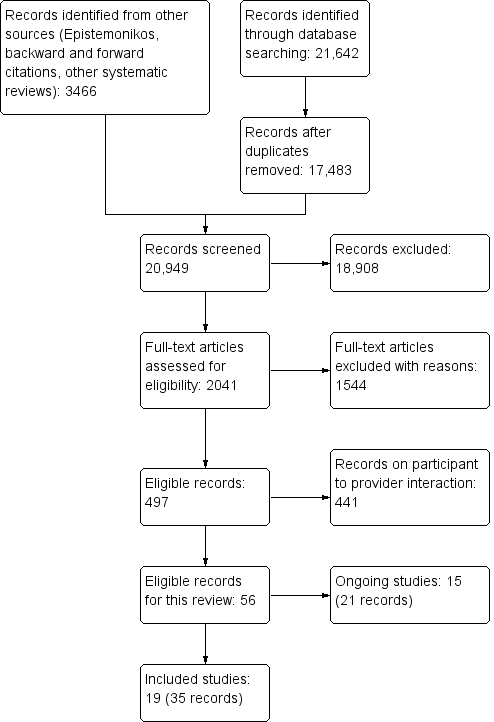
Tecnologías móviles para apoyar la comunicación y la gestión de la atención médica entre los profesionales sanitarios
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel assignment) Unit of allocation: Participant (GP) | |
| Participants | Providers Number: 296 participants randomised (same number ITT); number of professionals not described Type: GPs consulting with dermatologists in secondary care Other relevant characteristics: none reported Participants Number: Eligible: I: 148; C: 148 Analysed: same number (ITT) Mean age (SD): 49 years (14) Gender (% female): 50% Inclusion criteria: Adults diagnosed with plaque psoriasis, access to Internet and digital camera or mobile phone with camera Exclusion criteria: No diagnosis of plaque psoriasis, living outside the designated catchment area Other relevant characteristics: Mainly white (63%), with college education or more (88%), and working full‐time (47%). Baseline scores for severity of the condition were lower than anticipated, as several participants were receiving other therapies Location and study setting: USA, 3 regions Recruitment method: Participants recruited from practice‐based research networks, federally‐qualified health centres, and university‐based clinics, national groups and general public Duration: Number of sessions varied by participant, 12‐months follow‐up. Study ran between 2 February 2015 and 18 August 2017 Withdrawals: 6% of randomised participants were lost to follow‐up and 4% withdrew | |
| Interventions | Intervention components: Online, collaborative connected‐health model between participant, PCP and dermatologist. PCP could communicate with the dermatologist using the consultation function, sending digital photographs and clinical history for discussion. The dermatologist would assess the data and reply to the PCP within 2 business days, recommending treatment as well as educational materials for the participant. With the PCP permission, the dermatologist could also contact the participant directly. The PCP could also request for the dermatologist to become the main HCP. All communication was done through a secure web‐based platform. Participants were paid for participating in the study, through gift cards Comparison: Usual care: in‐person care as needed, frequency established by participants and their providers Technical equipment used: Secure safety policy–compliant web‐based connected‐health platform; mobile phone or digital camera for collecting images Fidelity assessment: Not reported; protocol states that protocol deviations will be noted, but not how they will be assessed | |
| Outcomes | Main outcome: Self‐reported psoriasis severity Other outcomes: Quality of life, access to care; depression; disease severity Time points reported: Baseline, 3‐, 6‐, 9‐, 12‐months | |
| Notes | Funding: Patient‐Centered Outcomes Research Institute Award (IHS‐071502‐IC) Ethical approval: Approved by university institutional review boards. Trial registry NCT02358135 Conflicts of interest: "Dr Armstrong reported serving as an investigator, consultant, advisor, and/or speaker for AbbVie, Janssen, Lilly, Novartis, Sanofi, Regeneron, Leo, Science 37, Modmed, Pfizer, Ortho Dermatologics, and Modernizing Medicine. Dr Gelfand reported serving as a consultant for and receiving honoraria from BMS, Coherus (DSMB), Dermira, GSK, Janssen Biologics, Menlo Therapeutics, Novartis Corp, Regeneron, Dr Reddy’s Laboratories, Sanofi, and Pfizer Inc; receiving research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Janssen, Novartis Corp, Regeneron, Sanofi, Celgene, Ortho Dermatologics, and Pfizer Inc; receiving payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly, Ortho Dermatologics, and AbbVie; and being a co–patent holder of resiquimod for treatment of cutaneous T‐cell lymphoma. Dr Wong reported being an employee of DirectDerm. No other disclosures were reported." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated random block sizes (p.3) |
| Allocation concealment (selection bias) | Low risk | Comment: Independent statistician (p.4) |
| Baseline outcome measurements similar (selection bias) | Low risk | Comment: Baseline data provided for main outcome and similar between groups (Table 1) |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline characteristics provided and similar between groups (Table 1) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants or personnel |
| Blinding of objective outcome assessment (detection bias) | Low risk | Comment: Data analyst blinded |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: Patient‐reported outcome measures, unblinded participants and personnel |
| Protection against contamination | High risk | Comment: Patients were randomised to the groups |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Comment: Not all outcomes specified in the protocol reported in publications (distance travel, wait time) |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel assignment) Unit of allocation: Participant (GP) | |
| Participants | Providers Number: 58 GPs randomised (31 analysed), number of hospital‐based physicians not reported Type: GPs consulting with physicians in secondary care Other relevant characteristics: GPs' median age was approximately 57 years, on average 34 km away from the hospital Participants Number: Eligible: I: 92; C: 164 Analysed: I: 72; C: 101 Median age: I: 56 years; C: 55 years Gender (% female): I: 59%; C: 60% Inclusion criteria: Adults who consulted with the GP and required a referral to secondary care Exclusion criteria: People who required or preferred an in‐person appointment Other relevant characteristics: Not reported Location and study setting: Spain, 6 primary care practices Recruitment method: Not reported Duration: Single session; 3‐months follow‐up. Study ran between March and December 2016 Withdrawals: 38% (N = 19) of eligible GPs were excluded from analysis (IG: 6 GPs excluded as they did not request phone consultations with physicians; CG: 13 GPs excluded as they did not collect data for at least 50% of their eligible participants); of those receiving care by GPs allocated to the IG, 50% were excluded from analysis as they were given in‐person appointments (either because they required or preferred it) | |
| Interventions | Intervention components: If during an initial appointment the GP considered a referral for a speciality appointment was needed, the GP would request an eConsult with the specialist, which would take place at the primary care practice. The GP would call the consultant at a convenient time, while the participant was still in the room. The 2 healthcare professionals would agree on the treatment, whether further investigations were required, and book follow‐up appointments Comparison: Usual care ‐ participant was given a referral for an in‐person appointment at secondary care Technical equipment used: Hands‐free telephone Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Waiting days between the GP referring the participant for an appointment and the appointment being provided; number of avoided/avoidable face‐to‐face referrals; waiting days for the resolution of the process Time points reported: Post‐intervention (3‐months follow‐up) | |
| Notes | Funding: Andalusian Society of Family and Community Medicine ‐SAMFyC‐ (Record ref. 157/18) Ethical approval: Regional research ethics committee. Trial registry ACTRN12617001536358 Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Random‐number table (p. 3) |
| Allocation concealment (selection bias) | Low risk | Comment: Unit of allocation was by primary care practices, and allocation was performed on all units at the start of the study (p.3) |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | High risk | Comment: Baseline differences between groups about participants distance to hospital and geographical living area (table 2) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants or personnel |
| Blinding of objective outcome assessment (detection bias) | Low risk | Comment: Automatically extracted from the EMR (p.4) |
| Protection against contamination | Low risk | Comment: Allocation by healthcare providers |
| Incomplete outcome data (attrition bias) | High risk | Comment: High attrition rates (almost 40% randomised GPs excluded from analysis; p.4) |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes listed in Methods reported in Results |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Cluster‐randomised trial (parallel assignment) Unit of allocation: Cluster (clinics) | |
| Participants | Providers Number: 20 GPs, number of hospital‐based physicians not reported Type: GPs consulting with physicians in secondary care Other relevant characteristics: Not reported Participants Number: Eligible: I: 221; C: 229 Analysed: same number Median age: Not reported Gender (% female): Not reported Inclusion criteria: Adults who consulted with the GP and required a referral to secondary care for skin lesions and problems Exclusion criteria: Not reported Other relevant characteristics: Not reported Location and study setting: Mongolia, 20 rural health clinics in 1 of the least densely‐population countries in the world. Recruitment method: Not reported Duration: 5‐month follow‐up. Study ran between September 2013 and January 2014 Withdrawals: Not reported | |
| Interventions | Intervention components: A primary care provider in a rural health clinic used a smartphone camera to collect images and clinical history of participants with skin lesions and problems. The PCP attended a 2‐day training session to learn how to take images and use the medical record system and software on mobile phones. The information was sent along with a teleconsultation request using the electronic medical record. The dermatologist reviewed the information and sent feedback within 24 hours. Comparison: Usual care ‐ GPs referred participants to district hospitals or the National Dermatology Centre Technical equipment used: Android‐based system Fidelity assessment: GPs from clinics allocated to the intervention attended a 2‐day training session on how to take pictures using the devices provided and how to operate the electronic medical record | |
| Outcomes | Main outcomes: Tertiary care referrals; costs Time points reported: Post‐intervention (5‐months follow‐up) | |
| Notes | Funding: National science Council Project no. NSC 101‐2923‐E‐038 ‐001 ‐MY2, Ministry of Health and Welfare (MOHW), Taiwan, under grant MOHW103‐TD‐B‐111‐01 and Taipei Medical University under grant 101TMUSHH‐21 Ethical approval: Not reported Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Comment: Cluster randomisation, all done at the start of the study |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | Unclear risk | Comment: Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Not reported |
| Blinding of objective outcome assessment (detection bias) | Unclear risk | Comment: Not reported how it was collected |
| Protection against contamination | Low risk | Comment: Allocation was by practice, unlikely that the control group received the intervention |
| Incomplete outcome data (attrition bias) | Low risk | Comment: There was no attrition |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes mentioned in Methods are presented in Results |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Cluster‐randomised trial (parallel) Unit of allocation: Cluster (10 clinics; I: 4, C: 6) | |
| Participants | Providers Number: I: 13, C: 16 Type: Community‐based peer health workers consulting with clinic staff Other relevant characteristics: None reported Participants Number: Randomised: 970 (I: 446, C: 524), Analysed: ITT analysis Median age (range): 35 years (15‐76) Gender (% female): 66% Inclusion criteria: Adults attending eligible clinics who were receiving or started receiving ART Exclusion criteria: None reported Other relevant characteristics: None reported Location and study setting: Uganda, 10 clinic sites Recruitment method: Not applicable; participants were not informed about the study as PHWs were performing routine care functions Duration: Intervention lasted 26 weeks, median follow‐up time was 103 weeks (97 ‐ 111 weeks), study was conducted between May 2006 and July 2008 Withdrawals: 11% and 12% of participants allocated to Intervention and Control were lost to follow‐up; main reason was death (8.3% and 10.1% of all participants lost, respectively) | |
| Interventions | Intervention components: mHealth intervention: Periodic home visits, supported by mobile phone. PHWs participated in a 1‐day residential training and were given a mobile phone and an hour‐long field‐based practicum. After each home visit the PHW would message data on adherence and other clinical information to a centralised database, which was staffed by clinic staff. Once a message had been received, clinic staff could provide care instructions, send a higher‐level care provider, or arrange for the participant to be taken to a healthcare facility. PHWs could also call a hotline if they had questions Comparison: No mobile phone, additional training or access to the hotline. All PHWs had previously been enrolled in a study of ART provision, where they received a 2‐day residential training on HIV‐related topics, as well as adherence counselling, patient confidentiality and filling out home visit forms. The main goal of the home visits was to evaluate and encourage adherence to ART therapy Technical equipment used: mobile phones (no further details provided) Fidelity assessment: PHWs were supervised by a member of staff (part‐time worker) | |
| Outcomes | Main outcomes: Participants’ cumulative risk of virologic failure | |
| Notes | Funding: Doris Duke Charitable Foundation, The Division of Intramural Research, The National Institute for Allergy and Infectious Diseases, National Institutes of Health, and a National Institutes of Health Training Grant and Career Development Grant Ethical approval: Institutional review boards at the Uganda Virus Research Institute’s Safety and Ethics Committee, the Uganda National Council for Science and Technology, and Johns Hopkins University Conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Inadequate information reported, no description of sequence generation ‐ Quote: "The 10 sites (...) randomised 2:3 to PHWs receiving a mHealth support intervention or not" (p.3) |
| Allocation concealment (selection bias) | Low risk | Comment: Cluster randomisation, all clinics done at the start of the study |
| Baseline outcome measurements similar (selection bias) | Low risk | Quote: "[K]ey predictors of clinical outcomes appeared well balanced between arms." (p.4) |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Reported and similar between groups (Table 1) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants or personnel |
| Blinding of objective outcome assessment (detection bias) | Unclear risk | Comment: No information provided on how objective outcomes were collected |
| Protection against contamination | Low risk | Comment: Cluster‐randomised trial with participants/peer health workers with mobile phone intervention access in separate districts |
| Incomplete outcome data (attrition bias) | High risk | Comment: High attrition rate |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes reported in different publications |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: Two Type: Primary care provider at the rural primary practice consulting with ophthalmologist in the university setting Other relevant characteristics: None reported Participants Number: Randomised: 59 (I: 30, C: 29), Analysed: same number Mean age (SD): Not reported Gender (% female): Not reported Inclusion criteria: Adults with diabetes diagnosed by a physician Exclusion criteria: Not reported Other relevant characteristics: Mainly African‐Americans Location and study setting: USA, 1 rural primary practice and 1 urban university hospital Recruitment method: Not reported Duration: Not reported Withdrawals: Not reported; all participants analysed | |
| Interventions | Intervention components: A primary care provider in a rural primary care practice used a nonmydriatic retinal camera and video‐conferencing to send real‐time images to an ophthalmologist located in an urban university setting. The ophthalmologist assessed the retinal photograph and communicated with the participant and the primary care professional Comparison: Usual care ‐ participants were reminded to schedule examinations with their usual eye‐care provider Technical equipment used: nonmydriatic retinal camera (Topcon with IMAGEnet software) and video‐conferencing (no further details provided) Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Frequency of eye examinations Time points reported: Post‐intervention | |
| Notes | Funding: Not reported Ethical approval: Not reported Conflicts of interest: Not reported Notes: short reports (letter and abstract), limited information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of objective outcome assessment (detection bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of subjective outcome assessment (detection bias) | Unclear risk | Comment: Not enough information provided |
| Protection against contamination | Unclear risk | Comment: Not enough information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Not enough information provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not enough information provided |
| Other bias | Unclear risk | Comment: Not enough information provided |
| Study characteristics | ||
| Methods | Study design: Cluster‐randomised trial (parallel) Unit of allocation: Cluster (36 GP practices; I: 19; C: 17) | |
