Intervenciones de cuidados paliativos para pacientes con esclerosis múltiple
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | 4 publications One centre cross‐over trial: open randomised fast‐track phase II controlled trial Randomisation ratio 1:1, stratified by gender, age, date of diagnosis and communication capacity N = 52 / Available for analysis = 46 Treatment duration: 12 weeks Follow‐up duration: 24 weeks | |
| Participants | Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention: Palliative Care Team ‐ Fast track
Control: Standard Best Practice
| |
| Outcomes | Primary outcome:
Secondary outcomes:
Time points: baseline, six weeks from baseline, twelve weeks from baseline, eighteen weeks from baseline, twenty‐four weeks from baseline | |
| Notes | Contact made with authors. Dr Nilay Hepgul, on behalf of Dr Irene Higginson and Dr Polly Edmonds, provided requested data. Patient consent: obtained Recruitment: from August 2004 to August 2005 Ethics approval: King's College Hospital NHS Trust Local Research Ethics Committee (Protocol number: 01‐04‐018) Funding: MS Society 676/01 Authors declared no competing interests. MRC Framework for the Evaluation of Complex Interventions Detailed protocol registered at clinicaltrials.gov: NCT00364936 (https://clinicaltrials.gov/ct2/show/NCT00364936?term=NCT00364936&rank=1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was conducted by independent statistical colleagues using the minimization method immediately after baseline interview." The sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Statistical colleagues, independent of the research and clinical team, registered the patients, conducted the randomisation and informed the research team, who then informed the patients, and if patients were "fast track" passed their details to the clinical team." The allocation concealment was not specified. |
| Blinding of participants and personnel (performance bias) | High risk | Given the nature of the intervention, there was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "We were unable to blind the interviewers...." Unblinded outcome assessor |
| Incomplete outcome data (attrition bias) | High risk | Data missing in MSIS physical subscale at 3 months for 10/26 (38%) participants in the fast‐track group and 19/26 (73%) participants in the control group |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively in August 2006 (enrolment started in March 2004). Available from: https://clinicaltrials.gov/ct2/show/NCT00364936?term=NCT00364936&rank=1). As the protocol was retrospectively registered, we were not able to assess if all planned outcomes were reported. |
| Other bias | Low risk | There was no imbalance at baseline. The authors reported results on the first and the second period. |
| Methods | 2 publications Monocentric, phase II, pilot randomised controlled trial Parallel design No sample size was calculated. Randomisation ratio 1:1, stratified by same diagnosis (ALS, MS or PD) and similar clinical necessities MS patients = 18 / Available for analysis = 16 Intervention group = 10 / Control group = 8 Treatment duration: 16 weeks Follow‐up duration: 16 weeks | |
| Participants | Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention: Specialist Palliative Care Service ‐ Fast track
Control: (Standard treatment)
| |
| Outcomes | Primary outcomes:
Secondary outcomes:
Time points: baseline, 16 weeks from baseline | |
| Notes | Contact made with authors. Simone Veronese provided all available data. Design followed the Medical Research Council Framework. No protocol was registered. Competing interests: none declared Patient consent: obtained Ethical approval: Ethics Committee of Department of Neuroscience, University of Torino and S. Luigi Gonzaga Hospital, Orbassano and University of Kent Ethics Committee, University of Kent at Canterbury, UK Funding: no funding declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomisation was undertaken by placing two unrecognisable white folders, containing the two patients charts, on a secretary' s desk. The secretary was asked to pick up one randomly and this was chosen as the Fast Track patient, the other patient went into the Standard Treatment group." The method for randomisation was inadequate. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not done. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Team members and study personnel could not be blind to participants, as they were involved in care." Given the nature of the intervention, there was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | Quotes: "The team can assess symptoms, prescribe medications, provide nursing care and physical therapies as well as psychological support and bereavement care." The same team that provided care also assessed the outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Data provided by the authors (email): 2/10 (20%) losses for palliative care group and no loss for control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found to compare planned and reported outcomes. |
| Other bias | Low risk | There was no imbalance at baseline. No other source of bias was found. |
| Methods | 4 publications Multicentric phase II/III single‐blind randomised controlled trial and nested qualitative study Three Italian centres: Milan, Rome and Catania Dyad: patient and caregiver Randomisation ratio 2:1, stratified for EDSS score (8.0‐8.5, 9.0‐9.5), cognitive status and centre N = 78 / Available for analysis = 76 Treatment duration: 6 months Follow‐up duration: 6 months | |
| Participants | Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention: HPA (Home‐based palliative approach)
Control: usual care (UC)
At the end of the study, control group dyads were offered the home palliative care program. | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Time points: baseline, three months from baseline and six months from baseline | |
