Tecnologías móviles para apoyar la comunicación y la gestión de la atención médica entre los profesionales sanitarios
Resumen
Antecedentes
El uso generalizado de las tecnologías móviles puede ampliar el uso de los enfoques de la telemedicina para facilitar la comunicación entre los profesionales sanitarios, lo que podría aumentar el acceso al asesoramiento especializado y mejorar los desenlaces de salud de los pacientes.
Objetivos
Evaluar los efectos de las tecnologías móviles para apoyar la comunicación y las consultas entre los profesionales sanitarios, en comparación con la atención habitual, sobre el rendimiento, la aceptabilidad y la satisfacción de los profesionales sanitarios, el uso de los servicios de salud, los desenlaces de salud de los pacientes, la aceptabilidad y la satisfacción, los costos y las dificultades técnicas.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL, MEDLINE, Embase y otras tres bases de datos desde el 1 de enero de 2000 hasta el 22 de julio de 2019. Se realizaron búsquedas en los registros de ensayos clínicos, se verificaron las referencias de las revisiones sistemáticas pertinentes y de los estudios incluidos, y se estableció contacto con expertos en el tema.
Criterios de selección
Ensayos aleatorizados que compararon las tecnologías móviles de apoyo a la comunicación y las consultas de los profesionales sanitarios en comparación con la atención habitual.
Obtención y análisis de los datos
Se siguieron los procedimientos metodológicos estándar previstos por Cochrane y EPOC. Se utilizaron los criterios GRADE para evaluar la certeza de la evidencia.
Resultados principales
Se incluyeron 19 ensayos (5766 participantes cuando se informó), la mayoría de los cuales se realizaron en países de ingresos altos. La tecnología móvil más utilizada fue el teléfono móvil, a menudo acompañado de capacitación si se utilizaba para transferir imágenes digitales. En los ensayos se reclutaron participantes con diferentes afecciones, y las intervenciones variaron en cuanto a la entrega, los componentes y la frecuencia de contacto. Se consideró que la mayoría de los ensayos tuvo alto riesgo de sesgo de realización y aproximadamente la mitad tuvo un alto riesgo de sesgo de detección, desgaste e informe. Dos estudios que informaron datos sobre los problemas técnicos, indicaron pocas dificultades.
Tecnologías móviles utilizadas por los profesionales sanitarios para consultar a los especialistas de los hospitales
La certeza de la evidencia en este grupo de ensayos se consideró de moderada a baja.
Las tecnologías móviles:
‐ probablemente dan lugar a poca o ninguna diferencia en el cumplimiento de las guías por los profesionales sanitarios en pacientes con enfermedad renal crónica (enfermedad renal crónica [ERC]; un ensayo, 47 consultorios generales, 3004 participantes);
‐ probablemente reducen el tiempo entre la presentación y el tratamiento de los pacientes con afecciones cutáneas, los pacientes con síntomas que requieren una ecografía o que son remitidos a una cita con un especialista después de acudir a la atención primaria (cuatro ensayos, 656 participantes);
‐ pueden reducir las derivaciones y las visitas clínicas entre los pacientes con algunas enfermedades cutáneas, y aumentar la probabilidad de recibir una prueba de detección de la retinopatía entre los pacientes con diabetes, o una ecografía en los remitidos con síntomas (nueve ensayos, 4810 participantes cuando se informó);
‐ probablemente dan lugar a poca o ninguna diferencia en la calidad de vida informada por los pacientes y en la calidad de vida relacionada con la salud (dos ensayos, 622 participantes) o en la recuperación clínica evaluada por el médico (dos ensayos, 769 participantes) entre los individuos con afecciones cutáneas;
‐ pueden dar lugar a poca o ninguna diferencia para el profesional sanitario (dos ensayos, 378 participantes) o en la aceptación y la satisfacción de los participantes (cuatro ensayos, 972 participantes) cuando los profesionales de atención primaria consultan a los dermatólogos;
‐ pueden dar lugar a poca o ninguna diferencia en los costos totales o previstos por participante en los adultos con algunas afecciones cutáneas o con ERC (seis ensayos, 5423 participantes).
Tecnologías móviles utilizadas por los médicos de urgencias para consultar a los especialistas del hospital sobre pacientes que acuden al servicio de urgencias
La certeza de la evidencia en este grupo de ensayos se consideró moderada.
Las tecnologías móviles:
‐ probablemente reducen ligeramente el tiempo de consulta entre los médicos de urgencias y los especialistas del hospital (diferencia de medias ‐12 minutos; IC del 95%: ‐19 a ‐7; un ensayo, 345 participantes);
‐ probablemente reducen la duración de la estancia de los participantes en el servicio de urgencias en unos pocos minutos (diferencia de medias ‐30 minutos; IC del 95%: ‐37 a ‐25; un ensayo, 345 participantes).
No se identificaron ensayos que informaran sobre la adherencia de los profesionales, el estado de salud y el bienestar de los participantes, la aceptabilidad y la satisfacción de los profesionales y los participantes, o los costos.
Tecnologías móviles utilizadas por el personal sanitario de la comunidad o el personal de atención domiciliaria para consultar con el personal clínico
La certeza de la evidencia en este grupo de ensayos se consideró de moderada a baja.
Las tecnologías móviles:
‐ probablemente dan lugar a poca o ninguna diferencia en el número de consultas clínicas ambulatorias y con enfermeras comunitarias en los pacientes con diabetes o en las personas de edad avanzada tratadas con nutrición enteral en el domicilio (dos ensayos, 370 participantes) o en la hospitalización de las personas de edad avanzada tratadas con nutrición enteral en el domicilio (un ensayo, 188 participantes);
‐ pueden dar lugar a poca o ninguna diferencia en la mortalidad entre los pacientes con VIH (RR 0,82; IC del 95%: 0,55 a 1,22) o diabetes (RR 0,94; IC del 95%: 0,28 a 3,12) (dos ensayos, 1152 participantes);
‐ pueden dar lugar a poca o ninguna diferencia en la actividad de la enfermedad o en la calidad de vida relacionada con la salud de los participantes con artritis reumatoide (un ensayo, 85 participantes);
‐ probablemente dan lugar a poca o ninguna diferencia en cuanto a la aceptabilidad y la satisfacción de los pacientes con diabetes y los pacientes con artritis reumatoide (dos ensayos, 178 participantes).
No se identificaron ensayos que informaran sobre la adherencia de los profesionales, el tiempo entre la presentación y el tratamiento, la aceptabilidad y la satisfacción de los profesionales sanitarios, o los costos.
Conclusiones de los autores
La confianza en la estimación del efecto es limitada. Las intervenciones que incluyen un componente de tecnología móvil para apoyar la comunicación y la gestión de la atención médica entre los profesionales sanitarios pueden reducir el tiempo que transcurre entre la presentación y el tratamiento de la afección de salud cuando los profesionales de atención primaria o los médicos de urgencia las utilizan para consultar a los especialistas, y pueden aumentar la probabilidad de recibir un examen clínico entre los pacientes con diabetes y los que necesitan una ecografía. Pueden disminuir el número de personas que acuden a la atención primaria y que son remitidas a la atención secundaria o terciaria en el caso de algunas afecciones, como algunas enfermedades cutáneas y ERC. Hubo poca evidencia de los efectos sobre el estado de salud, el bienestar y la satisfacción de los participantes o los costos.
PICO
Resumen en términos sencillos
Uso de tecnologías móviles para promover la comunicación y la gestión de la atención médica entre los profesionales sanitarios
¿Cuál es el objetivo de esta revisión?
El objetivo de esta revisión fue determinar si el personal sanitario que utiliza los servicios de mHealth a través de teléfonos móviles u otros dispositivos móviles para comunicarse con otros trabajadores sanitarios logra un acceso más rápido a la atención sanitaria y mejora los desenlaces de salud de los pacientes. Se obtuvieron y analizaron todas las investigaciones pertinentes y se encontraron 19 estudios.
Mensajes clave
Es probable que las tecnologías móviles reduzcan ligeramente el tiempo para brindar los servicios de atención sanitaria, así como el número de citas presenciales, en comparación con la atención habitual, y probablemente aumenten el número de personas que se someten a exámenes clínicos por algunas afecciones, lo que incluye el examen de la vista en pacientes con diabetes. Las tecnologías móviles pueden tener poco o ningún impacto en la satisfacción, el estado de salud o el bienestar del personal sanitario y de los participantes.
¿Qué se estudió en la revisión?
Muchos trabajadores sanitarios trabajan solos o tienen poco acceso a colegas y especialistas. Se trata de un problema común en el personal sanitario de zonas rurales o en los países de ingresos bajos.
Una posible solución a este problema es ofrecer al personal sanitario asesoramiento y apoyo mediante tecnologías móviles que les permitan obtener ayuda de colegas que no están en el mismo lugar. Por ejemplo, los trabajadores sanitaros pueden establecer contacto con especialistas o colegas con más experiencia a través de un teléfono o de Internet. El personal sanitario también puede utilizar sus teléfonos móviles u otros dispositivos móviles como las tabletas. A medida que más trabajadores sanitarios utilizan teléfonos móviles y otros dispositivos como parte de su trabajo, se podría facilitar especialmente el uso de los servicios de mHealth.
¿Cuáles son los principales resultados de la revisión?
Se encontraron 19 estudios relevantes que incluyeron a más de 5766 personas que necesitaban atención médica. Dieciséis estudios se realizaron en países de ingresos altos. Dos estudios informaron sobre problemas técnicos y no refirieron muchas dificultades.
Cuando el personal sanitario utiliza las tecnologías móviles para consultar con los especialistas del hospital:
‐ probablemente no influye en absoluto en el cumplimiento de las guías en los pacientes con enfermedad renal crónica, ni en el estado de salud o la calidad de vida de los enfermos de psoriasis.
‐ puede aumentar la probabilidad de que se realicen pruebas de detección de la retinopatía en pacientes con diabetes, o de que se les realice una ecografía si son remitidos con síntomas, y puede reducir las derivaciones o la visita a la clínica de pacientes con una afección cutánea o remitidas para un seguimiento clínico por diferentes problemas de salud.
‐ puede no influir en la satisfacción del personal sanitario o de los pacientes, o en el costo de la atención médica.
Cuando los médicos de urgencias utilizan las tecnologías móviles para consultar con los especialistas del hospital:
‐ probablemente los pacientes son tratados un poco más rápido.
No se encontraron estudios que examinaran el efecto de las tecnologías móviles en el cumplimiento de las guías por los médicos de urgencias, en la salud y el bienestar de los pacientes, la satisfacción del personal de salud o de los pacientes, o en los costos.
Cuando el personal sanitario de la comunidad o el personal de atención domiciliaria utilizan tecnologías móviles para consultar con el personal de la clínica:
‐ probablemente no influye en el número de veces que los pacientes con una nueva úlcera en el pie relacionada con la diabetes tienen que ver a una enfermera, o las personas de edad avanzada que se alimentan por sonda tienen que ver a una enfermera o ir al hospital.
‐ puede no influir en el número de personas que viven con VIH o diabetes que mueren; y puede no influir en el estado de salud o la calidad de vida de los pacientes con artritis reumatoide.
‐ probablemente no influyen en la satisfacción de los pacientes con diabetes o artritis reumatoide.
No se encontraron estudios que examinaran el efecto de las tecnologías móviles sobre el cumplimiento de las guías por parte del personal sanitario de la comunidad, la rapidez con la que los pacientes reciben atención, la satisfacción del personal sanitario, los costos o las dificultades técnicas.
¿Cuál es el grado de actualización de esta revisión?
Se buscaron estudios hasta el 22 de julio de 2019.
