherapy are, however, a significant burden for oropharyngeal cancer patients, with rates of acute and late grade 3 or higher toxicity at approximately 80% and 25% to 60% respectively (Kelly 2016). Recognised toxicities include: gastric tube dependence, pain, scarring, fibrosis, dysphagia, xerostomia, dental decay, osteoradionecrosis, hypothyroidism, carotid stenosis and stroke (Lee 2011). A relationship between the radiation dose to the constrictor muscles and long‐term swallowing difficulties has been well established: patients in whom more than 78% of their cricopharyngeus inlet receives over 60 Gy have a 50% risk of developing a stricture (Chen 2010). The addition of chemotherapy to radiotherapy worsens toxicity as demonstrated by the Intergroup trial, which found rates of grade 3 or higher toxicity of 89.5% in the chemoradiotherapy cohort compared to 52% in the radiotherapy alone cohort (Adelstein 2003). Furthermore, late toxicities may be under‐recognised as the 10‐year results of RTOG 91‐11 found increased non‐cancer mortality in the concurrent chemoradiotherapy arm (30.8%) compared to the induction chemotherapy arm (20.8%) or radiotherapy alone arm (16.9%) (Forastiere 2013). The incidence of dysphagia and feeding tube dependence post chemoradiotherapy cannot be entirely attributed to the treatment as there is evidence that part of the effect is due to disuse atrophy and resultant adverse remodelling of aerodigestive tract muscles (Hutcheson 2013).
Both open surgery and radiotherapy/concurrent chemoradiotherapy have drawbacks in terms of cost, overall survival and patient quality of life (Haigentz 2009; Machtay 2008). The benefit of a novel treatment regimen with lower toxicity is therefore clear, especially given the younger patient cohort who will have to live with treatment sequelae for longer.
As the biological differences in viral/non‐viral associated OPSCC are further elaborated (Masterson 2015; Pyeon 2007; Slebos 2006), radical change in most therapeutic interventions has taken place. In radiotherapy, intensity modulation and computerised planning have been introduced to external beam therapy. In surgery, the focus has shifted to the use of minimally invasive procedures such as transoral laser microsurgery or transoral robotic surgery, which demonstrate reduced immediate postoperative toxicity, reduced length of hospital stay and faster functional recovery compared with open surgery (Holsinger 2015). Furthermore, these techniques have the potential to improve organ preservation and function, and ameliorate the economic burden of treatment. Additionally, with regard to control of lymphatic spread, neck dissections have become more selective (resulting in the removal of fewer normal structures and therefore lower morbidity) (Adelstein 2012).
We have reviewed the evidence from studies comparing transoral minimally invasive surgery with (chemo)radiotherapy in the related Cochrane Review: 'Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small‐volume primary oropharyngeal carcinoma' (Howard 2016).
De‐intensified treatment strategies
The use of minimally invasive surgery for the primary site provides two principal areas of benefit. Firstly, it results in less morbidity for the patient compared to traditional open surgery. Secondly, in the context of HPV‐related OPSCC, it raises the potential option of 'de‐intensification therapy' with a concomitant reduction in radiation‐related morbidity (Masterson 2014a; Masterson 2014b; Moore 2009). So far this has been borne out by observational studies that suggest that transoral minimally invasive surgery may have an advantage by improving patient quality of life and functional outcome, and reducing the need for adjuvant concurrent chemoradiotherapy (Leonhardt 2012; Moore 2013).
De‐intensified treatment strategies fall into two groups. For primary concurrent chemoradiotherapy the options are to replace cisplatin with the epidermal growth factor receptor (EGFR) inhibitor cetuximab or to reduce the dose of radiation. These strategies have been covered by the Cochrane Review 'De‐escalation treatment protocols for human papillomavirus‐associated oropharyngeal squamous cell carcinoma' (Masterson 2014a). For primary surgery the options include:
-
administration of a lower dose of adjuvant radiotherapy; or
-
omission of chemotherapy from the adjuvant treatment regimen (radiotherapy alone).
It is these strategies that will be covered by this review.
The most appropriate option is decided based upon histopathological examination of the surgical specimen. This allows risk stratification of the individual patient, although it is worth noting that this stratification relies on traditional histopathological risk factors, which may not apply in HPV‐positive disease (Huang 2012; Masterson 2014b).
Patients are usually stratified into three cohorts:
-
Low‐risk: pathological findings associated with a low risk of locoregional relapse, which usually has no adjuvant treatment.
-
Medium‐risk: locoregional disease (or early disease with adverse histological features), which is usually treated with adjuvant radiotherapy.
