Endovascular versus conventional open surgical repair for thoracoabdominal aortic aneurysms
Abstract
Background
Thoracoabdominal aortic aneurysms (TAAAs) are a life‐threatening condition which remain difficult to treat. Endovascular and open surgical repair (OSR) provide treatment options for patients, however, due to the lack of clinical trials comparing these, the optimum treatment option is unknown.
Objectives
To assess the effectiveness and safety of endovascular repair versus conventional OSR for the treatment of TAAAs.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and AMED databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 26 April 2021. We also searched references of relevant articles retrieved from the electronic search for additional citations.
Selection criteria
We considered all published and unpublished randomised controlled trials (RCTs) and controlled clinical trials (CCTs) comparing endovascular repair to OSR for TAAAs for inclusion in the review. The main outcomes of interest were prevention of aneurysm rupture (participants without aneurysm rupture up to 5 years from intervention), aneurysm‐related mortality (30 days and 12 months), all‐cause mortality, spinal cord ischaemia (paraplegia, paraparesis), visceral arterial branch compromise causing mesenteric ischaemia or renal failure, and rate of reintervention.
Data collection and analysis
Two review authors independently screened all titles and abstracts identified from the searches to identify those that met the inclusion criteria. We planned to undertake data collection, risk of bias assessment, and analysis in accordance with Cochrane recommendations. We planned to assess the certainty of the evidence using GRADE.
Main results
No RCTs or CCTs met the inclusion criteria for this review.
Authors' conclusions
Due to the lack of RCTs or CCTs, we were unable to determine the safety and effectiveness of endovascular compared to OSR in patients with TAAAs and are unable to provide any evidence on the optimal surgical intervention for this cohort of patients. High‐quality RCTs or CCTs addressing this objective are necessary, however conducting such studies will be logistically and ethically challenging for this life‐threatening disease.
PICO
Plain language summary
Are patient outcomes superior after endovascular or open surgical repair of thoracoabdominal aortic aneurysms?
Background
A thoracoabdominal aortic aneurysm (TAAA) is a widening or swelling of a blood vessel 50% greater than the original vessel diameter, affecting the thoracic and abdominal aorta simultaneously. A TAAA greater than 6.0 cm to 6.5 cm in diameter (depending on the gender of the patient, or the presence of an underlying inherited weakness in the aortic wall), or a TAAA expanding faster than 1 cm per year, is regarded as a life‐threatening condition if left untreated, and presents a complex challenge for surgeons in deciding on the best treatment path for each patient. The treatment options include open surgical repair which requires the surgical team to open both the chest and the abdomen to replace the diseased aorta with a material graft; endovascular repair which involves inserting a series of stents covered with material (endografts) within the aneurysm through small groin incisions using X‐rays to guide the endografts into place; and non‐interventional management which requires counselling patients and their family and, when necessary, prescribing patients medications to control risk factors. Significant complications including death exist after endovascular and open surgical repair. However, endovascular repair is conceptually less invasive, and for this reason, we aimed to determine if endovascular repair is a safer option for patients compared to open surgical repair.
Study characteristics and key results
We searched the literature for randomised controlled trials and controlled clinical trials to evaluate the effectiveness and safety of endovascular compared to open surgical repair for treating thoracoabdominal aortic aneurysms. Randomised controlled trials and controlled clinical trials help inform healthcare professionals, policymakers, and consumers about the best possible treatment option for patients with TAAAs. These types of trials aim to control confounding factors, for example patient fitness or surgeon experience, so that the methods of treatment are fairly compared. Our search up to April 2021 did not identify any randomised controlled trials or controlled clinical trials that met our inclusion criteria.
Certainty of the evidence
We found no studies that addressed our objective.
Conclusion
To assess the safety and effectiveness of endovascular compared to open surgical repair effectively, randomised controlled trials and controlled clinical trials would be helpful, but there are logistical and ethical challenges in undertaking such trials.