| Participants | Providers Number: GPs: I: 59, C: 51; Dermatologists: 5 Type: GPs consulting with dermatologists Other relevant characteristics: GPs: I: 29% female, C: 35% female Participants Number: Randomised: 631 (I: 327, C: 304), Analysed: 605 (I: 312, C: 293) Mean age (SD): I: 42 years (23); C: 44 years (20) Gender (% female): I: 56%; C: 64% Inclusion criteria: Practices were required to have facilities to send digital images over the Internet; participants were eligible if they were referred to a dermatologist by their GP Exclusion criteria: GPs who already used teledermatology; patients were excluded if they required an urgent dermatology appointment Other relevant characteristics: None reported Location and study setting: The Netherlands, 36 primary care practices Recruitment method: Dermatologists working in eligible areas were invited to participate; GPs working in practices that referred participants to those dermatologists were then invited to participate Duration: Intervention was 1 teleconsultation, with 1 month follow‐up; study conducted between February 2004 and January 2006 Withdrawals: 5% (I) and 7% (C) of participants randomised were lost to follow‐up, main reasons were problems with data entry and participants visiting another dermatologist; for 39% of participants, information on the main outcome was missing, mainly because GPs did not complete study forms | |
| Interventions | Intervention components: GPs allocated to the intervention group received detailed instructions on how to take digital images and use the web‐based form. GPs took 4 digital images of the skin problems and completed a structured form (which included questions about duration and location of the skin lesion) on a secure website; the form was sent to the dermatologist along with the main reason for referral (diagnosis, advice, reassurance). At this stage GPs could also refer the participant to another dermatologist. Within 48 hours the dermatologist assessed the images and replied to the GP using the same system, providing advice and whether further investigations or urgent referrals were required. After a month the dermatologist saw the participant in person, regardless of the outcome of the online consultation Comparison: Usual care ‐ participants saw a dermatologist according to the usual procedures, usually by being referred by the GP who would give the participant a letter to take to the clinic Technical equipment used: digital cameras (Kodak EasyShare CX6230 2.0 megapixel) and a secure teledermatology website Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Proportion of and reasons for preventable consultations Other outcomes: Participant satisfaction in general and about interpersonal aspects of the consultation; costs Time points reported: 1 month follow‐up | |
| Notes | Funding: Senter Novem (Agency of the Dutch Ministry of Economic Affairs) and ZonMw (Dutch Organization for Health Research and Development). KSYOS Health Management Research provided the digital cameras and teleconsultation software Ethical approval: The ethics committee deemed this study to be exempt from review because the research did not interfere with usual care. Trial registration ISRCTN57478950 Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using dedicated randomisation software, practices were assigned to teledermatologic consultation or standard care." (p.559) |
| Allocation concealment (selection bias) | Low risk | Quote: "A special allocation concealment procedure (...) was followed to ensure that no allocation bias could occur." (p.559) |
| Baseline outcome measurements similar (selection bias) | Low risk | Comment: Diagnostic categories well‐balanced between groups (Table 4) |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline characteristics reported and similar between groups (Table 2) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Providers could not be blinded, no information on blinding of participants |
| Blinding of objective outcome assessment (detection bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: Subjective assessment of whether in‐person consultations could have been prevented done by consulting dermatologist, who knew which participants they had seen |
| Protection against contamination | Unclear risk | Comment: Treatment and diagnosis of GPs in intervention clinics may have been influenced cumulatively by consultations and contact with dermatologists |
| Incomplete outcome data (attrition bias) | High risk | Comment: High rate of missing data for the primary outcome |
| Selective reporting (reporting bias) | High risk | Comment: Trial registration states outcomes that were not reported (diagnostic accuracy, delay in treatment, learning effect GPs) |
| Other bias | Unclear risk | Comment: There were considerable problems following up participants and several methods have been reported to gather outcome data and analyse in different ways |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participants | |
| Participants | Providers Number: Not provided Type: Emergency physicians consulting with specialist physicians Other relevant characteristics: Not provided Participants Number: Randomised: 345 (IG: 173, CG: 172), Analysed: same number Mean age (SD): 48.5 years (22.1) Gender (% female): 33% Inclusion criteria: Participants: adults attending the emergency department; Physicians: owned a smartphone and were familiarised with secure messaging applications Exclusion criteria: Not reported Other relevant characteristics: Not reported Location and study setting: Turkey, 1 hospital Recruitment method: Not reported Duration: Intervention was consultation request using 2 different methods; study was conducted between November 2015 and February 2016 Withdrawals: No withdrawals or losses to follow‐up | |
| Interventions | Intervention components: Emergency physician requested consultation with a specialist physician using a secure messaging service (Whatsapp). Any additional medical information (e.g. blood pressure, x‐rays, ultrasounds, photographs) was sent through the same service Comparison: Usual care; consultations were requested by telephone, with any additional medical information (e.g. blood pressure, sensory‐motor findings, Glasgow Coma Score) sent verbally Technical equipment used: Smartphone with Whatsapp (owned by the healthcare professionals) Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Difference between groups for emergency department length of stay Other outcomes: Difference between groups in consult time (time when consultation was requested minus time when a bed was requested or discharge time); termination of consultation between groups Time points reported: Baseline, post‐intervention | |
| Notes | Funding: Not reported Ethical approval: Medical Ethics Committee. Trial registry NCT02586779 Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer programme (p.744) |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not enough information |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline characteristics provided and similar between groups (Table 1) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants or healthcare professionals, even though consulting physicians were blinded to the purpose of the study (p.744) |
| Blinding of objective outcome assessment (detection bias) | Low risk | Comment: Data collector was blinded (p.744) |
| Protection against contamination | Unclear risk | Comment: Healthcare professionals within the same hospital randomised and it is not clear whether communication occurred between them |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No attrition |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes stated in protocol are different from outcomes reported |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Cluster‐randomised trial (parallel) Unit of allocation: Cluster (42 sites, 21 each arm) | |
| Participants | Providers Number: Not reported Type: Community nurses consulting with diabetes specialist nurses and podiatrists Other relevant characteristics: Not reported Participants Number: Randomised: 182 (I: 94, C: 88), Analysed: same number Mean age (SD): I: 67.2 years (16.7); C: 65.5 years (16.5) Gender (% female): I: 75%; C: 74% Inclusion criteria: Adults aged ≥ 20 years with new diabetes‐related foot ulcers Exclusion criteria: Repeated ulcer treated in the past 6 months, mental illness, life expectancy < 1 year Other relevant characteristics: Diagnosed with diabetes for on average 20 years Location and study setting: Norway, 2 hospitals Recruitment method: Eligible patients attending 1 of 2 hospitals were invited to participate Duration: Intervention length could vary according the clinical needs, participants seen every 6 weeks, maximum follow‐up time as 12 months; not study conducted between September 2012 and June 2016 Withdrawals: For objective measures, all participants were followed‐up and included in the analysis; for subjective measures attrition rates were 29% for the IG and 35% for the CG | |
| Interventions | Intervention components: Telemedicine application composed by a mobile phone and an interactive web‐based ulcer record. Consultations happened every 6 weeks and included a written assessment and images taken using the mobile phone, which were then sent through the web to the specialist nurse or the podiatrist, who provided feedback. Any doubts could be further discussed. All staff received training in the use of the web‐based system, as well as in‐person access to hospital clinics to improve their practical skills Comparison: Usual care ‐ provided by outpatient clinic, usually scheduled every second week Technical equipment used: Smartphone (no further description) Fidelity assessment: Functionality was assessed yearly and minor adjustments introduced (protocol); all personnel received training and were following standardised guidelines for treatment of diabetes‐related foot ulcers | |
| Outcomes | Main outcomes: Ulcer healing time Other outcomes: Amputation; mortality; consultations; participant satisfaction; participant and healthcare professionals' experiences (qualitative) Time points reported: Baseline, post‐intervention (12 months post‐baseline) | |
| Notes | Funding: Norwegian Directorate of Health and Innovation Norway, Western Norway Regional Health Authority, Norwegian Diabetes Association, Western Norway University of Applied Sciences, Norwegian Research Council Ethical approval: Western Norway Regional Committee for Medical and Health Research Ethics. Trial registry: NCT01710774 Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Randomisation using computer programme (p.97) |
| Allocation concealment (selection bias) | Low risk | Comment: Done by a person independent of the study (p.97) |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Higher proportion of participants in the intervention group had ulcers in the toe area (60.6%) compared with control (38.6%) (p.99) |
| Baseline characteristics similar (selection bias) | Unclear risk | Comment: Higher proportion of participants in the intervention group had type II diabetes (86.2%) compared with control (71.6%) (p.99) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants or healthcare professionals |
| Blinding of objective outcome assessment (detection bias) | Low risk | Comment: Electronic records (protocol) |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: Participants and healthcare professionals not blinded, self‐reported |
| Protection against contamination | Unclear risk | Comment: Not enough information provided |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No attrition for objective outcomes; high attrition for the subjective outcome, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Comment: Several outcomes mentioned in protocol and not reported (e.g. quality of life, depression, anxiety) |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participants (HCP) | |
| Participants | Providers Number: Randomised: IG: 57; CG: 56 Type: Primary care practitioners consulting with specialist physicians Other relevant characteristics: PCP practicing in Ontario and not currently using eConsult. Mostly male (65%), 20 years since graduation Participants Number: Specific number of participants not reported; each PCP allocated to IG saw on average 724 participants during the pre‐intervention period (range 11 to 1692), whereas those in CG saw 828 participants during the same period (range 93 to 1971) Mean age (SD): Not reported Gender (% female): Not reported Inclusion criteria: Not reported Exclusion criteria: Not reported Other relevant characteristics: Not reported Location and study setting: Ontario, Canada Recruitment method: Eligible PCPs were sent an information pack and invited to participate by a third party; those interested could get directly in touch with the research team Duration: Trial conducted between 31 January 2014 and 26 September 2014, 12 months follow‐up from baseline Withdrawals: Approximately 12% of PCPs were not analysed at baseline and follow‐up due to lack of referral data | |
| Interventions | Intervention components: Champlain BASE™ eConsult service, a web‐based application that the PCP used to submit participant‐specific clinical questions to specialists. The specialists responded within 7 days, with recommendations, further questions, or recommendation for a face‐to‐face referral. Specialists received financial incentives for each eConsult they undertook Comparison: Usual care ‐ standard referral practices Technical equipment used: The application could be accessed through smartphone, laptop or desktop; most users accessed it through their smartphones Fidelity assessment: Participant PCPs underwent an orientation session and brief training, without which they could not access the system | |
| Outcomes | Main outcomes: Specialist referral rate per 100 participants seen to all medical specialties available through eConsult service Other outcomes: Referral rate to all medical specialties Time points reported: Baseline, follow‐up | |
| Notes | Funding: Ontario Ministry of Health and Long‐Term Care (MOHLTC) and Health Services Research Fund, Ministry Grant #06547, Province of Ontario, Primary Health Care Program (INSPRE‐PHC). Ethical approval: Hospital Research Ethics Board and the Institutional Review Board. Trial registry NCT02053467 Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated random list of numbers ("Randomization") |
| Allocation concealment (selection bias) | Low risk | Comment: Done by an independent research staff member, using opaque sealed envelopes ("Randomization") |
| Baseline outcome measurements similar (selection bias) | Low risk | Comment: Baseline outcome measurements provided and similar between groups, although IG had slightly lower referral rates at baseline (Table 2, Table 3) |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline characteristics provided (Table 1); differences between groups for model of practice and practice size, practice model and location adjusted for in the analysis |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible due to the nature of the intervention |
| Blinding of objective outcome assessment (detection bias) | Low risk | Comment: Automatically extracted from electronic medical records ("Data sources") |
| Protection against contamination | Unclear risk | Comment: PCPs were randomised to intervention and control groups; unclear whether they could be located in the same practice |
| Incomplete outcome data (attrition bias) | High risk | Comment: Authors note that for missing outcomes, due to limitations related to the use of administrative health databases, they did not end up with 50 providers per arm, resulting in an underpowered trial |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes reported as per protocol |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: Primary care professionals not reported, 2 experienced investigators Type: Primary care professionals consulting with experienced investigators based at an eye institute Other relevant characteristics: Participants Number: Randomised: 567 (I: 296, C: 271), Analysed (12 months follow‐up): same number Mean age (SD): I: 50.2 years (12.3); C: 51.7 years (11.3) Gender (% female): I: 52%; C: 51% Inclusion criteria: Adults diagnosed with diabetes who were scheduled to visit the primary care provider Exclusion criteria: Cognitive impairment Other relevant characteristics: On average, diagnosed with diabetes for 10 years. The overall prevalence of diabetic retinopathy (21.5%) was lower than the national average (28.5%) Location and study setting: USA, 2 primary care clinics Recruitment method: Research assistants called potentially eligible patients and invited them to participate Duration: Follow‐up lasted 48 months; not reported when study was conducted Withdrawals: 100% response rate at 12 months follow‐up, approximately 76% response rate at 48 months follow‐up | |