-
High‐risk: presence of positive (< 1 mm) margins, extracapsular nodal spread or advanced disease, which is usually treated with adjuvant chemoradiotherapy.
How the intervention might work
Toxicities (both acute and late) as a result of chemotherapy and radiotherapy to the head and neck are well recognised. If survival outcomes can be maintained, de‐intensification of adjuvant therapy provides the opportunity for a reduction in these toxicities and an improved quality of life for patients. This is increasingly important, especially considering the younger age of some patients who will have to live with the consequences of treatment for many years.
Why it is important to do this review
The oropharynx plays an essential role in swallowing, speech and protecting the airway as it is situated at the bifurcation of the respiratory and digestive tract. The toxicities from standard‐dose chemotherapy and radiotherapy are well recognised and treatment modalities are therefore heavily influenced by the aim of reducing the risk of functional disability where possible. In the context of an expanding cohort of younger patients the potential effects of de‐intensification of adjuvant (chemo)radiotherapy facilitated by a minimally invasive surgical approach should be systematically reviewed.
Objectives
To assess the effects of de‐intensified adjuvant (chemo)radiotherapy in comparison to standard adjuvant (chemo)radiotherapy in patients treated with minimally invasive transoral surgery (transoral robotic surgery or transoral laser microsurgery) for resectable HPV‐positive oropharyngeal squamous cell carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We planned to exclude quasi‐randomised and cluster‐randomised trials.
Types of participants
We planned to include patients with HPV‐positive oropharyngeal carcinoma (subsites C09 and C10 as defined by the World Health Organization classification). Cancers included were T1‐4a with or without nodal disease with no evidence of distant metastatic spread. All patients had received minimally invasive transoral surgery.
We excluded carcinoma of the oral cavity (C01‐06), nasopharynx (C11), hypopharynx (C13) and larynx (C32) (WHO 2000).
We excluded patients receiving open surgery.
Types of interventions
Intervention
-
De‐intensified adjuvant (chemo)radiotherapy.
De‐intensified radiotherapy, total dose 50 Gy given in 25 fractions.
De‐intensified chemoradiotherapy (= standard dose radiotherapy with omission of concurrent chemotherapy): radiotherapy alone, total dose 60 Gy given in 30 fractions.
Control
-
Standard adjuvant (chemo)radiotherapy.
Radiotherapy, total dose 60 Gy given in 30 fractions.
+/‐
Chemotherapy: platinum‐based agent (usually cisplatin) administered concurrently with radiotherapy either weekly or three‐weekly.
The comparisons were:
-
Low‐dose adjuvant radiotherapy versus standard‐dose adjuvant radiotherapy.
-
Standard‐dose adjuvant radiotherapy alone versus adjuvant chemoradiotherapy.
Types of outcome measures
We planned to analyse the following outcomes in the review, but we did not use them as a basis for including or excluding studies. Primary outcomes focus on survival whilst the secondary outcomes focus on quality of life indices that may be affected by de‐intensification of treatment.
Primary outcomes
-
Overall survival/total mortality (disease‐related mortality was also to be studied if possible).
-
Disease‐free survival.
We planned to measure these outcomes at one, two, three and five years.
Secondary outcomes
-
Swallowing ability, as measured by:
-
the proportion of people with a gastrostomy tube (at one year);
-
the MD Anderson Dysphagia Inventory (MDADI);
-
modified barium swallow ratings.
-
return to normal diet, measured with the Performance Status Scale for Head and Neck cancer (PSS‐HN) normalcy of diet scale.
-
-
Voice, measured with the Voice Handicap Index (VHI).
Apart from the proportion of people with a gastrostomy tube we planned to measure these outcomes at one, six, 12 and 24 months.
Despite the increasing focus on quality of life the optimal patient‐reported outcome instrument that should be used to measure the impact of cancer therapy for the HPV‐associated OPSCC patient population is not clearly defined. Moreover, we feel that it is important to distinguish between patient‐reported outcomes and quality of life measures that may be more subjective. Finally, there is a danger that subtle differences in particular areas (e.g. dysphagia) may be lost within more global scoring systems, adding further complexity to the comparison (dilution effect).
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 26 April 2018.