Authors' conclusions
Background
Description of the condition
Conventionally, an aneurysm is considered a widening, or dilation of a blood vessel 50% greater than the original vessel diameter. Aneurysms affecting the aorta can be regionally classified into three main categories: isolated thoracic; isolated abdominal; and thoracoabdominal (where the aneurysm crosses the diaphragm and affects both the thoracic and abdominal aorta simultaneously). Aortic aneurysms are known to rupture at various sizes, and rupture risk is influenced by numerous factors including wall stress, growth rate, gender, location of the aneurysm along the aorta and the presence of a connective tissue disorder (Bäck 2013; Kontopodis 2013). For further details, see the Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) (Riambau 2017). In the USA alone, approximately 16,000 deaths per year are attributed to aortic aneurysms and up to 10% of these are thoracoabdominal in pathology, while the overall estimated prevalence of thoracoabdominal aortic aneurysms (TAAAs) rests at 5.9 cases per 100,000 person years (Barbour 2007; Choong 2010; Youssef 2014).
TAAAs were first classified in Crawford 1986, and amended in Safi 1999. The classifications include type, or extent, I to V based on the anatomical extension of the aneurysm or involvement of the thoracic and abdominal aorta (Fernandez‐Moure 2011; Safi 1999). Type I affects just below the left subclavian artery (LSA) to above the renal arteries, type II originates distal to the LSA and ends below the renal arteries, type III presents between the sixth intercostal space and ends inferior to the renal arteries, type IV affects the aorta between the 12th intercostal space and the iliac bifurcation, and type V presents inferior to the sixth intercostal space and superior to the renal arteries (Fernandez‐Moure 2011).
Unlike aneurysms of the abdominal aorta, for which many countries have national screening programmes, there are no screening programmes for thoracic aortic aneurysms, and these are often detected incidentally when a patient is having cross‐sectional imaging for other indications. Aneurysms affecting the thoracic aorta are asymptomatic in approximately 95% of people and remain asymptomatic while the TAAA silently increases in size. Consequently, for aneurysms exceeding 6.0 cm in diameter, the associated overall mortality rate for rupture is 97% to 100% (Elefteriades 2015; Rolph 2015).
Description of the intervention
Currently, three surgical approaches to treat TAAAs exist; these include conventional open surgical repair (OSR), endovascular repair, and hybrid repair (Clough 2012; Fehrenbacher 2012; Oderich 2012). The principle of OSR is to operate directly on the aorta by means of surgical exposure, while endovascular repair relies upon aneurysm exclusion through the delivery of a stent graft intravascularly from a peripheral access point to the local site of the aneurysm. The effectiveness of hybrid repair will not be investigated in this review as it will be investigated in another Cochrane Review.
Open surgical repair
OSR of TAAAs involves opening both the chest and abdomen through a long incision. The procedure requires cross‐clamping of the aorta and replacement of the aneurysmal segment with a synthetic graft with branches to the visceral arteries, or in some cases the visceral branches can be preserved as a pedicle with a patch of aortic wall around its proximal aortic origin used to surgically implant the visceral branch into the synthetic aortic graft. The latter is contracted in the presence of connective tissue disorders. Due to the clamping and excision of the diseased section of the aorta, distal reperfusion may be required to offer protection from visceral, lower extremity and spinal cord ischaemia. Conventional OSR of TAAAs has been the gold standard since its introduction in 1955 (Kheirelseid 2014). Various centres have published mortality rates as high as 48.4% and postoperative spinal cord ischaemia rates up to 8.0% (Greenberg 2008a; Rigberg 2006). Modern advances in surgical techniques for OSR including left heart bypass, distal reperfusion and hypothermic circulatory arrest have helped reduce complications; however, issues relating to respiratory compromise, brain, spinal cord, cardiac and visceral ischaemia continue to pose significant concern (Riambau 2017).
Endovascular repair
Endovascular repair is considered a less invasive method of excluding an aneurysm by means of deploying stents inside the artery at the site of the aneurysm. The stents are within a delivery sheath which is guided from the arterial access site to the site of the aneurysm using a guidewire and fluoroscopic imaging techniques. Imaging is used throughout the procedure to locate important branches, which may necessitate either protection or stenting. Balloon inflation is often used to mould the sealing zones to the aorta and to improve apposition where stents overlap. Finally, completion angiography is used to confirm accurate placement, as well as the absence of type I endoleaks at the sealing zones of the endograft between the aorta or iliac arteries, or type III endoleaks which can occur due to malapposition of the various modular components of the endografts and/or visceral stents (Singh 2014).