Authors' conclusions
Summary of findings
| Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared with usual care | ||||
| Population: Primary care providers consulting with dermatologists (6 studies), ophthalmologists (2 studies), radiologists (1 study), nephrologists (1 study), or different specialists (1 study) | ||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement |
|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols: Adherence to the advised monitoring criteria Follow‐up not specified | 1 trial of telenephrology (Van Gelder 2017), using a web‐based platform with access to the electronic medical record reported OR of 1.23 (95% CI 0.89 to 1.70) for monitoring of disease and 0.61 (0.22 to 1.72) for monitoring of metabolic parameters | 3004 (1 cluster‐randomised trial, 47 general practices) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with a hospital‐based specialist probably make little or no difference to primary care providers’ adherence to the advised monitoring criteria for participants with chronic kidney disease (CKD), when compared with usual care |
| Time between presentation and management of the health condition Follow‐up: 3 to 6 months | 2 trials of teledermatology (Piette 2017; Whited 2002) reported that participants allocated to IG received the required treatment in less time than those allocated to CG (median delay 4 days for IG and 40 days for CG; MD −40.5 days, 95% CI −23 to −58) 1 trial of telemedicine using a portable ultrasound (Sutherland 2009) for people presenting with symptoms that required an ultrasound reported little or no difference between groups. 1 trial of eConsult for people attending primary care (Azogil‐López 2019) reported that participants allocated to IG had an appointment in less time than those allocated to CG (median difference −27 days, 99% CI −20 to −33) | 656 (4 randomised trials) | ⊕⊕⊕⊝ | The intervention probably reduces time between participants presenting and management among individuals with some skin conditions, symptoms requiring an ultrasound, or requiring an appointment with a specialist after attending primary care |
| Healthcare use Follow‐up: 3 to 12 months | 4 trials of teledermatology (Byamba 2015; Piette 2017; Whited 2002; Whited 2013; RRs ranged from to 0.28 (95% CI 0.13 to 0.63) to 0.82 (95% CI 0.75 to 0.88)) reported that those participants allocated to the intervention group were less likely to be referred for clinic follow‐up or attend an appointment at a clinic 2 trials of eConsults for nephrology (Van Gelder 2017) and different specialties (Liddy 2019a) reported little or no difference between groups (OR 0.61, 95% CI 0.31 to 1.23 and RR 0.93, 95% CI 0.85 to 1.03, respectively) 2 trials of telemedicine for retinopathy screening (Davis 2003; Mansberger 2015) and 1 trial for people presenting with symptoms that required an ultrasound (Sutherland 2009; RR 3.92, 95% CI 2.11 to 7.31) reported that those participants allocated to the intervention group were more likely to receive a clinical examination | 4810 (9 randomised trials) | ⊕⊕⊕⊝ Moderatec | Mobile technologies used by primary care providers to consult with hospital‐based specialists may reduce referrals and clinic visits among people with skin conditions, and increase the likelihood of receiving retinopathy screening among participants with diabetes, and an ultrasound in those referred with symptoms, when compared with usual care 1 trial did not specifically report the number of participants involved |
| Participants' health status and well‐being | Patient‐reported quality of life and health‐related quality of life (Follow‐up: 9 to 12 months) | |||
| 2 trials of teledermatology (Armstrong 2018; Whited 2013) found little or no difference between groups For health status (EQ‐5D‐5L): MD 0 (95% CI −0.003 to 0.003) For quality of life (Skindex‐16): IG: MD −12.0 (SD 24.5, 160 participants), CG: MD −13.2 (SD 21.6, 164 participants) For health‐related quality of life (SF‐12), results reported as little or no difference between groups | 622 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with hospital‐based specialists probably make little or no difference to quality of life and health‐related quality of life among individuals with skin conditions | |
| Clinician‐assessed clinical course (follow‐up: 4 to 9 months) | ||||
| 2 trials of teledermatology (Pak 2007; Whited 2013) found little or no difference between groups | 769 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists probably make little or no difference to clinical improvement among individuals with skin conditions | |
| Acceptability and satisfaction | Healthcare provider acceptability and satisfaction (follow‐up immediately after the intervention) | |||
| 1 trial of teledermatology (Piette 2017) reported little or no difference between groups 1 trial of teledermatology (Whited 2002) reported that GPs allocated to the intervention were more likely to agree that participants received timely appointments and to be satisfied with the consult process than GPs allocated to the control group | 378 | ⊕⊕⊝⊝ | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists may make little or no difference to healthcare provider acceptability and satisfaction with the intervention | |
| Participant acceptability and satisfaction (follow‐up: 1 to 9 months) | ||||
| 4 trials of teledermatology (Eminović 2009; Piette 2017; Whited 2002; Whited 2013) reported little or no difference between groups 1 trial reported MD 0.0 (95% CI −0.12 to 0.12; PSQ III), another trial reported that 87% of participants allocated to the intervention group were overall satisfied with treatment received, compared with 92% of those allocated to the control group* 2 trials reported the results as little or no difference only (VSQ9; *) | 972 (4 randomised trials) | ⊕⊕⊝⊝ Lowg | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists may make little or no difference to acceptability and satisfaction of participants with skin conditions | |
| Costs Follow‐up: 1 to 9 months | 2 teledermatology trials (Eminović 2009; Whited 2013) and 1 telenephrology trial (Van Gelder 2017) reported little or no difference between groups 2 teledermatology trials (Pak 2007; Whited 2002) reported that when loss of productivity was considered, the cost per participant was higher for those allocated to the intervention 1 trial of teledermatology (Byamba 2015) reported that total costs were lower for those allocated to the intervention group. | 5423 (6 randomised trials) | ⊕⊕⊝⊝ Lowh | The intervention may make little or no difference to total or expected costs per participant for adults with skin conditions or chronic kidney disease |
| Technical problems | 1 trial recruiting GPs consulting with dermatologists about images they took (Pak 2007) reported that there was little or no difference between groups for technical problems | 698 (1 randomised trial) | ⊕⊕⊕⊝ Moderatei | The intervention probably results in few or no technical difficulties |
| CG: Control group; CI: Confidence interval; EQ5D: EuroQol five dimensions questionnaire; GPs: General practitioners; IG: Intervention group; MD: Median difference; OR: Odds ratio; PSQ III: Shortened version of the Patient Satisfaction Questionnaire; RR: Risk ratio; SD: Standard deviation; SF‐12: Short‐Form Health Survey 12; VSQ9: Visit‐specific satisfaction questionnaire (VSQ9) * Questions developed by the authors for the specific trial | ||||
| GRADE Working Group grades of evidence | ||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to performance and detection bias, and lack of protection against contamination. | ||||
| Mobile technologies for use in the emergency department compared with usual care | |||||
| Patient or population: Emergency physicians consulting with hospital specialists about adults attending the emergency department | |||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement | |
|---|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols | ‐ | ‐ | ‐ | No studies were identified | |
| Time between presentation and management of the health condition Follow‐up not reported | 1 trial (Gulacti 2017) reported that those allocated with the intervention group were admitted to hospital or discharged more quickly from the emergency department (median difference −12 minutes, 95% CI −19 to −7 minutes) | 345 (1randomised trial) | ⊕⊕⊕⊝ | The intervention probably reduces time between participants presenting and management by a few minutes among individuals visiting the emergency department | |
| Healthcare use: length of stay in the emergency department Follow‐up not reported | 1 trial (Gulacti 2017) reported that participant allocated to the intervention group participants had a shorter stay in the emergency department (median difference −30 minutes, 95% CI: −37 to −25 minutes) | 345 (1 randomised trial) | ⊕⊕⊕⊝ | The intervention probably slightly reduces length of stay among individuals visiting the emergency department | |
| Participants' health status and well‐being | ‐ | ‐ | ‐ | No studies were identified | |
| Participant and provider acceptability or satisfaction | ‐ | ‐ | ‐ | No studies were identified | |
| Costs | ‐ | ‐ | ‐ | No studies were identified | |
| Technical problems | 1 trial (Gulacti 2017) reported that there were no technical problems during the course of the trial | 345 (1 randomised trial) | ⊕⊕⊕⊝ | The intervention probably results in few or no technical difficulties | |
| CI: Confidence interval | |||||
| GRADE Working Group grades of evidence | |||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to high risk of performance and reporting bias. | |||||
| Mobile technologies used by community health or home‐care workers compared with usual care | ||||
| Patient or population: Community‐based peer health workers consulting with clinic staff about receiving antiretroviral therapy, community nurses consulting with diabetes specialist nurses or podiatrists about adults with Type 2 diabetes, home‐care nurses consulting with hospital specialists about home enteral nutrition, rural‐based physical therapists consulting with urban‐based rheumatologists | ||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement |
|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols | ‐ | ‐ | ‐ | No studies were identified |
| Time between presentation and management of the health condition | ‐ | ‐ | ‐ | No studies were identified |
| Healthcare use | Outpatient clinic and community nurse consultations (follow‐up: 12 months) | |||
| 2 trials (Iversen 2018; Orlandoni 2016) reported little or no difference between groups for outpatient visits (MD −0.48, 95% CI −1.46 to 0.49) or community nurse consultations (MD 0.92, 95% CI −0.70 to 2.53) | 370 (2 randomised trials) | ⊕⊕⊕⊝ Moderatea | Mobile technologies used by community health or home‐care workers probably make little or no difference for outpatient clinic and community nurse consultations of participants with new diabetes‐related foot ulcer and older individuals treated with home enteral nutrition | |
| Hospitalisation (Follow‐up: 12 months) | ||||
| 1 study (Orlandoni 2016) reported that the incidence rate ratio for hospitalisations was similar between groups among older individuals treated with home enteral nutrition (95% CI 0.54 to 1.19, P = 0.26) | 188 (1 randomised trial) | ⊕⊕⊝⊝ | Mobile technologies for communication between home‐visiting nursing staff consulting with a hospital physician may have little or no effect on hospitalisations among older individuals treated with home enteral nutrition | |
| Participants' health status and well‐being | Mortality among individuals living with HIV or diabetes (Follow‐up: 11 to 12 months) | |||
| 2 trials reported little or no differences between groups. 1 study (Chang 2011) recruited peer health workers who consulted with clinic staff (RR: 0.82, 95% CI 0.55 to 1.22), and another study (Iversen 2018) recruited community nurses who consulted with diabetes specialist nurses (RR: 0.94, 95% CI 0.28 to 3.12). | 1157 | ⊕⊕⊝⊝ | The intervention may make little or no difference in mortality among people living with HIV or diabetes | |
| Disease activity or health‐related quality of life (Follow‐up: 9 months) | ||||
| 1 trial of rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis (Taylor‐Gjevre 2018) reported little or no difference between groups for disease activity (DAS28‐CRP MD 0.9, 95% CI −1.2 to 3.1; mHAQ MD 0.2, 95% CI −0.1 to 0.5; RADAI MD 0.9, 95% CI −0.5 to 2.4) or health‐related quality of life (EQ5D MD −0.1, 95% CI −0.4 to 0.1) | 85 (1 randomised trial) | ⊕⊕⊝⊝ | Mobile technologies used by community health or home‐care workers may make little or no difference for disease activity and health‐related quality of life in participants with rheumatoid arthritis | |
| Participant and provider acceptability or satisfaction | Healthcare provider acceptability and satisfaction | |||
| ‐ | ‐ | ‐ | No studies were identified | |
| Participant acceptability and satisfaction (Follow‐up: 9 to 12 months) | ||||
| 2 trials on diabetes (Iversen 2018) and arthritis (Taylor‐Gjevre 2018) reported little or no difference between groups for participants' experience with healthcare (GS‐PEQ MD 0.0, 95% CI −0.18 to 0.18) and satisfaction (VSQ9 results reported narratively) with the intervention. | 178 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by community health or home‐care workers probably make little or no difference for participant acceptability and satisfaction for participants with new diabetes‐related foot ulcer and participants with rheumatoid arthritis | |
| Costs | ‐ | ‐ | ‐ | No studies were identified |
| Technical difficulties | ‐ | ‐ | ‐ | No studies were identified |
| CI: Confidence interval; DAS28‐CRP: Disease activity score for Rheumatoid Arthritis; EQ5D: EuroQol five dimensions questionnaire; GS‐PEQ: Generic Short Patient Experiences Questionnaire; MD: Mean difference; mHAQ: Modified health assessment questionnaire; RADAI: Rheumatoid arthritis disease activity index; RR: Risk ratio; VSQ9: Visit‐specific satisfaction questionnaire | ||||
| GRADE Working Group grades of evidence | ||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (2 studies), attrition (1 study) and reporting (1 study) bias. bWe downgraded one point for imprecision because the 95% CI shows potential effect on both sides of “no effect” line and that there were few events. cWe downgraded one point for risk of bias due to high risk of performance, detection, and attrition bias. dWe downgraded one point for imprecision because the 95% CI shows potential effect on both sides of “no effect” line . eWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (1 study), attrition (1 study) and reporting (2 studies) bias. fWe downgraded one point for risk of bias due to high risk of performance, detection, attrition, and reporting bias. gWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (2 studies), attrition (1 study), and reporting (2 studies) bias. | ||||
Background
Effective communication with other healthcare providers and access to specialist expertise is essential for increasing health services capacity and providing optimal care, especially in areas where there is a shortage of healthcare providers (AAP 2015). The widespread use of information and communication technologies (ICT) can potentially increase the capacity of health services by supporting communication between different providers, and providing rapid access to specialist expertise.
Description of the condition
By 2035 there will be a worldwide shortage of approximately 12.9 million skilled healthcare providers (Campbell 2013). The biggest gaps will occur in Southeast Asia and sub‐Saharan Africa, but elsewhere too this will be a problem due to larger ageing populations, the rising prevalence of non‐communicable diseases, migration patterns and high turnover of healthcare providers. Remote and rural areas, where populations are likely to be poorer, sicker and less educated, are particularly at risk (OPHI 2017; Wu 2016). Healthcare providers in those settings can be isolated and have limited interaction with colleagues and specialists, with few opportunities for mentoring, consultation with experts, or referrals to other healthcare providers.
Description of the intervention
Digital technologies are increasingly used to support health systems (WHO 2018) by providing flexible options for communication and the exchange of information. These technologies can be used for medical diagnostic, monitoring and therapeutic purposes, when participants are separated by distance or time or both, with the ultimate goal of improving the health of individuals and communities (Steinhubi 2013). Provision of health care at a distance is usually referred to as telemedicine (WHO 2018), and can be implemented through mobile or fixed devices.
The exchange of information can happen synchronously (when interactions happen in real time) or asynchronously (when there is a lag between the clinical information being transmitted and the response), and through different channels, including video‐conferencing, mobile applications, and secure messaging (Kruse 2017; WHO 2016). The use of mobile technologies can improve access to specialty care (Liddy 2019b), particularly for underserved communities (Källander 2013). Widespread mobile broadband connectivity means that even healthcare providers in remote areas can access and communicate with their peers, improving co‐operation (Aceto 2018). The World Health Organization (WHO) Global Observatory for eHealth conducted a survey of the WHO Member States on the use of eHealth (WHO 2016), and reported that of the 122 countries surveyed 70% reported on the use of mobile health devices for consultation between healthcare professionals. The most common areas were teleradiology, telepathology, and teledermatology (WHO 2016), with teleradiology programmes being widely used. Within this review our focus was on mobile technologies to support provider‐to‐provider communication and management of care.
In a bid to maximise the coverage of healthcare services and to decrease the cost of providing health care, governments and healthcare agencies in some countries have funded some type of telehealth programme for provision of care, including promoting communication and management of care between providers. Some examples include the Technology Enabled Care Services programme in England (NHS Commissioning Assembly 2015), the Scottish Centre for Telehealth and Telecare (SCTT 2017), the telehealth services provided within the Medicare programme in the USA (MedPAC 2016), the Asia eHealth Information Network (AeHIN 20017), the KwaZulu‐Natal Experience in South Africa (Mars 2012), and the Aga Khan Development Network Digital Health Programme, which covers remote communities in South‐Central Asia and East Africa (AKDN 2019).
How the intervention might work
The use of mobile technologies between healthcare providers for communication, consultations and patient management might contribute to developing professional skills and expertise, as well as optimising multidisciplinary communication (AAP 2015) and evidence‐based clinical practice. This is particularly relevant for settings where there is a shortage of healthcare providers, for instance in low‐ and middle‐income countries and in rural and remote areas (Källander 2013). By enabling healthcare providers who are geographically separated to exchange clinical information and knowledge, mobile technology can facilitate universal health coverage by increasing access to health care. In 2018 the WHO published a classification of digital health interventions to categorise the functionality of the different applications; using this classification as a guide we include interventions that are portable and facilitate remote healthcare provider communication or co‐ordination of referrals, or both (WHO 2018).
Despite the possibilities, telehealth applications have been inconsistently implemented, with varying degrees of success due to technological challenges, legal considerations, human and cultural factors, and uncertainty around economic benefits and cost effectiveness (WHO 2016), although this is changing. Overcoming these barriers requires evidence‐based implementation of guidelines, driven both by governmental and professional medical organisations; legislation on confidentiality, privacy and liability; and the involvement of stakeholders in designing, implementing and evaluating telemedicine applications, focusing on the safety and the effectiveness of applications (Agboola 2016).
Why it is important to do this review
The rapid progress of information and communication technologies is accelerating the evolution of remote communication between providers for the management of care. This review is one of a suite of 11 Cochrane Reviews that contributed to the WHO guideline on digital interventions for health systems strengthening (WHO 2019), and focuses on the effectiveness of mobile technologies for communication and management of care between healthcare providers who are in different locations. The effectiveness of mobile technologies to support patient‐to‐healthcare provider communication is being assessed in another review (Gonçalves‐Bradley 2018a). The rationale for conducting this review is to assess the effectiveness of mobile health technologies as a method for healthcare providers to communicate, diagnose and manage patients; and to assess acceptability, satisfaction, resource use and technical difficulties. Research into the latter has been particularly neglected (Coiera 2016), and can provide crucial information for successful implementation.
Objectives
To assess the effects of mobile technologies versus usual care for supporting communication and consultations between healthcare providers on healthcare providers' performance, acceptability and satisfaction, healthcare use, patient health outcomes, acceptability and satisfaction, costs, and technical difficulties.
Methods
Criteria for considering studies for this review
Types of studies
We include randomised trials reported as full‐text studies, conference abstracts and unpublished data, irrespective of their publication status and language of publication.
Types of participants
All types of healthcare providers (i.e. professionals, healthcare assistants, and lay health workers) providing patient care through mobile technologies. We included trials targeting people with any condition, regardless of their location, setting, diagnoses, or demographic factors such as age.
Types of interventions
We include trials comparing health care delivered through a mobile device versus usual care. We defined 'usual care' by the setting in which the trial took place, including face‐to‐face exchanges and communication through other non‐digital channels. We include trials of healthcare providers who were geographically separated and used information and communication technologies. We have focused exclusively on the exchange of clinical information over wireless and mobile technologies, mobile phones of any kind (but not analogue land‐line telephones), tablets, personal digital assistants and smartphones, and when the healthcare provider enquiry received a response in real‐time or as immediate as clinically appropriate. Communication channels through a mobile device can include text messaging, video messaging, social media, voice calls, voice‐over Internet protocol (VoIP), and video‐conferencing, through software such as Skype, WhatsApp or Google Hangouts.
