Vacuna MVA85A para mejorar la actividad del BCG en la prevención de la tuberculosis
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials
#1 tuberculosis or TB:ti,ab,kw (Word variations have been searched)
#2 MeSH descriptor: [Tuberculosis] explode all trees
#3 MeSH descriptor: [BCG Vaccine] explode all trees
#4 "BCG vaccin*":ti,ab,kw (Word variations have been searched)
#5 bacill* Calmette‐Guerin
#6 #1 or #2 or #3 or #4 or #5
#7 "antigen 85A" or Ag85A or "modified vaccinia ankara" or MVA85A
#8 MVA85*
#9 #7 or #8
#10 #9 and #6
MEDLINE (PubMed)
| #12 | Search #7 and #11 |
| #11 | Search ((#8) OR #9) OR #10 |
| #10 | Search "drug therapy" [Subheading] |
| #9 | Search randomized or placebo or randomly or trial or groups Field: Title/Abstract |
| #8 | Search "Randomized Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] |
| #7 | Search #3 and #6 |
| #6 | Search 4 or 5 |
| #5 | "antigen 85A" OR Ag85A OR "modified vaccinia ankara" OR MVA85A Field: Title/Abstract |
| #4 | "antigen 85A, Mycobacterium tuberculosis" [Supplementary Concept] or "MVA 85A" [Supplementary Concept]) |
| #3 | Search 1 or 2 |
| #2 | (("BCG Vaccine"[Mesh]) OR (“bcg vaccin*” or “bacille Calmette‐Guérin” )Field: Title/Abstract |
| #1 | "Tuberculosis"[Mesh] or (tuberculosis or TB) Field: Title/Abstract |
Embase
1 (tuberculosis or tuberculous or TB).mp.
2 tuberculosis/
3 1 or 2
4 BCG vaccine/ or BCG vaccin*.mp. or BCG vaccination/
5 3 or 4
6 MVA85A.mp.
7 antigen 85A.mp.
8 Ag85A.mp.
9 modified vaccinia virus ankara.mp.
10 modified vaccine ankara.mp.
11 6 or 7 or 8 or 9 or 10
12 5 and 11
13 (randomized or randomised or placebo or double‐blind* or single‐blind*).mp.
14 randomized controlled trial/ or controlled clinical trial/
15 crossover procedure/
16 13 or 14 or 15
17 12 and 16
CINAHL (EBSCOHost)
| # | Search terms |
| S1 | TX ( tuberculosis or TB or BCG ) |
| S2 | TX ( (MVA85A or "antigen 85A" or "modified vaccinia ankara" ) |
| S3 | TX ( (randomized trial or controlled trial or placebo or double‐blind* or single‐blind* ) |
| S4 | S1 AND S2 AND S3 |
Web of Science
| # 2 | TOPIC: (tuberculosis or TB or BCG) ANDTOPIC: (MVA85A or "antigen 85A" or "modified vaccinia ankara") ANDTOPIC: (randomized trial or controlled trial or placebo or double‐blind* or single‐blind*) Timespan=All years Search language=Auto |
| # 1 | TOPIC: (tuberculosis or TB or BCG) ANDTOPIC: (MVA85A or "antigen 85A" or "modified vaccinia ankara") Timespan=All years |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
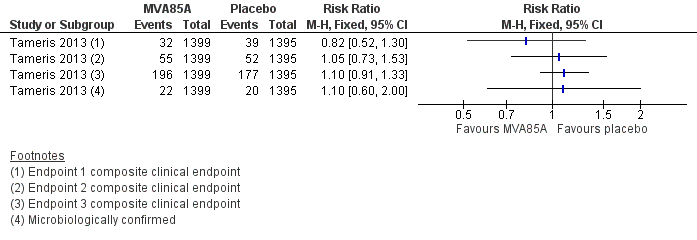
Forest plot of comparison: 2 Comparison of endpoints, outcome: 2.1 Tameris 2013: incidence of tuberculosis according to post‐hoc endpoints.

Forest plot of comparison: 1 MVA85A Vs Placebo, outcome: 1.1 Tuberculosis confirmed by culture or Xpert® MTB/RIF longest reported follow‐up.

Forest plot of comparison: 1 MVA85A versus placebo, outcome: 1.2 Active tuberculosis: started on tuberculosis treatment.