| Notes | To date, authors have not answered our contact for requesting data. All randomised participants were included in the ITT analysis for the primary outcomes. Protocol registered at WHO‐ICTRP: http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN73082124 Study in accordance with the Declaration of Helsinki Recruitment: from January 2015 to November 2015 Patient consent: obtained Ethical approval: Foundation IRCCS Neurological Institute 'C. Besta' Milan, Foundation 'S. Lucia' Hospital Roma and University Hospital Catania Funding source: the Fondazione Italiana Sclerosi Multipla (FISM) funded the trial (Grant No. 2014/S/1 to A.S.). All authors declared no competing interest, although some of them received speaker honoraria, research funding, speaking fees or travel grants from different companies (Bayer Schering, Biogen Idec, Novartis, Genzyme, Merck Serono, Sanofi Aventis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation to treatment groups was done using a third‐party, web‐based computerized randomisation procedure with stratified minimization for EDSS score (8.0‐8.5, 9.0‐9.5), presence of severe cognitive compromise (clinical judgement), and centre." The method for randomisation was adequate (web‐based computerised randomisation). |
| Allocation concealment (selection bias) | Low risk | Quote: "Following randomisation, an email informing on dyad assignment is sent to the Principal Investigator and the HPA team." The method for allocation concealment was adequate. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The team informs the dyad about assignment. The team also contacts the patient's caring physician (GP, neurologist or other physician responsible for the patient's care) to inform him/her about the study and (for dyads assigned to HPA) define a common agenda." Given the nature of the intervention, participants and personnel could not be blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: "To reduce measurement bias, the baseline and follow‐up assessments are performed by independent examiners blind to treatment assignment (one examiner plus backup at each centre, both trained, neither involved in HPA delivery). Prior to each follow‐up visit, study dyads will be reminded not to disclose their allocation by mentioning any contact with the HPA team to the blind examiner." "The blind examiners used a web‐based case report form (eCRF), so that visit 1‐3 data were available to HPA teams and coordination unit." The outcome assessors were blind to participants allocation and participants were oriented not to mention their group allocation to the assessors. |
| Incomplete outcome data (attrition bias) | High risk | The authors reported results of ITT analysis using an imputation method for the SEIQOL‐DW (lost participants 11/52, 21% in the HPA group and 5/26, 19% in the UC group). |
| Selective reporting (reporting bias) | Low risk | Prospective protocol available at http://isrctn.com/ISRCTN73082124. All planned outcomes were properly reported. |
| Other bias | Low risk | There was no imbalance at baseline. No other source of bias was found. |
ADL: Activity of Daily Living
ALS: Amiotrophic Lateral Sclerosis
ALSFRS‐R: Amyotrophic Lateral Sclerosis Functional Rating Scale‐Revised
CBI: Caregiver Burden Inventory
EDSS: Expanded Disability Status Scale
EQ‐5D‐3L: European Quality of Life Five Dimensions
FIM: Functional Independence Measure
HADS: Hospital Anxiety and Depression Scale
H&Y: Hoehn & Yahr
HPA: Home‐based palliative approach
IADL: Instrumental Activity of Daily Living
ITT: Intention to Treat
MS: Multiple Sclerosis
MSIS: Multiple Sclerosis Impact Scale
NRS: Numerical Rating Scales
PD: Parkinson Disease
POS: Palliative Outcome Scale
POS‐S‐MS: Palliative Outcome Scale ‐ Symptoms ‐ Multiple Sclerosis
SEIQoL: Schedule for the Evaluation of Individual Quality of Life
SEIQoL‐DW: Schedule for the Evaluation of Individual Quality of Life ‐ Direct Weighting
SF‐36: Short–Form Health Survey: 36 Items
UC: Usual care
ZBI: Zarit Burden Interview
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This study is a narrative review. | |
| This study is a narrative review. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 12 weeks Show forest plot | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 6.07 [‐4.91, 17.06] |
| Analysis 1.1  Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 1 Health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 12 weeks. | ||||
| 2 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in physical score from baseline to 12 weeks Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 6.8 [‐11.16, 24.76] |
| Analysis 1.2  Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 2 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in physical score from baseline to 12 weeks. | ||||
| 3 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in psychological score from baseline to 12 weeks Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.9 [‐3.12, 4.92] |
| Analysis 1.3  Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 3 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in psychological score from baseline to 12 weeks. | ||||
| 4 Disability (EDSS, higher scores mean worst symptoms) ‐ Change in disability score from baseline to 16 weeks Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.44, 0.70] |
| Analysis 1.4  Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 4 Disability (EDSS, higher scores mean worst symptoms) ‐ Change in disability score from baseline to 16 weeks. | ||||
| 5 Anxiety (HADS, higher scores means worst symptoms) ‐ Change in anxiety from baseline to 16 weeks Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐0.94, 5.94] |
| Analysis 1.5  Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 5 Anxiety (HADS, higher scores means worst symptoms) ‐ Change in anxiety from baseline to 16 weeks. | ||||
| 6 Depression (HADS, higher scores means worst symptoms) ‐ Change in depression from baseline to 16 weeks Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐4.14, 3.14] |
| Analysis 1.6  Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 6 Depression (HADS, higher scores means worst symptoms) ‐ Change in depression from baseline to 16 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in the health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 24 weeks Show forest plot | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 4.8 [‐12.32, 21.92] |
| Analysis 2.1  Comparison 2 Palliative care versus usual care (long‐term, ≥ six months post‐intervention), Outcome 1 Change in the health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 24 weeks. | ||||
| 2 Serious adverse events Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.44, 2.12] |
| Analysis 2.2  Comparison 2 Palliative care versus usual care (long‐term, ≥ six months post‐intervention), Outcome 2 Serious adverse events. | ||||
| 3 Hospital admission Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.24, 2.52] |
| Analysis 2.3  Comparison 2 Palliative care versus usual care (long‐term, ≥ six months post‐intervention), Outcome 3 Hospital admission. | ||||