We include:
-
trials in which the healthcare provider used mobile technologies, such as telemedicine applications, to seek clinical guidance and support from other qualified healthcare providers in order to deliver direct patient care. This included co‐ordination of referrals and requests for expert opinion and diagnosis;
-
trials in which the provider(s) seeking guidance was at a different location from the provider(s) offering guidance; and
-
trials in which the provider(s) seeking guidance transmitted clinical information using a mobile device and the provider(s) offering guidance responded on any device, including stationary devices.
We include trials of telemedicine interventions if they were portable/mobile. We include trials assessing unspecified types of communication devices for transmitting clinical information, so long as they were mobile, since trials often failed to report this detail.
We include all health issues and did not restrict the content of clinical health information exchanged. We include trials where the digital component of the intervention was delivered as part of a wider package if we judged it to be the core component of the intervention.
We excluded:
-
pilot and feasibility studies (pilot study defined as "a version of the main study that is run in miniature to test whether the components of the main study can all work together" and feasibility studies as "pieces of research done before a main study"; Arain 2010);
-
trials that compared different technical specifications of telecommunication technologies (e.g. different communication channels, software, etc.);
-
trials in which the use of telecommunications technology was not directly linked to patient care;
-
trials in which the primary purpose of the intervention was education/training;
-
trials assessing the accuracy of a portable medical device.
Types of outcome measures
Main outcomes
-
Providers' adherence to recommended practice, guidelines or protocols.
-
Time between presentation and management of the health condition.
Other outcomes
-
Healthcare use, including referrals, clinical examinations and hospitalisations.
-
Participants' health status and well‐being, to include mortality and measures of health status such as the Nottingham Health Profile or the SF‐36 (McDowell 2006).
-
Healthcare provider acceptability and satisfaction; this includes self‐reported acceptability and satisfaction, measured with a validated scale, such as the Physician Worklife Survey (Konrad 1999).
-
Participant acceptability and satisfaction; this included self‐reported acceptability and satisfaction, measured with a validated scale, such as the Patient Satisfaction Scale (La Monica 1986).
-
Costs, including cost to the user and cost to the service (e.g. human resources/time, training, supplies and equipment).
-
Unintended consequences; these could include errors in interpreting the data; transmission of inaccurate data, loss of verbal and non‐verbal communication cues, issues of privacy and disclosure that might affect interpersonal relationships, negative impacts on equity, and technical difficulties, for example failure or delay in the message delivery.
Search methods for identification of studies
Electronic searches
An Information Specialist developed the search strategies in consultation with the review authors and WHO content experts. We used a minimum cut‐off search date of 2000, based on the increased availability and penetration of mobile devices from that date onwards (ITU 2019). Appendix 1 lists the search strategies and results.
We searched the following databases until 22 July 2019:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 7), in the Cochrane Library;
-
MEDLINE Ovid;
-
Embase Ovid;
-
POPLINE;
-
WHO Global Health Library.
Searching other resources
Trial registries
We searched clinicaltrials.gov (clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (who.int/ictrp).
Grey literature
We conducted a grey literature search in August 2017, to identify trials not indexed in the databases listed above. We searched for relevant systematic reviews and primary studies on similar topics using Epistemonikos (epistemonikos.org), a database of health evidence and health‐related systematic reviews. We searched the content in mHealthEvidence (mhealthevidence.org), a database of global literature on mHealth. We contacted authors of relevant trials/reviews to clarify reported published information and to seek unpublished results/data, as well as researchers with expertise relevant to the review topic. Moreover, WHO issued a call for papers through popular digital health communities of practice such as the Global Digital Health Network and Implementing Best Practices, to identify additional primary trials as well as grey literature. We performed a backward and forward search of the primary reference identified for each eligible trial.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to reference management databases (Distiller and Covidence) and removed duplicates. For title and abstract screening, we used a machine‐learning classifier that is able to assign a probability score that a given record describes or does not describe a randomised trial (Wallace 2017). Two review authors (from AM, BB, DGB, GV, IRC, and NH) screened titles and abstracts of trials with at least a 10% probability of being a randomised trial, and one review author screened those with less than a 10% probability. We retrieved the full‐text trial reports/publication of all potentially eligible reports, and two review authors (from AM, BB, DGB, GV, IRC, and NH) screened the full text to identify trials for inclusion and to identify and record reasons for excluding the ineligible trials. We resolved any disagreement through discussion, and if required consulted a third review author (DGB or SS).
We listed trials that initially appeared to meet the inclusion criteria but that we later excluded in the Characteristics of excluded studies table. We collated multiple reports of the same trial so that each trial rather than each report was the unit of interest in the review. We also provided any information we could obtain about ongoing studies. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Data extraction and management
We used the EPOC standard data collection form and adapted it for trial characteristics and outcome data (EPOC 2017a); we piloted the form on five trials. One review author extracted the following characteristics and a second review author cross‐checked data (from AM, BB, DGB, GV, IRC, and NH).
-
Methods: trial design, unit of allocation, location and trial setting, withdrawals.
-
Participants: number, mean age, age range, sex, inclusion criteria, exclusion criteria, dates conducted, other relevant characteristics.
-
Interventions: function of the intervention (monitoring, consultation, therapy), intervention components (including type of technology and mode of delivery, frequency of data transmission), comparison, fidelity assessment. For this review, we defined monitoring as the continuous evaluation of the progress of symptoms or a condition over a period of time; consultation as an exchange between the healthcare provider and the participant, where the provider discusses the participant's health status and provides guidance, support, or information; and therapy as the ongoing management and care of a participant, to counteract a disease or disorder.
-
Outcomes: main outcomes specified and collected, time points reported.
-
Notes: funding for trial, ethical approval.
We contacted authors of included trials to seek missing data. We noted in the Characteristics of included studies table if outcome data were reported in an unusable way. We resolved disagreements by consensus or by involving a third review author (DGB or SS). We used Review Manager 5 (RevMan 5.3) for data management.
Assessment of risk of bias in included studies
One review author assessed risks of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), plus the guidance from the EPOC group (EPOC 2017b), and a second review author cross‐checked data (from AM, BB, DGB, GV, IRC, and NH). We resolved any disagreement by discussion or by involving a third review author (DGB or SS). We assessed the risks of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Baseline outcomes measurement.
-
Baseline characteristics.
-
Other bias.
We judged the risk of each potential source of bias as being high, low or unclear, and provide a quotation from the trial report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different trials for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a participant‐reported pain scale). We assessed incomplete outcome data separately for different outcomes. Where information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table. We did not exclude trials on the grounds of their risk of bias but clearly reported the risk of bias when presenting the results of the trials.
When considering treatment effects, we took into account the risk of bias for the trials that contributed to that outcome.
We conducted the review according to the published protocol (Gonçalves‐Bradley 2018b) and reported any deviations from it in 'Differences between protocol and review'.
Measures of treatment effect
We estimated the effect of the intervention using risk ratios (RRs) and associated 95% confidence intervals (CIs) for dichotomous data. For continuous measures, we analysed the data based on the mean, standard deviation (SD) and number of people assessed to calculate the mean difference (MD) and 95% CI (Higgins 2019). We ensured that readers could interpret an increase in scores for continuous outcomes in the same way for each outcome, explained the direction of effect, and reported where the direction was reversed if this was necessary.
Unit of analysis issues
Six trials used a cluster design (Byamba 2015; Chang 2011; Eminović 2009; Iversen 2018; Piette 2017; Van Gelder 2017). Of those trials, all except one had controlled for unit‐of‐analysis errors by adjusting for clustering, and thus were not further re‐analysed.
We had planned to control for unit of analysis errors by re‐analysing the data after adjusting for clustering, using the intracluster correlation coefficient reported by the trials. When not reported, we calculated intracluster correlation coefficients estimates (Campbell 2000) and the formula 1+(M‐1)xICC, where M is the average cluster size (Higgins 2019). However, it was not possible to obtain average cluster size for Byamba 2015 and as such it is possible that there are potential unit of analysis errors associated with the effect estimates of that trial.
Dealing with missing data
We contacted investigators in order to verify key trial characteristics and obtain missing outcome data where possible (e.g. when a trial report was only available as an abstract). Whenever it was not possible to obtain data, we reported the level of missingness and considered how that might have impacted the certainty of the evidence.
Assessment of heterogeneity
We conducted meta‐analyses and calculated the I2 statistic to measure heterogeneity among the trials in each analysis. We considered an I2 value of 50% or more to represent substantial levels of heterogeneity, but this value was interpreted in light of the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi2 test (Deeks 2017). We identified substantial heterogeneity for one of the outcomes (mortality), but were not able to explore it by prespecified subgroup analysis as there were not enough trials.
Assessment of reporting biases
We attempted to contact trial authors, asking them to provide missing outcome data. Where this was not possible, and we considered that the missing data might have introduced serious bias, we explored the impact of including such trials in the overall assessment of results. We were not able to explore possible publication bias through a funnel plot (Sterne 2011), as we did not combine a sufficient number of trials.
Data synthesis
We undertook meta‐analyses for outcomes when the interventions, participants, and underlying clinical question were similar enough for pooling to make sense (Borenstein 2009). As there was considerable heterogeneity, we applied a random‐effect model (Deeks 2017). A common way that trialists indicate the presence of skewed data is by reporting medians and interquartile ranges. When we encountered this we noted that the data were skewed and considered the implications.
'Summary of findings' table
Two review authors (DGB and MF) assessed the certainty of the evidence (high, moderate, low, and very low) using the five GRADE considerations: risk of bias, inconsistency, imprecision, indirectness, and publication bias) (Guyatt 2008). We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017) and the EPOC worksheets (EPOC 2017c), using GRADEpro software (GRADEpro GDT). We resolved disagreements on certainty ratings by discussion and provided justification for decisions to down‐ or upgrade the ratings using footnotes in the table, making comments to aid readers' understanding of the review where necessary. We used plain language statements to report these findings in the review (EPOC 2017d).
We created 'Summary of findings' tables for the following outcomes in order to draw conclusions about the certainty of the evidence within the text of the review:
-
Providers' adherence to recommended practice, guidelines or protocols;
-
Time between presentation and management of the health condition;
-
Healthcare use;
-
Participants' health status and well‐being;
-
Participant and provider acceptability or satisfaction with the intervention;
-
Costs;
-
Technical problems.
We created three 'Summary of findings' tables, according to the setting where the intervention was delivered (primary, secondary and community care), as the populations in those settings, both healthcare providers and participants, are substantially different.
We considered whether there was any additional outcome information that we were not able to incorporate into meta‐analyses, noted this in the tables and stated whether it supports or contradicts the information from the meta‐analyses. When it was not possible to meta‐analyse the data, we summarised the results in the text and in the 'Comments' section of the 'Summary of findings' tables.
Subgroup analysis and investigation of heterogeneity
We categorised trials by setting (community, primary and secondary care), according to healthcare provider type, e.g. primary care doctors' or nurses' communication with hospital‐based specialists, or community health workers consulting with clinic staff.
We planned to use the following outcomes in subgroup analysis.
-
Time between presentation and management of the health condition.
-
Participants' health status and well‐being.
We planned to use the formal statistical techniques of Mantel‐Haenszel and regression to test for subgroup interactions (Mantel 1959) but due to the limited number of studies we could not use this technique.
Sensitivity analysis
We planned to perform sensitivity analyses defined a priori to assess the robustness of our conclusions and explore the impact on effect sizes. This would have involved restricting the analysis to published trials and to trials at low risk of bias. We did not perform sensitivity analyses as there were no unpublished trials and within the pooled analyses all the trials had the same risk of bias for the relevant 'Risk of bias' criteria.
Results
Description of studies
We identified 19 published randomised trials of mobile technologies to support healthcare provider to healthcare provider communication and management of care (see Characteristics of included studies).
Results of the search
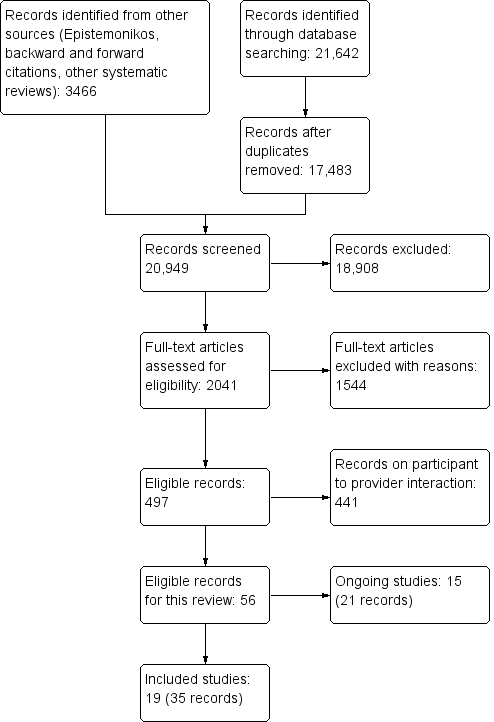
We retrieved 20,949 records for title and abstract screening, screened the full‐text of 2041 citations and included 19 trials (35 citations) (Armstrong 2018; Azogil‐López 2019; Byamba 2015; Chang 2011; Davis 2003; Eminović 2009; Gulacti 2017; Iversen 2018; Liddy 2019a; Mansberger 2015; Orlandoni 2016; Pak 2007; Piette 2017; Riordan 2015; Sutherland 2009; Taylor‐Gjevre 2018; Van Gelder 2017; Whited 2002; Whited 2013). In addition, we identified 15 ongoing trials (ACTRN12617000389303; ACTRN12618001007224; Gervès‐Pinquié 2017; Jeandidier 2018; Källander 2015; Koch 2018; Nakayama 2016; Stevanovic 2017; NCT02821143; NCT02986256; NCT03137511; Done 2018; NCT03559712; NCT03662256; Xu 2017). A total of 441 records were eligible for the associated review on mobile technologies to support patient to healthcare provider communication and management of care (Gonçalves‐Bradley 2018a). Figure 1 presents the results of the search.

Flow diagram
Included studies
Trial populations
Seventeen trials included 5766 participants, while two trials did not report the specific number of participants (Liddy 2019a; Riordan 2015). The number of healthcare professionals recruited ranged from one general practitioner (GP) consulting with one ophthalmologist (Davis 2003), to another trial that randomised 113 GPs consulting with several specialty physicians (Liddy 2019a). Most of the trials involved primary care professionals consulting with specialists, namely dermatologists (Armstrong 2018; Byamba 2015; Eminović 2009; Pak 2007; Piette 2017; Whited 2002; Whited 2013), ophthalmologists (Davis 2003; Mansberger 2015), nephrologists (Van Gelder 2017) or radiologists (Sutherland 2009). In two studies more than one type of specialist was involved (Azogil‐López 2019; Liddy 2019a). The GPs mainly worked in urban settings and consulted with specialists also located in urban settings (N = 11). In four studies the GPs were located in rural settings, and consulted with providers in urban settings. There was one trial each for community‐based peer health workers consulting with clinic staff (Chang 2011), home‐visiting nursing staff consulting with a hospital physician (Orlandoni 2016), rural‐based physical therapists consulting with rheumatologists (Taylor‐Gjevre 2018), and community nurses consulting with specialist nurses or podiatrists (Iversen 2018). Two trials reported on emergency physicians consulting with hospital‐based specialists (Gulacti 2017; Riordan 2015).