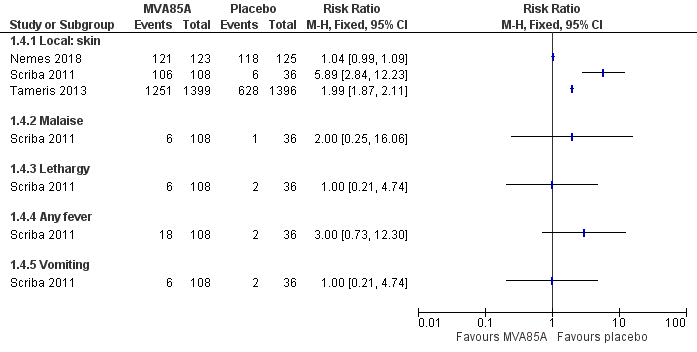
Forest plot of comparison: 1 MVA85A versus placebo, outcome: 1.4 Adverse effects of any severity.
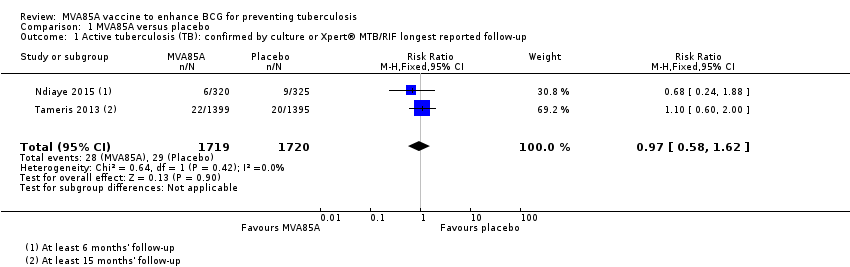
Comparison 1 MVA85A versus placebo, Outcome 1 Active tuberculosis (TB): confirmed by culture or Xpert® MTB/RIF longest reported follow‐up.
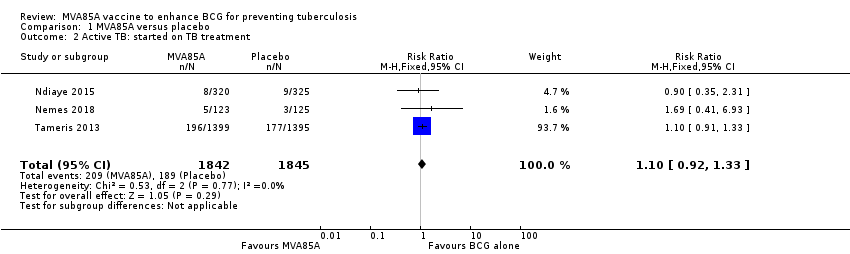
Comparison 1 MVA85A versus placebo, Outcome 2 Active TB: started on TB treatment.

Comparison 1 MVA85A versus placebo, Outcome 3 Latent TB.

Comparison 1 MVA85A versus placebo, Outcome 4 Adverse effects of any severity.
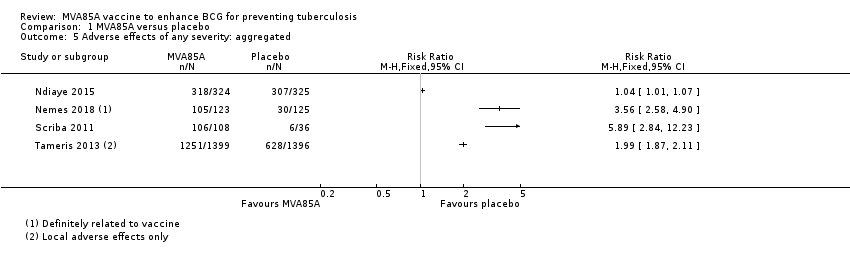
Comparison 1 MVA85A versus placebo, Outcome 5 Adverse effects of any severity: aggregated.

Comparison 1 MVA85A versus placebo, Outcome 6 Serious adverse effects.

Comparison 1 MVA85A versus placebo, Outcome 7 Adverse events of any severity.

Comparison 1 MVA85A versus placebo, Outcome 8 Abnormal biochemical tests.