Study flow diagram.
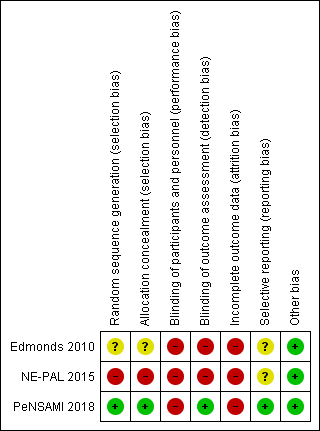
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 1 Health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 12 weeks.

Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 2 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in physical score from baseline to 12 weeks.

Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 3 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in psychological score from baseline to 12 weeks.

Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 4 Disability (EDSS, higher scores mean worst symptoms) ‐ Change in disability score from baseline to 16 weeks.
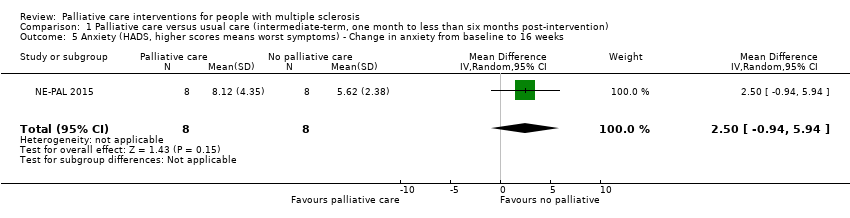
Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 5 Anxiety (HADS, higher scores means worst symptoms) ‐ Change in anxiety from baseline to 16 weeks.