All trials recruited adults, with Sutherland 2009 also recruiting adolescents and Azogil‐López 2019 recruiting participants aged seven years and older, and Orlandoni 2016 specifically recruiting participants aged 65 years and older. Three trials recruited participants with diabetes (Davis 2003; Mansberger 2015; Iversen 2018), and one with rheumatoid arthritis (Taylor‐Gjevre 2018). Seven trials recruited participants with a range of conditions seeking referral to a dermatologist (Armstrong 2018; Byamba 2015; Eminović 2009; Pak 2007; Piette 2017; Whited 2002; Whited 2013), two trials recruited participants attending the emergency department (Gulacti 2017; Riordan 2015) or requiring a hospital referral (Azogil‐López 2019; Liddy 2019a), and one trial each recruited participants requiring a trans‐abdominal or trans‐vaginal ultrasound (Sutherland 2009) or with chronic kidney disease (Van Gelder 2017). The two remaining trials recruited participants receiving antiretroviral therapy (Chang 2011) and home enteral nutrition (Orlandoni 2016).
Setting
Trials were mainly conducted in North America (9 trials) and Europe (six trials), with one trial each conducted in the Dominican Republic, Turkey, and Uganda, and Mongolia.
Interventions
The trials included in the review evaluated interventions that varied in mode of delivery, number of sessions, and healthcare providers involved. All trials used a portable device, 10 of them using a portable device to obtain clinical images which were then transmitted for further assessment (Armstrong 2018; Byamba 2015; Davis 2003; Eminović 2009; Mansberger 2015; Pak 2007; Piette 2017; Sutherland 2009; Whited 2002; Whited 2013). Four trials used mobile phones for text messages and voice calls (Chang 2011), secure messaging (Gulacti 2017), audio‐conferencing system (Azogil‐López 2019), and for interactive web‐based record and voice calls (Iversen 2018). Two trials used a tablet for secure messaging (Riordan 2015) or video consultation (Orlandoni 2016), whereas one trial employed a laptop for video consultation (Taylor‐Gjevre 2018). The remaining trials used an electronic health record system for eConsults, which could also be implemented through mobile phones (Liddy 2019a; Van Gelder 2017).
The trials also varied in the frequency and duration of contacts between the healthcare providers, with most trials consisting of a single consultation (e.g. Eminović 2009).
Although the control group was always described as receiving usual care, the description of the specific care received varied. For trials conducted in primary care, 'usual care' generally consisted of a referral for a face‐to‐face appointment in secondary care (Byamba 2015; Eminović 2009; Liddy 2019a; Pak 2007; Whited 2002; Whited 2013) or a reminder to book an appointment (Davis 2003; Mansberger 2015; Piette 2017; Sutherland 2009). For one trial that used a social media platform for emergency department physicians to communicate with specialists within the same hospital (Gulacti 2017), 'usual care' was to consult by phone, sending all clinical information verbally. For trials conducted in the community, 'usual care' was typically face‐to‐face appointments with specialists, either at the participant's home (Orlandoni 2016) or at outpatient clinics (Iversen 2018; Taylor‐Gjevre 2018).
Several trials reported on additional components of the intervention (Table 1). Nine reported the delivery of training (Armstrong 2018; Byamba 2015; Chang 2011; Eminović 2009; Iversen 2018; Mansberger 2015; Piette 2017; Sutherland 2009; Taylor‐Gjevre 2018), which usually focused on how to acquire digital images or use the web‐based system. For one trial of eConsult, the specialists received financial incentives for each eConsult they undertook (Liddy 2019a), and two trials provided monetary incentives for participants to take part (Armstrong 2018) or to complete follow‐up assessment (Mansberger 2015). Two trials reported that participants whose healthcare providers were allocated to the intervention group had increased access to health care, either directly (Armstrong 2018) or indirectly (Chang 2011).
| Study | Incentives | Specific training |
|---|---|---|
| Participants were paid for participating in the study, through gift cards (main paper, p.3, end 1st paragraph) | Participants and their carers were taught how to take standardised images of skin lesions, as well as how to communicate with the dermatologist using a secure web‐based system. PCPs also had access to the training materials. (Protocol, p.19, 2nd paragraph) | |
| ‐ | GPs attended a 2‐day training session to learn how to take images and use the medical record system and software on mobile phones (p.1, top 2nd column) | |
| PHWs were given a bicycle, t‐shirts, basic supplies, and an initial monthly allowance (parent trial) | PHWs allocated to the intervention group were given a mobile phone, and attended a 1‐day residential training and a brief field‐based practical training on the intervention (main paper, p.3, 2nd paragraph) | |
| ‐ | GPs allocated to the intervention group received detailed instructions on how to take digital images and use the web‐based form (main paper, p.559, bottom 1st column) | |
| ‐ | All staff received training in the use of the web‐based system, as well as in‐person access to hospital clinics to improve their practical skills (main paper, pp.97‐8) | |
| Specialists received financial incentives for each eConsult they undertook (support paper, under 8. Payment) | ‐ | |
| Participants received monetary incentive to complete follow‐up questionnaire (associated paper, p.524, bottom 1st column) | Technicians performing imaging attended a 3‐day training session to learn how to take images and ongoing feedback as needed (main paper, p.943, bottom 1st column) | |
| ‐ | GPs received training and a workbook on how to take photographs (p.2, top 2nd column) | |
| ‐ | The on‐site investigator received sonographic training over a 2‐month period, as well as practice guidelines for trans‐abdominal ultrasound scanning (P. 192, mid 1st column and top 2nd column) | |
| ‐ | Physical therapists and rheumatologists received an orientation and education session about rheumatoid arthritis and the study protocol and methods (main paper, p.2, top 2nd column) |
GP: general practitioner; PCP: primary care provider; PHW: peer health workers
Funding, ethical approval, and conflict of interest
Sixteen trials reported funding sources, all of which were provided by medical research institutes or university funding bodies. One of the trials also received funding from a biopharmaceutical company (Van Gelder 2017). Three trials did not report ethical or institutional review board approval (Byamba 2015, letter; Davis 2003, short report; Riordan 2015, conference abstract).
For three trials one or more members of the author team reported financial support from pharmaceutical companies (Armstrong 2018, 3/29 authors; Van Gelder 2017 1/10 authors; Whited 2013, 1/18 authors). The lead author of Pak 2007 was the co‐founder of a web‐based consultation service identical to that used in the intervention. Six studies did not report conflicts of interest (Chang 2011; Davis 2003; Riordan 2015; Sutherland 2009; Taylor‐Gjevre 2018; Whited 2002), and for the remaining nine studies the authors had no known conflict of interest.
Excluded studies
We excluded 1544 full texts, of which we report on 22 excluded trials (See Characteristics of excluded studies). The most frequent reason for excluding trials was the explicit use of non‐mobile equipment (eight trials).
Risk of bias in included studies
Figure 2 presents a graph for risk of bias and Figure 3 summarises risk of bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fourteen trials described the generation of the randomisation schedule, and were judged at low risk of bias (Armstrong 2018; Azogil‐López 2019; Byamba 2015; Eminović 2009; Gulacti 2017; Iversen 2018; Liddy 2019a; Mansberger 2015; Orlandoni 2016; Pak 2007; Piette 2017; Taylor‐Gjevre 2018; Van Gelder 2017; Whited 2013), one trial that 'tossed a coin' was judged as high risk of bias (Sutherland 2009), and we rated the remaining trials at unclear risk of bias. Fifteen trials were judged at low risk of bias for allocation concealment (Armstrong 2018; Azogil‐López 2019; Byamba 2015; Chang 2011; Eminović 2009; Iversen 2018; Liddy 2019a; Mansberger 2015; Orlandoni 2016; Pak 2007; Piette 2017; Taylor‐Gjevre 2018; Van Gelder 2017; Whited 2002; Whited 2013), one at high risk (Sutherland 2009), and the remaining trials were unclear due to a lack of information.
Eight trials reported baseline outcome measurements that were similar between groups, thus being assessed at low risk of bias (Armstrong 2018; Chang 2011; Eminović 2009; Liddy 2019a; Orlandoni 2016; Taylor‐Gjevre 2018; Whited 2002; Whited 2013), and the remaining 11 trials were assessed as being at unclear risk of bias. Ten trials reported similar baseline characteristics between groups and we judged them to be at low risk of bias (Armstrong 2018; Chang 2011; Eminović 2009; Gulacti 2017; Liddy 2019a; Mansberger 2015; Orlandoni 2016;Taylor‐Gjevre 2018; Van Gelder 2017; Whited 2013), three trials reported differences between groups at baseline and we judged them to be at high risk of bias (Azogil‐López 2019; Pak 2007; Piette 2017), and the remaining six trials were unclear.
Blinding
Due to the nature of the intervention it was often not possible to blind participants or healthcare professionals. We judged 16 trials to be at high risk of performance bias, and three at unclear (Byamba 2015; Davis 2003; Riordan 2015).
For objective outcomes we assessed six trials to be at high risk of detection bias (Mansberger 2015; Orlandoni 2016; Piette 2017; Taylor‐Gjevre 2018; Van Gelder 2017; Whited 2013), eight trials to be at low risk of bias and five trials to have an unclear risk of bias (Byamba 2015; Chang 2011; Davis 2003; Eminović 2009; Riordan 2015). For subjective outcomes we assessed eight trials to be at high risk of detection bias (Armstrong 2018; Eminović 2009; Iversen 2018; Piette 2017; Taylor‐Gjevre 2018; Van Gelder 2017; Whited 2002; Whited 2013), one trial to be at low risk of bias (Pak 2007), and two trials to have an unclear risk of bias (Davis 2003; Riordan 2015). Eight trials did not collect data on subjective outcomes.
Incomplete outcome data
Eight trials had high rates of incomplete outcome data and we judged them to be at high risk of attrition bias (Azogil‐López 2019; Chang 2011; Eminović 2009; Liddy 2019a; Orlandoni 2016; Pak 2007; Taylor‐Gjevre 2018; Whited 2013), and nine trials at low risk of attrition bias and were unclear about two trials (Davis 2003; Riordan 2015).
Selective reporting
We judged nine trials to be at high risk of reporting bias, as either outcomes were not reported per protocol (Armstrong 2018; Eminović 2009; Gulacti 2017; Iversen 2018; Taylor‐Gjevre 2018; Whited 2013) or publications were found for the same trial without cross‐reference (Chang 2011; Pak 2007; Whited 2002). For three trials it was not possible to make a judgement due to a lack of information (Davis 2003; Mansberger 2015; Riordan 2015), and seven trials had a low risk of reporting bias.
Other potential sources of bias
We judged other potential sources bias as unclear in three trials, two because there was not enough information (Davis 2003; Riordan 2015), and the other due to several methods being reported to collect outcome data due to problems with follow‐up (Eminović 2009). We judged one trial to have a high risk of other potential sources of bias, as data collection methods differed for the two trial groups and were not clearly reported (Mansberger 2015). There was no other apparent source of bias for the remaining trials and we judged them to be at a low risk of bias.
Effects of interventions
See: Summary of findings 1 Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared with usual care; Summary of findings 2 Mobile technologies for use in the emergency department compared with usual care; Summary of findings 3 Mobile technologies used by community health or home‐care workers compared with usual care
Comparison 1: Mobile technologies used by primary care providers to consult with hospital based specialists
Thirteen trials reported on mobile technologies used by primary care providers to consult with hospital‐based specialists. The studies involved GPs consulting with dermatologists (Armstrong 2018; Byamba 2015; Eminović 2009; Pak 2007; Piette 2017; Whited 2002; Whited 2013), ophthalmologists (Davis 2003; Mansberger 2015), radiologists (Sutherland 2009), nephrologists (Van Gelder 2017), or different specialists (Azogil‐López 2019; Liddy 2019a). The mobile component of the interventions consisted of a non‐mydriatic camera for retinal screening (Davis 2003; Mansberger 2015), portable ultrasound (Sutherland 2009), teledermatology to send digital images (Armstrong 2018; Byamba 2015; Eminović 2009; Pak 2007; Piette 2017; Whited 2002; Whited 2013), and eConsult through audio‐conferencing or secure direct messaging between healthcare providers, with a mobile component (Azogil‐López 2019;Liddy 2019a; Van Gelder 2017). For an overview of the evidence please refer to summary of findings Table 1.
Main outcomes
1. Providers' adherence to recommended practice, guidelines or protocols
One trial reported on the use of telenephrology by nephrologists to communicate with primary care providers for people with chronic kidney disease (CKD) (Van Gelder 2017). The authors found little or no difference for providers' adherence to the advised monitoring criteria from national CKD guidelines, as measured by monitoring of disease progression and metabolic parameters (3004 participants; moderate‐certainty evidence; Analysis 1.1). Follow‐up was not reported.
2. Time between presentation and management of the health condition
Four trials reported on time between presentation and management of the health condition (656 participants; moderate‐certainty evidence; Analysis 2.1). Two trials recruited GPs who collected digital images from people with a skin condition and consulted with hospital‐based dermatologists on how to interpret them, reporting that people received the required treatment from their dermatologist in less time than those allocated to the control group: for Whited 2002 mean difference −40.5 days, 95% CI −23 to −58 days (275 participants); Piette 2017 reported a median of 4 days for the intervention group (IG) and 40 days for the control group (CG), with an adjusted hazard ratio (HR) of 2.55, P = 0.01 (103 participants). A third trial recruited GPs who shared ultrasound images with radiologists, finding little or no difference between groups on median time to participant follow‐up or diagnosis (Sutherland 2009; 105 participants). Azogil‐López 2019 recruited GPs who either referred their participants to an in‐person hospital appointment (control group) or to an audio‐consultation (intervention group), finding that those allocated to the audio‐consultation waited for less time (median −27 days, 99% CI −20 to −33 days; 173 participants). Follow‐up, when provided, ranged between three and six months.
Other outcomes
1. Healthcare use
Nine trials reported on various forms of healthcare use, including referrals, screening examinations, outpatient visits and hospitalisations (4810 participants; moderate‐certainty evidence; Analysis 3.1).
Four trials recruited GPs who consulted with dermatologists through the use of digital images (Byamba 2015; Piette 2017; Whited 2002; Whited 2013; 4 trials, 1075 participants; follow‐up between three and nine months, when reported), finding that those participants allocated to the intervention group were less likely to subsequently receive a referral for an appointment with a dermatologist, visit a dermatology clinic, or be referred to tertiary care: risk ratio (RR) ranged from 0.28 (95% CI 0.21 to 0.38) to 0.82 (0.75 to 0.88). We did not retain the meta‐analysis because of high statistical heterogeneity (Analysis 3.2; I2 = 91%).
One trial of eConsults between PCPs and nephrologists reported that there was little or no difference between groups for referral rate (odds ratio (OR) 0.61, 95% CI 0.31 to 1.23; Van Gelder 2017; 3004 participants). Another trial of eConsults between PCPs and a range of specialists also found little or no difference between groups for face‐to‐face referral (RR 0.93, 95% CI 0.85 to 1.03; Liddy 2019a).
Two trials of retinopathy screening for participants with diabetes (Davis 2003; Mansberger 2015) reported that those allocated to the intervention group were more likely to receive a screening examination (2 trials, 626 participants; 12 months follow‐up when reported). High statistical heterogeneity precluded retaining the meta‐analysis (Analysis 3.3; I2 = 85%). Another trial of GPs consulting with radiologists about participants requiring a trans‐abdominal or trans‐vaginal ultrasound found that participants allocated to the intervention group were more likely to receive an ultrasound (RR 3.92, 95% CI 2.11 to 7.31; Sutherland 2009; 105 participants).