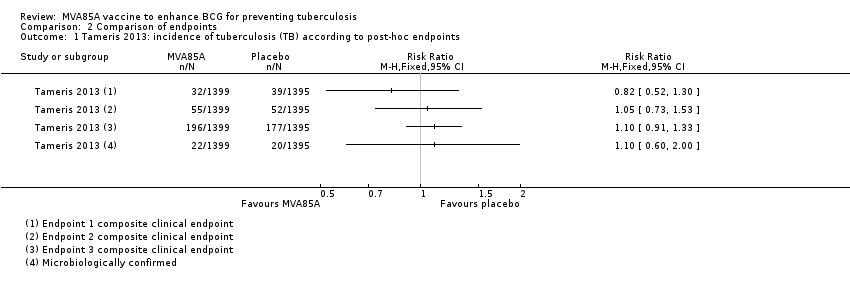
Comparison 2 Comparison of endpoints, Outcome 1 Tameris 2013: incidence of tuberculosis (TB) according to post‐hoc endpoints.

Comparison 2 Comparison of endpoints, Outcome 2 Ndiaye 2015: incidence of TB according to post hoc defined endpoints.
| MVA85A compared to placebo for preventing tuberculosis | ||||||
| Patient or population: HIV‐positive and ‐negative adults and children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with MVA85A | |||||
| Active tuberculosis: confirmed by culture or Xpert® MTB/RIF longest reported follow‐up | 17 per 1000 | 16 per 1000 | RR 0.97 | 3439 | ⊕⊕⊕⊝ | Vaccinating people with MVA85A in addition to BCG probably made little or no difference to the risk of developing active tuberculosis. |
| Active tuberculosis: started on tuberculosis treatment | 102 per 1000 | 112 per 1000 | RR 1.10 | 3687 | ⊕⊕⊕⊝ | Vaccinating people with MVA85A in addition to BCG probably made little or no difference to the risk of needing to start tuberculosis treatment. |
| Latent tuberculosis | 114 per 1000 | 115 per 1000 | RR 1.01 | 3831 | ⊕⊕⊕⊝ | Vaccinating people with MVA85A in addition to BCG probably made little or no difference to the risk of developing latent tuberculosis. |
| Serious adverse effects | 1 per 1000 | 1 per 1000 | RD 0.00 | 3692 | ⊕⊕⊕⊕ | Vaccinating people with MVA85A in addition to BCG did not cause life‐threatening serious adverse effects. |
| Adverse effects of any severity (local reactions of the skin) | Vaccination with MVA85A was associated with more reactions at the site of the injection.g | — | 3187 | ⊕⊕⊕⊝ | Vaccinating people with MVA85A in addition to BCG probably increased the risk of having an adverse reaction related to vaccination at the site of the injection. | |
| Adverse effects of any severity (systemic symptoms) | Adverse events reported included malaise, lethargy, fever, and vomiting although differences between groups were not significant at a 95% CI level.g | — | 144 (1 RCT) | ⊕⊕⊝⊝ | Vaccinating people with MVA85A in addition to BCG may not have been associated with an increase in adverse effects related to vaccination. | |
| Adverse events of any severity | 808 per 1000 | 849 per 1000 | RR 1.05 | 3836 | ⊕⊕⊕⊕ | Vaccination with MVA85A alone slightly increased the risk of having an adverse event. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCG: Bacillus Calmette‐Guérin; CI: confidence interval; RCT: randomized controlled trial; RD: risk difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aNot downgraded for risk of bias. The largest trial was at unclear risk of bias due to selective reporting; however, the outcomes presented were unlikely to be affected by this (Tameris 2013). | ||||||
| NCT trial number | Route | Dates | Intervention and schedule details | Country | Participants (age) | HIV | Adverse events | Reference |
| ID | 2002–2003 | MVA85A; 1 dose | UK | 14 adults (18–45 years) | –ve | 7 trials (112 participants); combined in 1 report: no serious AE attributable to the vaccine | ||
| ID | 2003–2005 | MVA85A; 1 dose, 2 doses (5 × 107 pfu) | Gambia | 21 adults | NR | No serious AE attributable to the vaccine | ||
| ID | 2003–2005 | MVA85A; 1 dose (5 × 107 pfu) | UK | 21 adults | –ve | No serious AE attributable to the vaccine | McShane 2004; Pathan 2012; Rowland 2012; Tanner 2014; Whelan 2009 | |
| ID | 2003–2005 | MVA85A; 1 dose (5 × 107 pfu) | UK | 10 adults | –ve | No serious AE attributable to the vaccine | ||
| ID | 2005–2007 | MVA85A, (5 × 107 pfu) | UK | 12 adults with latent tuberculosis | –ve | No vaccine‐related serious AEs 7 trials (112 participants; data combined in 1 report) | ||
| ID | 2005–2007 | MVA85A; 1 dose (1 × 108 pfu for 12 participants, and 1 × 107 pfu for 12 participants) | UK | 24 adults | –ve | No serious AE attributable to the vaccine | ||
| ID | 2005–2008 | MVA85A (5 × 107 pfu) | South Africa | 36 adults and adolescents | –ve | No vaccine‐related serious AEs | ||
