Protease activity as a prognostic factor for wound healing in venous leg ulcers
Abstract
Background
Venous leg ulcers (VLUs) are a common type of complex wound that have a negative impact on people's lives and incur high costs for health services and society. It has been suggested that prolonged high levels of protease activity in the later stages of the healing of chronic wounds may be associated with delayed healing. Protease modulating treatments have been developed which seek to modulate protease activity and thereby promote healing in chronic wounds.
Objectives
To determine whether protease activity is an independent prognostic factor for the healing of venous leg ulcers.
Search methods
In February 2018, we searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Ovid Embase and CINAHL.
Selection criteria
We included prospective and retrospective longitudinal studies with any follow‐up period that recruited people with VLUs and investigated whether protease activity in wound fluid was associated with future healing of VLUs. We included randomised controlled trials (RCTs) analysed as cohort studies, provided interventions were taken into account in the analysis, and case‐control studies if there were no available cohort studies. We also included prediction model studies provided they reported separately associations of individual prognostic factors (protease activity) with healing. Studies of any type of protease or combination of proteases were eligible, including proteases from bacteria, and the prognostic factor could be examined as a continuous or categorical variable; any cut‐off point was permitted. The primary outcomes were time to healing (survival analysis) and the proportion of people with ulcers completely healed; the secondary outcome was change in ulcer size/rate of wound closure. We extracted unadjusted (simple) and adjusted (multivariable) associations between the prognostic factor and healing.
Data collection and analysis
Two review authors independently assessed studies for inclusion at each stage, and undertook data extraction, assessment of risk of bias and GRADE assessment. We collected association statistics where available. No study reported adjusted analyses: instead we collected unadjusted results or calculated association measures from raw data. We calculated risk ratios when both outcome and prognostic factor were dichotomous variables. When the prognostic factor was reported as continuous data and healing outcomes were dichotomous, we either performed regression analysis or analysed the impact of healing on protease levels, analysing as the standardised mean difference. When both prognostic factor and outcome were continuous data, we reported correlation coefficients or calculated them from individual participant data.
We displayed all results on forest plots to give an overall visual representation. We planned to conduct meta‐analyses where this was appropriate, otherwise we summarised narratively.
Main results
We included 19 studies comprising 21 cohorts involving 646 participants. Only 11 studies (13 cohorts, 522 participants) had data available for analysis. Of these, five were prospective cohort studies, four were RCTs and two had a type of case‐control design. Follow‐up time ranged from four to 36 weeks. Studies covered 10 different matrix metalloproteases (MMPs) and two serine proteases (human neutrophil elastase and urokinase‐type plasminogen activators). Two studies recorded complete healing as an outcome; other studies recorded partial healing measures. There was clinical and methodological heterogeneity across studies; for example, in the definition of healing, the type of protease and its measurement, the distribution of active and bound protease species, the types of treatment and the reporting of results. Therefore, meta‐analysis was not performed. No study had conducted multivariable analyses and all included evidence was of very low certainty because of the lack of adjustment for confounders, the high risk of bias for all studies except one, imprecision around the measures of association and inconsistency in the direction of association. Collectively the research indicated complete uncertainty as to the association between protease activity and VLU healing.
Authors' conclusions
This review identified very low validity evidence regarding any association between protease activity and VLU healing and there is complete uncertainty regarding the relationship. The review offers information for both future research and systematic review methodology.
Plain language summary
Protease activity and its association with future healing of venous leg ulcers
What is the aim of this review?
The aim of this Cochrane Review was to find out if there is a link between different levels of protease in venous leg ulcers (open skin wounds on the lower leg caused by problems with the way blood flows through the veins) now and the healing of wounds at some time in the future. Protease is an enzyme, a chemical naturally produced by the body that breaks down proteins and which may affect wound healing. We wanted to know whether having higher protease levels meant that wounds were less likely to heal or to heal more slowly. If so, this could help find the most useful treatments for each person with a leg ulcer. Review authors from Cochrane collected and analysed all relevant studies to answer this question and found 19 studies.
Key messages
At the moment, there is complete uncertainty about any association between protease activity and venous leg ulcer healing, but this review did give pointers on what may be important for future research on natural chemicals present in wounds and their effect on healing.
What was studied in the review?