How the intervention might work
Although to date, trial results using an endovascular repair technique for TAAA are promising (Ferrer 2015), surgical opinion is still divided on the overall benefits of this technique over OSR among the wider vascular community. OSR is considered the gold standard of treatment for TAAAs (Kheirelseid 2014), but even in high volume experienced centres complications are still substantial, including mortality (11.26%), paraplegia (5%), cardiac events (4.41%), respiratory complications (23%), stroke (3.11%) and permanent haemodialysis (7.9%) (Moulakakis 2018). Operative mortality does relate to the extent of the aneurysm, with mortality rates of 6.97% and 7.20% for the less extensive Crawford types I and IV, respectively, while mortality rates are higher for the more extensive Crawford types II and III, at 10.32% and 8.02%, respectively. Reduced mortality rates are seen in higher volume centres and mortality rates fall further as surgeons and centres become more experienced (Moulakakis 2018). However, regardless of the experience of the surgeon and centre, open surgery is rarely indicated in patients older than 70 years of age, especially in extent II aneurysms (Harky 2021). Intervention for TAAAs via an endovascular approach offers an alternative to the highly invasive OSR, while distal reperfusion, laparotomy or thoracotomy, aortic clamping and long hospital stay can be avoided. However, there remains concern regarding delays from planning to implantation for patients who require manufacture of customised endografts, high rates of reintervention, paraplegia, renal failure and visceral ischaemia as a result of branch occlusions following endovascular repair (Greenberg 2008; Rocha 2021). As with OSR, outcomes with endovascular repair improve with operator experience and centre volume, and are relative to the extent of the aneurysm. However, recent registry data demonstrate that serious perioperative complications are not insignificant for TAAAs (extent I, II and III), including mortality (5%), cardiac complications (7%), pulmonary morbidity (8%), renal failure (13%), bowel ischaemia (1%), and permanent paraplegia (3%) (Gallitto 2021), and not all patients with TAAA have anatomy amenable for endovascular repair.
Why it is important to do this review
We are undertaking this review as there is a need within the vascular community for greater information regarding the best surgical approach for the treatment of TAAAs. The management of this condition is complex due to its often late diagnosis, patient anatomy and fitness, and the availability of surgeon and centre expertise in repair procedures. In conjunction with the significantly high rates of postoperative complications, management continues to challenge vascular interventionalists worldwide. Evidence for endovascular and open surgical treatment has yet to demonstrate the most clinically effective method (Zanow 2014). To date, no Cochrane Review has assessed the effectiveness and safety of endovascular repair compared to the gold standard of conventional OSR.
Objectives
To assess the effectiveness and safety of endovascular repair versus conventional OSR for the treatment of TAAAs.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) and controlled clinical trials (CCTs) comparing endovascular to open surgical repair (OSR) for thoracoabdominal aortic aneurysms (TAAAs) for inclusion in the review. We placed no limitations on publication date, language, or status.
Types of participants
All participants with TAAAs, diagnosed using conventional methods, such as computed tomography (CT) or magnetic resonance imaging (MRI), or both were to be included in the review. We planned to consider people with a primary TAAA of any type (Crawford Classifications Type I to V; see Description of the condition) and any morphology (e.g. fusiform, in which the entire circumference of the aneurysmal portion of the aortic wall is dilated, as opposed to a saccular aneurysm in which there is an eccentric outpouching of the aortic wall). We did not consider aneurysm formation post‐aortic dissection.
Types of interventions
We planned to include all studies comparing endovascular repair versus OSR for TAAAs. For both endovascular repair and OSR, a number of devices are available, and we planned to include all device types.
Types of outcome measures
The selection of primary and secondary outcomes was guided by the Society for Vascular Surgery (SVS) reporting standards for thoracic endovascular aortic repair (Fillinger 2010). We planned to report outcomes on time points such as 30 days, 12 months and 5 years unless otherwise stated.