| ID | 2006–2009 | MVA85A; 1 dose MVA85A (2.5 × 107 pfu, 5 × 107 pfu) Groups
| The Gambia | 214 infants (4 months) | NR | No serious AE judged to be related to the vaccine | ||
| ID | 2006–2010 | MVA85A; 1 dose (5 × 107pfu for 10 participants, and 1 × 108 pfu for 10 participants) | UK | 20 adults | +ve | No serious AE attributable to the vaccine | ||
| ID | 2007–2011 | MVA85A; 1 dose (5 × 107 pfu) 4 groups with background of
| South Africa | 48 adults (18–50 years) | +ve | No vaccine‐related serious AEs | ||
| ID | 2007–2010 | FP85A, MVA85A (5 × 107 pfu) | UK | 31 adults | –ve | No serious AE attributable to the vaccine | ||
| ID | 2007–2010 | MVA85A; 1 dose (1 × 108 pfu), administered as 2 injections (5 × 107 pfu each injection) | UK | 12 adults | –ve | 7 trials (112 participants); data combined in 1 report: no serious AE attributable to the vaccine | Porter (unpublished data: source Rowland 2012) | |
| ID | 2008–2011 | MVA85A; 2 doses (spaced by 6–12 months) (1 × 108 pfu) | Senegal | 24 adults | +ve | No serious AE attributable to the vaccine | ||
| ID IM | 2010–2011 | MVA85A; 1 dose (1 × 108 pfu) | UK | 24 adults | –ve | No serious AE attributable to the vaccine | ||
| ID | 2010–2012 | MVA85A, BCG; 1 dose (1 × 108 pfu) Group A: BCG naive, no MVA85A Group C: BCG vaccinated, no MVA85A Group D: BCG vaccinated, MVA85A. | UK | 49 adults recruited; 48 completed study | –ve | No serious AE attributable to the vaccine | ; Harris 2014b; | |
| Aerosol ID | 2011–2013 | MVA85A; 1 dose: 1 × 108, 1 × 107 pfu | UK | 24 adults | –ve | No vaccine related serious adverse effects. | ||
| ID | 2012–2014 | AERAS‐402 MVA85A; Group A: 2 doses AERAS‐402 then MVA85A Group B: 1 dose AERAS‐402 then MVA85A | UK | 40 adults | –ve | No vaccine related serious AEs | ||
| ID | 2013–2014 | MVA85A IMX313; Group A: low‐dose MVA85A‐IMX313 (1 × 107 pfu) Group B: dose MVA85A‐IMX313 (5 × 107 pfu) Group C: MVA85A (5 × 107 pfu) | UK | 30 BCG vaccinated adults | –ve | No vaccine‐related serious AE | ||
| IM | 2013–2016 | MVA85A, ChAdOx1 85A; Group A: 1 dose ChAdOx1 85A Group B: 1 dose ChAdOx1 85A then MVA85A Group C: 2 doses ChAdOx1 85A then MVA85A (1 × 108 pfu) | UK | 42 adults | –ve | No data reported yet | No publication | |
| Aerosol ID | 2013–2016 | MVA85A; Group 1: aerosol then ID Group 2: ID then aerosol Group 3: ID then ID (5 × 107 pfu) | UK | 37 adults | –ve | No data reported yet | (conference abstract) | |
| Aerosol ID | 2015–2018 | MVA85A; 1 × 107 pfu aerosol inhaled, 5 × 107 aerosol and ID | UK | 15 adults | –ve | No data reported yet | ||
| –ve: negative; +ve: positive; AE: adverse event; ART: antiretroviral therapy; BCG: bacillus Calmette‐Guérin; EPI: Expanded Programme on Immunization; ID: intradermal; IM: intramuscular; MTB: Mycobacterium tuberculosis; NR: not reported; pfu: plaque‐forming unit. | ||||||||
| Criterion | Assessment | Explanation |
| Participant‐reported symptoms | ||
| Was monitoring active or passive? | Active Passive Unclear | We classified monitoring as 'active' when authors reviewed participants at set time points and enquired about symptoms. |
| Was blinding for participants and outcome assessors adequate? | Adequate Inadequate Unclear | We classified blinding as 'adequate' when both participants and outcome assessors were blinded to the intervention group, and the methods of blinding (including use of a placebo) were described. |
| Was outcome data reporting complete or incomplete? | Complete Incomplete | We classified outcome data reporting as 'complete' when data were presented for all the time points where it was collected. |
| Were all participants included in reporting? | Yes No | We reported the percentage of randomized participants included in adverse event reporting. |
| Was the analysis independent of study sponsor? | Yes No Unclear | We classified the analysis of trials sponsored by pharmaceutical companies as independent of the sponsor when it was clearly stated that the sponsor had no input to the trial analysis |