Venous leg ulcers can last weeks, months or years. Leg ulcers can be painful, may become infected, and may affect mobility and quality of life. The usual treatment for venous leg ulcers is compression therapy (e.g. compression (elastic) bandages), but even this does not work for everyone (about a third of people still have wounds that have not healed after six months). We wanted to find out why these wounds often do not heal, and whether there are factors in the wound (called biomarkers) that can indicate which wounds are unlikely to heal. It has been suggested that wounds are slow to heal when there are high levels of protease. In this review, we investigated whether there was any evidence that higher protease levels at the start of a study were associated with slower healing leg ulcers or less healing at a future time point (such as six months).
In February 2018, we searched for relevant studies that had a reliable design and that investigated links between protease levels and future healing of venous leg ulcers. We found 19 studies involving 646 people. Not all studies reported the age and sex of participants. In those that did, the average age of the participants varied from 51 to 75 years. Eleven studies gave results we could use, involving 13 groups of people. Most people had wounds that had been there for at least three months.
What were the main results of the review?
There were many differences among the included studies: for example, how they defined healing, the type of proteases and how they measured them, the types of treatment and how they reported results. This lack of consistency meant we could not combine and compare the results, so we summarised the findings in a general way.
A bigger problem was that none of the studies had analysed the data appropriately as they did not take into account the impact of age or infection or treatments, and so we could not be sure that it was the protease levels that were important for healing, rather than age or other factors. Most studies were small and could have been better conducted, so it was difficult to be sure how meaningful the results were. Overall, the certainty of the evidence was very low. Further studies are needed to explore the importance of biomarkers for wound healing.
How up to date is this review?
We searched for studies that had been published up to February 2018.
Authors' conclusions
Background
Description of the condition
Venous leg ulcers (VLUs) are open skin ulcers (wounds) on the lower leg (from below the ankle up to mid‐calf), that can last weeks, months or years, and are a consequence of problems in either the superficial or deep veins (or both) of the legs. Damage to the valves or vein blockages results in malfunctioning of the venous system, reducing the efficient return of blood to the heart and increasing the pressure in the leg veins (Ghauri 2010; Vlajinac 2014), which, if prolonged, may result in VLUs. The precise chain of events that links the high venous pressures with skin breakdown and a chronic wound is not fully understood (Coleridge Smith 1988; Valencia 2001). Leg ulcers are frequently associated with venous disease in combination with vascular disease, which impairs arterial blood supply, and such ulcers are said to have a 'mixed aetiology' (Marston 2011).
Accurate current estimates of leg ulcer prevalence are difficult to identify because most surveys do not differentiate between causes of leg ulceration, or do so per limb but not per participant (Moffatt 2004; Srinivasaiah 2007; Vowden 2009). One 2011 estimate suggested that venous ulceration has a point prevalence of 0.29 cases per 1000 in the UK, whilst mixed arterial/venous leg ulceration has a point prevalence of 0.11 per 1000 (Hall 2014). One systematic review also reported the point prevalence of leg ulcers in non‐UK studies as: 0.39 per 1000 in New Zealand in 2004, 1.4 per 1000 in Portugal in 2005 and 2.4 per 1000 in Sweden in 2008 (Cullum 2016).
Venous disease is a chronic condition characterised by periods of ulceration (i.e. an open wound) followed by healing and then recurrence, though published contemporary data are lacking: one cross‐sectional study from the 1980s reported that half of current or recent ulcers had been open for up to nine months and that 35% of people with leg ulcers had experienced four or more episodes (Callam 1987). This picture was supported by a subsequent (1988) cross‐sectional study (Nelzén 1994).
Several prognostic factors have been independently associated with slower or reduced healing of VLUs, for example, wound area, duration of ulcer, age, number of wounds, lack of mobility (especially ankle) and weight (Ashby 2014; Barwell 2000; Barwell 2001; Chaby 2013; Gohel 2005; Harrison 2011; Iglesias 2004; Lantis 2013; Margolis 1999; Margolis 2004; Milic 2009; Moffatt 2010; Scotton 2014).
The first‐line treatment for VLUs is compression therapy in the form of bandages, stockings or mechanical devices (O'Meara 2012). This application of external pressure around the lower leg assists venous return and reduces the pooling of blood in the legs (venous reflux) (Fletcher 2013; O'Meara 2012). Alongside compression, dressings are almost always applied to open ulcers (O'Meara 2014). Other treatments for VLUs include venous surgery (removal of incompetent superficial veins) (SIGN 2010), and drugs such as pentoxifylline (Jull 2012).
