Computer and mobile technology interventions to promote medication adherence and disease management in people with thalassemia
Abstract
Background
Thalassemia syndromes are inherited hemoglobin disorders that result when the synthesis of normal hemoglobin is lacking or significantly reduced. For people with thalassemia, long‐term red blood cell transfusion remains the mainstay of therapy, which may lead to iron overload causing severe complications and damage in different body organs. Long‐term iron chelation therapy is essential for people with thalassemia to minimize the ongoing iron‐loading process. In addition, suboptimal adherence can increase adverse events associated with iron overload and result in increased morbidity, mortality, healthcare utilization and cost of care.
Objectives
To identify and assess the effects of computer and mobile technology interventions designed to facilitate medication adherence and disease management in individuals with thalassemia, including:
‐ evaluating the effects of using computer and mobile technology interventions for medication adherence and disease management on health and behavioral outcomes;
‐ identifying and assessing the effects of computer and mobile technology interventions specific to different age groups (children, adolescents and adults) and type of modality (e.g. cell phone, the Internet).
Search methods
We searched CENTRAL (the Cochrane Library), MEDLINE, Embase, CINAHL, PsycINFO, ProQuest Dissertations & Theses Global, Psychology and Behavioral Sciences Collection, Web of Science Science & Social Sciences Conference Proceedings Indexes, IEEE Xplore and ongoing trial databases (22 February 2018). We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group’s Haemoglobinopathies Trials Register (20 June 2019). We also searched for unpublished work in the abstract book of nine major conferences in the related field.
Selection criteria
Randomized controlled trials (RCT) and quasi‐RCTs comparing single‐ or multi‐component interventions versus no intervention, placebo or standard care, with adherence to iron chelation as the primary outcome were eligible for inclusion. Non‐randomized studies of interventions, controlled before‐after studies, and interrupted‐time‐series studies were also eligible for inclusion.
Data collection and analysis
Three authors independently assessed study eligibility. If we had included any studies, we would have independently assessed risk of bias and extracted data; we planned to assess the quality of the evidence using GRADE.
Main results
We did not identify any eligible studies for inclusion in the review.
Authors' conclusions
Due to lack of evidence, we cannot comment on the efficacy or effectiveness of computer and mobile technology intervention strategies to promote disease management and adherence to iron chelation therapy in people with thalassemia.
We concluded that RCTs are needed to examine a variety of computer and mobile technology intervention strategies that may be useful for promoting disease management and increasing adherence to iron chelation therapy in individuals with thalassemia.
PICO
Plain language summary
Strategies to improve disease management and increase adherence to iron chelation therapy in people with thalassaemia
Review question
We wanted to see if there were any interventions using computer and mobile technology (e.g. cell phone, the Internet) that would help people manage their thalassemia better and adhere more to their iron chelation therapy.
Background
People with thalassaemia, who receive regular transfusions, are exposed to excess iron in their body. This can result in medical complications, including organ damage and death. Some medications are used to remove excess iron in the body, but these treatment schedules can be difficult to follow and have undesirable side effects.
Search date
The evidence is current to 20 June 2019.
Study characteristics
We searched the literature for both randomized and non‐randomized studies, and found none which were eligible for inclusion.
Key results
We found no eligible studies of computer and mobile technology intervention strategies for individuals with thalassemia.
Quality of evidence
We did not identify any evidence for inclusion in the review.
Authors' conclusions
Background
Description of the condition
Thalassemia syndromes are inherited hemoglobin disorders that result when the synthesis of normal hemoglobin is lacking or significantly reduced (Martin 2013). Approximately 5% of the world's populations are estimated to carry one variant globin allele with over 300,000 newborns affected with the disease (i.e. thalassemia syndrome) every year (Weatherall 2012).
Thalassemia syndromes include either alpha (α) thalassemia or beta (β) thalassemia. α‐thalassemia is caused by either absent or decreased production of α‐globin chains and its clinical severity varies based on the number of alleles affected as well as the type of genetic mutation (Higgs 2010). β‐thalassemia is caused by either absent or decreased production of β‐globin chains and its clinical presentation can be as early as the first six months of life with moderate to severe anemia, or in early childhood with symptoms such as anemia, jaundice, abdominal distention, hepatosplenomegaly and poor growth (Rund 2005). People with transfusion‐dependent thalassemia (TDT) require blood transfusions on a regular basis, on average 8 to 12 times or more per year. In contrast, those with non‐transfusion‐dependent thalassemia (NTDT) can maintain an adequate hemoglobin level and require packed red blood cell (RBC) transfusions only in times of physiologic stress, or fewer than eight times per year (Martin 2013; Rund 2005). For people with thalassemia, stem cell transplantation is the only curative treatment option (Angelucci 2010) and long‐term RBC transfusion remains the mainstay of therapy, which may lead to iron overload causing severe complications and damage in different body organs (Martin 2013).
Long‐term iron chelation is essential for people with thalassemia to minimize the ongoing iron loading process (Rachmilewitz 2011; Ware 2013). Three iron‐chelating agents, deferoxamine, deferiprone, and deferasirox, are approved by the USA's Food & Drug Administration (FDA) and are commercially available. Routine monitoring may vary with different iron chelators and as a minimum should include serum ferritin levels (every three months) and measurements of cardiac and liver iron burden with annual magnetic resonance imaging (MRI) scans (Badawy 2016a; Martin 2013). Recent studies have shown the need for iron chelation therapies has an impact on the quality of life of people with thalassemia and results in low levels of personal satisfaction (Abetz 2010; Cappellini 2007; Payne 2008; Porter 2012; Taher 2010; Trachtenberg 2012a; Trachtenberg 2014). In addition, suboptimal adherence can increase adverse events associated with iron overload and result in increased morbidity, mortality, healthcare utilization and cost of care (DiMatteo 2002; Sabate 2003; Vekeman 2016).