2. Participants' health status and well‐being
Two trials reported on a dermatologist providing feedback to GPs based on digital images, finding similar scores between those allocated to the intervention and the control group, for general health status at 12‐month follow‐up (Armstrong 2018), as well as quality of life and health‐related quality of life as reported by the participants, at nine‐month follow‐up (Whited 2013) (2 trials, 622 participants; moderate‐certainty evidence; Analysis 4.1). Two teledermatology trials reported on clinical course as assessed by dermatologists at four‐ (Pak 2007) and nine‐month follow‐up (Whited 2013), finding little or no difference between groups in clinical course (2 trials, 769 participants; moderate‐certainty evidence; Analysis 4.2).
3. Healthcare provider acceptability and satisfaction
Two trials (378 participants) recruited GPs who consulted with dermatologists using digital images (low‐certainty evidence); Piette 2017 reported little or no difference between groups for acceptability or satisfaction, and Whited 2002 reported that GPs allocated to the intervention were more likely to agree that participants received timely appointments and to be satisfied with the consult process than GPs allocated to the control group. One additional trial (Van Gelder 2017) reported on satisfaction for healthcare professionals allocated to the intervention group (Analysis 5.1).
4. Participant acceptability and satisfaction
Four trials (972 participants, low‐certainty evidence; Analysis 5.2) recruiting GPs who consulted with dermatologists through the use of digital images reported little or no difference in participant satisfaction between those allocated to the intervention or to care as usual (Eminović 2009; Piette 2017; Whited 2002; Whited 2013).
5. Costs
Six trials reported costs (5423 participants; low‐certainty evidence; Analysis 6.1). One teledermatology trial reported that the expected cost per participant per visit was higher for the intervention group (Whited 2002; 275 participants); a second teledermatology trial reported that the total direct costs were lower for the comparison group (Pak 2007; 698 participants; MD USD −4678, 95% CI −4720 to −4635), and that this difference was offset by the lost productivity for participants allocated to the control group (MD USD 14,409, 95% CI 14,398 to 14,419). Another teledermatology trial reported little or no difference between groups for total costs per participant from the healthcare perspective (MD USD 30, 95% CI USD −79 to 20), and from the societal perspective that included the cost of loss of productivity (MD USD −82, 95% CI −12 to −152) per participant allocated to the intervention (Whited 2013; 391 participants). Two trials (teledermatology and telenephrology, respectively) reported little or no difference between groups for costs (Eminović 2009, 605 participants; MD EUR 32.5, 95% CI −29.0 to 74.7; Van Gelder 2017; 3004 participants; IG: EUR 453.86, 95% CI 392.98 to 514.74; CG EUR 433.74, 95% CI 387.64 to 479.84, P = 0.60). One teledermatology trial set in rural areas in Mongolia reported lower costs associated with the intervention group, mainly explained by the long distances that those allocated to the control group had to travel, which was avoided with teledermatology (Byamba 2015; 450 participants, IG: USD 320, CG: 3174, difference USD 2854).
6. Unintended consequences
Four trials reported on the quality of the data transmitted (Analysis 7.1). However, only one trial recruiting GPs consulting with dermatologists about images they took from their participants reported data for both groups (Pak 2007), reporting that 10 images from each group were lost due to technical problems (1 trial, 698 participants; moderate‐certainty evidence). The remaining trials reported results for the intervention group only (Piette 2017, Sutherland 2009, Whited 2002).
One trial where GPs could consult with dermatologists about people with psoriasis collected data about mortality as part of adverse events, reporting one death for each group (IG: 1/148; CG: 1/148; Armstrong 2018).
Comparison 2: Mobile technologies for communication between specialists in the emergency department
Two trials reported on mobile technologies for communication between physicians and specialists in the emergency department (Gulacti 2017; Riordan 2015), using a smartphone application for secure messaging. For an overview of the evidence please refer to summary of findings Table 2.
Main outcomes
1. Providers' adherence to recommended practice, guidelines or protocols
Neither of the trials of mobile technologies for communication between specialists in the emergency department reported data on providers' adherence.
2. Time between presentation and management of the health condition
One trial that recruited emergency physicians who consulted with specialist physicians using a smartphone application reported that participants allocated to the intervention group were probably either admitted to hospital or discharged in slightly less time from the emergency department (median difference −12 minutes, 95% CI −19 to −7; 345 participants; moderate‐certainty evidence) (Gulacti 2017; Analysis 8.1).
Other outcomes
1. Healthcare use
One trial reported that participants seen by emergency physicians allocated to the intervention group probably had a shorter length of emergency department stay (median difference −30 minutes, 95% CI −37 to −25 minutes; 345 participants; moderate‐certainty evidence; Analysis 9.1, Gulacti 2017).
2. Participants' health status and well‐being
Neither of the trials of mobile technologies for communication between specialists in the emergency department reported data on participants' health status and well‐being.
3. Healthcare provider acceptability and satisfaction
Neither of the trials on mobile technologies for communication between specialists in the emergency department reported data on healthcare provider acceptability or satisfaction.
4. Participant acceptability and satisfaction
Neither of the trials on mobile technologies for communication between specialists in the emergency department reported on participant acceptability and satisfaction.
5. Costs
Neither of the trials on mobile technologies for communication between specialists in the emergency department reported data on costs.
6. Unintended consequences
Gulacti 2017 reported that there were no technical problems during the course of the trial (Analysis 10.1).
Comparison 3: Mobile technologies used by community health or home‐care workers
Four trials reported on mobile technologies used by community‐based health workers or home‐care workers. The professionals involved were community‐based peer health workers consulting with clinic staff about receiving antiretroviral therapy (Chang 2011); community nurses consulting with diabetes specialist nurses or podiatrists about adults with Type 2 diabetes (Iversen 2018); home‐care nurses consulting with hospital specialists about home enteral nutrition (Orlandoni 2016); and rural‐based physical therapists consulting with urban‐based rheumatologists (Taylor‐Gjevre 2018). The mobile‐based component of the interventions consisted of mobile phone, teledermatology, video‐consultations, and interactive web‐based records, respectively. For an overview of the evidence please refer to summary of findings Table 3.
Main outcomes
1. Providers' adherence to recommended practice, guidelines or protocols
None of the trials of mobile technologies used by community‐based health workers reported data on providers' adherence.
2. Time between presentation and management of the health condition
None of the trials of mobile technologies used by community health workers reported data on time between presentation and management of the health condition.
Other outcomes
1. Healthcare use
Two studies reported on outpatient clinic and community nurse consultations (370 participants, moderate‐certainty evidence). Iversen 2018 recruited community nurses consulting with diabetes specialist nurses and podiatrists about adults with new diabetes‐related foot ulcers, reporting little or no difference between groups for outpatient consultations (0.48 fewer consultations in the intervention group, 95% CI −1.46 to 0.49) or community nurse consultations (0.92 more consultations in the intervention group, 95% CI −0.70 to 2.53). One trial (188 participants) that recruited home‐visiting staff who consulted with hospital physicians through video‐conferencing about older adults treated with home enteral nutrition reported little or no difference for healthcare use, as measured by outpatient visits (Incidence rate ratio 95% CI 0.65 to 1.30, P = 0.62) and hospitalisations (Incidence rate ratio 95% CI 0.54 to 1.19, P = 0.26) (Orlandoni 2016; low‐certainty evidence; Analysis 11.1).
2. Participants' health status and well‐being
Two trials, one recruiting community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy (Chang 2011) and another recruiting community nurses consulting with diabetes specialist nurses and podiatrists about adults with new diabetes‐related foot ulcers (Iversen 2018), reported mortality at 11‐ to 12‐month follow‐up (RR 0.82, 95% CI 0.55 to 1.22 and RR 0.94, 95% CI 0.28 to 3.12, respectively; 1157 participants; low‐certainty evidence; Analysis 12.1).
One trial (85 participants) of rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis reported little or no difference between groups for health‐related quality of life and disease activity (low‐certainty evidence) (Taylor‐Gjevre 2018; Analysis 12.1).
3. Healthcare provider acceptability and satisfaction
None of the trials of mobile technologies used by community health workers reported data on healthcare provider acceptability or satisfaction.
4. Participant acceptability and satisfaction
Two trials (178 participants) reported on participants' experience with healthcare (Iversen 2018) and satisfaction with the intervention (Taylor‐Gjevre 2018), reporting little or no difference between those allocated to the intervention or the control groups (moderate‐certainty evidence) (Analysis 13.1).
5. Costs
One trial reported the total cost of running the intervention and cost per participant for the intervention group only (Chang 2011, Analysis 14.1).
6. Unintended consequences
A trial that recruited community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy reported that healthcare professionals allocated to the intervention were not always able to charge the mobile phone, and that some mobile phones were stolen (Chang 2011; Analysis 15.1). Another trial where community nurses consulted with diabetes specialist nurses and podiatrists about adults with new diabetes‐related foot ulcers through videoconference, reported that images were not always transmitted (Taylor‐Gjevre 2018).
Equity considerations
Some of the included trials were designed and implemented to address geographical (Byamba 2015; Chang 2011; Davis 2003; Taylor‐Gjevre 2018) or socio‐economic limitations (Mansberger 2015; Sutherland 2009) on access to health care, and thus to promote equity for rural‐based and other disadvantaged populations who would have less access to health care (Table 2).
| Study ID | Population | Disadvantaged populations included/excluded? | Notes |
|---|---|---|---|
| General practitioner consulting with dermatologists about adults with psoriasis | Participants without access to the Internet and a digital camera or smartphone with camera features were excluded | ‐ | |
| GP consulting with hospital physicians about participants (aged ≥ 7 years) | Participants deemed as complex were not eligible for receiving the intervention | Complex participants defined as those lacking a specific diagnosis or requiring further clinical assessment | |
| GP consulting with dermatologists about adults with skin lesions | Intervention was set in rural health clinics in Mongolia | ‐ | |
| Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Specifically targeted HIV‐positive participants in rural Uganda. However, many participants had limited access to mobile phones*, which might have limited the benefits of the intervention. For the healthcare providers, the costs of the intervention were also a factor, as although they were given a monthly stipend it was not always enough Charging the mobile phone was often challenging, as access to electricity was limited | * Current mobile phone penetration in Uganda at the time the trial was conducted was 39% | |
| PCPs at the rural primary practice consulting with ophthalmologist in the university setting about adults with Type 2 diabetes | Specifically targeted rural‐based ethnic minorities, 35% of whom did not have health insurance | ‐ | |
| Emergency physicians consulting with specialists about adults attending the emergency department | Only consultants who owned a smartphone and were familiarised with the secure messaging service were included | ‐ | |
| PCPs consulting with experienced investigators based at an eye institute about adults with Type 2 diabetes | Primary clinics that served a large number of ethnic minorities, including a high percentage of participants with transient housing | ‐ | |
| General practitioners consulting with dermatologists about adults with skin lesions | Participants who were not able to attend in‐person appointments at the dermatologist office were excluded, i.e. participants unable to travel or those residing in nursing homes. | ‐ | |
| GP consulting with radiologists about participants aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Sample was composed mainly of low‐skilled workers relying on government‐supported primary clinics for their health care | ‐ | |
| Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Specifically targeted rural‐based adults | ‐ | |
| GP consulting with dermatologists about adults with skin condition | Participants who could not speak or read English or who failed a single‐question literacy assessment* were excluded | *Single‐Item Literacy Screener (SILS), which identifies limited reading ability (Morris 2006) |
GP: General practitioner; PCP: primary care provider; PHW: Peer health workers
Even when a trial was specifically designed to address inequities identified a priori, it might still exclude the most vulnerable elements of the targeted population. Chang 2011 recruited peer health workers in rural Uganda, giving them access to experienced clinical staff through text messages and mobile phone calls, in order to provide better health care to HIV‐positive people. The peer health workers could interact with the participants using the mobile phone. The authors concluded that the relatively low penetration of mobile phones in Uganda, which at the time was 39%, alongside the challenges posed with phone‐charging in a setting where access to electricity is limited, might not only have limited the benefits of the intervention but also increased inequities. Furthermore, the authors also noted that the costs of the intervention could have been a limiting factor for the peer health workers, as the monthly stipend given for mobile phone credits was not always enough.
Whited 2013 excluded people who could not speak or read English, as well as those who failed a single‐question literacy assessment.
Gulacti 2017 assessed the use of a messaging system for communication between emergency physicians in the emergency department and physicians working elsewhere in the hospital, excluding consultants who did not own a smartphone with a secure messaging service.
Armstrong 2018 excluded people without access to the Internet and either a digital camera or a mobile phone with camera features.
Discussion
Summary of main results
We included 19 randomised trials of mobile technologies that recruited more than 5766 participants with varied conditions and health problems. Healthcare professionals included general practitioners, community‐based peer health workers, nurses and physiotherapists, who consulted with specialist healthcare professionals in another healthcare facility, and emergency physicians who consulted colleagues within the same facility. Most trials reported on the use of mobile technologies by general practitioners to consult with specialists, and reported that mobile technologies reduced the time between presentation and management of the health problem (4 trials, 656 participants; moderate‐certainty evidence). Accessing healthcare services through mobile technologies may reduce referrals and clinic visits among people with skin conditions and those with chronic kidney disease, and increase the likelihood of receiving an eye examination among people with diabetes and people referred for an ultrasound (9 trials, 4810 participants when reported, moderate‐certainty evidence). There was little evidence of a difference to patient‐reported quality of life outcomes (2 trials, 622 participants), clinician‐reported outcomes of disease progression (2 trials, 769 participants); or to healthcare providers and participants' satisfaction and acceptability, or cost (6 trials, 5423 participants, low‐certainty evidence). One trial reported on images being lost during transmission, when using mobile technologies and also in usual care; and one trial reported a few experiences of mobile phones not being charged or being lost. However, most trials did not measure or report technical problems.
Four studies reported on the use of mobile technologies by community health or home‐care workers to consult with clinic staff, there was little evidence of an effect on consultations in the trials that recruited participants with new diabetes‐related foot ulcer or older individuals treated with home enteral nutrition (2 trials, 370 participants; moderate‐certainty evidence). There was little or no difference for hospitalisations among older individuals treated with home enteral nutrition (1 trial, 188 participants; low‐certainty evidence), or mortality among people living with HIV or diabetes (2 trials, 1157 participants), for disease activity and health‐related quality of life in participants with rheumatoid arthritis (1 trial, 85 participants) or participant acceptability and satisfaction in people with new diabetes‐related foot ulcer or rheumatoid arthritis (178 participants).
Overall completeness and applicability of evidence
Most trials did not report data on providers' adherence, five trials reported on time between presentation and management of the health condition for the main comparison, and for the remaining comparisons and outcomes we identified very little evidence. A third of the trials recruited adults seeking care for dermatological conditions, reflecting current use of mobile technologies in healthcare settings.
The use of mobile technologies for communication between healthcare professionals and patient management might be particularly relevant for settings where there is a shortage of healthcare providers. However, most of the trials were conducted in high‐income (eleven trials in North America and six trials in Europe) or upper‐middle‐income countries (two trials, one in North America and one in Asia), with one trial each conducted in a lower‐middle rural country (Mongolia) and a low‐income country (Uganda). A similar range of countries was reported in a review of mobile technologies for healthcare service delivery processes (Free 2013). Specific challenges might arise when implementing trials in those contexts, such as the lack of access to power sockets to charge the mobile phones, highlighted by the peer health workers interviewed by Chang 2011 in Uganda but not in the study conducted in Mongolia.