| Interventions | Intervention components: Telemedicine ‐ digital images were captured with a non‐mydriatic camera by clinic technicians and sent to a specialist for review and report generation. Technicians performing imaging attended a 3‐day training session to learn how to take images and ongoing feedback as needed. Communication was done through private encrypted software, which transferred images and participant data to a secure database. Experienced investigators would receive an alert once the images were available, and grade them based on international standardised criteria, completing online reports that were automatically sent to clinic staff. Participants were also encouraged to see an eye care provider early as the camera‐based exam is not considered to be a replacement for a comprehensive eye exam. Participants received monetary incentive to complete follow‐up questionnaire Comparison: Usual care ‐ during their primary care visit, participants were encouraged to see an eye care provider yearly. If a participant did not have an eye care provider, the primary care professional would refer them. The study investigators contacted all the providers the participants could be referred to, asking them to complete the same assessment forms as done for those allocated to the intervention group. Participants in this group were also offered telemedicine screening after 48 months enrolment Technical equipment used: Digital non‐mydriatic fundus camera (model NM‐1000); Internal software for data transmission; Screen‐Vu stereoscope Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Percentage of participants receiving annual diabetic retinopathy screening examinations; percentage of eyes with worsening diabetic retinopathy; percentage of telemedicine participants who would require referral to an eye care professional for follow‐up care Time points reported: Baseline, follow‐up (12, 24, 36, and 48 months post‐baseline) | |
| Notes | Funding: National Eye Institute(NEI 3 K23 EY0155501‐01), the Centers for Disease Control and Prevention (CDCU48DP000024‐01 and 1U48DP002673‐01), and the Good Samaritan Foundation at Legacy Health Ethical approval: Institutional Review Boards of Legacy Health (Portland, OR),Oregon Health and Science University (Portland), and the Northwest Portland Area Indian Health Board (Portland). Trial registry: NCT01364129 Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a random number generator to randomly assign participants to the telemedicine group or the traditional surveillance group" (p.519) |
| Allocation concealment (selection bias) | Low risk | Quote: "We used a random number generator to randomly assign participants to the telemedicine group or the traditional surveillance group" (p.519) |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Baseline measurements relating to service use outcome (i.e. previous attendance for screening) not reported. Baseline measurements relating to the clinical outcome (diabetic retinopathy) not reported |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline characteristics reported and similar between groups (Table 2) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: No information on blinding of central study personnel; not possible to blind primary care providers as they conducted the screening examinations; not possible to blind participants who received examinations in different settings |
| Blinding of objective outcome assessment (detection bias) | High risk | Comment: Due to the nature of the intervention it was not possible to blind participants or personnel |
| Protection against contamination | Unclear risk | Comment: No specific mention of measures to prevent contamination |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Low attrition rates for first 2 data points. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Telemedicine was offered exclusively to the telemedicine group only to 2 years after recruitment. At 2 years standard‐care group also offered telemedicine. Yet follow‐up of exclusive telemedicine reported only to 18 months. Outcomes for worsening retinopathy reported for intervention and control groups combined |
| Other bias | High risk | Comment: Data collection methods differed for the 2 study groups. Whereas outcome data (screening attendance and clinical) in the telemedicine group were recorded by study staff (telemedical assessors, both report authors), the study relies upon eye‐care professionals in the community to report these for the standard care group. The researchers telephoned the community eye‐care professionals to introduce the project and request their participation in completing data collection forms for the study. It is uncertain how community eye‐care professional would know who was recruited in the study. Researchers also reviewed medical charts to search for eye examination data |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: Not reported Type: Home‐visiting nursing staff consulting with a hospital physician Other relevant characteristics: Not reported Participants Number: Randomised: 188 (I: 100, C: 88), Analysed: unclear Mean age (SD): I. 86.5 (7.0), C: 84.4 (7.1) Gender (% female): I: 72%; C: 76% Inclusion criteria: Adults aged ≥ 65 years, attending the Department of Clinical Nutrition, treated with home enteral nutrition Exclusion criteria: Not reported Other relevant characteristics: Most had multiple morbidities Location and study setting: Italy, 1 hospital Recruitment method: Not reported Duration: Intervention lasted 12 months; study conducted between January and December 2013 Withdrawals: 38% and 33% of participants were lost to follow‐up (I and C, respectively), reasons not provided | |
| Interventions | Intervention components: Usual care plus video consultation ‐ during the monthly home visits, the home‐visiting staff called the hospital physician using the tablet. The latter would visually examine the participant for different clinical signs (e.g. hydration, oedema). Video calls lasted on average 2 minutes. If necessary, nutrition and pharmacological therapy would be adjusted Comparison: Usual care ‐ regular monthly home visits done by nurses to perform scheduled evaluations, which included an electrocardiogram, pulse oximetry, and blood glucose and pressure measurement. Data collected were logged online and reviewed by the hospital physician 2 to 3 days after the visit Technical equipment used: Tablet (Samsung Galaxy) Fidelity assessment: Video‐consultation followed a specific protocol; no further details provided | |
| Outcomes | Main outcomes: Frequency and type of complications; frequency and reason for outpatient visits and hospitalisations; modification of nutrition therapy; frequency and duration of video‐consultations (intervention group only) Time points reported: Baseline, follow‐up (12 months post‐baseline) | |
| Notes | Funding: Not reported Ethical approval: Hospital Ethics Committee Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation (...) was carried out by the statistical service of [the hospital] using computer‐generated allocation" (p.763) |
| Allocation concealment (selection bias) | Low risk | Comment: Block randomisation was used |
| Baseline outcome measurements similar (selection bias) | Low risk | Comment: Baseline medical measurements that could impact the clinical outcome (main indicators of nutritional status, comorbidities, general health status) reported and similar between groups (Table 1) |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline characteristics provided and overall similar between groups; participants allocated to intervention were slightly older than those allocated to control |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants and personnel |
| Blinding of objective outcome assessment (detection bias) | High risk | Comment: Main outcome is overall complications. The home‐visit staff, who were not blinded, collected the outcome data |
| Protection against contamination | Unclear risk | Comment: Contamination between groups is possible as all home‐visiting staff and physicians had tablets which could be used with control participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: High rates of attrition, not explained |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes presented in the Methods are reported in the Results |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: Not reported Type: Primary care professional consulting with dermatologist Other relevant characteristics: Not reported Participants Number: Randomised: 698 (I: 351, C: 347), Analysed: 508: 236 in usual care and 272 in teledermatology Mean age (SD): I: 43.6 years; C: 46.8 years Gender (% female): I: 71%; C: 66% Inclusion criteria: Adults referred from Department of Defence primary care clinics Exclusion criteria: Urgent condition, multiple complaints Other relevant characteristics: Mainly white Location and study setting: USA, 4 primary care clinics (Department of Defence owned) Recruitment method: Eligible participants were invited to participate Duration: Single consultation with 4 months follow‐up; not reported when study was conducted Withdrawals: 33% of randomised participants did not complete follow‐up: 15% were withdrawn, mainly due to deployment or loss of privileges; 6% withdrew, mainly due to resolution of skin problem; 4% could not be contacted and were lost to follow‐up | |
| Interventions | Intervention components: A teledermatology appointment was scheduled, unclear how this was done. A dermatologist would then review the consultation and the images, and could either schedule a face‐to‐face appointment with the participant or send a diagnosis and management plan to the primary care professional Comparison: Usual care ‐ a dermatology appointment was scheduled at a clinic Technical equipment used: Digital camera (Coolpix 990, 3.3 megapixel); images were transferred using a web‐based secure server purposively developed Fidelity assessment: Data | |
| Outcomes | Main outcomes: Clinical improvement based on serial cutaneous examination; costs Time points reported: Baseline, post‐intervention (4 months post‐baseline) | |
| Notes | Funding: Telemedicine and Advanced Technology Research Center Ethical approval: Appropriate committees Conflicts of interest: "HSP is Chairman and Co‐founder of TeledermSolutions, Inc., a Web‐based teledermatology consultation service, is Co‐editor of Teledermatology: A user’s guide, published by Cambridge University Press, and is slated to receive royalties based on sales. JDW is Co‐editor of Teledermatology: A user’s guide, published by Cambridge University Press, and is slated to receive royalties based on sales." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Authors report that block randomisation was used (p.27) |
| Allocation concealment (selection bias) | Low risk | Comment: Following informed consent the sealed envelope was opened to reveal the randomisation assignment |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | High risk | Comment: Partciipants allocated to the control group were older than those allocated to intervention group (Table 1) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants or personnel as care pathway was different |
| Blinding of objective outcome assessment (detection bias) | Low risk | Comment: Calculated using repayment rates |
| Blinding of subjective outcome assessment (detection bias) | Low risk | Quote: "A dermatologist blinded to randomisation assignment reviewed the images." (p.27) |
| Protection against contamination | Unclear risk | Comment: No information on strategies to prevent contamination or evidence suggesting contamination |
| Incomplete outcome data (attrition bias) | High risk | Comment: High attrition rates |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes reported in different papers |
| Other bias | Low risk | Comment: No other apparent risk of bias |
| Study characteristics | ||
| Methods | Study design: Cluster‐randomised trial (parallel assignment) Unit of allocation: Cluster (8 primary care practices, 4 allocated to the intervention group and 4 allocated to the control group) | |
| Participants | Providers Number: 39 GPs, 3 dermatologists Type: GPs consulting with dermatologists Other relevant characteristics: Not reported Participants Number: Randomised: 109 (I:55, C:54), Analysed: 103 (I:53, C:50) Mean age: I: 44 years; C: 43.5 years Gender (% female): I: 70%; C: 50% Inclusion criteria: Adults with a skin condition for which the GP required a dermatologist's advice Exclusion criteria: Urgent medical care Other relevant characteristics: Not reported Location and study setting: France, 8 urban primary care practices Recruitment method: Participants identified by the GPs and invited to participate Duration: Single session; 90 days follow‐up Withdrawals: 5.5% of participants were excluded after being assessed as eligible (reasons provided) | |
| Interventions | Intervention components: GPs received training and a workbook on how to take photographs (p.2, top 2nd column). GPs took at least 3 photos of skin lesions and sent them with a standardised written message (date of symptoms, symptomatology, topography, description and extension of lesions, drug intake) through secure e‐mail to dermatologists. Dermatologists provided a diagnosis or possible differential diagnoses, and if necessary a management plan, which was implemented by the GP. The dermatologists could also book an appointment to see the participant in person Comparison: Usual care ‐ participants were given a standardised printed referral letter, which they could use to book an appointment with a dermatologist Technical equipment used: Photos were taken using either a mobile phone or digital camera (minimum 3 megapixels) Fidelity assessment: GPs received 2 hours training on how to take photos and were given a workbook explaining the detailed procedures to take photos compliant with the American Telemedicine Association recommendations | |
| Outcomes | Main outcomes: Days lapsed between the GP’s consultation and the dermatologist’s reply that allowed for the GP to begin treatment; participant's satisfaction; physicians' and participants' satisfaction; number of non‐usable photographs taken Time points reported: Post‐intervention (3 months post‐baseline) | |
| Notes | Funding: Pole de Santé Universitaire Gennevilliers Villeneuve la Garenne Ethical approval: Hospital Institutional Review Board. Trial registry: NCT02122432 Conflicts of interest: None known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated random list (p.2) |
| Allocation concealment (selection bias) | Low risk | Comment: Investigator generated the list at the start of the study for all primary practices; investigator did not have contact with physicians or participants (p.2) |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | High risk | Comment: Baseline characteristics provided, groups different for sex distribution and dermatologist final diagnosis (Table 1) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Participants, GPs, and study personnel were not blinded to group allocation (p.2) |
| Blinding of objective outcome assessment (detection bias) | High risk | Comment: Days between consultations reported by the participant, who was not blinded to group allocation |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: Reported by GPs and participants who were not blinded to group allocation, and dermatologists (intervention group only) |
| Protection against contamination | Low risk | Comment: Allocation by GP practices |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Low attrition rates (Figure 1) |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes specified in the protocol were reported in the published article |
| Other bias | Low risk | Comment: No other apparent risk of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (cross‐over) Unit of allocation: Participant | |
| Participants | Providers Number: 8 emergency department (ED) residents Type: ED residents consulting with consultants Other relevant characteristics: Not reported Participants Number: Not reported Mean age (SD): Not reported Gender (% female): Not reported Inclusion criteria: Adults attending the ED Exclusion criteria: Not reported Other relevant characteristics: Not reported Location and study setting: USA, 1 ED Recruitment method: Not reported Duration: Single consultation with 1 month follow‐up; not reported when study was conducted Withdrawals: Not reported | |
| Interventions | Intervention components: Electronic consultation application, used by the ED resident to communicate with consultants Comparison: Usual care Technical equipment used: Tablets (iPad) Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Conciseness; pertinence of information presented; flow; effectiveness of communication skills; overall quality of physician to physician consultations Time points reported: Post‐intervention (1 month post‐baseline) | |
| Notes | Funding: Not reported Ethical approval: Not reported Conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of objective outcome assessment (detection bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of subjective outcome assessment (detection bias) | Unclear risk | Comment: Not enough information provided |
| Protection against contamination | Unclear risk | Comment: Not enough information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Not enough information provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not enough information provided |
| Other bias | Unclear risk | Comment: Not enough information provided |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: 1 primary care physician, 6 radiologists Type: Primary care physician consulting with radiologists Other relevant characteristics: Primary care physician received sonographic training at 3 US medical centres Participants Number: Randomised: 105 (I: 53, C: 52), Analysed: same number Mean age (SD): I: 27 years; C: 29 years Gender (% female): I: 90; C: 94% Inclusion criteria: Participants aged ≥ 13 years attending a primary care clinic, with symptoms requiring a trans‐abdominal or trans‐vaginal ultrasound Exclusion criteria: Not reported Other relevant characteristics: Low‐income setting Location and study setting: Dominican Republic, 1 rural clinic Recruitment method: All eligible patients were invited to participate Duration: Intervention was 1 consultation; not reported when study was conducted Withdrawals: No withdrawals | |