Description of the intervention
Mobile technology interventions for promoting medication adherence and disease management include delivery of education, reminders, or behavioral skills through cell (mobile) phones (e.g. text‐messaging and mobile applications), the Internet (e.g. web‐based interventions), or other mobile technology tools. Mobile technology interventions could:
-
enhance the communication between patients and healthcare providers,
-
facilitate management and self‐monitoring of thalassemia;
-
provide education about thalassemia, iron chelators and other related medications;
-
support adherence to iron chelators or medications using reminders;
-
offer a network for communication among patients with thalassemia;
-
support decision making for people with thalassemia and their parents or caregivers; or
-
collect or capture users' data (from the individuals concerned and their parents or caregivers).
We conducted a brief scoping review of the literature in PubMed and found two published studies that used mobile‐based interventions to improve adherence to iron chelation in people with thalassemia (Leonard 2017; Ward 2016). The first study included 35 people with thalassemia (aged 18 to 34 years), who were part of a Delphi process to inform the development of a mobile app to improve disease self‐management, including adherence to chelation therapy (Ward 2016). The research team was able to develop and test the mobile app with participants who perceived it as highly favorable with improved adherence to iron chelation and positive experience using it, especially the adherence pledge functionality and customized treatment goals (Ward 2016). The second study included 11 people (β‐thalassemia major and sickle cell anemia) receiving chronic blood transfusions and evaluated a mobile app intervention as part of an intensive training program to improve disease self‐management, including adherence to iron chelation therapy (Leonard 2017). The authors reported on the feasibility of the mobile app intervention and that there was high acceptability and improved disease knowledge, as well as adherence to iron chelation therapy (using medication possession ration with pharmacy records and laboratory markers of adherence) with serum ferritin levels trending downwards (Leonard 2017).
How the intervention might work
Mobile technology interventions for promoting medication adherence and disease management could:
-
enhance an individual's self‐efficacy, organizational skills, or change adherence behavior (e.g. reminders for daily iron chelators, clinic appointment reminders, transfusion reminders, feedback on adherence to iron chelation therapy);
-
provide a form of communication (e.g. health professionals);
-
establish social support networks (e.g. advocacy groups, peer‐to‐peer networks); or
-
provide education about thalassemia, iron chelation therapy and necessary steps for optimal disease management.
By increasing self‐efficacy (Bandura 1977) and providing support mechanisms (Christakis 2004; Cobb 1976; Cohen 1985), computer and mobile technology interventions may influence health behaviors and enhance self‐management of long‐term illnesses. These interventions may facilitate education on self‐management problem solving skills, and in this way increase the individual's confidence in carrying out the behaviors necessary to achieve the desired goal of optimum disease management. A recently developed taxonomy of behavior change techniques (BCT) has been published with 93 consensually agreed, distinct BCTs, which will help to further define specific intervention effects, including mobile technology interventions (Michie 2013).
Why it is important to do this review
Management of thalassemia represents a challenge for people with the disease and their families (Badawy 2016a; Evangeli 2010). Recent reports demonstrated a high prevalence of poor self‐management and non‐adherence in thalassemia in both children and adults (Cappellini 2007; Evangeli 2010; Haghpanah 2014; Lee 2011; Porter 2012; Porter 2011; Trachtenberg 2011; Vekeman 2016) similar to other chronic health conditions (Loiselle 2016; Modi 2012; Walsh 2014). Nevertheless, effective self‐management is essential to maximize treatment efficacy, optimize clinical outcomes, and reduce unnecessary health care utilization and costs (Modi 2012). However, people with thalassemia and their families are responsible for managing complex and time‐consuming treatment regimens, including daily medications (e.g. iron chelators, vitamins, hormonal supplements), laboratory monitoring (e.g. serum ferritin, hemoglobin concentration), imaging studies (e.g. MRI of liver R2*, MRI of heart T2*), and attendance at regular transfusions as well as routine clinic appointments with a variety of healthcare professionals (Badawy 2016a; Rachmilewitz 2011; Rund 2005). These treatment regimens could place substantial burden on individuals with thalassemia and their families, and effective management of these regimens represents a challenge to them. Moreover, in relation to industrialised countries, given that thalassemia predominantly affects individuals who are of ethnic minorities (Rund 2005; Weatherall 2012), disparities in access to care and resources may also serve as further barriers to effective self‐management.
People with thalassemia and their families have adopted computer and mobile technology such as standard cell phones, smartphones, the Internet, and social networking at a rapid rate across levels of social position and status (Badawy 2016b; Lenhart 2015; Shah 2014; Smith 2015a; Ward 2016). Therefore, the widespread availability and frequent use of these technologies by those with thalassemia and their families across age groups present an opportunity to develop high‐quality, efficacious, and cost‐effective interventions to facilitate self‐management, promote medication adherence, link people with thalassemia with their physicians, and improve health outcomes (Badawy 2016b; Leonard 2017; Shah 2014; Ward 2016). There has been growing interest and evidence to support the utilization of technology‐based strategies, including mobile technology interventions, to improve medication adherence and self‐management in people with chronic health conditions (Badawy 2017a; Badawy 2017b; Badawy 2017c; Badawy 2018; Bonoto 2017; de Jongh 2012; Gurol‐Urganci 2013; Lin 2014; Liu 2014; Pfaeffli 2016; Smith 2015b; Thakkar 2016; Whitehead 2016) including sickle cell disease (Crosby 2017; Jonassaint 2015) and thalassemia (Leonard 2017; Ward 2016), though the cost‐effectiveness of such interventions warrants further investigation (Badawy 2016c). To date, the evidence for the use of computer and mobile technology interventions in people with thalassemia remains unclear. Although this systematic review may not yet identify any eligible studies, it is important to identify and report this knowledge gap in the field of thalassemia to highlight where future research efforts can be directed using computer and mobile technology interventions.