| Laboratory tests | ||
| Number of tests undertaken | — | We extracted the type and number of laboratory tests were taken. |
| Timing of tests: was number and timing of tests adequate? | Adequate Inadequate | We classified the number and timing of tests as 'adequate,' when tests were taken at baseline, plus 2 other time points within the first week after treatment, plus the last day of the study. We classified the number of test taken as 'inadequate,' if either the laboratory controls in the first week or controls at 4 weeks were not performed. |
| Reporting of test results: was reporting of test results complete? | Complete Incomplete | We classified reporting as 'complete' when test results of all time points were reported. For the trials with inadequate number of tests taken, we considered completeness of reporting as inconsequential, and therefore did not record a judgement. |
| Independence of data analysis: was data analysis independent? | Yes No Unclear | We classified the analysis of trials sponsored by pharmaceutical companies as independent of the sponsor when it is clearly stated that the sponsor had no input to the trial analysis. |
| Adapted from Bukirwa 2014. | ||
| Study | Endpoint 1 | Endpoint 2 | Endpoint 3 |
| Any of the following criteria.
| "Included all infants who met endpoint 1 criteria; had marginally less stringent criteria to define TB infection and household exposure." Any of the following numerical categories.
| All participants placed on treatment for TB by a health professional with the intent of treating TB regardless of whether they have met the other efficacy endpoints. | |
| Revised endpoint 1 from Tameris 2013 that removed QFT conversion from the diagnostic criteria to avoid bias towards association with QFT status. | Not used | Not used | |
| Not used | Not used | Same definition as for Tameris 2013. | |
| Any of the following numerical categories.
| Any of the following numerical categories:
| Same definition as for Tameris 2013. | |
| Not applicable | Not applicable | Not applicable | |
| Outcomes not specified in the methods section. In results, authors specified that 8 participants were diagnosed as TB: "of whom one was M.tb [Mycobacterium tuberculosis] culture positive and 7 were diagnosed on clinical/ radiographic grounds and TB contact history. Two of the TB cases were QFT positive." | Not used | Not used | |
| AFB: acid‐fast bacilli; CSF: cerebrospinal fluid; CT: computerized tomography; QFT: quantiFERON; TB: tuberculosis. | |||
| Study | Protocol | Published findings | Differences between protocol and published findings | ||
| Stated outcomes published prior to commencement of trial that differ to published outcomes | Measurement of outcome as stated a priori | Measurement of outcome as stated in published findings | Reported findings | ||
| No protocol published. | |||||
| No protocol published (extended post‐trial follow‐up of Tameris 2013). | |||||
| Adverse events: blood tests a | "Percentage of participants with adverse events" AEs measured up to day 28 SAEs measured up to 6 months. | "Phlebotomy for routine haematological and biochemical analysis was done at screening, before booster vaccination, and on days 7 and 28 after each vaccination." | "Routine haematological and biochemical test results did not differ between study groups (data not shown)." | Haematological and biochemical blood tests not outlined as a measure of safety in the study protocol. Blood test findings reported unclearly. | |
| Safety | Clinicaltrials.gov – local, regional, and systemic AEs and SAEs which would be reported as cumulative 12‐month incidences. | "Infants followed for safety end points at weeks 1, 4, 6, and 8 after MVA85A/control vaccination and thereafter, at weeks 9, 12, and 16 (corresponding to weeks 1, 4, and 8 following delayed BCG vaccination at 8 weeks of age), and at week 52." | Reported total events for AEs per group after MVA85A and before BCG and for whole follow‐up period. Data including for laboratory AEs were not disaggregated as prespecified. | Data including for laboratory AEs were not disaggregated as prespecified. | |