Electronic searches
We identified published, unpublished and ongoing studies by searching the following databases from their inception:
-
the Cochrane ENT Trials Register (searched via the Cochrane Register of Studies to 26 April 2018);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies to 26 April 2018);
-
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 26 April 2018);
-
Ovid EMBASE (1974 to 26 April 2018);
-
LILACS, lilacs.bvsalud.org (searched 2 April 2018);
-
KoreaMed (searched via Google Scholar 27 April 2018);
-
Web of Knowledge, Web of Science (1945 to 26 April 2018);
-
ClinicalTrials.gov (searched via the Cochrane Register of Studies to 27 April 2018);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched to 26 April 2018).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). The search strategy for CENTRAL is provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
Selection of studies
Two authors (JH and LM) independently screened each abstract. We independently assessed full texts against the inclusion criteria. Any conflict was resolved through discussion with a senior author.
Data extraction and management
Two authors (JH and LM) planned to independently extract data using a specifically designed data extraction form. We planned to pilot the data extraction form on several studies and adjust it as necessary prior to use. Any disagreements were resolved through consultation with a senior author. Where required we contacted study authors for clarification or missing information.
For each study we planned to record the following:
-
Year of publication, country of origin and source of study funding.
-
Details of the participants, including demographic characteristics and criteria for inclusion and exclusion.
-
Details of the type of intervention, timing and duration (including type of surgery).
-
Details of survival outcomes reported, with time intervals.
-
Details of treatment‐related morbidity, categorised as acute (less than 90 days after treatment) or late (more than 90 days) and classified according to the Common Terminology Criteria for Adverse Events (CTCAE 4.03).
-
Details of all other outcomes reported, including method of assessment and time intervals.
Assessment of risk of bias in included studies
Had suitable studies been identified JH and LM would have assessed the risk of bias of the included studies independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
-
sequence generation;
-
allocation concealment;
-
blinding;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other sources of bias.
We planned to use the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
Measures of treatment effect
We planned to express continuous outcomes as a mean endpoint (or change from baseline) for each group with standard deviation and number of people. For continuous outcomes measured using different (but compatible) scales we planned to express treatment effects as a standardised mean difference (SMD).
We planned to express dichotomous outcomes as a risk ratio (RR) with the number of people with the outcome and number of participants.
We planned to express time‐to‐event outcomes as a hazard ratio (HR) with standard deviation (SD).
We planned to preferentially report ordinal data as continuous, however if studies only reported data dichotomously then we would have expressed as dichotomous.
The analysis would have been on an intention‐to‐treat basis. Where useful we planned to calculate the number needed to treat to benefit/harm (NNTB/NNTH) to aid clinical interpretation of the findings.
Unit of analysis issues
We planned to use data only from individually randomised controlled trials to avoid unit of analysis issues. We excluded cluster‐randomised trials.
Dealing with missing data
Where standard deviations or hazard ratios were not reported we planned to impute these from other reported data.
We planned to contact study authors:
-
where a study protocol suggested that an outcome of interest had been measured but was not reported;
-
if not all data required for meta‐analysis were reported;
-
if standard deviation data or hazard ratios were not available or estimable from other reported data (using the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011)).
Assessment of heterogeneity
We intended to assess clinical heterogeneity by examining the types of participants, interventions and outcomes in each study. We planned to conduct formal assessment using the Chi² test (with a significance level of α = 0.1 in view of the low power of this test) and the I² statistic (with 75% or more indicating a considerable level of inconsistency), both available in RevMan 5.3 (RevMan 2014). If meta‐analysis was performed we planned to further assess heterogeneity by inspecting the overlap of confidence intervals for the results of individual studies within a forest plot.
Assessment of reporting biases
We planned to assess reporting bias as within‐study (outcome reporting) bias and between‐study (publication) bias.
Outcome reporting bias
Bias can occur if outcomes are not adequately reported to allow further analysis. We planned to assess outcome reporting bias by comparing the reported outcomes against the outcomes listed in the trial protocol or methods section. Where the protocol, methods or results indicated that an outcome had been measured but the results were not presented sufficiently we planned to contact the study authors. If no further information was found we planned to judge this a 'high' risk of bias. If insufficient information was found to allow adequate judgement we planned to judge this an 'unclear' risk of bias.
Publication bias
If sufficient studies were available we planned to create funnel plots for the outcomes overall survival and dysphagia (MDADI scores). If asymmetry was found we planned to investigate this further according to the methodology in the Cochrane Handbook of Systematic Reviews of Interventions (Handbook 2011).
Data synthesis
We planned to extract data from the included studies and enter the data into RevMan 5.3 for statistical analysis. In the event of incomplete data, we intended to contact the study authors to obtain further information and to seek statistical advice where necessary.