Objectives
To identify and assess the effects of computer and mobile technology interventions designed to facilitate medication adherence and disease management in individuals with thalassemia, including:
-
evaluating the effects of using computer and mobile technology interventions for medication adherence and disease management on health and behavioral outcomes;
-
identifying and assessing the effects of computer and mobile technology interventions specific to different age groups (children, adolescents and adults) and type of modality (e.g. cell phone, the Internet).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCT) and quasi‐RCTs comparing single‐ or multi‐component interventions versus no intervention, placebo or standard care. We also planned to include non‐randomized studies of interventions (NRSI): controlled before‐after (CBA) studies, and interrupted‐time‐series (ITS) studies. We used the Cochrane Effective Practice and Organization of Care (EPOC) Group’s definition of study designs to consider studies for inclusion (EPOC 2017)*.
Given the growing evidence in the field and the possible paucity of research studies, in particular RCTs, evaluating technology interventions in this population, we wanted to broaden our eligibility criteria to be able to report the up‐to‐date current status of the field and identify gaps. Therefore, we planned to include NRSI in this review.
We planned to include cluster‐RCTs, non‐randomized cluster studies and CBA studies if they have at least two intervention sites and two control sites. However, we planned to exclude such studies with only one intervention or control site since the intervention (or comparison) could be confounded by the study site, thus making it difficult to attribute any observed differences to the intervention rather than to other site‐specific variables (EPOC 2017).
We also planned to include ITS and repeated‐measures studies which have a clearly defined point in time when the intervention occurred and at least three data points before and at least three data points after the intervention. We planned to exclude ITS studies that do not have a clearly defined point in time when the intervention occurred, fewer than three data points before and after the intervention, or if the ITS study has ignored secular (trend) changes, performed a simple t‐test of the pre‐ versus post‐intervention periods and re‐analysis of the data is not possible (EPOC 2017).
We planned to evaluate for the possible bias introduced by confounding in the different types of included studies, and we will adjust for confounder variables. Possible confounders include age and education of the participant, self‐management skills, personal behavior or personality traits, health literacy, parental involvement in medical care, parental education, household income, insurance status, access to healthcare services, type of thalassemia (TDT versus NTDT), associated co‐morbidities or complications (or both) affecting clinical course, and iron chelation regimen.
We planned to exclude studies with a cross‐over design since any intervention focused on improving disease knowledge will leave that knowledge with a residual 'carry‐over' effect.
* Please refer to 'Differences between protocol and review'.
Types of participants
People with TDT or NTDT of all ages (children, adolescents and adults) or their parents or caregivers.
Types of interventions
Interventions delivered via cell phones (including smart phones), the Internet or other technology or mobile devices compared to other technology interventions or no interventions or standard of care or placebo.
We planned to include remote and Web 2.0‐based interventions delivered via technologies that give people with thalassemia access to e‐health information to promote medication adherence and disease management. These e‐health technologies included personal computers (PCs) and applications (apps) for mobile technology such as iOS tablets (iPads), Android tablets and smartphones.
We planned to include e‐health or technology‐based interventions that are focused on people with thalassemia and parents or caregivers to promote individual medication adherence and disease management and are self‐administered or user‐centred (where the intervention design process focuses on user needs).
We also planned to include as possible comparison groups (whenever reported): face‐to‐face support; educational material (either as hard copy or digital documents); or self‐management tools (either as hard copy or digital documents).
We planned to exclude studies that focus on monitoring devices, telemonitoring or telemedicine or other technologies that involve the participation of healthcare professionals.
Types of outcome measures
Primary outcomes
-
Medication adherence
-
directly observed adherence rates with individuals recording their daily iron chelator use on their cell phones
-
radiological markers of iron overload (MRI of liver R2* and MRI of heart T2*)
-
laboratory markers of iron overload (serum ferritin levels)
-
iron chelation adherence rates by pharmacy records (i.e. medication possession ratio)
-
self‐reported iron chelator adherence rate
-
Secondary outcomes
-
Knowledge about thalassemia
-
Health outcomes (e.g. health‐related quality of life)
-
User evaluation of the mobile technology interventions, including acceptability and satisfaction
-
Adverse events (e.g. issues of privacy and disclosure, or failure or delay in the intervention delivery)
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
We searched the Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: (thalassaemia OR (haemoglobinopathies AND general)) AND (mobile technology).
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Haemoglobinopathies Trials Register: 20 June 2019.
In addition to the above, we conducted a search of the following databases:·
-
CENTRAL, Other Reviews (DARE) and Technology Assessments (HTA) Databases (the Cochrane Library) (22 February 2018) (www.cochranelibrary.com/);
-
PubMed (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, for recent records not yet added to MEDLINE) (22 February 2018) (www.ncbi.nlm.nih.gov/sites/entrez);
-
MEDLINE (OvidSP, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE, 1946 to 22 February 2018);
-
Embase (OvidSP, 1974 to 22 February 2018);
-
CINAHL (EBSCOHost, 1937 to 22 February 2018);
-
PsycInfo (EBSCOHost, 1900 to 22 February 2018);
-
ProQuest Dissertations & Theses Global (ProQuest, 1861 to 22 February 2018);
-
Web of Science & Social Sciences Conference Proceedings Indexes (CPSI‐S & CPSSI, 1990 to 22 February 2018);
-
Institute of Electrical and Electronics Engineers Explore (IEEE Xplore, 1963 to 22 February 2018).