Similar contextual factors might contribute to the applicability of the evidence. One factor often mentioned was the variation in healthcare professionals' willingness to use mHealth (Azogil‐López 2019; Liddy 2019a), to attend training (Liddy 2019a), to invite people to participate (Eminović 2009), to select participants for electronic referrals (Van Gelder 2017), or to hold face‐to‐face appointments (Iversen 2018), or provide the required feedback (Sutherland 2009). Four trials reported that recruited participants might not have been representative of the general population, as the study population was more educated (Armstrong 2018), more likely to be male (Mansberger 2015; Whited 2002; Whited 2013), and more likely to be healthier (Armstrong 2018; Mansberger 2015). There was also variation associated with participants' location, as those allocated to the intervention who lived closer to the referral setting were less likely to accept a telephone appointment (Azogil‐López 2019) and more likely to be referred to a face‐to‐face appointment from their healthcare provider (Iversen 2018). One trial reported that participants allocated to the control group had to travel on average 98 km to receive face‐to‐face care, thus indicating that the intervention might provide particular benefits in settings with a low‐density population (Byamba 2015).
Some of the included trials were designed and implemented to address geographical limitations on access to health care and thus allowed for healthcare providers who were geographically separated to exchange clinical information, promoting equity for rural‐based and other disadvantaged populations who would have less access to healthcare. Two trials were conducted in rural settings: South Carolina, USA (Davis 2003) and Saskatchewan, Canada (Taylor‐Gjevre 2018). Two trials recruited participants from socio‐economically disadvantaged areas (Mansberger 2015; Sutherland 2009).
Certainty of the evidence
The included randomised trials were mostly at low or unclear risk of selection bias. We downgraded the evidence for almost all of the outcomes due to a high risk of performance bias, and almost half of the trials were also at risk of detection, attrition, and reporting biases. We also downgraded some of the evidence for imprecision, due to the relatively small size of the trials. Our confidence in the effect estimates overall is moderate, although due to the relatively low number of trials, different uses of mHealth interventions and small numbers of participants recruited there is a possibility that the estimate of the effect is substantially different.
Potential biases in the review process
We limited the risk of publication bias by conducting a comprehensive literature search of different databases, including published articles, clinical trials registries and unpublished mHealth evidence. The WHO issued a call for papers through popular digital health communities of practice to identify additional primary trials as well as grey literature, all of which have contributed to limit publication bias. Two review authors screened records, extracted data and assessed the certainty of the evidence using GRADE, with discussion with the author team whenever there were any discrepancies.
Agreements and disagreements with other studies or reviews
Hasselberg 2014 conducted a review on image‐based medical expert teleconsultation, with 24 studies, including non‐randomised and feasibility studies. The overall results were similar to ours. A review on asynchronous electronic consultations that included 36 trials, seven of which were randomised trials, reported that healthcare providers were generally satisfied with the timely advice received and the health care provided to the participants (Liddy 2016). When updating the review, Liddy 2019b included non‐randomised evidence and concluded that eConsults were expanding beyond teledermatology and that providers from other specialties were also satisfied. We found limited evidence from randomised trials about how satisfied healthcare providers are with mHealth to communicate with other providers. For both reviews the authors concluded that there was limited research on morbidity and mortality, which is consistent with our results (Liddy 2016; Liddy 2019b).
A Cochrane qualitative evidence synthesis (QES) on healthcare providers' perceptions and experiences of using mHealth technologies to deliver primary care healthcare services found that while providers thought that mobile technologies improved their work and relationships with other providers as well as participants, they also highlighted specific challenges such as access to electricity and network coverage (Odendaal 2020). Similarly, an unpublished overview of factors influencing the acceptability, feasibility and implementation of mobile health technologies also reported problems with installation and usability, as well as issues with electricity and connection (Glenton 2019). This is consistent with our results, especially for settings where constant access to electricity might be an issue (Chang 2011).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Providers' adherence to recommended guidelines | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Complete monitoring of disease progression Complete monitoring of metabolic parameters | OR 1.23 (0.89 to 1.70) OR 0.61 (0.22 to 1.72) | Follow‐up not specified OR: Odds ratio; IG: intervention group; CG: control group * Multilevel analysis for IG compared to CG; model with a random intercept keeping the independent variable (General Practice Information System) fixed |
Comparison 1: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Providers' adherence to recommended practice, guidelines or protocols, Outcome 1: Providers' adherence to recommended guidelines
| Time between presentation and management | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Azogil‐López 2019 | General practitioner consulting with hospital physicians about participants (aged ≥ 7 years) | Median time from referral request to appointment with hospital physician Median time from referral request to resolution of the process | IG: 17 days (IQR 8 to 32, N = 72) CG: 51 days, (IQR 35 to 57 days, N = 101) Median difference: −27 days (99% CI −20 to −33 days)* IG: 105 days (IQR 40 to 169); CG: 147 days (IQR 74 to 228) Median difference: −47 days (95% CI −74 to −17 days)* | IG: Intervention group; CG: Control group; IQR: Interquartile range 3‐month follow‐up * As reported by the authors |
| Piette 2017 | General practitioner consulting with dermatologists about adults with skin lesions | Median delay between | IG: 4 days (N = 53) CG: 40 days (N = 50) Adjusted HR 2.55 (P = 0.01)* | 3‐month follow‐up Reported in days Data also provided for number of participants not receiving an appointment (15 days, 1‐, 2‐ and 3‐month follow‐up) Adjusted hazard ratio (HR) as provided by the authors (adjusting for clustering of GPs and identities of dermatologists) |
| Sutherland 2009 | General practitioner consulting with radiologists about clients aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Median time to participant follow‐up Median time to final diagnosis | IG: 67.1 hours (IQR: 45.9 to 113.7, N = 53) CG: 76.7 hours (IQR 65.8 to 144.7, N = 52) IG: 17.8 hours (IQR: 12.2 to 27.1, N = 53) CG: 23.9 (IQR 21.4 to 48.1, N = 52) | Duration not provided |
| Whited 2002 | General practitioner consulting with dermatologists about adults with skin condition | Mean time to intervention | IG: 73.8 days (SD 71.6, N = 135) CG: 114.3 days (SD 72.3, N = 140) MD: −40.5 days (95% CI −23.41 to −57.89) | Duration not provided SD: standard deviation; MD: mean difference |
Comparison 2: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Time between presentation and management of the health condition, Outcome 1: Time between presentation and management
| Healthcare use | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Healthcare use | ||||
| Byamba 2015 | General practitioner consulting with dermatologists about adults with skin lesions | Participant referred to tertiary‐care centres for consultation | IG: 7/221 CG: 28/229 RR: 0.28, 95% CI 0.13 to 0.63 | IG: Intervention group; CG: Control group RR: risk ratio; CI: confidence interval 5 months follow‐up Note: there was no evidence of clustering taken into account in the analysis, and we were not able to re‐analyse the data. It is possible there are potential unit of analysis errors. |
| Davis 2003 | Primary care provider at the rural primary practice consulting with ophthalmologist at the university setting about adults with diabetes | Participant received diabetic | IG: 23/30 CG: 4/29 RR 5.56 (95% CI 2.19 to 14.10) | Follow‐up not reported RR: risk ratio; CI: confidence interval |
| Liddy 2019a | Primary care provider consulting with specialists for a range of different conditions | Participants referred for face‐to‐face visits to all medical specialties available through eConsult service during the study period | Mean number of participants seen (SD, range) IG: 608 (258, 90 to 1134) CG: 724 (370, 11 to 1692) RR 0.93, 95% CI 0.85 to 1.03* | 12‐month follow‐up RR: risk ratio; CI: confidence interval * Adjusted for covariates |
| Mansberger 2015 | Primary care providers consulting with experienced investigators based at an eye institute about adults with diabetes | Participant received diabetic | IG: 157/296 CG: 90/271 RR 1.60 (95% CI 1.31 to 1.95) | 12‐month follow‐up (24, 36 and 48 months also reported; during these periods telemedicine was offered to all participants) |
| Piette 2017 | General practitioner consulting with dermatologists about adults with skin lesions | Participant referred for clinic follow‐up | IG: 14/39*; CG: 50/50 RR: 0.36 (95% CI 0.24 to 0.55) | 3‐month follow‐up * Only includes participants for whom dermatologists were able to elaborate a treatment plan based on transmitted photographs; for approx. 1/5 of participants allocated to IG the photographs were not usable |
| Sutherland 2009 | General practitioner consulting with radiologists regarding clients aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Participant received ultrasound | IG: 36/53 CG: 9/52 RR 3.92 (95% CI 2.11 to 7.31) | Follow‐up not specified RR: risk ratio; CI: confidence interval |
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Participant referred for clinic follow‐up | IG: 29/1277 CG: 52/1727 OR 0.61 (95% CI 0.31 to 1.23)* | Follow‐up not specified OR: Odds ratio; CI: confidence interval * Multilevel analysis for IG compared to CG; model with a random intercept keeping the independent variable (General Practice Information System) fixed |
| Whited 2002 | General practitioners consulting with dermatologists about adults with skin condition | Participant referred for clinic follow‐up | IG: 110/135; CG: 140/140 RR: 0.82 (95% CI 0.75 to 0.88) | Follow‐up not specified RR: risk ratio; CI: confidence interval |
| Whited 2013 | General practitioner consulting with dermatologists about adults with skin condition | Client visited dermatology clinic | IG: 78/125 CG: 120/136 RR 0.71 (95% CI 0.61 to 0.82) | Proportion of participats who had at least 1 visit to the dermatology clinic during the 9‐month follow‐up |
Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 1: Healthcare use

Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 2: Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up

Comparison 3: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Healthcare use, Outcome 3: Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up
| Health‐related quality of life | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Armstrong 2018 | General practitioner consulting with dermatologists about adults with psoriasis | General health status: Description General health status: Evaluation | MD 0 (95% CI −0.003 to 0.003) MD −0.002 (95% CI −2.75 to 2.75) | General health status ‐ Description assessed with EuroQol‐5D‐5L. Scores converted into an index number, with values ranging from −0.109 (worst) to 1 (best). General health status ‐ Evaluation assessed with EuroQol‐Visual Analogue Scale. Higher scores represent better perceived health status Mean difference from baseline to 12 months follow‐up, 296 participants. MD: mean difference; CI: confidence interval |
| Whited 2013 | General practitioner consulting with dermatologists about adults with skin condition | Quality of life: Composite Health‐related quality of life | IG: MD −12.0 (SD 24.5, N = 160) CG: MD −13.2 (SD 21.6, N = 166) Similar scores between groups throughout the trial | Quality of life assessed with Skindex‐16, 0 ‐ 100 Higher scores represent worse quality of life Health‐related quality of life (HRQoL) assessed with Short‐Form Health Survey 12 (SF‐12) Higher scores represent better HRQoL Mean difference from baseline to 9‐month follow‐up IG: intervention group; CG: control group; MD: mean difference; SD: standard deviation |
Comparison 4: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Participant's healthcare status and well‐being, Outcome 1: Health‐related quality of life
| Clinical course | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Pak 2007 | Primary care professional consulting with dermatologist about adults with skin condition | Clinical course ratings | Improved IG: 173/272, CG: 154/236 No change IG: 89/272; CG: 76/236 Worse IG: 10/272; CG: 6/236 | Based on dermatologist's assessment, at four‐month follow‐up There was little or no difference between groups |
| Whited 2013 | General practitioner consulting with dermatologists about adults with skin condition | Clinical course ratings | Resolved IG: 31/125; CG: 35/136 Improved IG: 59/125; CG: 63/136 Unchanged (not clinically relevant) IG: 13/125; CG: 15/136 Unchanged (clinically relevant) IG: 13/125; CG: 17/136 Worse IG: 9/125; CG: 6/136 | Based on dermatologist's assessment, at nine‐month follow‐up There was little or no difference between groups |
Comparison 4: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Participant's healthcare status and well‐being, Outcome 2: Clinical course
| Healthcare provider satisfaction with the intervention | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Piette 2017 | General practitioners consulting with dermatologists about adults with a skin condition | Satisfaction | Global satisfaction Same proportion of GPs in both groups were satisfied or very satisfied (69%) Time to treatment satisfaction Similar proportion of GPs in both groups considered the time for resolution to be short or very short (IG: 77%; CG: 54%) | Response rate: 65% (N = 26) 2 questions with a Likert scale response (1 very satisfied to 4 very unsatisfied) Results provided narratively |
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Exprience with the intervention | Content of information sent was good Yes: 71%; No: 13%; Did not use: 16% Ease of use Good: 39%; Reasonable: 37%; Insufficient: 8%; Did not use: 16% Added to knowledge of kidney disease Yes: 68%; No: 16%; Did not use: 16% Pleased with feasibility of telenephrology Yes: 79%; No: 5%; Did not use: 16% | Intervention group only (general practitioners) Response rate: 66% (N = 36) |
| Whited 2002 | General practitioners consulting with dermatologists about adults with a skin condition | Satisfaction with the intervention | N = 275 participants Timely appointments (GPs) IG: 95% agreed, 5% neutral CG: 7% agreed, 70% disagreed Consultant sent back information (GPs) IG: 87% agreed, 13% neutral CG: 68% agreed, 17% neutral Educational benefit from the referral (GPs) IG: 55% agreed, 45% neutral CG: 34% agreed, 41% neutral Satisfied with the consult process (GPs) IG: 92% agreed, 3% disagreed CG: 23% agreed, 35% disagreed Less confident with TD than FtF (CD) 75% agree, 12.5% disagree TD consultation takes longer (CD) 100% disagree TD makes it easier to triage clients (CD) 100% agree Satisfied with using TD (CD) 75% agree, 25% neutral | IG: intervention group; CG: control group; TD: teledermatology; FtF: face‐to‐face; CD: consulting dermatologists GPs: 4 questions relating to timeliness, information transfer, education, and overall satisfaction; score agree, neutral, disagree Referring GPs (N = 60) Dermatologists: confidence in using TD for diagnostic and management, resource use, and overall satisfaction; score agree, neutral, disagree CD (N = 8) |
Comparison 5: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Acceptability or satisfaction, Outcome 1: Healthcare provider satisfaction with the intervention
| Participant satisfaction with care | ||||
| Study | Population | Outcomes | Results | Notes |
|---|---|---|---|---|
| Eminović 2009 | General practitioners consulting with dermatologists about adults with skin condition | General satisfaction Interpersonal aspects of care | IG: Mean 3.8 (SD 0.59, N = 191) CG: Mean 3.8 (SD 0.59, N = 159) MD: 0.0 (95% CI −0.12 to 0.12) IG: Mean 4.13 (SD 0.62, N = 191) CG: Mean 4.15 (SD 0.73, N = 159) MD: 0.2 (95% CI −0.12 to 0.16) | Shortened version of the Patient Satisfaction Questionnaire (PSQ III) 1 ‐ 5, higher scores indicate more satisfaction with the care received 1 month follow‐up IG: Intervention group; CG: Control group; SD: standard deviation; MD: mean difference; CI: confidence interval |
| Piette 2017 | General practitioner consulting with dermatologists regarding adults with skin lesions | Global satisfaction Time to treatment satisfaction | Similar proportion of participants in both groups were satisfied or very satisfied (IG: 85%; CG: 94%) Higher proportion of participants in the IG considered the time for resolution to be short or very short, compared to the CG (46%)* | Response rate: 100% (N = 103) 2 questions with a Likert scale response (1 very satisfied to 4 very unsatisfied) Results provided narratively P = 0.20, as provided by the authors |