| Interventions | Intervention components: Primary care professional performed scans according to current practice guidelines, which were then emailed to US‐based radiologists along with forms with any relevant clinical information. The on‐site investigator received sonographic training over a 2‐month period, as well as practice guidelines for trans‐abdominal ultrasound scanning. The radiologists interpreted the scans and returned the forms, along with an assessment of the scan's quality. Participants were instructed to return to the primary care clinic within 48 hours Comparison: Usual care ‐ received regular ultrasound referral and were instructed to return the diagnostic report in hand as soon as possible Technical equipment used: Portable ultrasound scanner (SonoSite Titan with 5.2 MHz curvilinear transducer), images sent by email as attachment Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Time to final diagnosis; time to follow‐up appointments; number of successful follow‐ups; number of delivered reports Time points reported: Post‐intervention | |
| Notes | Funding: Global Health Leadership Fellowship, sponsored by the Edward Via Virginia College of Osteopathic Medicine Ethical approval: Appropriate ethics committee Conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: Coin tossing (p.192) |
| Allocation concealment (selection bias) | High risk | Comment: Coin tossing is at high risk of allocation being predictable. |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Baseline characteristics similar (selection bias) | Unclear risk | Comment: Not enough information provided |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Only 1 primary care physician, not possible to blind; participants were aware of group allocation as it implied different actions |
| Blinding of objective outcome assessment (detection bias) | Low risk | Comment: The radiologists were blinded from one another’s interpretations (p.194) |
| Protection against contamination | Low risk | Comment: Not possible for contamination to occur |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No attrition |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes mentioned in the Methods are reported in the Results section |
| Other bias | Low risk | Comment: No other apparent risk of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: Type: 3 rural‐based physical therapists consulting with 3 urban‐based rheumatologists Other relevant characteristics: Not reported Participants Number: Randomised: 85 (I: 54, C: 31), Analysed: 54 (I: 31, C: 23) Mean age (SD): I: 58.4 years (10.7); C: 53.1 years (12.2) Gender (% female): I: 80%; C: 81% Inclusion criteria: Adults with a clinical diagnosis of rheumatoid arthritis, living more than 100 km away from the urban centres Exclusion criteria: Not reported Other relevant characteristics: Mean duration of rheumatoid arthritis was 1.9 years Location and study setting: Canada, 1 urban clinic, 5 rural clinics Recruitment method: Identified through the clinic databases Duration: Intervention was 1 consultation every 3 months, follow‐up lasted 9 months; not reported when study was conducted Withdrawals: 43% (I) and 26% (C) of participants did not complete the study; main reason provided by participants allocated to the intervention group was a preference for travelling into town for their appointment | |
| Interventions | Intervention components: Video‐consultations between physical therapist and rheumatologist; the participants were present for part of the consultation, during which they were examined by the rheumatologist. Physical therapists and rheumatologists received an orientation and education session about rheumatoid arthritis and the study protocol and methods Comparison: Usual care ‐ in‐person rheumatology clinics Technical equipment used: Laptops with video‐conferencing software (VidyoDesktop software); detachable external web camera with remote pan, tilt and zoom functions Fidelity assessment: All healthcare professionals attended an education session about the study protocol | |
| Outcomes | Main outcomes: Disease activity metrics; health assessment; participant satisfaction Time points reported: Baseline, post‐intervention (9 months post‐baseline) | |
| Notes | Funding: Canadian Initiative for Outcomes in Rheumatology cAre (CIORA) Ethical approval: University of Saskatchewan Biomedical Research Ethics Board; trial registry NCT02371915 Conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[S]tratified block randomisation algorithm" (p.2) |
| Allocation concealment (selection bias) | Low risk | Quote: "Clinicians were not involved in or aware of the outcome of the randomisation allocation, which was overseen by the research coordinator" (p.2) |
| Baseline outcome measurements similar (selection bias) | Low risk | Comment: Baseline outcome measurements reported and similar between groups (Fig. 2) |
| Baseline characteristics similar (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Due to the nature of the intervention participants and personnel could not have been blinded and no attempts at blinding were described |
| Blinding of objective outcome assessment (detection bias) | High risk | Comment: Physical examination data were collected by on‐site physical therapists and by urban rheumatologists, due to the nature of the intervention they could not have been blinded |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: Self‐reported outcomes were quality of life and satisfaction with care, and participants were not blinded |
| Protection against contamination | Unclear risk | Comment: Many of the dropouts were reportedly due to preference for standard treatment |
| Incomplete outcome data (attrition bias) | High risk | Comment: High attrition rates |
| Selective reporting (reporting bias) | High risk | Comment: Reports all outcomes mentioned in Methods section of paper, but not all outcomes stated in online trial record (NCT02371915), e.g. change in healthcare use |
| Other bias | Low risk | Comment: No other apparent risk of bias |
| Study characteristics | ||
| Methods | Study design: Cluster‐randomised trial (parallel) Unit of allocation: Cluster (47 primary care practices, 23 allocated to the intervention group and 24 to the control group) | |
| Participants | Providers Number: 128 GPs (number of nephrologists not provided) Type: GPs consulting with nephrologists Other relevant characteristics: Not reported Participants Number: I: 1277; C: 1727 Mean age (SD): I: 68.0 years (13.6); C: 66.4 years (13.2) Gender (% female): I: 67%; C: 65% Inclusion criteria: Adults with a clinical diagnosis of chronic kidney disease who qualified for consultation or referral to nephrology specialist care Exclusion criteria: Receiving secondary renal care Other relevant characteristics: Most had at least 1 comorbid chronic condition Location and study setting: The Netherlands, 47 primary care practices across the country Recruitment method: GPs were invited to participate while attending a CKD management course; eligible patients were identified through EMR Duration: Intervention was implemented between March 2011 and June 2012; follow‐up duration unclear Withdrawals: Approximately 3% of eligible patients did not start the trial (reasons provided); 7.7% of participants did not complete follow‐up (deceased: n = 181; moved: n = 50; unknown: n = 1) | |
| Interventions | Intervention components: Telenephrology was added to the EMR as an add‐on application, which was activated by the GP for each specific participant. The nephrologist was then notified about the consultation by e‐mail or text message and advised the GP about further treatment required, including referrals if needed Comparison: Usual care ‐ conventional consultation methods Technical equipment used: Encrypted EMR, accessed with a direct single sign‐on Fidelity assessment: Not reported | |
| Outcomes | Main outcomes: Difference in referral rate between intervention and control groups Other outcomes: Difference in consultation rates by telephone or telenephrology; adherence to the advised monitoring criteria; GP's compliance with coding renal impairment as a separate entity; achievement of blood pressure targets; main related medical costs; incidence of CKD; GPs experience with using telenephrology Time points reported: Unclear | |
| Notes | Funding: Dutch Kidney Foundation and Amgen Ethical approval: Not required according to the accredited Medical Research Ethics Committee Arnhem/Nijmegen. Clinicians and participants were informed electronic medical data were being used for research purposes and could opt‐out. Netherlands Trial Registration 2242 Conflicts of interest: "The Department of Primary and Community Care received a non‐conditional grant from Amgen. Jack Wetzels received research grants from Amgen, Genzyme and Pfizer for the Masterplan study. All other authors have no conflicting interests" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Stratified block randomisation (p.432) |
| Allocation concealment (selection bias) | Low risk | Comment: Independent statistician performed randomisation by institution at the start of the study (p.432) |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Comment: Not enough information provided to make a decision |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline characteristics provided and similar between groups (table 1) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Due to the nature of the intervention participants and personnel could not have been blinded and no attempts at blinding were described |
| Blinding of objective outcome assessment (detection bias) | High risk | Comment: All referrals were reported by both the GPs and the nephrologists in an online survey system |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: GPs in the intervention group answered a survey about their experience with the intervention (p.432) |
| Protection against contamination | High risk | Comment: GPs allocated to the CG participated in a training course about CKD (p.435) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Low attrition rates |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes specified in the protocol were reported in the published article |
| Other bias | Low risk | Comment: No other apparent risk of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: 60 GPs, 8 dermatologists Type: GPs consulting with dermatologists Other relevant characteristics: Dermatologists were mainly third‐year residents Participants Number: Randomised: 274 (I: 134, C: 140), Analysed: ITT analysis Mean age (SD): I: 60.9 years (7.8); C: 66.9 years (8.5) Gender (% female): 5% Inclusion criteria: Adults referred to the Dermatology service from primary care clinics Exclusion criteria: Urgent conditions that required immediate attention Other relevant characteristics: Mainly white Location and study setting: USA, 4 clinics at a Veteran Affairs Medical Centre Recruitment method: Not reported Duration: 1 consultation/referral; not reported when study was conducted Withdrawals: Not reported | |
| Interventions | Intervention components: GPs submitted digital images of skin lesions with a standardised medical history and any additional relevant information. The consultant dermatologist reviewed all the data and replied either by scheduling a clinic‐based appointment or sending a diagnosis and management plan to the GP, without further need for a clinic‐based appointment Comparison: Usual care ‐ GPs referred participants to the dermatology service as needed Technical equipment used: Fujix DS‐515 digital camera Fidelity assessment: As a quality‐control measure, images were assessed on a laptop computer while acquiring them | |
| Outcomes | Main outcomes: Time to intervention; costs; participant and healthcare professional satisfaction Time points reported: Baseline, resolution of the problem (variable) | |
| Notes | Funding: VA Health Services Research and Development Service and VA Health Services Research and Development Service Ethical approval: Research and Development Committee and the Human Studies Subcommittee of the Department of Veterans Affairs Medical Center, Durham, North Carolina Conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not enough information provided |
| Allocation concealment (selection bias) | Low risk | Quote: "Referring primary care clinicians contacted the research assistants during the course of clinic visits when a dermatology consult was considered appropriate. Research assistants were blinded to the study arm in which prospective patients were randomised." (p.314) |
| Baseline outcome measurements similar (selection bias) | Low risk | Comment: Lesion characteristics were similar between groups (Table 2) |
| Baseline characteristics similar (selection bias) | Unclear risk | Comment: Baseline characteristics reported and similar between groups (Table 2) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible to blind participants and personnel |
| Blinding of objective outcome assessment (detection bias) | Low risk | Quote: "Research assistants were blinded to the study arm in which prospective patients were randomised." (p.314) |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: Self‐reported satisfaction |
| Protection against contamination | Unclear risk | Comment: Contamination unlikely to be a risk at participant level. Risks of or strategies to prevent contamination at the level of responding dermatologists unclear |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Data analysed for all participants |
| Selective reporting (reporting bias) | High risk | Comment: Different outcomes reported in different publications |
| Other bias | Low risk | Comment: No other apparent risk of bias |
| Study characteristics | ||
| Methods | Study design: Randomised trial (parallel) Unit of allocation: Participant | |
| Participants | Providers Number: Not reported Type: GPs consulting with dermatologists Other relevant characteristics: Not reported Participants Number: Randomised: 392 (I: 196, C: 196), Analysed: 261 (I: 136; C: 125) Mean age (SD): I: 62.9 years (13.9); C: 61.7 years (14.9) Gender (% female): 2% (Veteran Affairs clinics) Inclusion criteria: Adults referred to the Dermatology service from primary care clinics Exclusion criteria: More than 1 skin condition, required full‐body examination, could not read or speak English, low health literacy Other relevant characteristics: Mainly white Location and study setting: USA, 2 outpatient community‐based Veteran Affairs clinics Recruitment method: Eligible participants were identified whenever the GP generated a request for a consultation with the dermatology department Duration: 1 consultation/referral (unless participant required further treatment), 9 months follow‐up; study conducted between November 2008 and March 2011 Withdrawals: 33% of participants randomised did not complete follow‐up (reasons provided, similar numbers for I and C) | |
| Interventions | Intervention components: Alongside the request for a referral, GPs submitted digital images of skin lesions with a standardised medical history and any additional relevant information. The consultant dermatologist reviewed all the data and replied either by scheduling a clinic‐based appointment or sending a diagnosis and management plan to the GP, without further need for a clinic‐based appointment Comparison: Usual care ‐ GPs referred participants to the dermatology service as needed using the electronic medical record Technical equipment used: 8‐megapixel digital camera with an integrated flash; if required, digital ring flash for short focal length or macro images Fidelity assessment: Imaging protocol | |
| Outcomes | Main outcomes: Quality of life; health status; comorbidity assessment; cost; satisfaction with care Time points reported: Baseline, follow‐up (3 and 9 months post‐baseline assessment) | |
| Notes | Funding: US Department of Veterans Affairs Health Services Research and Development Service; National Institutes of Health Ethical approval: Approved by institutional review boards. Trial registry: NCT00488293 Conflicts of interest: "Drs Whited and Edison are coeditors of the book Teledermatology: A User’s Guide published by Cambridge University Press and receive royalties based on sales. Dr Chren is a consultant to Genetech Inc (on patient‐reported outcomes)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Simple randomisation scheme stratified by site (p.586) |
| Allocation concealment (selection bias) | Low risk | Comment: Off‐site statistical co‐ordinating centre (p.586) |
| Baseline outcome measurements similar (selection bias) | Low risk | Comment: Baseline outcome measurements provided and similar between groups (table 1) |
| Baseline characteristics similar (selection bias) | Low risk | Comment: Baseline outcome characteristics provided and similar between groups (table 1) |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not possible due to the nature of the intervention |
| Blinding of objective outcome assessment (detection bias) | High risk | Comment: Costs partially calculated based on site reports, which are variable; clinical staff not blinded to group allocation |
| Blinding of subjective outcome assessment (detection bias) | High risk | Comment: Participants, clinical staff and research personnel not blinded to group allocation |
| Protection against contamination | Unclear risk | Comment: Contamination unlikely to be a risk at participant level. Risks of or strategies to prevent contamination at the level of responding dermatologists unclear |
| Incomplete outcome data (attrition bias) | High risk | Comment: High attrition rates (67% participants randomised were included in primary analysis) |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes differ between protocol and publications |
| Other bias | Low risk | Comment: No other apparent risk of bias |
Empty cells in the "Risk of bias" tables refer to instances where the specific risk of bias criterion did not apply, e.g. the type of outcome was not collected by the study.