Comparison 1 Palliative care versus usual care (intermediate‐term, one month to less than six months post‐intervention), Outcome 6 Depression (HADS, higher scores means worst symptoms) ‐ Change in depression from baseline to 16 weeks.

Comparison 2 Palliative care versus usual care (long‐term, ≥ six months post‐intervention), Outcome 1 Change in the health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 24 weeks.

Comparison 2 Palliative care versus usual care (long‐term, ≥ six months post‐intervention), Outcome 2 Serious adverse events.

Comparison 2 Palliative care versus usual care (long‐term, ≥ six months post‐intervention), Outcome 3 Hospital admission.
| Palliative care compared to usual care (long time) for people with multiple sclerosis | ||||||
| Patient or population: Adults people with multiple sclerosis (including RRMS, SPMS, PPMS, PRMS)1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control group (long time) | Risk with Palliative care | |||||
| Health‐related quality of life Assessed by: SeiQoL‐DW (higher scores mean better quality of life) Follow‐up: long‐term (24 weeks) | The mean change of the SeiQoL score in the control group was ‐4.0 | The mean difference of the SeiQoL score in palliative group was 4.8 higher (‐12.43 lower to 22.03 higher) | 58 | ⊕⊝⊝⊝ | ||
| Serious adverse events Assessed by: number of participants presenting at least one serious adverse event Follow‐up: long‐term (24 weeks) | 269 per 1.000 | 261 per 1.000 | RR 0.97 | 76 | ⊕⊕⊝⊝ LOW 3.4 | |
| Hospital admission Assessed by: number of participants admitted in hospital Follow‐up: long‐term (24 weeks) | 154 per 1.000 | 120 per 1.000 | RR 0.78 | 76 | ⊕⊕⊝⊝ LOW 3,4 | |
| Fatigue ‐ Not reported at long term follow‐up | ||||||
| Disability ‐ Not reported at long term follow‐up | ||||||
| Anxiety ‐ Not reported at long term follow‐up | ||||||
| Depression ‐ Not reported at long term follow‐up | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 RRMS: Relapsing‐remitting Multiple Sclerosis; SPMS: Secondary‐progressive Multiple Sclerosis; PPMS: Primary‐progressive Multiple Sclerosis; PRMS: Progressive‐relapsing Multiple Sclerosis. 2 Downgraded two levels due to risk of bias (unblinded participants and outcome liable to participant subjective judgement and incomplete outcome data). 3 Downgraded one level due to imprecision (low number of participants and wide confidence interval crossing the null). 4 Downgraded one level for risk of selective reporting (2/3 studies did not report health‐related quality of life measured by SeiQoL‐DW at 24 weeks). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 12 weeks Show forest plot | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 6.07 [‐4.91, 17.06] |
| 2 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in physical score from baseline to 12 weeks Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 6.8 [‐11.16, 24.76] |
| 3 Health‐related quality of life (Multiple Sclerosis Impact Scale, higher scores means worst quality of life) ‐ Change in psychological score from baseline to 12 weeks Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.9 [‐3.12, 4.92] |
| 4 Disability (EDSS, higher scores mean worst symptoms) ‐ Change in disability score from baseline to 16 weeks Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.44, 0.70] |
| 5 Anxiety (HADS, higher scores means worst symptoms) ‐ Change in anxiety from baseline to 16 weeks Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐0.94, 5.94] |
| 6 Depression (HADS, higher scores means worst symptoms) ‐ Change in depression from baseline to 16 weeks Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐4.14, 3.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in the health‐related quality of life (SeiQoL‐DW, higher scores means better quality of life) ‐ Change from baseline to 24 weeks Show forest plot | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 4.8 [‐12.32, 21.92] |
| 2 Serious adverse events Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.44, 2.12] |
| 3 Hospital admission Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.24, 2.52] |