| Whited 2002 | General practitioner consulting with dermatologists regarding adults with skin condition | Satisfaction | There was little or no difference between IG (N = 101) and CG (N = 93)* | Visit‐specific satisfaction questionnaire (VSQ), 1 ‐ 5, higher scores indicate more satisfaction 1 month follow‐up * As reported by study authors, no usable data |
| Whited 2013 | General practitioner consulting with dermatologists regarding adults with skin condition | Overall satisfied with the care received for skin problem | Agree/strongly agree: IG: 86.8%; CG: 92% Neutral: IG: 8.8%; CG: 6.7% Disagree/Strongly disagree: IG: 4.5%; CG: 1.2% | Single question assessing global satisfaction with the care received 9 months follow‐up N = 159 (IG) and 166 (CG) |
Comparison 5: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Acceptability or satisfaction, Outcome 2: Participant satisfaction with care
| Costs | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Byamba 2015 | General practitioners consulting with dermatologists about adults with skin lesions | Total mean costs | IG: USD 320 CG: USD 3174 Difference: USD 2854* | IG: intervention group; CG: control group Costs calculated in USD (2014) *Data as provided by the authors; no further information available 5 months follow‐up Note: there was no evidence of clustering taken into account in the analysis, and we were not able to re‐analyse the data. It is possible there are potential unit of analysis errors. |
| Eminović 2009 | General practitioners consulting with dermatologists about adults with a skin condition | Total mean costs | IG: EUR 387 (95% CI 281 to 502.5, N = 312) CG: EUR 354 (95% CI 228 to 484, N = 293) MD: EUR 32.5 (95% CI −29.0 to 74.7)* | Costs calculated in EUR (2003) 1‐month follow‐up MD: mean difference; CI: confidence interval * Data as provided by authors |
| Pak 2007 | Primary care professional consulting with dermatologist about adults with skin condition | Total mean costs | Total direct cost IG: USD 103,043 (SD:294, N = 351), CG: 98,365 (283, N = 347) MD: USD −4678 (95% CI −4720 to −4635) Lost productivity IG: USD 16,359 (SD:47, N = 351) CG: USD 30,768 (SD 89, N = 347) MD: USD 14,409 (95% CI 14,398 to 14,419) | Total direct costs include consultations, laboratory analyses and procedures and medications Costs calculated in USD (2006) 4‐month follow‐up |
| Van Gelder 2017 | General practitioners consulting with nephrologists about adults with chronic kidney disease | Mean cost per participant | IG: EUR 453.86 (95% CI 392.98 to 514.74; N = 1277) CG: EUR 433.74 (95% CI 387.64 to 479.84; N = 1727) (P = 0.60) | Main related medical costs, including number of contacts between healthcare providers and participant, as well as between healthcare providers; lab costs; prescriptions; referrals to secondary for renal care. Costs calculated in EUR (2017) Follow‐up not specified |
| Whited 2002 | General practitioners consulting with dermatologists about adults with skin condition | Mean expected cost per participant per visit | Using basic technology IG: USD 40.35; CG: USD 26.50 Using more advanced technology IG: USD 33.10; CG: USD 21.40 | Follow‐up not specified Costs calculated in USD (2002) N = 275 participants |
| Whited 2013 | General practitioners consulting with dermatologists about adults with skin condition | Mean total costs per participant | Healthcare system perspective* IG: USD 308 (SD 298; N = 195) CG: USD 338 (SD 291; N = 196) MD: USD 30 (95% CI USD −79 to 20) Societal perspective** IG: USD 460 (SD 428; N = 195) CG: USD 542 (SD 403; N = 196) MD USD −82 (95% CI USD −12 to −152) | * Includes intervention costs (healthcare providers input, dermatology visits, medication, travel reimbursement) ** Travel, loss of productivity, other dermatology care USD Follow‐up 9 months Costs calculated in USD (2011) |
Comparison 6: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Costs, Outcome 1: Costs
| Technical difficulties | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Quality of the data transmitted | ||||
| Pak 2007 | Primary care providers consulting with dermatologist about adults referred to the dermatology service from primary care clinics | Technical problems | 20/528 participants’ images were lost | 10 images in each group |
| Piette 2017 | General practitioner consulting with dermatologists about adults with skin lesions | Technical quality of the images received | 11/53 participants' images did not have enough quality as to allow diagnosis or treatment or both | Intervention group only The dermatologist was able to make a decision about the need of an in‐person appointment for 8 of the clients, based on the clinical notes sent along with the images |
| Sutherland 2009 | General practitioner consulting with radiologists about clients aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Technical quality of the images received | Mean 4.6 (standard deviation 0.5) Procedural quality Mean 4.7 (standard deviation 0.6) | As rated by 6 radiologists based on 53 scans, delivered by email; 1 ‐ 5, higher scores represent better quality of the images and the procedure Intervention group only |
| Whited 2002 | General practitioner consulting with dermatologists about adults referred to the dermatology service from primary care clinics | Technical quality of the images received | Due to the bad quality of the images transmitted, 1/134 clients allocated to the IG required an in‐person consultation | Intervention group only |
Comparison 7: Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared to usual care: Technical difficulties, Outcome 1: Technical difficulties
| Time between presentation and management | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Gulacti 2017 | Emergency physicians consulting with specialists about adults attending the emergency department; duration not provided | Median consult time* | IG: 158 minutes (IQR:133 to 177.25, 95% CI:150 to169, N = 173) CG: 170 minutes (IQR:165 to 188.5, 95% CI: 170 to 171, N = 172) Median difference: −12 minutes (95% CI: −19 to −7), P < 0.0001** | * Time when consultation was requested minus time when a bed was requested (for admission to hospital) or discharge time IG: intervention group; CG: control group; CI: confidence interval ** Data as provided by the authors |
Comparison 8: Mobile technologies for use in the emergency department compared to usual care: Time between presentation and management of the health condition, Outcome 1: Time between presentation and management
| Healthcare use | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Gulacti 2017 | Emergency physicians consulting with specialists about adults attending the emergency department | Median emergency department length of stay | IG: 240 minutes (IQR: 230 to 270, 95% CI: 240 to 255.2, N = 173) CG: 277 minutes (IQR: 270 to 287.8, 95% CI:277 to 279, N = 172) Median difference −30 minutes, 95% CI −37 to −25* | IG: intervention group; CG: control group; IQR: interquartile range; CI: confidence interval Follow‐up not specified * Data provided by study authors |
Comparison 9: Mobile technologies for use in the emergency department compared to usual care: Healthcare use, Outcome 1: Healthcare use
| Technical difficulties | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Quality of the data transmitted | ||||
| Gulacti 2017 | Emergency physicians consulting with specialists about adults attending the emergency department | Technical problems | There were no problems reported | |
Comparison 10: Mobile technologies for use in the emergency department compared to usual care: Technical difficulties, Outcome 1: Technical difficulties
| Healthcare use | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Iversen 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Outpatient clinic consultations Community nurse consultations | IG: Mean 2.8 (SD 1.9, N = 94), CG: Mean 2.5 (SD 3.0, N = 88) MD −0.48 (95% CI −1.46 to 0.49) IG: M 6.7 (SD 3.4, N = 94), CG: M 5.9 (SD 4.6, N = 88) MD 0.92 (95% CI −0.70 to 2.53) | 12‐month follow‐up SD: standard deviation; MD: mean difference; CI: confidence interval |
| Orlandoni 2016 | Home‐visiting nursing staff consulting with a hospital physician about older adults treated with home enteral nutrition | Outpatient visits Hospitalisations | Incidence rate ratio 95% CI: 0.65 to 1.30, P = 0.62 Incidence rate ratio 95% CI: 0.54 to 1.19, P = 0.26* | 12‐month follow‐up * Data as provided by the authors |
Comparison 11: Mobile technologies used by community health workers or home‐care workers compared to usual care: Healthcare use, Outcome 1: Healthcare use
| Participant healthcare status and well‐being | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Chang 2011 | Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Mortality | IG: 37/446; CG: 53/524 RR 0.82, 95% CI 0.55 to 1.22 | Average follow‐up: 103 weeks |
| Iversen 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Mortality | IG: 5/99; CG 5/88 RR 0.94, 95% CI 0.28 to 3.12 | 12 months follow‐up |
| Taylor‐Gjevre 2018 | Rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis | Disease activity Health‐related quality of life | DAS28‐CRPa MD 0.9 (95% CI −1.2 to 3.1, P = 0.33) mHAQb MD 0.2 (95% CI −0.1 to 0.5, P = 0.14) RADAIc MD 0.9 (95% CI −0.5 to 2.4, P = 0.19) EQ5Dd MD −0.1 (95% CI −0.4 to 0.1, P = 0.29)* | aDisease activity score for rheumatoid arthritis, higher scores represent greater disease activity b Modified health assessment questionnaire, 0 ‐ 3, higher scores represent greater impairment cRheumatoid arthritis disease activity index, 0 ‐ 10, higher scores represent greater disease activity dEuroQol 5 dimensions questionnaire (EQ5D), 0 ‐ 1, higher scores represent better health‐related quality of life Mean difference (MD) between groups, (Control (N = 31), Intervention (N = 54)), from baseline to 9‐month follow‐up All data as provided by the study authors |
Comparison 12: Mobile technologies used by community health workers or home‐care workers compared to usual care: Participant's healthcare status and well‐being, Outcome 1: Participant healthcare status and well‐being
| Participant satisfaction with care | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Iversen 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Experience with healthcare | IG: M 4.4 (SD 0.5, N = 67) CG: M 4.4 (SD 0.5, N = 57) MD: 0.0 (95% CI −0.18 to 0.18) | Generic Short Patient Experiences Questionnaire (GS‐PEQ), 1 ‐ 5, higher scores indicate more satisfaction 12‐month follow‐up |
| Taylor‐Gjevre 2018 | Rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis | Participant satisfaction | There was little or no difference between IG (N = 31) and CG (N = 23)* | Visit specific satisfaction questionnaire (VSQ9), 1 ‐ 5, higher scores indicate more satisfaction 9‐month follow‐up * As reported by study authors, no usable data |
Comparison 13: Mobile technologies used by community health workers or home‐care workers compared to usual care: Acceptability or satisfaction, Outcome 1: Participant satisfaction with care
| Costs | ||||
| Study | Population | Outcome | Results | Notes |
|---|---|---|---|---|
| Chang 2011 | Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Yearly total cost of running the mHealth intervention Cost per participant | N = 29 clusters, 970 participants. USD 1046 USD 2.35 | Intervention arm only, costs calculated in Ugandan shillings and converted to USD (2011). Does not include cost of a previously set‐up intervention to train peer health workers, to which the mHealth was an add‐on Average follow‐up: 103 weeks |
Comparison 14: Mobile technologies used by community health workers or home‐care workers compared to usual care: Costs, Outcome 1: Costs
| Technical difficulties | ||||
| Study | Population | Outcomes | Results | Notes |
|---|---|---|---|---|
| Quality of the data transmitted | ||||
| Taylor‐Gjevre 2018 | Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Technical problems | For 10 video‐conferencing visits images were not transmitted and only an audio‐link was available | Unclear how many visits were conducted in total Intervention group only |
| Technical difficulties reported by the healthcare professionals | ||||
| Chang 2011 | Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Problems with the equipment | Healthcare professionals were not always able to charge the mobile phone Some mobile phones were stolen | Qualitative outcomes based on a small number of interviews (4) Intervention group only |
Comparison 15: Mobile technologies used by community health workers or home‐care workers compared to usual care: Technical difficulties, Outcome 1: Technical difficulties
| Mobile technologies used by primary care providers to consult with a hospital‐based specialist compared with usual care | ||||
| Population: Primary care providers consulting with dermatologists (6 studies), ophthalmologists (2 studies), radiologists (1 study), nephrologists (1 study), or different specialists (1 study) | ||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement |
|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols: Adherence to the advised monitoring criteria Follow‐up not specified | 1 trial of telenephrology (Van Gelder 2017), using a web‐based platform with access to the electronic medical record reported OR of 1.23 (95% CI 0.89 to 1.70) for monitoring of disease and 0.61 (0.22 to 1.72) for monitoring of metabolic parameters | 3004 (1 cluster‐randomised trial, 47 general practices) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with a hospital‐based specialist probably make little or no difference to primary care providers’ adherence to the advised monitoring criteria for participants with chronic kidney disease (CKD), when compared with usual care |
| Time between presentation and management of the health condition Follow‐up: 3 to 6 months | 2 trials of teledermatology (Piette 2017; Whited 2002) reported that participants allocated to IG received the required treatment in less time than those allocated to CG (median delay 4 days for IG and 40 days for CG; MD −40.5 days, 95% CI −23 to −58) 1 trial of telemedicine using a portable ultrasound (Sutherland 2009) for people presenting with symptoms that required an ultrasound reported little or no difference between groups. 1 trial of eConsult for people attending primary care (Azogil‐López 2019) reported that participants allocated to IG had an appointment in less time than those allocated to CG (median difference −27 days, 99% CI −20 to −33) | 656 (4 randomised trials) | ⊕⊕⊕⊝ | The intervention probably reduces time between participants presenting and management among individuals with some skin conditions, symptoms requiring an ultrasound, or requiring an appointment with a specialist after attending primary care |
| Healthcare use Follow‐up: 3 to 12 months | 4 trials of teledermatology (Byamba 2015; Piette 2017; Whited 2002; Whited 2013; RRs ranged from to 0.28 (95% CI 0.13 to 0.63) to 0.82 (95% CI 0.75 to 0.88)) reported that those participants allocated to the intervention group were less likely to be referred for clinic follow‐up or attend an appointment at a clinic 2 trials of eConsults for nephrology (Van Gelder 2017) and different specialties (Liddy 2019a) reported little or no difference between groups (OR 0.61, 95% CI 0.31 to 1.23 and RR 0.93, 95% CI 0.85 to 1.03, respectively) 2 trials of telemedicine for retinopathy screening (Davis 2003; Mansberger 2015) and 1 trial for people presenting with symptoms that required an ultrasound (Sutherland 2009; RR 3.92, 95% CI 2.11 to 7.31) reported that those participants allocated to the intervention group were more likely to receive a clinical examination | 4810 (9 randomised trials) | ⊕⊕⊕⊝ Moderatec | Mobile technologies used by primary care providers to consult with hospital‐based specialists may reduce referrals and clinic visits among people with skin conditions, and increase the likelihood of receiving retinopathy screening among participants with diabetes, and an ultrasound in those referred with symptoms, when compared with usual care 1 trial did not specifically report the number of participants involved |
| Participants' health status and well‐being | Patient‐reported quality of life and health‐related quality of life (Follow‐up: 9 to 12 months) | |||
| 2 trials of teledermatology (Armstrong 2018; Whited 2013) found little or no difference between groups For health status (EQ‐5D‐5L): MD 0 (95% CI −0.003 to 0.003) For quality of life (Skindex‐16): IG: MD −12.0 (SD 24.5, 160 participants), CG: MD −13.2 (SD 21.6, 164 participants) For health‐related quality of life (SF‐12), results reported as little or no difference between groups | 622 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with hospital‐based specialists probably make little or no difference to quality of life and health‐related quality of life among individuals with skin conditions | |
| Clinician‐assessed clinical course (follow‐up: 4 to 9 months) | ||||
| 2 trials of teledermatology (Pak 2007; Whited 2013) found little or no difference between groups | 769 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists probably make little or no difference to clinical improvement among individuals with skin conditions | |
| Acceptability and satisfaction | Healthcare provider acceptability and satisfaction (follow‐up immediately after the intervention) | |||
| 1 trial of teledermatology (Piette 2017) reported little or no difference between groups 1 trial of teledermatology (Whited 2002) reported that GPs allocated to the intervention were more likely to agree that participants received timely appointments and to be satisfied with the consult process than GPs allocated to the control group | 378 | ⊕⊕⊝⊝ | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists may make little or no difference to healthcare provider acceptability and satisfaction with the intervention | |