ART: Antiretroviral Therapy; C: Control; CKD: Chronic kidney disease; EMR: Electronic medical record; GPs: General practitioners; HCP: Healthcare provider; HEW: Health extension workers; I: Intervention; PCP: Primary care provider; PHWs: Peer health workers; VA: Veteran Affairs
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Compares in‐person supervision with automated text messages | |
| Mobile technologies used for emergency referrals, not seeking guidance or providing care | |
| Not mobile | |
| Feasibility study | |
| Not mobile (desktop video‐conferencing system) | |
| Not mobile (desktop personal computers) | |
| Pilot study | |
| Not an intervention study (diagnostic accuracy) | |
| Not mobile | |
| Compares 2 Internet‐based interventions (clinical images vs. dermoscopic images) | |
| Not mobile (desktop personal computers) | |
| Multifaceted study with several components | |
| Pilot study | |
| Not mobile (desktop video‐conferencing telephone) | |
| Not mobile | |
| Not mobile (desktop personal computers, as well as laptops) | |
| Not mobile (desktop personal computers) | |
| Feasibility study | |
| Mainly educational | |
| Compares 2 devices (smartphone and pager) for routine communication | |
| Diagnostic accuracy study | |
| Compares 2 methods for fitting a Cochlear device, each participant has the device fitted remotely or face‐to‐face (not randomised) |
Characteristics of ongoing studies [ordered by study ID]
| Study name | Establishing the role of teleconsulting in the care of chronic conditions in rural areas of the Southern District Health Board (SDHB): A randomised controlled trial (RCT) in patients with Inflammatory Bowel Disease |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | Adults aged ≥ 18 years diagnosed with irritable bowel syndrome living in rural settings |
| Interventions | Intervention: remote consultation through teleconference with nurse facilitation Comparison: usual care |
| Outcomes | Main outcomes: disease control; disease‐specific quality of life Other outcomes: cost effectiveness; acceptability |
| Starting date | April 2017 (expected completion date June 2020) |
| Contact information | Ms Christine Ho ([email protected]) |
| Notes |
| Study name | A prospective randomised controlled study of telehealth specialist palliative care consultations in rural and metropolitan settings and the impact on patient and carer clinical outcomes and quality‐of‐life |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | Adults aged ≥ 18 years receiving community, inpatient or outpatient palliative care |
| Interventions | Intervention: in‐home consultation through teleconference with nurse facilitation Comparison: usual care |
| Outcomes | Main outcomes: clinical symptoms; quality of life; performance status Other outcomes: emergency department attendances; time to set up teleconference equipment; user experience; home visit duration; other participant‐reported symptoms |
| Starting date | June 2018 (expected completion date October 2020) |
| Contact information | A/Prof Peter Poon ([email protected]) |
| Notes |
| Study name | Teledermatology mobile apps: implementation and impact on veterans' access to dermatology |
| Methods | Randomised trial, cross‐over assignment, open‐label |
| Participants | All people receiving dermatology care at eligible clinics |
| Interventions | Intervention: Tablet loaded with app, which allows to capture and immediately upload images into the electronic records system, where it can be reviewed by referring providers and imagers Comparison: Usual care (for 3‐month blocks, until they also start using the app) |
| Outcomes | Main outcomes: consult completion time; appointment completion time; number of teledermatology appointments; fraction of appointments using teledermatology; travel distance Other outcomes: none specified |
| Starting date | March 2019 (estimated completion date September 2020) |
| Contact information | Dennis Oh ([email protected]) |
| Notes | Published protocol |
| Study name | CAPRI |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | People with metastatic cancer or haematological malignancy being treated with oral therapy, aged ≥ 18 years |
| Interventions | Intervention: Participants will be given access to nurse navigators and a web portal, which will also be used for nurses to communicate with other healthcare professionals Comparison: Usual care |
| Outcomes | Main outcomes: relative dose intensity Other outcomes: compliance; toxicity |
| Starting date | October 2016 (estimated completion date October 2020) |
| Contact information | Marie Ferrua ([email protected]) |
| Notes | Registered trial (NCT02828462) |
| Study name | Evaluation of the DIABEO system in poorly controlled DM1 or DM2 patients treated with a basal‐bolus insulin regimen |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | People aged ≥ 18 years with Type 1 or Type 2 diabetes |
| Interventions | Intervention: Participants are provided with an electronic diary system for monitoring glycaemic levels; results are uploaded to an online portal that can be accessed by HCP; clinical information can be exchanged between different HCPs Comparison: Usual care |
| Outcomes | Main outcomes: change in HbA1c Other outcomes: HbA1c levels; percent of responder participants; severe hypoglycaemia |
| Starting date | February 2013 (estimated completion date July 2018) |
| Contact information | Sylvia Franc |
| Notes | No published results found, contact authors emailed twice for further information, no reply Registered trial (NCT02287532) |
| Study name | TeleDerm study |
| Methods | Cluster‐randomised trial, parallel assignment, open‐label |
| Participants | Adults with a dermatologic problem and insured by a specific health insurance company |
| Interventions | Intervention: When faced with a dermatologic case, the GP can trigger a teleconsultation process with a dermatologist, based on high‐resolution pictures and clinical history Comparison: Usual care |
| Outcomes | Main outcomes: Number of physical referrals to dermatologists Other outcomes: Referral time; process quality; health‐related quality of life; costs |
| Starting date | July 2018 (expected completion date June 2019) |
| Contact information | Roland Koch ([email protected]‐tuebingen.de) |
| Notes | Registered trial (DRKS00012944) |
| Study name | inSCALE |
| Methods | Randomised trial (cluster parallel) |
| Participants | Community health workers (CHWs) working in districts with Integrated Community Case Management (Uganda and Mozambique) |
| Interventions | CHWs are equipped with smartphones that can be used to facilitate decision‐making, submit data, receive personal performance feedback and communicate with their supervisor |
| Outcomes | Main outcome: appropriate treatment of malaria, pneumonia and diarrhoea in children under 5 years of age at 12 months Other outcomes: CHWs with medicine stock‐out < 1 week each quarter; CHW retention |
| Starting date | April 2013 |
| Contact information | Karen Kalländer |
| Notes | Registered trial (NCT01972321) |
| Study name | Screening of cardiovascular, cerebrovascular, and renal disease for residents in rural areas using a medical IT network |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | Adults aged ≥ 65 years living in rural areas, with low‐to‐moderate risk of cardiovascular disease |
| Interventions | Intervention: Using clinical data from a medical information network, specialists in cardiology, nephrology and cerebrovascular disease assess patient data and make treatment recommendations to GPs Comparison: Usual care, participants are treated in‐person by physician |
| Outcomes | Main outcomes: Incidence of cardiovascular, cerebrovascular, or renal disease Other outcomes: Not reported |
| Starting date | May 2015 (no information about study completion) |
| Contact information | Masaharu Nakayama ([email protected]) |
| Notes | No published results found, contact authors emailed twice for further information, no reply Registered trial (UMIN000018552) |
| Study name | The impact of Telemedicine to support palliative care resident in nursing home (TELESM) |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | Nursing home residents aged ≥ 65 years, with palliative care needs |
| Interventions | Intervention: Telemedicine consultation ‐ multi‐professional consultation with healthcare professionals (participant and their families can also participate if desired) Comparison: Usual care |
| Outcomes | Main outcomes: hospitalisation rates Other outcomes: emergency hospitalisation rates; proportion of hospitalised participants; quality of life; caregiver satisfaction; costs |
| Starting date | January 2018 |
| Contact information | Sandrine Sourdet (sourdet.s@chu‐toulouse.fr) |
| Notes |
| Study name | Evaluation of the management of diabetic foot ulcers by telemedicine on the number of hospital days in diabetic patients (TELEPIED) |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | Patiients with diabetes and foot ulcer aged ≥ 18 years |
| Interventions | Intervention: During home visits the community nurse will photograph foot ulcers, which will be sent to the specialist nurse for assessment and follow‐up Comparison: Usual care |
| Outcomes | Main outcomes: number of hospitalisation days due to diabetic foot ulcers Other outcomes: total direct care costs; average duration of hospitalisation due to diabetic foot ulcers; ulcer recidivism rate; frequency of ulceration; duration of ulceration; healing rate; amputation rate; participant satisfaction score |
| Starting date | January 2017 (estimated completion date January 2021) |
| Contact information | Sylvia Franc ([email protected]) |
| Notes |
| Study name | OASE Melanome |
| Methods | Cluster‐randomised trial, open‐label |
| Participants | Adults aged ≥ 18 years consulting a GP for a suspicious cutaneous lesion who require a referral to a dermatologist |
| Interventions | Intervention: The GP sends the dermatologist 2 photos of skin lesions, along with relevant clinical information, after which the dermatologist assesses the photos and follows up with the participant as required Comparison: Usual care |
| Outcomes | Main outcomes: Time limit between consultation with GP and consultation with dermatologist Other outcomes: Proportion of participants who did have a consultation with a dermatologist 12 months after consulting with GP |
| Starting date | May 2017 (estimated completion date May 2018) |
| Contact information | Jean‐Michel Nguyen (jeanmichel.nguyen@chu‐nantes.fr) |
| Notes | No published results found, contact authors emailed twice for further information, no reply |
| Study name | Effectiveness of collaborative tele‐mental health services for ADHD in primary care: a randomised trial in Dubai (ECTSAP‐ Dubai Trial) |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | Children aged 6 to 12 years diagnosed with attention deficit hyperactivity disorder (ADHD) |
| Interventions | Intervention: remote consultation through teleconference with specialist supervision Comparison: usual care |
| Outcomes | Change in clinical symptoms |
| Starting date | June 2018 (expected completion date December 2018, personal communication with principal investigator 22 October 2019 confirmed it is ongoing) |
| Contact information | Ammar AlBanna ([email protected]) |
| Notes |
| Study name | Addressing early childhood hearing loss in rural Alaska: a community randomised trial |
| Methods | Randomised trial, parallel assignment, single masking (outcomes assessor) |
| Participants | Children aged 2 to 6 years, attending eligible schools |
| Interventions | Little information provided; intervention described as telemedicine referral and mHealth screening tool |
| Outcomes | Main outcomes: time to diagnosis Other outcomes: sensitivity and specificity of screening protocols; prevalence of hearing loss |
| Starting date | September 2018 (estimated completion date February 2020) |
| Contact information | Samantha Robler ([email protected]) |
| Notes |
| Study name | Telemedical support for prehospital Emergency Medical Service (TEMS) |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | All emergency calls that are assessed as non‐life‐threatening, which do not require an obligatory emergency medical service physician on scene and which do not solely require an ambulance staffed by paramedics |
| Interventions | Intervention: Tele‐EMS physician ‐ participants are treated by the paramedics, who will be supported by the tele‐EMS physicians based at a teleconsultation centre Comparison: Usual care, participants are treated by physician on scene |
| Outcomes | Main outcomes: adverse events Other outcomes: adherence to guidelines; quality of medical history; completeness and correctness of data; tracer diagnoses; mortality; intensive care unit length of stay; hospital length of stay; other outcomes |
| Starting date | July 2017 (estimated study completion date December 2019) |
| Contact information | Ana Stevanovic ([email protected]) |
| Notes | Published protocol |
| Study name | A coordinated PCP‐cardiologist telemedicine model (PCTM) in China's community hypertension care |
| Methods | Randomised trial, parallel assignment, open‐label |
| Participants | Adults aged ≥ 21 years, with a clinical diagnosis of hypertension with uncontrolled blood pressure in the past 3 months, currently taking or about to take anti‐hypertensive medications |
| Interventions | Intervention: Participants are given a blood pressure monitoring system for self‐management, which feeds data back to the primary care and cardiology team. Primary care providers and cardiologists use a web‐based system to communicate and manage care Comparison: Usual care ‐ based on national guidelines for hypertension management |
| Outcomes | Main outcomes: Changes in mean systolic blood pressure Other outcomes: Changes in mean diastolic blood pressure; hypertension control rate; medication adherence |
| Starting date | September 2016 (estimated completion date August 2018) |
| Contact information | Lei Xu ([email protected]) |
| Notes | No published results found, contact authors emailed twice for further information, no reply. Trial registry NCT02919033 |
DM1 or DM2: Type 1 or Type 2 Diabetes Mellitus; EMS: Emergency medical service; GP: General practitioner; HCP: Healthcare professionals
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||
| 1.1 Providers' adherence to recommended guidelines Show forest plot | 1 | Other data | No numeric data | ||||||||||||||||
| Analysis 1.1
Comparison 1: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Providers' adherence to recommended practice, guidelines or protocols, Outcome 1: Providers' adherence to recommended guidelines | |||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||
| 2.1 Time between presentation and management Show forest plot | 4 | Other data | No numeric data | |||||||||||||||||||||||||||||||
| Analysis 2.1
Comparison 2: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Time between presentation and management of the health condition, Outcome 1: Time between presentation and management | ||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 Healthcare use Show forest plot | 9 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.1
Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 1: Healthcare use | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1.1 Healthcare use | 9 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.2  Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 2: Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2.1 Referred to a dermatology clinic | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.3  Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 3: Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 4.1 Health‐related quality of life Show forest plot | 2 | Other data | No numeric data | |||||||||||||||||||||
| Analysis 4.1
Comparison 4: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Participant's healthcare status and well‐being, Outcome 1: Health‐related quality of life | ||||||||||||||||||||||||
| 4.2 Clinical course Show forest plot | 2 | Other data | No numeric data | |||||||||||||||||||||
| Analysis 4.2
Comparison 4: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Participant's healthcare status and well‐being, Outcome 2: Clinical course | ||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||
| 5.1 Healthcare provider satisfaction with the intervention Show forest plot | 3 | Other data | No numeric data | |||||||||||||||||||||||||||||||
| Analysis 5.1
Comparison 5: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Acceptability or satisfaction, Outcome 1: Healthcare provider satisfaction with the intervention | ||||||||||||||||||||||||||||||||||
| 5.2 Participant satisfaction with care Show forest plot | 4 | Other data | No numeric data | |||||||||||||||||||||||||||||||
| Analysis 5.2
Comparison 5: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Acceptability or satisfaction, Outcome 2: Participant satisfaction with care | ||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||