Additionally, we searched the following trial databases for trials (22 February 2018):
-
ISRCTN registry (www.isrctn.com/);
-
ClinicalTrials.gov (www.ClinicalTrials.gov);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/).
See Appendix 1 for the full search strategies.
Searching other resources
We aimed to further identify unpublished work by searching the abstract books of the International Society for Research on Internet interventions (ISRII); the NIH Wireless Health Conference; the Society for Behavioral Medicine (SBM) Annual Meeting; and the Connected Health Conference over the past 10 years (2007 to 2018).
Reference lists
The reference lists of all included articles and relevant systematic reviews were reviewed to identify any additional studies. We planned to contact the lead authors of the included studies to identify any unpublished material, missing data or information regarding ongoing relevant studies.
Data collection and analysis
Selection of studies
We selected studies according to chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Two review authors (SB and KM) independently screened all electronically derived citations and abstracts of papers identified by the search strategy for relevance. We excluded all articles that are clearly irrelevant at this stage based on the title and the abstract. Two review authors (SB and KM) independently assessed the full texts of all potentially relevant studies for eligibility against criteria outlined above. If a consensus could not be reached, we resolved any disagreements by discussion and consultation with a third review author (TP). We planned to seek further information from study authors if studies or abstracts contain insufficient data to make a decision about eligibility. We designed a study eligibility form which included ascertaining whether the participants have thalassaemia, if the study addressed mobile technology interventions to improve disease management, and whether the study was randomized or a NRSI or a CBA or an ITS study. We also recorded the reasons why potentially relevant studies failed to meet the eligibility criteria.
Data extraction and management
Two review authors (SB and KM) planned to conduct data extraction according to Cochrane guidelines (Higgins 2011a) and according to the criteria developed for NRSI as recommended in chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011). We aimed to resolve any disagreements by consensus. We planned to pilot data extraction forms for RCTs, NRSI or CBAs or ITS studies; thereafter, data extraction is planned to be conducted by two authors (SB and KM) independently for all the studies using templates modified to reflect the outcomes in this review. In addition, we planned to use the available tables in Review Manager 5 (RevMan 2014) to extract data on study characteristics as below.
General information
Review author’s name, date of data extraction, study ID, first author of study, author’s contact address (if available), citation of paper, and objectives of the study.
Study details
Study design, location, setting, sample size, power calculation, treatment allocation, inclusion and exclusion criteria, reasons for exclusion, comparability of groups, length of follow‐up, stratification, stopping rules described, statistical analysis, results, conclusion, and funding.
Characteristics of participants
Age, gender, total number recruited, total number randomized, total number analyzed, types of underlying disease, loss to follow‐up numbers, dropouts (percentage in each arm) with reasons, protocol violations (if any), and co‐morbidities.
Interventions
Details of the mobile technology interventions including purpose (e.g. adherence promotion), modality used (cell phone, the Internet or other technology), length of intervention, how the intervention is being delivered (i.e. electronically only or electronically in addition to group, face‐to‐face, written information) and by whom (i.e. clinicians, researchers, patients or parents) and where the intervention is being delivered (i.e. hospital, clinic, home or other settings). We planned to analyse data from different modes of interventions separately (e.g. cell phone versus standard care, Internet versus standard care, etc.).
Outcomes measured
Data on measures of medication adherence, including iron chelation adherence rates by self‐report or pharmacy records (or both) (i.e. medication possession ratio), laboratory markers of iron overload (serum ferritin level), radiological markers of iron overload (MRI of liver R2* and MRI of heart T2*), and other reported adherence indicators; also data on knowledge about thalassemia and health outcomes (e.g. health‐related quality of life).
Data on user perception of acceptability and satisfaction (e.g. the Likert ratings of satisfaction). Adverse events if reported and whether or not they were associated with interventions.
For NRSI, CBA or ITS studies, data on: confounding factors; the comparability of groups on confounding factors; methods used to control for confounding and on multiple effect estimates (both unadjusted and adjusted estimates) as recommended in chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011). We used both full‐text versions and abstracts as data sources and we used one data extraction form for each unique study. Where sources did not provide sufficient information, we planned to contact authors and study groups for additional details. We planned for one review author to enter data and for a second to check these data for accuracy.
Assessment of risk of bias in included studies
In future, two review authors (SB and KM) will assess included studies for possible risks of bias as described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011c). In our assessment, we will include information about the design, the conduct and the analysis of the study.
RCTs
We planned to assess each criterion using Cochrane's RoB (V2.0) tool for assessing the risk of bias for RCTs (classed as 'low risk', 'high risk' or 'some concerns' for bias) in the following areas (Higgins 2011c).
Bias arising from the randomization process
-
Was the allocation sequence random?
-
Was the allocation sequence concealed until participants were recruited and assigned to interventions?
-
Were there baseline imbalances that suggest a problem with the randomization process?
-
What is the predicted direction of bias arising from the randomization process?
Bias due to deviations from intended interventions
-
Were participants aware of their assigned intervention during the RCT?
-
Were carers and trial personnel aware of participants' assigned intervention during the RCT?
-
Were there deviations from the intended intervention beyond what would be expected in usual practice?
-
Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome?
-
Were any participants analysed in a group different from the one to which they were assigned?
-
Was there potential for a substantial impact (on the estimated effect of intervention) of analysing participants in the wrong group?
-
What is the predicted direction of bias due to deviations from intended interventions?
Bias due to missing outcome data
-
Were outcome data available for all, or nearly all, participants randomized?
-
Are the proportions of missing outcome data and reasons for missing outcome data similar across intervention groups?
-
Is there evidence that results were robust to the presence of missing outcome data?
-
What is the predicted direction of bias due to missing outcome data?