| Participant acceptability and satisfaction (follow‐up: 1 to 9 months) | ||||
| 4 trials of teledermatology (Eminović 2009; Piette 2017; Whited 2002; Whited 2013) reported little or no difference between groups 1 trial reported MD 0.0 (95% CI −0.12 to 0.12; PSQ III), another trial reported that 87% of participants allocated to the intervention group were overall satisfied with treatment received, compared with 92% of those allocated to the control group* 2 trials reported the results as little or no difference only (VSQ9; *) | 972 (4 randomised trials) | ⊕⊕⊝⊝ Lowg | Mobile technologies used by primary care providers to consult with hospital‐based dermatologists may make little or no difference to acceptability and satisfaction of participants with skin conditions | |
| Costs Follow‐up: 1 to 9 months | 2 teledermatology trials (Eminović 2009; Whited 2013) and 1 telenephrology trial (Van Gelder 2017) reported little or no difference between groups 2 teledermatology trials (Pak 2007; Whited 2002) reported that when loss of productivity was considered, the cost per participant was higher for those allocated to the intervention 1 trial of teledermatology (Byamba 2015) reported that total costs were lower for those allocated to the intervention group. | 5423 (6 randomised trials) | ⊕⊕⊝⊝ Lowh | The intervention may make little or no difference to total or expected costs per participant for adults with skin conditions or chronic kidney disease |
| Technical problems | 1 trial recruiting GPs consulting with dermatologists about images they took (Pak 2007) reported that there was little or no difference between groups for technical problems | 698 (1 randomised trial) | ⊕⊕⊕⊝ Moderatei | The intervention probably results in few or no technical difficulties |
| CG: Control group; CI: Confidence interval; EQ5D: EuroQol five dimensions questionnaire; GPs: General practitioners; IG: Intervention group; MD: Median difference; OR: Odds ratio; PSQ III: Shortened version of the Patient Satisfaction Questionnaire; RR: Risk ratio; SD: Standard deviation; SF‐12: Short‐Form Health Survey 12; VSQ9: Visit‐specific satisfaction questionnaire (VSQ9) * Questions developed by the authors for the specific trial | ||||
| GRADE Working Group grades of evidence | ||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to performance and detection bias, and lack of protection against contamination. | ||||
| Mobile technologies for use in the emergency department compared with usual care | |||||
| Patient or population: Emergency physicians consulting with hospital specialists about adults attending the emergency department | |||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement | |
|---|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols | ‐ | ‐ | ‐ | No studies were identified | |
| Time between presentation and management of the health condition Follow‐up not reported | 1 trial (Gulacti 2017) reported that those allocated with the intervention group were admitted to hospital or discharged more quickly from the emergency department (median difference −12 minutes, 95% CI −19 to −7 minutes) | 345 (1randomised trial) | ⊕⊕⊕⊝ | The intervention probably reduces time between participants presenting and management by a few minutes among individuals visiting the emergency department | |
| Healthcare use: length of stay in the emergency department Follow‐up not reported | 1 trial (Gulacti 2017) reported that participant allocated to the intervention group participants had a shorter stay in the emergency department (median difference −30 minutes, 95% CI: −37 to −25 minutes) | 345 (1 randomised trial) | ⊕⊕⊕⊝ | The intervention probably slightly reduces length of stay among individuals visiting the emergency department | |
| Participants' health status and well‐being | ‐ | ‐ | ‐ | No studies were identified | |
| Participant and provider acceptability or satisfaction | ‐ | ‐ | ‐ | No studies were identified | |
| Costs | ‐ | ‐ | ‐ | No studies were identified | |
| Technical problems | 1 trial (Gulacti 2017) reported that there were no technical problems during the course of the trial | 345 (1 randomised trial) | ⊕⊕⊕⊝ | The intervention probably results in few or no technical difficulties | |
| CI: Confidence interval | |||||
| GRADE Working Group grades of evidence | |||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to high risk of performance and reporting bias. | |||||
| Mobile technologies used by community health or home‐care workers compared with usual care | ||||
| Patient or population: Community‐based peer health workers consulting with clinic staff about receiving antiretroviral therapy, community nurses consulting with diabetes specialist nurses or podiatrists about adults with Type 2 diabetes, home‐care nurses consulting with hospital specialists about home enteral nutrition, rural‐based physical therapists consulting with urban‐based rheumatologists | ||||
| Outcomes | Impact | № of participants | Certainty of the evidence | Plain language statement |
|---|---|---|---|---|
| Providers' adherence to recommended practice, guidelines or protocols | ‐ | ‐ | ‐ | No studies were identified |
| Time between presentation and management of the health condition | ‐ | ‐ | ‐ | No studies were identified |
| Healthcare use | Outpatient clinic and community nurse consultations (follow‐up: 12 months) | |||
| 2 trials (Iversen 2018; Orlandoni 2016) reported little or no difference between groups for outpatient visits (MD −0.48, 95% CI −1.46 to 0.49) or community nurse consultations (MD 0.92, 95% CI −0.70 to 2.53) | 370 (2 randomised trials) | ⊕⊕⊕⊝ Moderatea | Mobile technologies used by community health or home‐care workers probably make little or no difference for outpatient clinic and community nurse consultations of participants with new diabetes‐related foot ulcer and older individuals treated with home enteral nutrition | |
| Hospitalisation (Follow‐up: 12 months) | ||||
| 1 study (Orlandoni 2016) reported that the incidence rate ratio for hospitalisations was similar between groups among older individuals treated with home enteral nutrition (95% CI 0.54 to 1.19, P = 0.26) | 188 (1 randomised trial) | ⊕⊕⊝⊝ | Mobile technologies for communication between home‐visiting nursing staff consulting with a hospital physician may have little or no effect on hospitalisations among older individuals treated with home enteral nutrition | |
| Participants' health status and well‐being | Mortality among individuals living with HIV or diabetes (Follow‐up: 11 to 12 months) | |||
| 2 trials reported little or no differences between groups. 1 study (Chang 2011) recruited peer health workers who consulted with clinic staff (RR: 0.82, 95% CI 0.55 to 1.22), and another study (Iversen 2018) recruited community nurses who consulted with diabetes specialist nurses (RR: 0.94, 95% CI 0.28 to 3.12). | 1157 | ⊕⊕⊝⊝ | The intervention may make little or no difference in mortality among people living with HIV or diabetes | |
| Disease activity or health‐related quality of life (Follow‐up: 9 months) | ||||
| 1 trial of rural‐based physical therapists consulting with urban‐based rheumatologists about adults with a clinical diagnosis of rheumatoid arthritis (Taylor‐Gjevre 2018) reported little or no difference between groups for disease activity (DAS28‐CRP MD 0.9, 95% CI −1.2 to 3.1; mHAQ MD 0.2, 95% CI −0.1 to 0.5; RADAI MD 0.9, 95% CI −0.5 to 2.4) or health‐related quality of life (EQ5D MD −0.1, 95% CI −0.4 to 0.1) | 85 (1 randomised trial) | ⊕⊕⊝⊝ | Mobile technologies used by community health or home‐care workers may make little or no difference for disease activity and health‐related quality of life in participants with rheumatoid arthritis | |
| Participant and provider acceptability or satisfaction | Healthcare provider acceptability and satisfaction | |||
| ‐ | ‐ | ‐ | No studies were identified | |
| Participant acceptability and satisfaction (Follow‐up: 9 to 12 months) | ||||
| 2 trials on diabetes (Iversen 2018) and arthritis (Taylor‐Gjevre 2018) reported little or no difference between groups for participants' experience with healthcare (GS‐PEQ MD 0.0, 95% CI −0.18 to 0.18) and satisfaction (VSQ9 results reported narratively) with the intervention. | 178 (2 randomised trials) | ⊕⊕⊕⊝ | Mobile technologies used by community health or home‐care workers probably make little or no difference for participant acceptability and satisfaction for participants with new diabetes‐related foot ulcer and participants with rheumatoid arthritis | |
| Costs | ‐ | ‐ | ‐ | No studies were identified |
| Technical difficulties | ‐ | ‐ | ‐ | No studies were identified |
| CI: Confidence interval; DAS28‐CRP: Disease activity score for Rheumatoid Arthritis; EQ5D: EuroQol five dimensions questionnaire; GS‐PEQ: Generic Short Patient Experiences Questionnaire; MD: Mean difference; mHAQ: Modified health assessment questionnaire; RADAI: Rheumatoid arthritis disease activity index; RR: Risk ratio; VSQ9: Visit‐specific satisfaction questionnaire | ||||
| GRADE Working Group grades of evidence | ||||
| Rationale for downgrading the evidence aWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (2 studies), attrition (1 study) and reporting (1 study) bias. bWe downgraded one point for imprecision because the 95% CI shows potential effect on both sides of “no effect” line and that there were few events. cWe downgraded one point for risk of bias due to high risk of performance, detection, and attrition bias. dWe downgraded one point for imprecision because the 95% CI shows potential effect on both sides of “no effect” line . eWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (1 study), attrition (1 study) and reporting (2 studies) bias. fWe downgraded one point for risk of bias due to high risk of performance, detection, attrition, and reporting bias. gWe downgraded one point for risk of bias due to high risk of performance (2 studies), detection (2 studies), attrition (1 study), and reporting (2 studies) bias. | ||||
| Study | Incentives | Specific training |
|---|---|---|
| Participants were paid for participating in the study, through gift cards (main paper, p.3, end 1st paragraph) | Participants and their carers were taught how to take standardised images of skin lesions, as well as how to communicate with the dermatologist using a secure web‐based system. PCPs also had access to the training materials. (Protocol, p.19, 2nd paragraph) | |
| ‐ | GPs attended a 2‐day training session to learn how to take images and use the medical record system and software on mobile phones (p.1, top 2nd column) | |
| PHWs were given a bicycle, t‐shirts, basic supplies, and an initial monthly allowance (parent trial) | PHWs allocated to the intervention group were given a mobile phone, and attended a 1‐day residential training and a brief field‐based practical training on the intervention (main paper, p.3, 2nd paragraph) | |
| ‐ | GPs allocated to the intervention group received detailed instructions on how to take digital images and use the web‐based form (main paper, p.559, bottom 1st column) | |
| ‐ | All staff received training in the use of the web‐based system, as well as in‐person access to hospital clinics to improve their practical skills (main paper, pp.97‐8) | |
| Specialists received financial incentives for each eConsult they undertook (support paper, under 8. Payment) | ‐ | |
| Participants received monetary incentive to complete follow‐up questionnaire (associated paper, p.524, bottom 1st column) | Technicians performing imaging attended a 3‐day training session to learn how to take images and ongoing feedback as needed (main paper, p.943, bottom 1st column) | |
| ‐ | GPs received training and a workbook on how to take photographs (p.2, top 2nd column) | |
| ‐ | The on‐site investigator received sonographic training over a 2‐month period, as well as practice guidelines for trans‐abdominal ultrasound scanning (P. 192, mid 1st column and top 2nd column) | |
| ‐ | Physical therapists and rheumatologists received an orientation and education session about rheumatoid arthritis and the study protocol and methods (main paper, p.2, top 2nd column) | |
| GP: general practitioner; PCP: primary care provider; PHW: peer health workers | ||
| Study ID | Population | Disadvantaged populations included/excluded? | Notes |
|---|---|---|---|
| General practitioner consulting with dermatologists about adults with psoriasis | Participants without access to the Internet and a digital camera or smartphone with camera features were excluded | ‐ | |
| GP consulting with hospital physicians about participants (aged ≥ 7 years) | Participants deemed as complex were not eligible for receiving the intervention | Complex participants defined as those lacking a specific diagnosis or requiring further clinical assessment | |
| GP consulting with dermatologists about adults with skin lesions | Intervention was set in rural health clinics in Mongolia | ‐ | |
| Community‐based peer health workers consulting with clinic staff about adults who were receiving or started receiving antiretroviral therapy | Specifically targeted HIV‐positive participants in rural Uganda. However, many participants had limited access to mobile phones*, which might have limited the benefits of the intervention. For the healthcare providers, the costs of the intervention were also a factor, as although they were given a monthly stipend it was not always enough Charging the mobile phone was often challenging, as access to electricity was limited | * Current mobile phone penetration in Uganda at the time the trial was conducted was 39% | |
| PCPs at the rural primary practice consulting with ophthalmologist in the university setting about adults with Type 2 diabetes | Specifically targeted rural‐based ethnic minorities, 35% of whom did not have health insurance | ‐ | |
| Emergency physicians consulting with specialists about adults attending the emergency department | Only consultants who owned a smartphone and were familiarised with the secure messaging service were included | ‐ | |
| PCPs consulting with experienced investigators based at an eye institute about adults with Type 2 diabetes | Primary clinics that served a large number of ethnic minorities, including a high percentage of participants with transient housing | ‐ | |
| General practitioners consulting with dermatologists about adults with skin lesions | Participants who were not able to attend in‐person appointments at the dermatologist office were excluded, i.e. participants unable to travel or those residing in nursing homes. | ‐ | |
| GP consulting with radiologists about participants aged ≥ 13 years requiring a trans‐abdominal or trans‐vaginal ultrasound | Sample was composed mainly of low‐skilled workers relying on government‐supported primary clinics for their health care | ‐ | |
| Community nurses consulting with diabetes specialist nurses and podiatrists about adults aged ≥ 20 years with new diabetes‐related foot ulcers | Specifically targeted rural‐based adults | ‐ | |
| GP consulting with dermatologists about adults with skin condition | Participants who could not speak or read English or who failed a single‐question literacy assessment* were excluded | *Single‐Item Literacy Screener (SILS), which identifies limited reading ability (Morris 2006) | |
| GP: General practitioner; PCP: primary care provider; PHW: Peer health workers | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Providers' adherence to recommended guidelines Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Time between presentation and management Show forest plot | 4 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Healthcare use Show forest plot | 9 | Other data | No numeric data | |
| 3.1.1 Healthcare use | 9 | Other data | No numeric data | |
| 3.2 Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2.1 Referred to a dermatology clinic | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Referred for clinic follow‐up or clinical examination, 3 to 12 months follow‐up Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Health‐related quality of life Show forest plot | 2 | Other data | No numeric data | |
| 4.2 Clinical course Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Healthcare provider satisfaction with the intervention Show forest plot | 3 | Other data | No numeric data | |
| 5.2 Participant satisfaction with care Show forest plot | 4 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Costs Show forest plot | 6 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Technical difficulties Show forest plot | 4 | Other data | No numeric data | |
| 7.1.1 Quality of the data transmitted | 4 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Time between presentation and management Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Healthcare use Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Technical difficulties Show forest plot | 1 | Other data | No numeric data | |
| 10.1.1 Quality of the data transmitted | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Healthcare use Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Participant healthcare status and well‐being Show forest plot | 3 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Participant satisfaction with care Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Costs Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Technical difficulties Show forest plot | 2 | Other data | No numeric data | |
| 15.1.1 Quality of the data transmitted | 1 | Other data | No numeric data | |
| 15.1.2 Technical difficulties reported by the healthcare professionals | 1 | Other data | No numeric data | |