| 6.1 Costs Show forest plot | 6 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||
| Analysis 6.1
Comparison 6: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Costs, Outcome 1: Costs | ||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||
| 7.1 Technical difficulties Show forest plot | 4 | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||
| Analysis 7.1
Comparison 7: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Technical difficulties, Outcome 1: Technical difficulties | |||||||||||||||||||||||||||||||||||||||
| 7.1.1 Quality of the data transmitted | 4 | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||
| 8.1 Time between presentation and management Show forest plot | 1 | Other data | No numeric data | ||||||||||||||||
| Analysis 8.1
Comparison 8: Mobile technologies for use in the emergency department compared to usual care: Time between presentation and management of the health condition, Outcome 1: Time between presentation and management | |||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||
| 9.1 Healthcare use Show forest plot | 1 | Other data | No numeric data | ||||||||||||||||
| Analysis 9.1
Comparison 9: Mobile technologies for use in the emergency department compared to usual care: Healthcare use, Outcome 1: Healthcare use | |||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 10.1 Technical difficulties Show forest plot | 1 | Other data | No numeric data | |||||||||||||||||||||
| Analysis 10.1
Comparison 10: Mobile technologies for use in the emergency department compared to usual care: Technical difficulties, Outcome 1: Technical difficulties | ||||||||||||||||||||||||
| 10.1.1 Quality of the data transmitted | 1 | Other data | No numeric data | |||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 11.1 Healthcare use Show forest plot | 2 | Other data | No numeric data | |||||||||||||||||||||
| Analysis 11.1
Comparison 11: Mobile technologies used by community health workers or home‐care workers compared to usual care: Healthcare use, Outcome 1: Healthcare use | ||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||
| 12.1 Participant healthcare status and well‐being Show forest plot | 3 | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 12.1
Comparison 12: Mobile technologies used by community health workers or home‐care workers compared to usual care: Participant's healthcare status and well‐being, Outcome 1: Participant healthcare status and well‐being | |||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 13.1 Participant satisfaction with care Show forest plot | 2 | Other data | No numeric data | |||||||||||||||||||||
| Analysis 13.1
Comparison 13: Mobile technologies used by community health workers or home‐care workers compared to usual care: Acceptability or satisfaction, Outcome 1: Participant satisfaction with care | ||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||
| 14.1 Costs Show forest plot | 1 | Other data | No numeric data | ||||||||||||||||
| Analysis 14.1
Comparison 14: Mobile technologies used by community health workers or home‐care workers compared to usual care: Costs, Outcome 1: Costs | |||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||
| 15.1 Technical difficulties Show forest plot | 2 | Other data | No numeric data | |||||||||||||||||||||||||||||||
| Analysis 15.1
Comparison 15: Mobile technologies used by community health workers or home‐care workers compared to usual care: Technical difficulties, Outcome 1: Technical difficulties | ||||||||||||||||||||||||||||||||||
| 15.1.1 Quality of the data transmitted | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||
| 15.1.2 Technical difficulties reported by the healthcare professionals | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Providers' adherence to recommended guidelines | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Complete monitoring of disease progression Complete monitoring of metabolic parameters | OR 1.23 (0.89 to 1.70) OR 0.61 (0.22 to 1.72) | Follow‐up not specified OR: Odds ratio; IG: intervention group; CG: control group * Multilevel analysis for IG compared to CG; model with a random intercept keeping the independent variable (General Practice Information System) fixed |
Comparison 1: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Providers' adherence to recommended practice, guidelines or protocols, Outcome 1: Providers' adherence to recommended guidelines
| Time between presentation and management | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Azogil‐López 2019 | General practitioner consulting with hospital physicians about participants (aged ≥ 7 years) | Median time from referral request to appointment with hospital physician Median time from referral request to resolution of the process | IG: 17 days (IQR 8 to 32, N = 72) CG: 51 days, (IQR 35 to 57 days, N = 101) Median difference: −27 days (99% CI −20 to −33 days)* IG: 105 days (IQR 40 to 169); CG: 147 days (IQR 74 to 228) Median difference: −47 days (95% CI −74 to −17 days)* | IG: Intervention group; CG: Control group; IQR: Interquartile range 3‐month follow‐up * As reported by the authors |
| Piette 2017 | General practitioner consulting with dermatologists about adults with skin lesions | Median delay between | IG: 4 days (N = 53) CG: 40 days (N = 50) Adjusted HR 2.55 (P = 0.01)* | 3‐month follow‐up Reported in days Data also provided for number of participants not receiving an appointment (15 days, 1‐, 2‐ and 3‐month follow‐up) Adjusted hazard ratio (HR) as provided by the authors (adjusting for clustering of GPs and identities of dermatologists) |
| Sutherland 2009 | General practitioner consulting with radiologists about clients aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Median time to participant follow‐up Median time to final diagnosis | IG: 67.1 hours (IQR: 45.9 to 113.7, N = 53) CG: 76.7 hours (IQR 65.8 to 144.7, N = 52) IG: 17.8 hours (IQR: 12.2 to 27.1, N = 53) CG: 23.9 (IQR 21.4 to 48.1, N = 52) | Duration not provided |
| Whited 2002 | General practitioner consulting with dermatologists about adults with skin condition | Mean time to intervention | IG: 73.8 days (SD 71.6, N = 135) CG: 114.3 days (SD 72.3, N = 140) MD: −40.5 days (95% CI −23.41 to −57.89) | Duration not provided SD: standard deviation; MD: mean difference |
Comparison 2: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Time between presentation and management of the health condition, Outcome 1: Time between presentation and management
| Healthcare use | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Healthcare use | ||||
| Byamba 2015 | General practitioner consulting with dermatologists about adults with skin lesions | Participant referred to tertiary‐care centres for consultation | IG: 7/221 CG: 28/229 RR: 0.28, 95% CI 0.13 to 0.63 | IG: Intervention group; CG: Control group RR: risk ratio; CI: confidence interval 5 months follow‐up Note: there was no evidence of clustering taken into account in the analysis, and we were not able to re‐analyse the data. It is possible there are potential unit of analysis errors. |
| Davis 2003 | Primary care provider at the rural primary practice consulting with ophthalmologist at the university setting about adults with diabetes | Participant received diabetic | IG: 23/30 CG: 4/29 RR 5.56 (95% CI 2.19 to 14.10) | Follow‐up not reported RR: risk ratio; CI: confidence interval |
| Liddy 2019a | Primary care provider consulting with specialists for a range of different conditions | Participants referred for face‐to‐face visits to all medical specialties available through eConsult service during the study period | Mean number of participants seen (SD, range) IG: 608 (258, 90 to 1134) CG: 724 (370, 11 to 1692) RR 0.93, 95% CI 0.85 to 1.03* | 12‐month follow‐up RR: risk ratio; CI: confidence interval * Adjusted for covariates |
| Mansberger 2015 | Primary care providers consulting with experienced investigators based at an eye institute about adults with diabetes | Participant received diabetic | IG: 157/296 CG: 90/271 RR 1.60 (95% CI 1.31 to 1.95) | 12‐month follow‐up (24, 36 and 48 months also reported; during these periods telemedicine was offered to all participants) |
| Piette 2017 | General practitioner consulting with dermatologists about adults with skin lesions | Participant referred for clinic follow‐up | IG: 14/39*; CG: 50/50 RR: 0.36 (95% CI 0.24 to 0.55) | 3‐month follow‐up * Only includes participants for whom dermatologists were able to elaborate a treatment plan based on transmitted photographs; for approx. 1/5 of participants allocated to IG the photographs were not usable |
| Sutherland 2009 | General practitioner consulting with radiologists regarding clients aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Participant received ultrasound | IG: 36/53 CG: 9/52 RR 3.92 (95% CI 2.11 to 7.31) | Follow‐up not specified RR: risk ratio; CI: confidence interval |
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Participant referred for clinic follow‐up | IG: 29/1277 CG: 52/1727 OR 0.61 (95% CI 0.31 to 1.23)* | Follow‐up not specified OR: Odds ratio; CI: confidence interval * Multilevel analysis for IG compared to CG; model with a random intercept keeping the independent variable (General Practice Information System) fixed |
| Whited 2002 | General practitioners consulting with dermatologists about adults with skin condition | Participant referred for clinic follow‐up | IG: 110/135; CG: 140/140 RR: 0.82 (95% CI 0.75 to 0.88) | Follow‐up not specified RR: risk ratio; CI: confidence interval |
| Whited 2013 | General practitioner consulting with dermatologists about adults with skin condition | Client visited dermatology clinic | IG: 78/125 CG: 120/136 RR 0.71 (95% CI 0.61 to 0.82) | Proportion of participats who had at least 1 visit to the dermatology clinic during the 9‐month follow‐up |
Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 1: Healthcare use

Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 2: Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up

Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 3: Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up
| Health‐related quality of life | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Armstrong 2018 | General practitioner consulting with dermatologists about adults with psoriasis | General health status: Description General health status: Evaluation | MD 0 (95% CI −0.003 to 0.003) MD −0.002 (95% CI −2.75 to 2.75) | General health status ‐ Description assessed with EuroQol‐5D‐5L. Scores converted into an index number, with values ranging from −0.109 (worst) to 1 (best). General health status ‐ Evaluation assessed with EuroQol‐Visual Analogue Scale. Higher scores represent better perceived health status Mean difference from baseline to 12 months follow‐up, 296 participants. MD: mean difference; CI: confidence interval |
| Whited 2013 | General practitioner consulting with dermatologists about adults with skin condition | Quality of life: Composite Health‐related quality of life | IG: MD −12.0 (SD 24.5, N = 160) CG: MD −13.2 (SD 21.6, N = 166) Similar scores between groups throughout the trial | Quality of life assessed with Skindex‐16, 0 ‐ 100 Higher scores represent worse quality of life Health‐related quality of life (HRQoL) assessed with Short‐Form Health Survey 12 (SF‐12) Higher scores represent better HRQoL Mean difference from baseline to 9‐month follow‐up IG: intervention group; CG: control group; MD: mean difference; SD: standard deviation |
Comparison 4: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Participant's healthcare status and well‐being, Outcome 1: Health‐related quality of life
| Clinical course | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Pak 2007 | Primary care professional consulting with dermatologist about adults with skin condition | Clinical course ratings | Improved IG: 173/272, CG: 154/236 No change IG: 89/272; CG: 76/236 Worse IG: 10/272; CG: 6/236 | Based on dermatologist's assessment, at four‐month follow‐up There was little or no difference between groups |
| Whited 2013 | General practitioner consulting with dermatologists about adults with skin condition | Clinical course ratings | Resolved IG: 31/125; CG: 35/136 Improved IG: 59/125; CG: 63/136 Unchanged (not clinically relevant) IG: 13/125; CG: 15/136 Unchanged (clinically relevant) IG: 13/125; CG: 17/136 Worse IG: 9/125; CG: 6/136 | Based on dermatologist's assessment, at nine‐month follow‐up There was little or no difference between groups |
Comparison 4: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Participant's healthcare status and well‐being, Outcome 2: Clinical course
| Healthcare provider satisfaction with the intervention | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Piette 2017 | General practitioners consulting with dermatologists about adults with a skin condition | Satisfaction | Global satisfaction Same proportion of GPs in both groups were satisfied or very satisfied (69%) Time to treatment satisfaction Similar proportion of GPs in both groups considered the time for resolution to be short or very short (IG: 77%; CG: 54%) | Response rate: 65% (N = 26) 2 questions with a Likert scale response (1 very satisfied to 4 very unsatisfied) Results provided narratively |
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Exprience with the intervention | Content of information sent was good Yes: 71%; No: 13%; Did not use: 16% Ease of use Good: 39%; Reasonable: 37%; Insufficient: 8%; Did not use: 16% Added to knowledge of kidney disease Yes: 68%; No: 16%; Did not use: 16% Pleased with feasibility of telenephrology Yes: 79%; No: 5%; Did not use: 16% | Intervention group only (general practitioners) Response rate: 66% (N = 36) |
| Whited 2002 | General practitioners consulting with dermatologists about adults with a skin condition | Satisfaction with the intervention | N = 275 participants Timely appointments (GPs) IG: 95% agreed, 5% neutral CG: 7% agreed, 70% disagreed Consultant sent back information (GPs) IG: 87% agreed, 13% neutral CG: 68% agreed, 17% neutral Educational benefit from the referral (GPs) IG: 55% agreed, 45% neutral CG: 34% agreed, 41% neutral Satisfied with the consult process (GPs) IG: 92% agreed, 3% disagreed CG: 23% agreed, 35% disagreed Less confident with TD than FtF (CD) 75% agree, 12.5% disagree TD consultation takes longer (CD) 100% disagree TD makes it easier to triage clients (CD) 100% agree Satisfied with using TD (CD) 75% agree, 25% neutral | IG: intervention group; CG: control group; TD: teledermatology; FtF: face‐to‐face; CD: consulting dermatologists GPs: 4 questions relating to timeliness, information transfer, education, and overall satisfaction; score agree, neutral, disagree Referring GPs (N = 60) Dermatologists: confidence in using TD for diagnostic and management, resource use, and overall satisfaction; score agree, neutral, disagree CD (N = 8) |
Comparison 5: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Acceptability or satisfaction, Outcome 1: Healthcare provider satisfaction with the intervention
| Participant satisfaction with care | ||||
| Study | Population | Outcomes | Results | Notes |
|---|---|---|---|---|
| Eminović 2009 | General practitioners consulting with dermatologists about adults with skin condition | General satisfaction Interpersonal aspects of care | IG: Mean 3.8 (SD 0.59, N = 191) CG: Mean 3.8 (SD 0.59, N = 159) MD: 0.0 (95% CI −0.12 to 0.12) IG: Mean 4.13 (SD 0.62, N = 191) CG: Mean 4.15 (SD 0.73, N = 159) MD: 0.2 (95% CI −0.12 to 0.16) | Shortened version of the Patient Satisfaction Questionnaire (PSQ III) 1 ‐ 5, higher scores indicate more satisfaction with the care received 1 month follow‐up IG: Intervention group; CG: Control group; SD: standard deviation; MD: mean difference; CI: confidence interval |
| Piette 2017 | General practitioner consulting with dermatologists regarding adults with skin lesions | Global satisfaction Time to treatment satisfaction | Similar proportion of participants in both groups were satisfied or very satisfied (IG: 85%; CG: 94%) Higher proportion of participants in the IG considered the time for resolution to be short or very short, compared to the CG (46%)* | Response rate: 100% (N = 103) 2 questions with a Likert scale response (1 very satisfied to 4 very unsatisfied) Results provided narratively P = 0.20, as provided by the authors |
| Whited 2002 | General practitioner consulting with dermatologists regarding adults with skin condition | Satisfaction | There was little or no difference between IG (N = 101) and CG (N = 93)* | Visit‐specific satisfaction questionnaire (VSQ), 1 ‐ 5, higher scores indicate more satisfaction 1 month follow‐up * As reported by study authors, no usable data |
| Whited 2013 | General practitioner consulting with dermatologists regarding adults with skin condition | Overall satisfied with the care received for skin problem | Agree/strongly agree: IG: 86.8%; CG: 92% Neutral: IG: 8.8%; CG: 6.7% Disagree/Strongly disagree: IG: 4.5%; CG: 1.2% | Single question assessing global satisfaction with the care received 9 months follow‐up N = 159 (IG) and 166 (CG) |