Primary outcomes
-
Prevention of aneurysm rupture (number of participants without aneurysm rupture up to 5 years from intervention)
-
Aneurysm‐related mortality (30 days and 12 months)
-
All‐cause mortality (5 years)
-
Spinal cord ischaemia (paraplegia, paraparesis) (5 years)
Secondary outcomes
(Adapted from Fillinger 2010)
-
Visceral arterial branch compromise causing mesenteric ischaemia or renal failure (5 years)
-
Renal failure
-
Mesenteric ischaemia
-
Rate of reintervention (5 years)
-
Presence of endoleak associated with aneurysm sac expansion
-
Conversion to open repair
Search methods for identification of studies
We did not restrict by language or publication status.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and CCTs without language, publication year or publication status restrictions.
-
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 26 April 2021)
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, issue 3) via the Cochrane Register of Studies Online (CRSO)
-
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched 26 April 2021)
-
Embase Ovid (searched 26 April 2021)
-
CINAHL Ebsco (searched 26 April 2021)
-
AMED (searched 26 April 2021)
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and CCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2021). Search strategies for major databases are provided in Appendix 1.
The Information Specialist also searched the following trials registries on 26 April 2021.
-
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch)
-
ClinicalTrials.gov (clinicaltrials.gov)
Searching other resources
We searched references of relevant articles retrieved from the electronic search for additional citations.
Data collection and analysis
Selection of studies
Two review authors (NH and SS) independently screened all titles and abstracts identified from the literature searches to identify those that met the inclusion criteria. We retrieved the full text of studies identified as potentially relevant by at least one review author. The same review authors independently screened the full‐text articles for inclusion or exclusion. We resolved any disagreements by discussion or, when necessary, we consulted a third review author (FJ). All studies excluded at the full text stage are listed as excluded studies and reasons for their exclusion are presented in the Characteristics of excluded studies table. The screening and selection processes are presented using the adapted PRISMA flowchart (Liberati 2009).
Data extraction and management
Had we included studies, we planned for two review authors (NH and SS) to independently extract data from eligible studies using an adapted data extraction form provided by Cochrane Vascular. We planned to resolve any disagreements by discussion or if necessary, consult a third review author (FJ). We planned for one review author (NH) to enter extracted data into Review Manager 5 (Review Manager 2020), and we planned for a second review author (FJ), to check for accuracy and consistency against the data extraction sheets.
We planned to describe the studies according to:
-
trial design;
-
diagnosis of TAAA;
-
demographic characteristics of participants;
-
type of intervention (OSR and endovascular repair);
-
frequency of primary and secondary outcomes;
-
treatment centre.
Assessment of risk of bias in included studies
Had we included studies, we planned that two review authors (NH and SS) would assess each study independently for risk of bias using RoB 1 according to the following criteria, as recommended by the Cochrane Handbook (Higgins 2017).
-
Random sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants and personnel (performance bias)*
-
Blinding of outcome assessment (detection bias)*
-
Incomplete outcome data (attrition bias)
-
Selective reporting (reporting bias)
-
Other sources of bias
We planned to judge all included studies as having low, high or unclear risk of bias because of these criteria. We planned to resolve disagreements by discussion or if necessary, by consulting with a third review author (FJ).
*Due to the nature of the type of intervention, blinding of participants and personnel (i.e. participants, clinicians and outcome assessors) is not possible, therefore, we intended to judge these domains at high risk of bias.
Measures of treatment effect
Dichotomous data
Had we included studies, we planned to express the results for dichotomous outcome measures using risk ratio (RR) and associated 95% confidence intervals (CIs) to reflect uncertainty of the point estimate of effects.
Continuous data
For continuous outcome measures, we planned to calculate mean difference (MD) and standard deviation (SD) with corresponding 95% CIs. We planned to use standardised mean difference (SMD) with 95% CIs to combine outcomes from trials that measure the same outcome using different scales (Higgins 2021).
Time‐to‐event data
In relation to survival analysis, we planned to report time‐to‐event data and the intervention effect expressed as a hazard ratio (HR) and associated 95% CIs. Methods used to analyse time‐to‐event data would have been guided by those described by Parmar 1998 and Tierney 2007.