Bias in measurement of the outcome
-
Were outcome assessors aware of the intervention received by participants?
-
Was the assessment of the outcome likely to be influenced by knowledge of intervention received?
-
What is the predicted direction of bias due to measurement of the outcome?
Bias in selection of the reported result(s)
-
Are the reported outcome data likely to have been selected, on the basis of the results, from multiple outcome measurements (e.g. scales, definitions, time points) within the outcome domain? Or from multiple analyses of the data?
-
What is the predicted direction of bias due to selection of the reported result?
Overall bias
An overview of the overall bias identified.
NRSI studies
We planned to use the ROBINS‐I tool (Risk Of Bias In Non‐randomized Studies of Interventions) to rate the quality of NRSI or CBA studies (Sterne 2016). The ROBINS‐I tool uses signalling questions and covers seven domains (listed below) where the quality of evidence is rated as 'low', 'moderate', 'serious', 'critical' or 'no information'.
Bias due to confounding
-
Is there potential for confounding of the effect of intervention in this study?
-
Was the analysis based on splitting participants’ follow‐up time according to intervention received?
-
Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome?
-
Did the authors use an appropriate analysis method that controlled for all the important confounding domains?
-
Were confounding domains that were controlled for measured validly and reliably by the variables available in this study?
-
Did the authors control for any post‐intervention variables that could have been affected by the intervention?
-
Did the authors use an appropriate analysis method that adjusted for all the important confounding domains and for time varying confounding?
-
Were confounding domains that were adjusted for measured validly and reliably by the variables available in this study?
Bias in the selection of participants into the study
-
Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention?
-
Were the post‐intervention variables that influenced selection likely?
-
Were the post‐intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome?
-
Do start of follow‐up and start of intervention coincide for most participants?
Bias in classification of interventions
-
Were intervention groups clearly defined?
-
Was the information used to define intervention groups recorded at the start of the intervention?
-
Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome?
-
What is the predicted direction of bias due to measurement of outcomes or interventions?
Bias due to deviation from intended interventions
-
Were there deviations from the intended intervention beyond what would be expected in usual practice?
-
Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome?
-
Were important co‐interventions balanced across intervention groups?
-
Was the intervention implemented successfully for most participants?
-
Did study participants adhere to the assigned intervention regimen?
-
Was an appropriate analysis used to estimate the effect of starting and adhering to the intervention?
-
What is the predicted direction of bias due to deviations from the intended interventions?
Bias due to missing data
-
Were outcome data available for all, or nearly all, participants?
-
Were participants excluded due to missing data on intervention status?
-
Were participants excluded due to missing data on other variables needed for the analysis?
-
Are the proportion of participants and reasons for missing data similar across interventions?
-
Is there evidence that results were robust to the presence of missing data?
-
What is the predicted direction of bias due to missing data?
Bias in measurement of outcomes
-
Could the outcome measure have been influenced by knowledge of the intervention received?
-
Were outcome assessors aware of the intervention received by study participants?
-
Were the methods of outcome assessment comparable across intervention groups?
-
Were any systematic errors in measurement of the outcome related to intervention received?
-
What is the predicted direction of bias due to measurement of outcomes?
Bias in the selection of the reported result
-
Is the reported effect estimate likely to be selected, on the basis of the results, from multiple outcome measurements within the outcome domain? Or from multiple analyses of the intervention outcome relationship? Or from different subgroups?
-
What is the predicted direction of bias due to selection of the reported result?
ITS studies
For ITS studies, we planned to use the risk of bias criteria below as suggested for EPOC reviews (EPOC 2017) as follows.
-
Was the intervention independent of other changes?
-
Was the shape of the intervention effect pre‐specified?
-
Was the intervention unlikely to affect data collection?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Were incomplete outcome data adequately addressed?
-
Was the study free from selective outcome reporting?
-
Was the study free from other risks of bias?
We planned to resolve disagreements on the assessment of quality of an included study by discussion until consensus is reached or by consultation with a third review author (TP).
Measures of treatment effect
RCTs
We planned to report the following continuous primary endpoints of medication adherence, including iron chelation adherence rates by self‐report or pharmacy records (i.e. medication possession ratio), laboratory markers of iron overload with serum ferritin level, radiological markers of iron overload (MRI of liver R2* and MRI of heart T2*), and other reported adherence indicators. We also planned to report the following continuous secondary endpoints (user evaluation of acceptability and satisfaction).
For continuous endpoints, we planned to extract and report the absolute change from baseline using statistical analysis, adjusting for baseline differences, in both the treatment and control groups. We planned to record the mean, standard deviation (SD) and total number of participants in both the treatment and control groups. For those using the same scale or outcome, we planned to perform analyses using the mean difference (MD) with 95% confidence intervals (CIs). For those reported using different scales or outcomes, we planned to use the standardized mean difference (SMD). We planned to extract and report the relative change adjusted for baseline differences in the outcome measures (i.e. the absolute post‐intervention difference between the intervention and control groups, as well as the absolute pre‐intervention difference between the intervention and control groups divided by the post‐intervention level in the control group) (EPOC 2017).
For binary outcomes we planned to record the number of events and the total number of participants in both the treatment and control groups and report the pooled risk ratio (RR) with a 95% CI (Deeks 2011). Where the number of observed events was small (less than 5% of sample per group), and where studies have balanced treatment groups, we planned to report the Peto odds ratio (OR) with 95% CI (Deeks 2011).
For cluster‐RCTs, we planned to extract and report direct estimates of the effect measure (e.g. RR with a 95% CI) from an analysis that accounts for the clustered design. We planned to obtain statistical advice to ensure the analysis is appropriate. If appropriate analyses are not available, we planned to make every effort to approximate the analysis with necessary adjustment using effective sample size recalculation, following the recommendations in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d).