| Safetya | Local and systemic AEs for the first week. | Diary cards | Local and systemic AEs reported on ≥ 1 day of the first 7 days after MVA85A vaccination. | None | |
| Blood tests (days 7, 28) | Biochemical and haematological tests (days 7, 28) | Reported number and percentages of participants with abnormal results and reported that, "all except one patient that had elevated liver enzymes remained unresolved by day 28." | |||
| Immunology | ESAT‐6/CFP‐10 | Infants converted – suggestive of TB infection but seemed to be reported as safety data not efficacy. | |||
| Safety profile – AEsa | AEs measured up to day 28 SAEs measured throughout follow‐up. | Collected data on solicited and unsolicited local and systemic AEs. Active surveillance for SAEs. | AEs broken down by type of event and reported in supplementary material. Only local events at the injection site were considered to be related to the vaccine. | Causal relationship with AEs other than local injection site reactions was not reported. | |
| Safety profile – blood testsa | Testing up to 28 days postvaccination. | "Peripheral blood for routine haematological and biochemical tests was taken at screening and on day 7 and day 28 after vaccination in an initial safety cohort of at least 330 infants." | Not reported | Primary outcome not reported | |
| Efficacy of MVA85Ab | Using an endpoint derived from epidemiological cohort surveys in BCG vaccinated infants. | Not reported – simply stated clinical endpoints 'developed.' | Composite clinical endpoints 1, 2, 3 (see Table 3) Microbiologically confirmed cases reported in appendix. | The "primary efficacy endpoint" was measured using an endpoint not derived from cohort studies. The endpoint definition differed from all other implied or reported ways of measuring efficacy in the other studies. The point estimate showed clinically significant benefit for endpoint 1 (no benefit seen at the 95% confidence level). This endpoint was reported as the main efficacy finding. All other point estimates show no clinically significant benefit or harm. | |
| AE: adverse events; BCG: bacillus Calmette‐Guérin; ESAT‐6/CFP‐10: early secretory antigenic‐6/culture filtrate protein‐10; SAE: severe adverse events; TB: tuberculosis. | |||||
| Study | Participant reported adverse events | Outcome data reporting | Laboratory tests | ||||||||
| Monitoring active or passive | Blinding of participants or outcome assessors | Times data collected | Times data reported | Complete/not complete | Percentage of participants reported on | Analysis independent of study sponsor | Number of tests taken | Timing of tests and adequacy | Complete reporting of test results | Independence of data analysis | |
| Active | Inadequate | 60 min, D 2, 7, 28, 84, and 168 | D 7, 28 | Incomplete | 100% | Unclear | Biochemistry and haematology | Inadequate | Inconsequential | Unclear | |
| Active | Inadequate | D 7, 28, and 84 after boost 3 monthly until end of study | NR | Incomplete | 99.8% | No | Haematology, chemistry, virological markers | Adequate | Incomplete | No | |
| Active | Adequate | Baseline, D 7 and 28, throughout up to D 84 | NR | Incomplete | 99.9% | No | Biochemistry and haematology | Inadequate | Incomplete | No | |
| Active | Adequate | Week 1, 4, 6, 8, 16, and 52 | NR | Incomplete | 85.9% | Unclear | Not specified | Adequate | Incomplete | Unclear | |
| D: day; min: minute; NR: not reported. | |||||||||||
| Active TB | ||||||||||||
| MVA85A | Placebo | MVA85A | Placebo | MVA85A | Placebo | MVA85A | Placebo | MVA85A | Placebo | MVA85A | Placebo | |
| Endpoint 1a | 32/1399 (2.3%) | 39/1395 (2.8%) | 58/2797 (2.1%) with NDD | N/A | N/A | 6/320 (1.9%) | 9/325 (2.8%) | N/A | N/A | 5/123 (4.1%) | 3/125 (2.4%) | |