Unit of analysis issues
Had we included studies, the unit of analysis would have been considered to be each individual participant.
Dealing with missing data
For studies with missing data, we planned to contact the corresponding study authors to try to obtain additional information. We planned to record missing and unclear data for each included study. We also aimed to perform all analyses using an intention‐to‐treat approach, that is, we planned to analyse all participants and their outcomes within the groups to which they were allocated too, regardless of whether they received the intervention or not.
Assessment of heterogeneity
Had we included studies, we planned to assess the degree of heterogeneity by visual inspection of forest plots and by examining the Chi2 test for heterogeneity. We planned to assess heterogeneity of the overall results for the main outcomes by use of Chi2, I2 and Tau2 statistics, according to the Cochrane Handbook (Higgins 2021). We planned to regard statistical heterogeneity as substantial if an I2 was greater than 50% and either the T2 was greater than zero, or there was a low P value (< 0.10) in the Chi2 test for heterogeneity.
Had we included studies and identified substantial clinical, methodological or statistical heterogeneity across the included trials, we planned not to pool results in a meta‐analysis but instead use a narrative approach to data synthesis. If we identified substantial heterogeneity, we planned to explore possible reasons by grouping trials with similar populations.
Assessment of reporting biases
If 10 or more studies were included in the review, we planned to investigate publication bias using funnel plots, as recommended by the Cochrane Handbook (Higgins 2021).
Data synthesis
Had we included studies, we planned to record data and carry out statistical analysis using Review Manager 5 (Review Manager 2020), and we planned to use a fixed‐effect meta‐analysis for synthesising data where it was reasonable to assume that trials were estimating the same underlying treatment effect. If clinical heterogeneity were sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity were detected, we planned to use random‐effects meta‐analysis to produce an overall summary were the average treatment effect clinically meaningful. If substantial clinical, methodological or statistical heterogeneity were identified across the included trials, we planned not to report pooled results from the meta‐analysis but instead use a narrative approach to data synthesis.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses limited to primary outcomes. Our planned subgroup analyses included:
-
type I OSR versus endovascular repair;
-
type II OSR versus endovascular repair;
-
type III OSR versus endovascular repair;
-
type IV OSR versus endovascular repair;
-
type V OSR versus endovascular repair;
-
connective tissue disorder versus non‐connective tissue disorder;
-
physician‐modified devices (chimney/parallel graft repairs) versus commercially‐available patient‐specific devices (branched/fenestrated repair);
-
intercentre efficacy.
Sensitivity analysis
For the purpose of this review, we planned to classify trials judged as ‘low risk of bias’ for sequence generation and allocation concealment as high‐quality trials, had we included studies. We planned to repeat the analyses to include only high‐quality trials. We had also planned to repeat the analyses including only RCTs.
Summary of findings and assessment of the certainty of the evidence
Had we included studies, we planned to prepare a summary of findings table to present the findings from this review using GRADEpro GDT (GRADEPro GDT). We planned to include the following outcomes: prevention of aneurysm rupture (number of participants without aneurysm rupture up to 5 years from intervention), aneurysm‐related mortality (30 days and 12 months), all‐cause mortality, spinal cord ischaemia (paraplegia, paraparesis), visceral arterial branch compromise causing mesenteric ischaemia or renal failure, and rate of reintervention. We would have graded the certainty of the evidence for each outcome using criteria devised by GRADE (Atkins 2004). The certainty of the evidence was planned to be assessed as high, moderate, low or very low, based on risk of bias, inconsistency, indirectness, imprecision and publication bias (Atkins 2004; Guyatt 2008; Higgins 2021; Schünemann 2006).