We planned to undertake quantitative assessments using the Review Manager (RevMan) software (RevMan 2014), if data allowed.
NRSI studies
For continuous variables in any included studies, if available, we planned to extract and report the absolute change from baseline using statistical analysis, adjusting for baseline differences (such as regression models, mixed models or hierarchical models). An adjusted change of outcomes was required in NRSI and confounding was considered and evaluated in the included articles. If available, we planned to extract and report the relative change adjusted for baseline differences in the outcome measures (i.e. the absolute post‐intervention difference between the intervention and control groups, as well as the absolute pre‐intervention difference between the intervention and control groups divided by the post‐intervention level in the control group) (EPOC 2017).
For binary outcomes, if available we planned to extract and report the RR with a 95%CI from statistical analyses, adjusting for baseline differences (such as Poisson regressions or logistic regressions) or the ratio of RRs (i.e. the RR post intervention / risk ratio pre intervention).
ITS studies
For ITS studies that fulfil the criteria of analysis, and from which relevant information can be extracted, we planned to standardize data by dividing the level (or time slope) and standard error (SE) by the SD of the pre‐intervention slope, in order to obtain the effect sizes. Where appropriate, we planned to report the number‐needed‐to‐treat‐to‐benefit (NNTB) and the number‐needed‐to‐treat‐to‐harm (NNTH) with 95% CIs. If we were not able to report the available data in any of the formats described above, we planned to produce a narrative report, and if appropriate, we planned to present the data in tables.
Unit of analysis issues
We planned to identify issues related to unit of analysis, given the inclusion of cluster‐RCTs or non‐randomized studies, and multiple observations for the same outcome. Therefore, if any of these study designs included in our review, we planned to treat these in accordance with the advice given in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d). For cluster designs, we planned to extract results adjusted for clustering, and if analyses have not been adjusted for clustering, we planned to re‐analyze the data taking clustering and and individual participant data (IPD) into account, if possible; if not possible, we planned to present data in a table. If participants were randomized more than once, we planned to contact the authors of the study to provide us with data on outcomes associated with the initial randomization. For studies with multiple treatment groups we planned to include subgroups that were considered relevant to the analysis. When appropriate, we planned to combine groups to create a single pair‐wise comparison. If not possible, we planned to select the most appropriate pair of interventions and exclude the others (Higgins 2011d). We planned to deal with any unit of analysis issues arising from the inclusion of ITS studies according to the EPOC recommendations (EPOC 2017). We planned to analyze ITS studies as a statistical comparison of time trends before and after the intervention. In time‐series analysis, there are a number of statistical techniques that could be used depending on the characteristics of the data, the number of data points available and whether autocorrelation was present (e.g. auto‐regressive integrated moving average 'ARIMA' model).
Dealing with missing data
If we identified incidences where we suspected data were missing or unclear, we planned to contact study authors directly. We planned to record the number of participants lost to follow‐up for each study. Where possible, we planned to analyze data on an intention‐to‐treat (ITT) basis, but if insufficient data had been available, we planned to present per protocol analyses (Higgins 2011c).
Assessment of heterogeneity
If the clinical and methodological characteristics of individual studies are sufficiently homogeneous, we planned to combine the data to perform a meta‐analysis. We planned to analyze the data from RCTs, NRSI, CBA and ITS studies separately. We planned to assess the statistical heterogeneity of treatment effects between studies using a Chi² test with a significance level at P < 0.1. We planned to use the I² statistic to quantify the degree of potential heterogeneity and classify it as moderate if I² is greater than 50%, or considerable if I² is greater than 75%. If statistical heterogeneity is moderate within the studies selected for inclusion; in such cases, we planned to use the random‐effects model. If statistical heterogeneity is considerable within the studies selected for inclusion, we planned not to report the overall summary statistic. When possible, we planned to assess the potential causes of heterogeneity by sensitivity and subgroup analyses (Deeks 2011).
Assessment of reporting biases
Where we identified at least 10 studies for inclusion in a meta‐analysis, we planned to explore potential publication bias with a funnel plot and using a linear regression test. We planned to consider a P value of less than 0.1 as significant for this test (Sterne 2011).
Data synthesis
If studies were sufficiently homogenous in their study design, we planned to conduct a meta‐analysis according to Cochrane recommendations (Deeks 2011). We planned to analyze RCTs and non‐RCTs separately using the random‐effects model as the true effects are expected to be related but not the same for included studies. If we cannot perform a meta‐analysis, we planned to include a narrative describing the results and the results from all studies presented in the tables.
For RCTs, where meta‐analysis was feasible, we planned to use the inverse variance method for continuous outcomes, or outcomes that included data from cluster‐RCTs. If we found heterogeneity to be above 75%, and we identified a possible cause for the heterogeneity, we planned to investigate these with subgroup analyses. If a cause for the heterogeneity cannot be found, then we planned not to perform a meta‐analysis.
If meta‐analysis was feasible for NRSI or CBA studies, we planned to analyze these separately. We planned only to analyze outcomes with adjusted effect estimates, if these are adjusted for the same factors using the inverse variance method as recommended in chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011).
If meta‐analysis was feasible for ITS studies, we planned to use the effect sizes (if reported in the included studies or obtained, as described earlier) and we planned to pool these using the generic inverse variance method in RevMan (RevMan 2014).
We planned to report data for our listed outcomes at the following time points: pre‐intervention; immediate post‐intervention; and at first follow‐up (up to 12 months).