Our analysis of survival and disease recurrence would have depended on the data available. We aimed to analyse the proportion surviving at one, two, three and five years as either:
-
proportion surviving; or
-
hazard ratios, for comparison in meta‐analysis if appropriate.
We planned to express the proportion of people with a gastrostomy tube as a risk ratio. The remaining secondary outcomes were to be restricted to assessment of validated assessment tools (where appropriate). If the data provided were in the form of means and standard deviations, we intended to display the effects on outcomes as a mean with standard deviation. If there was disparity in terms of scales we planned to express the data as a SMD with 95% confidence interval (CI). If hazard ratios were not quoted in studies, we planned to calculate them from available summary statistics such as observed events, expected events, variance, confidence intervals, P values or survival curves (Parmar 1998). If required, we planned to analyse the survival curves using the online tool: https://automeris.io/WebPlotDigitizer/.
We hoped to attempt a meta‐analysis if studies were available with similar comparisons and reporting the same outcome measures. If appropriate, we intended to calculate pooled estimates using a random‐effects model (Handbook 2011), as there is likely to be significant statistical or clinical heterogeneity (an I² value > 50%, as specified in the Cochrane Handbook for Systematic Reviews of Interventions).
Subgroup analysis and investigation of heterogeneity
We had no planned subgroup analyses.
We planned to assess heterogeneity using the methods advised in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Sensitivity analysis
If meta‐analysis had been performed we would have used sensitivity analysis. If we included studies with high risk of bias (e.g. poor follow‐up rate) we would have re‐calculated outcomes including these studies individually to see what influence this had on our presumed treatment effect.
Where thresholds had been set for inclusion or analysis we planned to re‐analyse the data using values either side of the set threshold to assess whether our decisions had influenced the outcomes.
GRADE and 'Summary of findings' table
We planned that three authors (JH, RD, LM) would ensure that each study was independently assessed twice by different authors using the GRADE approach to rate the overall quality of evidence. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we planned to apply this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision; and
-
publication bias.
We planned to include 'Summary of findings' tables, constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). The planned comparisons were:
-
low‐dose adjuvant radiotherapy versus standard‐dose adjuvant radiotherapy;
-
standard‐dose adjuvant radiotherapy alone versus adjuvant chemoradiotherapy.
We planned to include the following outcomes in the 'Summary of findings' tables:
Primary outcomes
-
Overall survival.
-
Disease‐free survival.
Secondary outcomes
-
Swallowing ability, as measured by:
-
the proportion of people with a gastrostomy tube (at one year);
-
the MD Anderson Dysphagia Inventory (MDADI);
-
modified barium swallow ratings;
-
return to normal diet, measured with the Performance Status Scale for Head and Neck cancer (PSS‐HN) normalcy of diet scale.
-
-
Voice, measured with the Voice Handicap Index (VHI).
Results
Description of studies
Results of the search
Our searches in April 2018 retrieved a total of 1959 records, which reduced to 1527 after the removal of duplicates. Following title and abstract screening we were able to discard 1502 irrelevant records. We screened 25 records in full text. We discarded 20 of these studies due to irrelevance (incorrect intervention, population, control, study design etc). We formally excluded two studies (see below).
We identified three ongoing RCTs that are eligible for inclusion in this review (see below).
The PRISMA diagram in Figure 1 shows our study search and selection process.
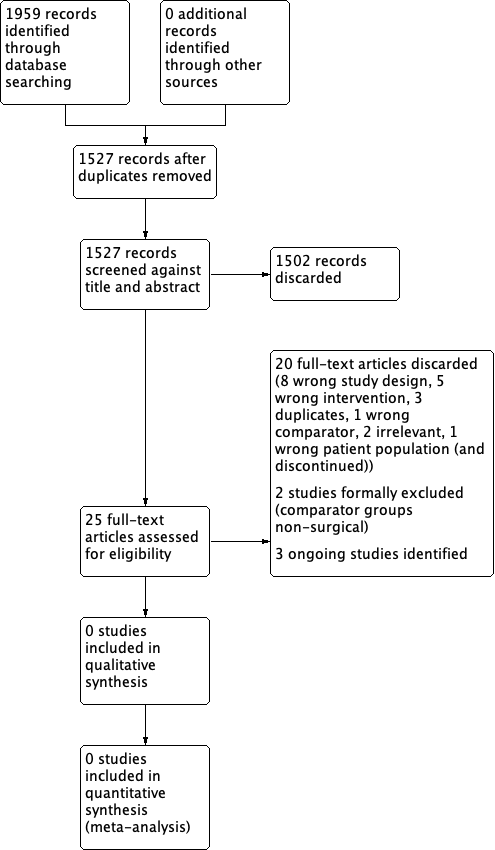
Study search and selection process (PRISMA diagram).