Comparison 5: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Acceptability or satisfaction, Outcome 2: Participant satisfaction with care
| Costs | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Byamba 2015 | General practitioners consulting with dermatologists about adults with skin lesions | Total mean costs | IG: USD 320 CG: USD 3174 Difference: USD 2854* | IG: intervention group; CG: control group Costs calculated in USD (2014) *Data as provided by the authors; no further information available 5 months follow‐up Note: there was no evidence of clustering taken into account in the analysis, and we were not able to re‐analyse the data. It is possible there are potential unit of analysis errors. |
| Eminović 2009 | General practitioners consulting with dermatologists about adults with a skin condition | Total mean costs | IG: EUR 387 (95% CI 281 to 502.5, N = 312) CG: EUR 354 (95% CI 228 to 484, N = 293) MD: EUR 32.5 (95% CI −29.0 to 74.7)* | Costs calculated in EUR (2003) 1‐month follow‐up MD: mean difference; CI: confidence interval * Data as provided by authors |
| Pak 2007 | Primary care professional consulting with dermatologist about adults with skin condition | Total mean costs | Total direct cost IG: USD 103,043 (SD:294, N = 351), CG: 98,365 (283, N = 347) MD: USD −4678 (95% CI −4720 to −4635) Lost productivity IG: USD 16,359 (SD:47, N = 351) CG: USD 30,768 (SD 89, N = 347) MD: USD 14,409 (95% CI 14,398 to 14,419) | Total direct costs include consultations, laboratory analyses and procedures and medications Costs calculated in USD (2006) 4‐month follow‐up |
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Mean cost per participant | IG: EUR 453.86 (95% CI 392.98 to 514.74; N = 1277) CG: EUR 433.74 (95% CI 387.64 to 479.84; N = 1727) (P = 0.60) | Main related medical costs, including number of contacts between healthcare providers and participant, as well as between healthcare providers; lab costs; prescriptions; referrals to secondary for renal care. Costs calculated in EUR (2017) Follow‐up not specified |
| Whited 2002 | General practitioners consulting with dermatologists about adults with skin condition | Mean expected cost per participant per visit | Using basic technology IG: USD 40.35; CG: USD 26.50 Using more advanced technology IG: USD 33.10; CG: USD 21.40 | Follow‐up not specified Costs calculated in USD (2002) N = 275 participants |
| Whited 2013 | General practitioners consulting with dermatologists about adults with skin condition | Mean total costs per participant | Healthcare system perspective* IG: USD 308 (SD 298; N = 195) CG: USD 338 (SD 291; N = 196) MD: USD 30 (95% CI USD −79 to 20) Societal perspective** IG: USD 460 (SD 428; N = 195) CG: USD 542 (SD 403; N = 196) MD USD −82 (95% CI USD −12 to −152) | * Includes intervention costs (healthcare providers input, dermatology visits, medication, travel reimbursement) ** Travel, loss of productivity, other dermatology care USD Follow‐up 9 months Costs calculated in USD (2011) |
Comparison 6: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Costs, Outcome 1: Costs
| Technical difficulties | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Quality of the data transmitted | ||||
| Pak 2007 | Primary care providers consulting with dermatologist about adults referred to the dermatology service from primary care clinics | Technical problems | 20/528 participants’ images were lost | 10 images in each group |
| Piette 2017 | General practitioner consulting with dermatologists about adults with skin lesions | Technical quality of the images received | 11/53 participants' images did not have enough quality as to allow diagnosis or treatment or both | Intervention group only The dermatologist was able to make a decision about the need of an in‐person appointment for 8 of the clients, based on the clinical notes sent along with the images |
| Sutherland 2009 | General practitioner consulting with radiologists about clients aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Technical quality of the images received | Mean 4.6 (standard deviation 0.5) Procedural quality Mean 4.7 (standard deviation 0.6) | As rated by 6 radiologists based on 53 scans, delivered by email; 1 ‐ 5, higher scores represent better quality of the images and the procedure Intervention group only |
| Whited 2002 | General practitioner consulting with dermatologists about adults referred to the dermatology service from primary care clinics | Technical quality of the images received | Due to the bad quality of the images transmitted, 1/134 clients allocated to the IG required an in‐person consultation | Intervention group only |
Comparison 7: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Technical difficulties, Outcome 1: Technical difficulties
| Time between presentation and management | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Gulacti 2017 | Emergency physicians consulting with specialists about adults attending the emergency department; duration not provided | Median consult time* | IG: 158 minutes (IQR:133 to 177.25, 95% CI:150 to169, N = 173) CG: 170 minutes (IQR:165 to 188.5, 95% CI: 170 to 171, N = 172) Median difference: −12 minutes (95% CI: −19 to −7), P < 0.0001** | * Time when consultation was requested minus time when a bed was requested (for admission to hospital) or discharge time IG: intervention group; CG: control group; CI: confidence interval ** Data as provided by the authors |
Comparison 8: Mobile technologies for use in the emergency department compared to usual care: Time between presentation and management of the health condition, Outcome 1: Time between presentation and management
| Healthcare use | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Gulacti 2017 | Emergency physicians consulting with specialists about adults attending the emergency department | Median emergency department length of stay | IG: 240 minutes (IQR: 230 to 270, 95% CI: 240 to 255.2, N = 173) CG: 277 minutes (IQR: 270 to 287.8, 95% CI:277 to 279, N = 172) Median difference −30 minutes, 95% CI −37 to −25* | IG: intervention group; CG: control group; IQR: interquartile range; CI: confidence interval Follow‐up not specified * Data provided by study authors |
Comparison 9: Mobile technologies for use in the emergency department compared to usual care: Healthcare use, Outcome 1: Healthcare use
| Technical difficulties | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Quality of the data transmitted | ||||
| Gulacti 2017 | Emergency physicians consulting with specialists about adults attending the emergency department | Technical problems | There were no problems reported | |
Comparison 10: Mobile technologies for use in the emergency department compared to usual care: Technical difficulties, Outcome 1: Technical difficulties
| Healthcare use | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Iversen 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Outpatient clinic consultations Community nurse consultations | IG: Mean 2.8 (SD 1.9, N = 94), CG: Mean 2.5 (SD 3.0, N = 88) MD −0.48 (95% CI −1.46 to 0.49) IG: M 6.7 (SD 3.4, N = 94), CG: M 5.9 (SD 4.6, N = 88) MD 0.92 (95% CI −0.70 to 2.53) | 12‐month follow‐up SD: standard deviation; MD: mean difference; CI: confidence interval |
| Orlandoni 2016 | Home‐visiting nursing staff consulting with a hospital physician about older adults treated with home enteral nutrition | Outpatient visits Hospitalisations | Incidence rate ratio 95% CI: 0.65 to 1.30, P = 0.62 Incidence rate ratio 95% CI: 0.54 to 1.19, P = 0.26* | 12‐month follow‐up * Data as provided by the authors |
Comparison 11: Mobile technologies used by community health workers or home‐care workers compared to usual care: Healthcare use, Outcome 1: Healthcare use
| Participant healthcare status and well‐being | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Chang 2011 | Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Mortality | IG: 37/446; CG: 53/524 RR 0.82, 95% CI 0.55 to 1.22 | Average follow‐up: 103 weeks |
| Iversen 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Mortality | IG: 5/99; CG 5/88 RR 0.94, 95% CI 0.28 to 3.12 | 12 months follow‐up |
| Taylor‐Gjevre 2018 | Rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis | Disease activity Health‐related quality of life | DAS28‐CRPa MD 0.9 (95% CI −1.2 to 3.1, P = 0.33) mHAQb MD 0.2 (95% CI −0.1 to 0.5, P = 0.14) RADAIc MD 0.9 (95% CI −0.5 to 2.4, P = 0.19) EQ5Dd MD −0.1 (95% CI −0.4 to 0.1, P = 0.29)* | aDisease activity score for rheumatoid arthritis, higher scores represent greater disease activity b Modified health assessment questionnaire, 0 ‐ 3, higher scores represent greater impairment cRheumatoid arthritis disease activity index, 0 ‐ 10, higher scores represent greater disease activity dEuroQol 5 dimensions questionnaire (EQ5D), 0 ‐ 1, higher scores represent better health‐related quality of life Mean difference (MD) between groups, (Control (N = 31), Intervention (N = 54)), from baseline to 9‐month follow‐up All data as provided by the study authors |
Comparison 12: Mobile technologies used by community health workers or home‐care workers compared to usual care: Participant's healthcare status and well‐being, Outcome 1: Participant healthcare status and well‐being
| Participant satisfaction with care | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Iversen 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Experience with healthcare | IG: M 4.4 (SD 0.5, N = 67) CG: M 4.4 (SD 0.5, N = 57) MD: 0.0 (95% CI −0.18 to 0.18) | Generic Short Patient Experiences Questionnaire (GS‐PEQ), 1 ‐ 5, higher scores indicate more satisfaction 12‐month follow‐up |
| Taylor‐Gjevre 2018 | Rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis | Participant satisfaction | There was little or no difference between IG (N = 31) and CG (N = 23)* | Visit specific satisfaction questionnaire (VSQ9), 1 ‐ 5, higher scores indicate more satisfaction 9‐month follow‐up * As reported by study authors, no usable data |
Comparison 13: Mobile technologies used by community health workers or home‐care workers compared to usual care: Acceptability or satisfaction, Outcome 1: Participant satisfaction with care
| Costs | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Chang 2011 | Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Yearly total cost of running the mHealth intervention Cost per participant | N = 29 clusters, 970 participants. USD 1046 USD 2.35 | Intervention arm only, costs calculated in Ugandan shillings and converted to USD (2011). Does not include cost of a previously set‐up intervention to train peer health workers, to which the mHealth was an add‐on Average follow‐up: 103 weeks |
Comparison 14: Mobile technologies used by community health workers or home‐care workers compared to usual care: Costs, Outcome 1: Costs
| Technical difficulties | ||||
| Study | Population | Outcomes | Results | Notes |
|---|---|---|---|---|
| Quality of the data transmitted | ||||
| Taylor‐Gjevre 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Technical problems | For 10 video‐conferencing visits images were not transmitted and only an audio‐link was available | Unclear how many visits were conducted in total Intervention group only |
| Technical difficulties reported by the healthcare professionals | ||||
| Chang 2011 | Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Problems with the equipment | Healthcare professionals were not always able to charge the mobile phone Some mobile phones were stolen | Qualitative outcomes based on a small number of interviews (4) Intervention group only |
Comparison 15: Mobile technologies used by community health workers or home‐care workers compared to usual care: Technical difficulties, Outcome 1: Technical difficulties
| Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared with usual care | ||||
| Population: Primary care providers consulting with dermatologists (6 studies), ophthalmologists (2 studies), radiologists (1 study), nephrologists (1 study), or different specialists (1 study) | ||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement |
|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols: Adherence to the advised monitoring criteria Follow‐up not specified | 1 trial of telenephrology (Van Gelder 2017), using a web‐based platform with access to the electronic medical record reported OR of 1.23 (95% CI 0.89 to 1.70) for monitoring of disease and 0.61 (0.22 to 1.72) for monitoring of metabolic parameters | 3004 (1 cluster‐randomised trial, 47 general practices) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with a hospital‐based specialist probably make little or no difference to primary care providers’ adherence to the advised monitoring criteria for participants with chronic kidney disease (CKD), when compared with usual care |
| Time between presentation and management of the health condition Follow‐up: 3 to 6 months | 2 trials of teledermatology (Piette 2017; Whited 2002) reported that participants allocated to IG received the required treatment in less time than those allocated to CG (median delay 4 days for IG and 40 days for CG; MD −40.5 days, 95% CI −23 to −58) 1 trial of telemedicine using a portable ultrasound (Sutherland 2009) for people presenting with symptoms that required an ultrasound reported little or no difference between groups. 1 trial of eConsult for people attending primary care (Azogil‐López 2019) reported that participants allocated to IG had an appointment in less time than those allocated to CG (median difference −27 days, 99% CI −20 to −33) | 656 (4 randomised trials) | ⊕⊕⊕⊝ | The intervention probably reduces time between participants presenting and management among individuals with some skin conditions, symptoms requiring an ultrasound, or requiring an appointment with a specialist after attending primary care |
| Healthcare use Follow‐up: 3 to 12 months | 4 trials of teledermatology (Byamba 2015; Piette 2017; Whited 2002; Whited 2013; RRs ranged from to 0.28 (95% CI 0.13 to 0.63) to 0.82 (95% CI 0.75 to 0.88)) reported that those participants allocated to the intervention group were less likely to be referred for clinic follow‐up or attend an appointment at a clinic 2 trials of eConsults for nephrology (Van Gelder 2017) and different specialties (Liddy 2019a) reported little or no difference between groups (OR 0.61, 95% CI 0.31 to 1.23 and RR 0.93, 95% CI 0.85 to 1.03, respectively) 2 trials of telemedicine for retinopathy screening (Davis 2003; Mansberger 2015) and 1 trial for people presenting with symptoms that required an ultrasound (Sutherland 2009; RR 3.92, 95% CI 2.11 to 7.31) reported that those participants allocated to the intervention group were more likely to receive a clinical examination | 4810 (9 randomised trials) | ⊕⊕⊕⊝ Moderatec | Mobile technologies used by primary care providers to consult with hospital‐based specialists may reduce referrals and clinic visits among people with skin conditions, and increase the likelihood of receiving retinopathy screening among participants with diabetes, and an ultrasound in those referred with symptoms, when compared with usual care 1 trial did not specifically report the number of participants involved |
| Participants' health status and well‐being | Patient‐reported quality of life and health‐related quality of life (Follow‐up: 9 to 12 months) | |||
| 2 trials of teledermatology (Armstrong 2018; Whited 2013) found little or no difference between groups For health status (EQ‐5D‐5L): MD 0 (95% CI −0.003 to 0.003) For quality of life (Skindex‐16): IG: MD −12.0 (SD 24.5, 160 participants), CG: MD −13.2 (SD 21.6, 164 participants) For health‐related quality of life (SF‐12), results reported as little or no difference between groups | 622 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with hospital‐based specialists probably make little or no difference to quality of life and health‐related quality of life among individuals with skin conditions | |
| Clinician‐assessed clinical course (follow‐up: 4 to 9 months) | ||||
| 2 trials of teledermatology (Pak 2007; Whited 2013) found little or no difference between groups | 769 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists probably make little or no difference to clinical improvement among individuals with skin conditions | |
| Acceptability and satisfaction | Healthcare provider acceptability and satisfaction (follow‐up immediately after the intervention) | |||
| 1 trial of teledermatology (Piette 2017) reported little or no difference between groups 1 trial of teledermatology (Whited 2002) reported that GPs allocated to the intervention were more likely to agree that participants received timely appointments and to be satisfied with the consult process than GPs allocated to the control group | 378 | ⊕⊕⊝⊝ | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists may make little or no difference to healthcare provider acceptability and satisfaction with the intervention | |