Subgroup analysis and investigation of heterogeneity
If adequate data were available, we planned to perform subgroup analyses according to Cochrane recommendations (Deeks 2011) for each of the following criteria, and separately for the different study design types included in the review in order to assess the effect on heterogeneity. We planned to base the subgroup analyses on:
-
delivery mode, phone‐based versus Internet‐based interventions;
-
age groups, children (8 to 11 years old) (parent‐focused) versus adolescents (12 to 17 years old) versus adults (18 years and above);
-
transfusion‐dependent versus non‐transfusion‐dependent thalassemia; and
-
iron‐chelation regimen, deferasirox versus deferoxamine versus deferiprone.
Sensitivity analysis
We planned to assess the robustness of the review findings by performing the following sensitivity analyses according to Cochrane recommendations where appropriate (Deeks 2011):
-
including only those studies with a 'low' risk of bias (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation);
-
including only those studies with less than a 20% dropout rate.
Summary of findings table
We plan to use the GRADE approach to generate a 'Summary of Findings' table as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a). We will rate the quality of the evidence as 'high', 'moderate', 'low', or 'very low' using the five GRADE considerations.
-
Risk of bias (serious or very serious)
-
Inconsistency (serious or very serious)
-
Indirectness (serious or very serious)
-
Imprecision (serious or very serious)
-
Publication bias (likely or very likely)
For non‐RCTs or CBA or ITS studies, we will also consider the following factors:
-
dose response (yes or no);
-
size of effect (large or very large);
-
confounding either reduces the demonstrated effect or increases the effect if no effect was observed (yes or no).
When using GRADE, we planned to initially rate NRSI or CBA or ITS studies as low quality and upgrade according to GRADE guidelines if appropriate. We planned to present the outcomes for these studies in separate tables from outcomes for the results of RCTs. We planned to report the following outcomes in each 'Summary of findings' table:
-
directly observed adherence rates with individuals recording their daily iron chelator use on their cell phones;
-
radiological markers of iron overload (MRI of liver R2* and MRI of heart T2*);
-
laboratory markers of iron overload (serum ferritin levels);
-
iron chelation adherence rates by pharmacy records (i.e. medication possession ratio);
-
self‐reported iron chelation adherence rate;
-
disease knowledge about thalassemia; and
-
health outcomes (e.g. health‐related quality of life).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See PRISMA flow diagram (Figure 1).
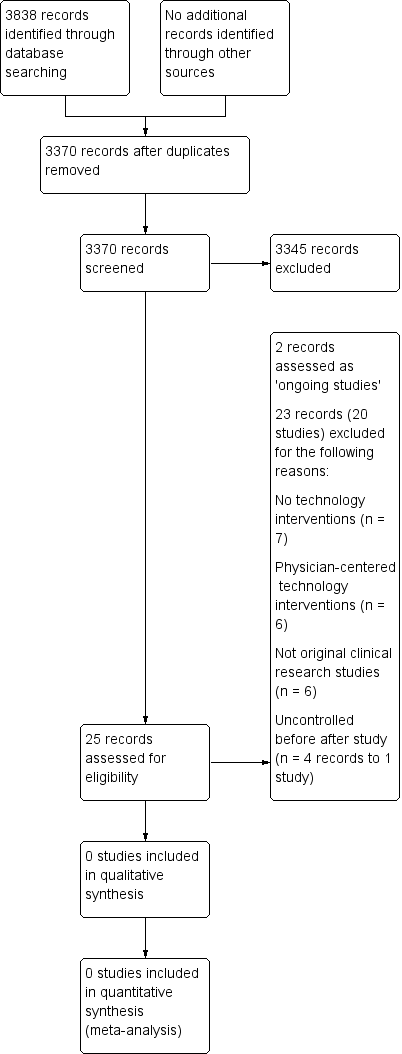
Study flow diagram.
In the searches for this review we identified a total of 3838 potentially relevant references. There were 3370 references after we removed duplicates and two review authors (SB and KM) excluded 3345 references on the basis of the title and the abstract. Two authors (SB and KM) reviewed 25 references for relevance. We did not identify any completed NRSI or any RCTs, cluster‐RCTs, CBA or ITS studies that met the inclusion criteria. We have listed two RCTs as ongoing studies and we excluded 20 studies (23 references) that did not meet our pre‐defined inclusion criteria. We resolved disagreements by discussion and consultation with a third senior review author (TP).
Included studies
No studies were identified for inclusion in the review.
Excluded studies
We excluded 20 studies after full‐text screening for the following reasons. Six studies did not use technology interventions (Abu Samra 2015; Dehkordi 2008; Gambari 2007; Olivieri 1991; Paholpak 2006; Trachtenberg 2012b). Six studies used physician‐centered technology interventions, defined as interventions developed for physicians or other healthcare providers to enhance their communications with patients and families or documentation of their medical evaluation and management, such as electronic health records (Antoniou 2015; Arbaiy 2017; Deftereos 2001; Lederer 2009; Piga 2013; Santini 2017). Six studies were not original clinical research studies (Bal 2014; Bobati 2016; Jonassaint 2012; Nawaz 2012; Paokanta 2014; Yamashita 1998). Two studies were an inappropriate design, uncontrolled before after pilot study (Leonard 2017) and intervention development and usability pilot study (Ward 2016).
Ongoing studies
We identified two ongoing RCTs that we plan to assess in a future update (IRCT20170826035907N1; IRCT20180207038655N1).
Risk of bias in included studies
No studies were identified for inclusion in the review.
Effects of interventions
No studies were identified for inclusion in the review.
Discussion
For people with thalassemia, long‐term RBC transfusion remains the mainstay of therapy. This may lead to iron overload causing severe complications and damage in different body organs, with the heart, liver and endocrine glands being particularly vulnerable. Using data from six state Medicaid programs (1997 to 2013), a recent research study has shown that adherence to iron chelation ranges from 20% to 66% in people with thalassemia, which varies based on the type of iron chelator (Vekeman 2016). Similar data were recently reported in two systematic reviews for iron chelation adherence rates in people with sickle cell disease, 22% to 89% (Loiselle 2016) and 16% to 89% (Walsh 2014).