Included studies
We did not identify any completed studies that met the inclusion criteria for the review.
Excluded studies
We excluded two studies from the review. See Characteristics of excluded studies.
ORATOR ('Early‐stage squamous cell carcinoma of the oropharynx: radiotherapy versus trans‐oral robotic surgery') is a randomised controlled trial comparing primary radiotherapy with primary transoral robotic surgery for early‐stage (T1‐2, N0‐2) OPSCC. It is currently in progress with an estimated completion date of June 2021 for part 1 (phase 2, 68 patients) and 2028 for part 2 (phase 3, 120 patients). Patients will be randomised to receive either primary radiotherapy or primary transoral robotic surgery. The primary outcome for part 1 is quality of life (at one year) and the secondary outcomes include overall and progression‐free survival (at three and five years), toxicity and swallowing function (at five years). The primary outcome for part 2 (phase III trial) is two‐year progression‐free survival and the secondary outcomes are overall survival and quality of life outcomes. We excluded this study because the participants in the comparator group will not have undergone surgery.
EORTC‐1420 (European Organisation for Research and Treatment of Cancer 1420) is a phase III, randomised study assessing the "best of" radiotherapy compared to the "best of" minimally invasive head and neck surgery in patients with T1‐T2, N0 squamous cell carcinoma (EORTC‐1420). The study is recruiting, with an estimated enrollment of 170 patients and an estimated completion in May 2026. The primary outcome will be the assessment of swallowing function within the first year after the two treatment strategies. We excluded this study because again the participants in the comparator group will not have undergone surgery.
Ongoing studies
See Characteristics of ongoing studies.
ADEPT is a phase III prospective trial of de‐escalated adjuvant treatment after minimally invasive head and neck surgery (transoral robotic surgery or transoral laser microsurgery) for HPV‐positive OPSCC (T1‐4a) patients noted to have extracapsular extension detected in the nodal disease. This study is currently in progress and has finished recruiting (41 patients recruited), with an estimated completion date of August 2021. Patients are allocated to two arms after minimally invasive head and neck surgery through randomisation or patient choice, to receive either postoperative radiotherapy (60 Gy) alone or with concurrent systemic cisplatin therapy.
The primary outcomes are disease‐free survival and locoregional control at two years. Secondary outcomes include overall survival and distant metastasis rate up to five years, quality of life and functional outcomes to two years, and toxicities up to 4.5 months.
ECOG‐E3311 is a phase II prospective randomised trial of reduced adjuvant treatment after transoral robotic surgery for HPV‐positive, locally advanced OPSCC (stages III‐IVa + IVb without distant metastases). The study is in progress but not recruiting (511 patients recruited), with an estimated completion date of February 2023. The primary foci for investigation are a feasibility study of risk‐adjusted adjuvant therapy and the oncologic outcomes for intermediate‐risk patients post transoral robotic surgery and standard or de‐escalated treatment.
Patients will be stratified into three groups depending on their surgical histology. The low‐risk group will have no adjuvant therapy as per standard treatment. The medium‐risk group will be randomised to receive either standard (60 Gy) or de‐escalated (50 Gy) postoperative radiotherapy. The high‐risk group will receive postoperative radiotherapy (60 Gy) with concurrent cisplatin.
The primary outcomes include progression‐free survival at two years, risk distribution and grade 3‐4 bleeding events during surgery. Secondary outcomes include overall survival, swallowing and voice function up to two years post treatment, and change in patient‐reported quality of life up to six months post radiation treatment.
PATHOS is a phase III prospective randomised controlled trial of reduced‐intensity adjuvant treatment for HPV‐positive OPSCC (T1‐3, N0‐2b) patients treated with transoral robotic or laser surgery, with an estimated recruitment of 242 patients and estimated completion in December 2019.
Patients will be stratified into three groups depending on their surgical histology. The low‐risk group will have no adjuvant therapy as per standard treatment. The medium‐risk group will be randomised to receive either standard (60 Gy) or de‐escalated (50 Gy) postoperative radiotherapy. The high‐risk group will be randomised to receive postoperative radiotherapy (60 Gy) with or without concurrent cisplatin.
The primary outcome measure is swallowing function (MDADI) at one year. Secondary outcomes include disease‐free and overall survival at six months, and swallowing function, quality of life and acute or late toxicities measured at up to 24 months.