| Participant acceptability and satisfaction (follow‐up: 1 to 9 months) | ||||
| 4 trials of teledermatology (Eminović 2009; Piette 2017; Whited 2002; Whited 2013) reported little or no difference between groups 1 trial reported MD 0.0 (95% CI −0.12 to 0.12; PSQ III), another trial reported that 87% of participants allocated to the intervention group were overall satisfied with treatment received, compared with 92% of those allocated to the control group* 2 trials reported the results as little or no difference only (VSQ9; *) | 972 (4 randomised trials) | ⊕⊕⊝⊝ Lowg | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists may make little or no difference to acceptability and satisfaction of participants with skin conditions | |
| Costs Follow‐up: 1 to 9 months | 2 teledermatology trials (Eminović 2009; Whited 2013) and 1 telenephrology trial (Van Gelder 2017) reported little or no difference between groups 2 teledermatology trials (Pak 2007; Whited 2002) reported that when loss of productivity was considered, the cost per participant was higher for those allocated to the intervention 1 trial of teledermatology (Byamba 2015) reported that total costs were lower for those allocated to the intervention group. | 5423 (6 randomised trials) | ⊕⊕⊝⊝ Lowh | The intervention may make little or no difference to total or expected costs per participant for adults with skin conditions or chronic kidney disease |
| Technical problems | 1 trial recruiting GPs consulting with dermatologists about images they took (Pak 2007) reported that there was little or no difference between groups for technical problems | 698 (1 randomised trial) | ⊕⊕⊕⊝ Moderatei | The intervention probably results in few or no technical difficulties |
| CG: Control group; CI: Confidence interval; EQ5D: EuroQol five dimensions questionnaire; GPs: General practitioners; IG: Intervention group; MD: Median difference; OR: Odds ratio; PSQ III: Shortened version of the Patient Satisfaction Questionnaire; RR: Risk ratio; SD: Standard deviation; SF‐12: Short‐Form Health Survey 12; VSQ9: Visit‐specific satisfaction questionnaire (VSQ9) * Questions developed by the authors for the specific trial | ||||
| GRADE Working Group grades of evidence | ||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to performance and detection bias, and lack of protection against contamination. | ||||
| Mobile technologies for use in the emergency department compared with usual care | |||||
| Patient or population: Emergency physicians consulting with hospital specialists about adults attending the emergency department | |||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement | |
|---|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols | ‐ | ‐ | ‐ | No studies were identified | |
| Time between presentation and management of the health condition Follow‐up not reported | 1 trial (Gulacti 2017) reported that those allocated with the intervention group were admitted to hospital or discharged more quickly from the emergency department (median difference −12 minutes, 95% CI −19 to −7 minutes) | 345 (1randomised trial) | ⊕⊕⊕⊝ | The intervention probably reduces time between participants presenting and management by a few minutes among individuals visiting the emergency department | |
| Healthcare use: length of stay in the emergency department Follow‐up not reported | 1 trial (Gulacti 2017) reported that participant allocated to the intervention group participants had a shorter stay in the emergency department (median difference −30 minutes, 95% CI: −37 to −25 minutes) | 345 (1 randomised trial) | ⊕⊕⊕⊝ | The intervention probably slightly reduces length of stay among individuals visiting the emergency department | |
| Participants' health status and well‐being | ‐ | ‐ | ‐ | No studies were identified | |
| Participant and provider acceptability or satisfaction | ‐ | ‐ | ‐ | No studies were identified | |
| Costs | ‐ | ‐ | ‐ | No studies were identified | |
| Technical problems | 1 trial (Gulacti 2017) reported that there were no technical problems during the course of the trial | 345 (1 randomised trial) | ⊕⊕⊕⊝ | The intervention probably results in few or no technical difficulties | |
| CI: Confidence interval | |||||
| GRADE Working Group grades of evidence | |||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to high risk of performance and reporting bias. | |||||
| Mobile technologies used by community health or home‐care workers compared with usual care | ||||
| Patient or population: Community‐based peer health workers consulting with clinic staff about receiving antiretroviral therapy, community nurses consulting with diabetes specialist nurses or podiatrists about adults with Type 2 diabetes, home‐care nurses consulting with hospital specialists about home enteral nutrition, rural‐based physical therapists consulting with urban‐based rheumatologists | ||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement |
|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols | ‐ | ‐ | ‐ | No studies were identified |
| Time between presentation and management of the health condition | ‐ | ‐ | ‐ | No studies were identified |
| Healthcare use | Outpatient clinic and community nurse consultations (follow‐up: 12 months) | |||
| 2 trials (Iversen 2018; Orlandoni 2016) reported little or no difference between groups for outpatient visits (MD −0.48, 95% CI −1.46 to 0.49) or community nurse consultations (MD 0.92, 95% CI −0.70 to 2.53) | 370 (2 randomised trials) | ⊕⊕⊕⊝ Moderatea | Mobile technologies used by community health or home‐care workers probably make little or no difference for outpatient clinic and community nurse consultations of participants with new diabetes‐related foot ulcer and older individuals treated with home enteral nutrition | |
| Hospitalisation (Follow‐up: 12 months) | ||||
| 1 study (Orlandoni 2016) reported that the incidence rate ratio for hospitalisations was similar between groups among older individuals treated with home enteral nutrition (95% CI 0.54 to 1.19, P = 0.26) | 188 (1 randomised trial) | ⊕⊕⊝⊝ | Mobile technologies for communication between home‐visiting nursing staff consulting with a hospital physician may have little or no effect on hospitalisations among older individuals treated with home enteral nutrition | |
| Participants' health status and well‐being | Mortality among individuals living with HIV or diabetes (Follow‐up: 11 to 12 months) | |||
| 2 trials reported little or no differences between groups. 1 study (Chang 2011) recruited peer health workers who consulted with clinic staff (RR: 0.82, 95% CI 0.55 to 1.22), and another study (Iversen 2018) recruited community nurses who consulted with diabetes specialist nurses (RR: 0.94, 95% CI 0.28 to 3.12). | 1157 | ⊕⊕⊝⊝ | The intervention may make little or no difference in mortality among people living with HIV or diabetes | |
| Disease activity or health‐related quality of life (Follow‐up: 9 months) | ||||
| 1 trial of rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis (Taylor‐Gjevre 2018) reported little or no difference between groups for disease activity (DAS28‐CRP MD 0.9, 95% CI −1.2 to 3.1; mHAQ MD 0.2, 95% CI −0.1 to 0.5; RADAI MD 0.9, 95% CI −0.5 to 2.4) or health‐related quality of life (EQ5D MD −0.1, 95% CI −0.4 to 0.1) | 85 (1 randomised trial) | ⊕⊕⊝⊝ | Mobile technologies used by community health or home‐care workers may make little or no difference for disease activity and health‐related quality of life in participants with rheumatoid arthritis | |
| Participant and provider acceptability or satisfaction | Healthcare provider acceptability and satisfaction | |||
| ‐ | ‐ | ‐ | No studies were identified | |
| Participant acceptability and satisfaction (Follow‐up: 9 to 12 months) | ||||
| 2 trials on diabetes (Iversen 2018) and arthritis (Taylor‐Gjevre 2018) reported little or no difference between groups for participants' experience with healthcare (GS‐PEQ MD 0.0, 95% CI −0.18 to 0.18) and satisfaction (VSQ9 results reported narratively) with the intervention. | 178 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by community health or home‐care workers probably make little or no difference for participant acceptability and satisfaction for participants with new diabetes‐related foot ulcer and participants with rheumatoid arthritis | |
| Costs | ‐ | ‐ | ‐ | No studies were identified |
| Technical difficulties | ‐ | ‐ | ‐ | No studies were identified |
| CI: Confidence interval; DAS28‐CRP: Disease activity score for Rheumatoid Arthritis; EQ5D: EuroQol five dimensions questionnaire; GS‐PEQ: Generic Short Patient Experiences Questionnaire; MD: Mean difference; mHAQ: Modified health assessment questionnaire; RADAI: Rheumatoid arthritis disease activity index; RR: Risk ratio; VSQ9: Visit‐specific satisfaction questionnaire | ||||
| GRADE Working Group grades of evidence | ||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (2 studies), attrition (1 study) and reporting (1 study) bias. bWe downgraded one point for imprecision because the 95% CI shows potential effect on both sides of “no effect” line and that there were few events. cWe downgraded one point for risk of bias due to high risk of performance, detection, and attrition bias. dWe downgraded one point for imprecision because the 95% CI shows potential effect on both sides of “no effect” line . eWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (1 study), attrition (1 study) and reporting (2 studies) bias. fWe downgraded one point for risk of bias due to high risk of performance, detection, attrition, and reporting bias. gWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (2 studies), attrition (1 study), and reporting (2 studies) bias. | ||||
| Study | Incentives | Specific training |
|---|---|---|
| Participants were paid for participating in the study, through gift cards (main paper, p.3, end 1st paragraph) | Participants and their carers were taught how to take standardised images of skin lesions, as well as how to communicate with the dermatologist using a secure web‐based system. PCPs also had access to the training materials. (Protocol, p.19, 2nd paragraph) | |
| ‐ | GPs attended a 2‐day training session to learn how to take images and use the medical record system and software on mobile phones (p.1, top 2nd column) | |
| PHWs were given a bicycle, t‐shirts, basic supplies, and an initial monthly allowance (parent trial) | PHWs allocated to the intervention group were given a mobile phone, and attended a 1‐day residential training and a brief field‐based practical training on the intervention (main paper, p.3, 2nd paragraph) | |
| ‐ | GPs allocated to the intervention group received detailed instructions on how to take digital images and use the web‐based form (main paper, p.559, bottom 1st column) | |
| ‐ | All staff received training in the use of the web‐based system, as well as in‐person access to hospital clinics to improve their practical skills (main paper, pp.97‐8) | |
| Specialists received financial incentives for each eConsult they undertook (support paper, under 8. Payment) | ‐ | |
| Participants received monetary incentive to complete follow‐up questionnaire (associated paper, p.524, bottom 1st column) | Technicians performing imaging attended a 3‐day training session to learn how to take images and ongoing feedback as needed (main paper, p.943, bottom 1st column) | |
| ‐ | GPs received training and a workbook on how to take photographs (p.2, top 2nd column) | |
| ‐ | The on‐site investigator received sonographic training over a 2‐month period, as well as practice guidelines for trans‐abdominal ultrasound scanning (P. 192, mid 1st column and top 2nd column) | |
| ‐ | Physical therapists and rheumatologists received an orientation and education session about rheumatoid arthritis and the study protocol and methods (main paper, p.2, top 2nd column) | |
| GP: general practitioner; PCP: primary care provider; PHW: peer health workers | ||
| Study ID | Population | Disadvantaged populations included/excluded? | Notes |
|---|---|---|---|
| General practitioner consulting with dermatologists about adults with psoriasis | Participants without access to the Internet and a digital camera or smartphone with camera features were excluded | ‐ | |
| GP consulting with hospital physicians about participants (aged ≥ 7 years) | Participants deemed as complex were not eligible for receiving the intervention | Complex participants defined as those lacking a specific diagnosis or requiring further clinical assessment | |
| GP consulting with dermatologists about adults with skin lesions | Intervention was set in rural health clinics in Mongolia | ‐ | |
| Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Specifically targeted HIV‐positive participants in rural Uganda. However, many participants had limited access to mobile phones*, which might have limited the benefits of the intervention. For the healthcare providers, the costs of the intervention were also a factor, as although they were given a monthly stipend it was not always enough Charging the mobile phone was often challenging, as access to electricity was limited | * Current mobile phone penetration in Uganda at the time the trial was conducted was 39% | |
| PCPs at the rural primary practice consulting with ophthalmologist in the university setting about adults with Type 2 diabetes | Specifically targeted rural‐based ethnic minorities, 35% of whom did not have health insurance | ‐ | |
| Emergency physicians consulting with specialists about adults attending the emergency department | Only consultants who owned a smartphone and were familiarised with the secure messaging service were included | ‐ | |
| PCPs consulting with experienced investigators based at an eye institute about adults with Type 2 diabetes | Primary clinics that served a large number of ethnic minorities, including a high percentage of participants with transient housing | ‐ | |
| General practitioners consulting with dermatologists about adults with skin lesions | Participants who were not able to attend in‐person appointments at the dermatologist office were excluded, i.e. participants unable to travel or those residing in nursing homes. | ‐ | |
| GP consulting with radiologists about participants aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Sample was composed mainly of low‐skilled workers relying on government‐supported primary clinics for their health care | ‐ | |
| Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Specifically targeted rural‐based adults | ‐ | |
| GP consulting with dermatologists about adults with skin condition | Participants who could not speak or read English or who failed a single‐question literacy assessment* were excluded | *Single‐Item Literacy Screener (SILS), which identifies limited reading ability (Morris 2006) | |
| GP: General practitioner; PCP: primary care provider; PHW: Peer health workers | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Providers' adherence to recommended guidelines Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Time between presentation and management Show forest plot | 4 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Healthcare use Show forest plot | 9 | Other data | No numeric data | |
| 3.1.1 Healthcare use | 9 | Other data | No numeric data | |
| 3.2 Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2.1 Referred to a dermatology clinic | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Health‐related quality of life Show forest plot | 2 | Other data | No numeric data | |
| 4.2 Clinical course Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Healthcare provider satisfaction with the intervention Show forest plot | 3 | Other data | No numeric data | |
| 5.2 Participant satisfaction with care Show forest plot | 4 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Costs Show forest plot | 6 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Technical difficulties Show forest plot | 4 | Other data | No numeric data | |
| 7.1.1 Quality of the data transmitted | 4 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Time between presentation and management Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Healthcare use Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Technical difficulties Show forest plot | 1 | Other data | No numeric data | |
| 10.1.1 Quality of the data transmitted | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Healthcare use Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Participant healthcare status and well‐being Show forest plot | 3 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Participant satisfaction with care Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Costs Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Technical difficulties Show forest plot | 2 | Other data | No numeric data | |
| 15.1.1 Quality of the data transmitted | 1 | Other data | No numeric data | |
| 15.1.2 Technical difficulties reported by the healthcare professionals | 1 | Other data | No numeric data | |