An example version of the summary of findings table is included in this review (see Table 1).
| Endovascular repair compared with conventional open surgery for TAAAs | ||||||
| Patient or population: people with a diagnosis of thoracoabdominal aortic aneurysm Settings: hospital ‐ elective and emergency Intervention: endovascular repair Comparison: conventional OSR | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with conventional OSR | Risk with endovascular repair | |||||
| Prevention of aneurysm rupture (number of participants without a rupture) (5 years) |
|
|
|
|
|
|
| Aneurysm‐related mortality (30 days) |
|
|
|
|
|
|
| Aneurysm‐related mortality (12 months) |
|
|
|
|
|
|
| All‐cause mortality (5 years) |
|
|
|
|
|
|
| Spinal cord ischaemia (paraplegia, paraparesis) (5 years) |
|
|
|
|
|
|
| Visceral arterial branch compromise causing mesenteric ischaemia or renal failure (5 years) |
|
|
|
|
|
|
| Rate of reintervention (5 years) |
|
|
|
|
|
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Results
Description of studies
Results of the search
The literature searches resulted in 1731 reports to trials. After deduplication we screened 1192 records by title and abstract. We identified all 1192 as irrelevant at title and abstract stage, and so did not identify any RCTs or CCTs relevant to this review (see Figure 1).
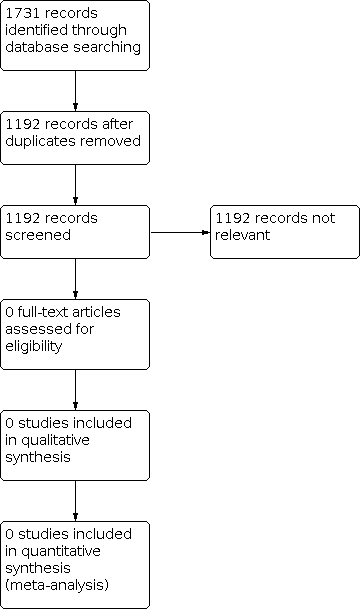
Study flow diagram
Included studies
We identified no RCTs or CCTs eligible for inclusion (see Figure 1).
Excluded studies
We identified no studies which could be brought forward to full‐text review and therefore had no studies to exclude.
Risk of bias in included studies
It was not possible to assess the risk of bias due to the absence of eligible studies for inclusion.
Effects of interventions
Due to the lack of published and unpublished studies eligible for inclusion, it was not possible to examine the effectiveness and safety of endovascular versus OSR for TAAAs.
Discussion
Summary of main results
Thoracoabdominal aortic aneurysms (TAAAs) continue to pose a daunting challenge clinically. Although advancements have been made in open surgical repair (OSR), high complication rates persist, with studies reporting 30‐day mortality incidences of up to 48% (Greenberg 2008a). Since its introduction, endovascular repair has progressed significantly and is now a viable treatment option for TAAAs. In certain populations, low levels of complications have been reported (Ferrer 2016; Orr 2014; Roselli 2007), although several studies continue to report high mortality and complication rates (Greenberg 2008b; Reilly 2009; Verhoeven 2015).
We found no published or unpublished randomised controlled trials (RCTs) or clinical controlled trials (CCTs) addressing the objective of this review. As a result, we cannot draw any conclusions regarding the safety and efficacy of endovascular repair compared with OSR in patients with TAAAs. It is evident from the results from this review, that there is a lack of high‐quality studies (RCTs and CCTs) comparing endovascular repair to OSR, particularly assessing aneurysm‐related mortality and major surgical complications. Current clinical information is based on single‐armed prospective studies, case series and observational studies.
Overall completeness and applicability of evidence
We found no evidence to suggest the superiority of endovascular repair to OSR in patients with TAAAs. Studies describing outcomes of endovascular repair and OSR for treating TAAAs, have been small observational studies or single‐armed prospective studies (Fiane 2003; Kang 2019; Safi 1994). These studies are typically underpowered to adequately detect differences in patient outcomes, limiting the generalisability of their findings (Miao 2017).
Quality of the evidence
It was not possible to assess methodological quality, risk of bias or the certainty of the evidence in the absence of studies eligible for inclusion.
Potential biases in the review process
We found no studies relevant for inclusion in this review. By establishing inclusion and exclusion criteria from the outset and preforming an extensive literature search we reduced the risk of bias.