| Endpoint 2a | 55/1399 (3.9%) | 52/1395 (3.7%) | N/A | N/A | N/A | N/A | 6/320 (1.9%) | 9/325 (2.8%) | N/A | N/A | N/A | N/A |
| Endpoint 3a | 196/1399 (14.0%) | 177/1395 (12.6%) | N/A | N/A | 3.3/100 pyo (95% CI 2.9 to 3.9) | 3.0/100 pyo (95% CI 2.6 to 3.5) | 8/320 (2.5%) | 9/325 (2.8%) | N/A | N/A | N/A | N/A |
| CI: confidence interval; N/A: not applicable; NDD: no disaggregated data; pyo: person‐years of observation; TB: tuberculosis. | ||||||||||||
| Study | MVA85A | Placebo | Breakdown | Author conclusions | ||||
| Number of participants with ≥ 1 event caused by the intervention | Total participants | Number of participants with ≥ 1 event caused by the control | Total participants | Detailed AEs | MVA85A | Placebo | ||
| 318 | 324 | 307 | 325 | Solicited AEsa | 288 | 235 | "Solicited adverse events were more common in MVA85A group and most were local injection site reactions." | |
| 105b | 123 | 30b | 125 | Not detailed | N/A | N/A | "Infants in MVA85A arm were more likely to experience an AE than in control arm. Injection site reactions were more frequent in MVA85A recipients and mild." | |
| 106c | 108c | 6 | 36 | Injection sited | 106 | 6 | "Desquamation significantly increased with greater vaccine dose." | |
| Malaise | 6 | 1 | ||||||
| Lethargy | 6 | 2 | ||||||
| Tactile fever | 18 | 0 | ||||||
| Documented fever | 13 | 2 | ||||||
| Vomiting | 6 | 2 | ||||||
| Elevated liver enzyme levels | 13 | 4 | ||||||
| Increased white cell count | 0 | 1 | ||||||
| Local 1251e | 1399 | Local 628e | 1396 | Not detailed | 1251 | 628 | None | |
| AE: adverse event; N/A: not applicable. | ||||||||
| Study | Adverse events of any severity | |||
| MVA85A | Placebo | |||
| 1120/1399 (80.1%) | 1059/1396 (75.9%) | |||
| NR | NR | |||
| NR | NR | |||
| 321/324 (99.1%) | 312/325 (96%) | |||
| 2.5 × 107 pfu = 35 μL | 5 × 107 pfu = 70 μL | 1 × 108 pfu = 135 μL | 1/36 | |
| 1/36 | 3/36 | 6/36 | ||
| Mild 122/123 (99.2%) | 121/125 (96.8) | |||
| Moderate 62/123 (50.4%) | 54/125 (3.6%) | |||
| Severe 11/123 (8.9%) | 14/125 (11.2%) | |||
| NR: not reported; pfu: plaque‐forming unit. | ||||
| Study | Haematological blood tests | Biochemical blood tests | ||||
| MVA85A | Placebo | MVA85A | Placebo | |||
| NR | NR | NR | NR | |||
| NR | NR | NR | NR | |||
| NR | NR | NR | NR | |||
| NRa | NRa | NRa | NRa | |||
| 0/108b | 1/36b | 2.5 × 107 pfu = 35 μL | 5 × 107 pfu = 70 μL | 1 × 108 pfu = 135 μL | 4/36 (11%) | |
| 1/36 (2.8%) | 3/36 (8.3%) | 9/36 (25%) | ||||
| NR | NR | 14/123 (11.4%) | 13/125 (10.4%) | |||
| NR: not reported; pfu: plaque‐forming unit. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Active tuberculosis (TB): confirmed by culture or Xpert® MTB/RIF longest reported follow‐up Show forest plot | 2 | 3439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.58, 1.62] |
| 2 Active TB: started on TB treatment Show forest plot | 3 | 3687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.92, 1.33] |
| 3 Latent TB Show forest plot | 4 | 3831 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.21] |
| 4 Adverse effects of any severity Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Local: skin | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Lethargy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects of any severity: aggregated Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Serious adverse effects Show forest plot | 3 | 3692 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.00, 0.00] |
| 7 Adverse events of any severity Show forest plot | 4 | 3836 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.02, 1.08] |
| 8 Abnormal biochemical tests Show forest plot | 2 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.60, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tameris 2013: incidence of tuberculosis (TB) according to post‐hoc endpoints Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Ndiaye 2015: incidence of TB according to post hoc defined endpoints Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