Risk of bias in included studies
No studies are included in the review.
Effects of interventions
See: Summary of findings for the main comparison Low‐dose adjuvant radiotherapy versus standard‐dose adjuvant radiotherapy; Summary of findings 2 Standard‐dose adjuvant radiotherapy alone versus adjuvant chemoradiotherapy
No studies are included in the review.
Discussion
Summary of main results
We identified no completed randomised controlled trials (RCTs) that met the inclusion criteria for this review. We are aware of three RCTs meeting our inclusion criteria; however, all are currently in progress (ADEPT; ECOG‐E3311; PATHOS).
Overall completeness and applicability of evidence
We believe this is a thorough and unbiased review of the current literature available on this subject. Unfortunately, there were no eligible studies with results for inclusion in our analysis; however, applicable studies are ongoing.
Quality of the evidence
We did not identify any completed studies that could be included in the review.
Potential biases in the review process
We have striven to design a protocol that would include all the highest‐quality evidence in this area (Howard 2018). The search strategy was designed and run by a qualified Cochrane Information Specialist so any bias here should be minimal. The search was not limited to the English language. It is possible that suitable studies have been carried out and the results published elsewhere in another language; however, we feel that this is unlikely, as all applicable studies are likely to have been registered with one of the central trial registries.
All studies that we discarded during our search and selection process were rejected based on study design (e.g. they were not randomised) or because they were not on the topic of interest. We formally excluded two studies because they compared a surgical intervention with a non‐surgical comparator and thus did not meet our inclusion criteria.
Agreements and disagreements with other studies or reviews
The debate regarding the relevance of traditional risk factors in human papillomavirus (HPV)‐positive disease and the necessity of adjuvant therapy is ongoing, with conflicting evidence from retrospective and non‐randomised trials.
In a combined analysis of the results from EORTC‐22931 and RTOG‐9501 Bernier et al demonstrated benefit from the addition of chemotherapy to radiotherapy with extracapsular extension status as one of the main risk factors warranting additional chemotherapy (Bernier 2005). Furthermore, in a meta‐analysis Blanchard et al showed improved survival with the addition of chemotherapy to radiotherapy (Blanchard 2011). More recent retrospective series, however, have questioned the relevance of extracapsular extension (Lewis 2011; Maxwell 2013; Sinha 2015a), with some suggesting that the total number of nodes is more prognostic than extracapsular extension or advanced N‐stage (Sinha 2015b).
Grant et al published a retrospective series showing no benefit in locoregional control from adjuvant treatment (HPV unknown population) (Grant 2009). By contrast, Pasalic et al published a retrospective series of 158 patients with intermediate or advanced disease showing a benefit from adjuvant therapy in patients with extracapsular extension compared to those without (hazard ratio (HR) 4.34, 95% confidence interval (CI) 1.540 to 12.213; P = 0.006) (Pasalic 2018). Of note, there was no difference in overall survival between the two groups; however, other series have again demonstrated benefit in disease‐free survival and overall survival from adjuvant therapy in advanced disease (T4 tumour burden) although the numbers were small (5 out of 62 treated surgically) (Zenga 2015).
The overall picture from retrospective and non‐randomised controlled data is unclear at present and we therefore await the results of the prospective randomised controlled trials currently ongoing.

Study search and selection process (PRISMA diagram).
| Low‐dose adjuvant radiotherapy compared with standard‐dose adjuvant radiotherapy for patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery | ||||||
| Patient or population: patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery Settings: post minimally invasive transoral surgery Intervention: low‐dose adjuvant radiotherapy Comparison: standard‐dose adjuvant radiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard‐dose adjuvant radiotherapy | Low‐dose adjuvant radiotherapy | |||||
| Overall survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Disease‐free survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Swallowing ability Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| Voice (Voice Handicap Index) Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Standard‐dose adjuvant radiotherapy alone compared with adjuvant chemoradiotherapy for patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery | ||||||
| Patient or population: patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery Settings: post minimally invasive transoral surgery Intervention: standard‐dose adjuvant radiotherapy alone Comparison: adjuvant chemoradiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Adjuvant chemoradiotherapy | Standard‐dose adjuvant radiotherapy alone | |||||
| Overall survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Disease‐free survival Follow‐up: at 1, 2, 3 and 5 years | No data available (no included studies) | |||||
| Swallowing ability Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| Voice (Voice Handicap Index) Follow‐up: at 1, 6, 12 and 24 months | No data available (no included studies) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||