Agreements and disagreements with other studies or reviews
Improved outcomes have been reported in TAAA populations treated with endovascular repair, however, significant complications remain, including renal failure (Weiss 2014), paraplegia (Rossi 2015), and stroke (Reilly 2012). A meta‐analysis of 71 studies included 24 studies reporting outcomes after endovascular repair of at least 10 cases of TAAA and 47 studies reporting outcomes following OSR of at least 10 cases of TAAA (Rocha 2020); none of the studies reported a direct comparison between OSR and endovascular repair. The review found that endovascular repair studies included higher‐risk patients with more comorbidities and higher rates of spinal cord ischaemia but similar rates of permanent paraplegia to OSR. On the other hand OSR studies had higher rates of postoperative dialysis but similar rates to endovascular studies of being discharged on permanent dialysis. An earlier multicentre propensity‐matched study comparing endovascular to OSR for a cohort of 341 TAAA patients found no statistical difference between groups in relation to 30‐day mortality or paraplegia (Ferrer 2016).
Despite major investment and advances in endovascular technologies there is no consensus on the most appropriate intervention in TAAA patients. When comparing endovascular repair to OSR, there are potential positives and negatives to the use of each treatment method, including higher secondary intervention rates following endovascular repair (Rocha 2021), and higher spinal cord ischaemia and renal insufficiency post‐OSR (Ferrer 2016); the optimum is likely determined on a case‐by‐case basis. Intuitively, one could perceive endovascular repair to be less invasive, because it is associated with a reduced recovery time and shorter length of hospital stay (Rocha 2018). However, endovascular repair of TAAA is often a complex and lengthy procedure, as reflected by the perioperative mortality rates, which in fact are no better than with open repair (Ferrer 2016; Rocha 2018). Furthermore, although the risk of renal insufficiency may be reduced with endovascular repair, this does not translate into reduced risk of dialysis (Rocha 2018). The growing field of computational engineering (finite element analysis and computational fluid dynamics) can provide further insights into preferential treatment options to minimise adverse outcomes in both patient‐specific situations (Vande Geest 2008), and population‐based frameworks (Conway 2012). Patient‐specific computational modelling, and in particular Fluid Structure Interaction models, allow for accurate predictive models of how aortic devices will perform in vivo, and the effects that these devices may have on the aorta itself, but also on cardiac function (Concannon 2021; van Bakel 2019). Additional factors should also be considered when deciding upon the most appropriate intervention for TAAA patients, including procedural costs, training, centre expertise, and postoperative surveillance capabilities and guidelines, which vary on a centre‐by‐centre basis (Chuter 2008; Locham 2018).
Several logistical and ethical challenges exist in conducting high‐quality RCTs and CCTs for TAAA populations including: the rarity of this specific aneurysm type, making recruiting sufficient trial numbers difficult; anatomical suitability, potentially making one intervention type more feasible than another and preventing randomisation; and surgeon expertise, where endovascular experts may not be as proficient in open surgery, and vice versa, ultimately affecting group outcomes on a site‐by‐site basis. Nonetheless, measures can be put in place to address such challenges, or alternative modes of objective assessment explored, as ultimately, objective evidence is required to effectively compare endovascular repair to OSR for TAAAs.

Study flow diagram
| Endovascular repair compared with conventional open surgery for TAAAs | ||||||
| Patient or population: people with a diagnosis of thoracoabdominal aortic aneurysm Settings: hospital ‐ elective and emergency Intervention: endovascular repair Comparison: conventional OSR | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with conventional OSR | Risk with endovascular repair | |||||
| Prevention of aneurysm rupture (number of participants without a rupture) (5 years) |
|
|
|
|
|
|
| Aneurysm‐related mortality (30 days) |
|
|
|
|
|
|
| Aneurysm‐related mortality (12 months) |
|
|
|
|
|
|
| All‐cause mortality (5 years) |
|
|
|
|
|
|
| Spinal cord ischaemia (paraplegia, paraparesis) (5 years) |
|
|
|
|
|
|
| Visceral arterial branch compromise causing mesenteric ischaemia or renal failure (5 years) |
|
|
|
|
|
|
| Rate of reintervention (5 years) |
|
|
|
|
|
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||