We identified two published studies that used mobile‐based interventions to improve adherence to iron chelation in people with thalassemia (Leonard 2017; Ward 2016). The Ward study included 35 people with thalassemia (aged 18 to 34 years), who were part of a Delphi process to inform the development of a mobile app to improve disease self‐management, including adherence to chelation therapy (Ward 2016). The research team was able to develop and test the mobile app with participants who perceived it as highly favorable with improved adherence to iron chelation and positive experience using it, especially the adherence pledge functionality and customized treatment goals (Ward 2016). The Leonard study included 11 people (β‐thalassemia major and sickle cell anemia) receiving chronic blood transfusions and evaluated a mobile app intervention as part of an intensive training program to improve disease self‐management, including adherence to iron chelation (Leonard 2017). The authors reported on the feasibility of the mobile app intervention and that there was high acceptability and improved disease knowledge, as well as adherence to iron chelation (using medication possession ratio with pharmacy records and laboratory markers of adherence) with serum ferritin levels trending downwards (Leonard 2017). However, these two studies were not included in this review because of inappropriate design, uncontrolled before after pilot study (Leonard 2017) and intervention development and usability pilot study (Ward 2016).
Summary of main results
We found no evidence in the form of RCTs, cluster‐RCTs, CBA or ITS studies that met the inclusion criteria. We identified two ongoing RCTs that will be assessed further in a future update of this review.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of the evidence for computer and mobile technology interventions designed to promote adherence to iron chelation therapy and disease management in people with thalassemia. The results of this review should be interpreted with caution and only in consideration of the following factors.
-
We did not identify any RCTs, cluster‐RCTs, CBA or ITS studies that met the inclusion criteria; we identified two ongoing RCTs that may be eligible in a future update of this review.
-
Due to lack of evidence, this review cannot comment on the efficacy or effectiveness of computer and mobile technology intervention strategies to promote disease management and adherence to iron chelation therapy in people with thalassemia.
Quality of the evidence
No studies were identified for inclusion in the review.
Potential biases in the review process
To our knowledge, our review process was free from bias. We conducted a comprehensive search: searching data sources (including multiple databases, and clinical trial registries) to ensure that all relevant studies would be captured. We had no restrictions for the language in which the paper was originally published. We carefully evaluated the relevance of each potentially‐eligible paper.
Agreements and disagreements with other studies or reviews
There have been limited data from published intervention studies to address adherence to iron chelation or disease management in people with thalassemia.
A study by Bahnasawy evaluated the effect of an intervention with clinical pharmacist services, including detection of drug‐related problems and their management, education about β‐thalassemia and iron chelators, and individually‐tailored medication charts, compared to standard of care in 48 people with β‐thalassemia (Bahnasawy 2017). At six months follow‐up, the authors reported a significant improvement in adherence to iron chelator therapy, in serum ferritin as a marker of iron overload, in participants' satisfaction and in quality of life, with fewer drug‐related problems in the intervention arm compared to the control arm (Bahnasawy 2017).
Pakpaz examined the impact of providing patient education about iron overload with assessment of adherence to iron chelation therapy (subcutaneous deferoxamine injections) using the numerical Likert Scale and liver iron concentration or overload status using SQUID biosusceptometer (Pakbaz 2005). The authors showed improvement in self‐report adherence rates to iron chelation therapy as well as liver iron concentration at 15 months follow‐up (Pakbaz 2005).
Koch evaluated the effect of contingency contracting with 23 people with β‐thalassemia as an intervention to improve their adherence level to iron chelation therapy (subcutaneous deferoxamine injections) and measured adherence by counting the number of empty vials (Koch 1993). At six months follow‐up, the authors reported success of the contingency contracting program with improvement in adherence rates in majority of participants.
Treadwell examined the impact of participating in Desferal or Deferoxamine Day Camp with educational strategies and peer support as an intervention to improve self‐management skills in 15 individuals with β‐thalassemia or sickle cell disease and their caregivers (Treadwell 2001). The authors showed adherence to iron chelation at follow‐up (i.e. number of days of deferoxamine administration) was associated with perceived level of support as well as patient and caregiver disease knowledge. The authors also reported that increased disease knowledge as well as shared responsibilities for care of home between patients and their caregivers have been associated with an improvement in markers of iron overload with lower serum ferritin and liver iron concentration (Treadwell 2001).
Further, a recent Cochrane Review identified no published data on psychological therapies for people with thalassemia as interventions to improve their ability to cope and self‐manage their condition, or improve both medical and psychosocial outcomes (Anie 2014).
Another Cochrane Review showed very limited evidence to suggest indications that mobile phone messaging interventions may be beneficial in promoting self‐management in people with chronic illnesses (de Jongh 2012). Similarly, Badawy has systematically evaluated the effects of text messaging and mobile phone apps as interventions to improve adherence to preventive behavior and to chronic medication regimens in adolescents (Badawy 2017a; Badawy 2017b). The authors reported significant improvement in adherence with moderate effect sizes, although most of the included studies were of low or moderate quality (Badawy 2017a; Badawy 2017b). Furthermore, Thakkar conducted a meta‐analysis of RCTs to assess the effect of mobile phone text messaging interventions on medication adherence in chronic disease (Thakkar 2016). The authors found that these interventions increased the odds of medication adherence by approximately two‐fold, with about an 18% increase in adherence rates; however, most were of short duration and used self‐report measures of medication adherence (Thakkar 2016).

Study flow diagram.