Leg ulcers are associated with considerable cost to patients and to healthcare providers.
Two systematic reviews summarised the literature on health‐related quality of life in people with leg ulcers (Herber 2007; Persoon 2004). Both included qualitative and quantitative evaluations, and reported that presence of leg ulceration was associated with pain, restriction of work and leisure activities, impaired mobility, sleep disturbance, reduced psychological well‐being and social isolation. Ulcers can be painful, malodorous, prone to infection, and may severely affect people's mobility and quality of life (Dumville 2009; Herber 2007). In severe cases, ulceration can lead to limb amputation, though this is more likely in people who also have arterial insufficiency (Dumville 2009; Nelzén 2008; Valencia 2001), or even to a type of cancer known as Marjolin's ulcer (Choa 2015). Research suggested that people with complex wounds, including people with VLUs, commonly see complete ulcer healing as the most important outcome to them (Madden 2014).
The financial cost of treating a person with an open VLU in the UK was estimated at around GBP 1700 per year at 2012 prices: the largest component of ulcer treatment cost is nursing time (Ashby 2014). Another evaluation estimated the mean cost of treating a person with a VLU in Sweden as between EUR 1332 and EUR 2585 (based on costs for material for dressing changes) and in the UK as between EUR 814 and EUR 1994 (price year 2002), with higher costs associated with larger and more chronic wounds (Ragnarson Tennvall 2005). Data from one German study, which estimated total costs including those classified as indirect or intangible costs, estimated mean annual costs of treating leg ulcers as EUR 9060 per person (2006 evaluation). This figure was higher than other estimates because it included non‐health service costs to the person and to society (Augustin 2012). One Australian cost‐effectiveness study of 905 people estimated the mean cost per person per week for treatment of a chronic leg or foot ulcer below the knee for 24 weeks was AUD 53.31 (which corresponds to AUD 2772 per year); costs included consultations with healthcare professionals, compression bandaging, other dressings and treatments, and community care services, such as Meals‐on‐Wheels and home help (Graves 2014).
Research has shown that not all VLUs heal, even under trial conditions: one large study of 453 participants showed that about 30% of VLUs did not heal following first‐line treatment with compression therapy over 12 months (Ashby 2014). There is interest in additional treatments that may improve wound healing, and one dressing option is the use of protease‐modulating dressings, which are suggested to reduce the activity of a group of enzymes known as proteases. As discussed below, it is thought that prolonged and elevated activity of proteases may be a feature of non‐healing wounds, such that appropriate use of protease‐modulating dressings could promote healing. However, evidence for the effectiveness of these dressings in VLUs is largely unclear (Westby 2016). Such dressings are a new development in wound care in that they are intended to be a 'targeted' treatment aimed at wounds with high protease activity. In this context, protease activity dictates subsequent treatment, but there is limited evidence supporting the use of a test‐and‐treat approach (Norman 2016). In fact, the prognostic nature of protease levels in relation to wound healing is unclear and this will be the focus of this Cochrane Review.
Description of the prognostic factor
A prognostic factor is any measure that, among people with a given health condition, is associated with a future clinical outcome; for example, in people with a VLU, lower body mass index (BMI) may be associated with less time to healing. The prognostic factor for this review was a biomarker, protease activity, which is sometimes elevated in open wounds. A biomarker is defined by the National Institutes of Health Biomarkers Definitions Working Group (1999) as "a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention" (Biomarkers Definitions Working Group 2001). Biomarkers are medical signs, as opposed to medical symptoms (which are indications of health or illness perceived by patients themselves) (Strimbu 2010). Biomarkers may predict health outcomes, but do not necessarily do so. However, there are still no accepted definitive biomarkers for making assessments of chronic wounds (Patel 2016; Yager 2007).
One possible biomarker type that has received some attention for wounds is proteases, which are enzymes that break down proteins into peptides and amino acids (Sittampalam 2017); in general, the various wound‐related proteases break down different proteins. The principal proteases involved in the wound healing process are the matrix metalloproteinases (MMPs) and the serine proteases, which break down extracellular matrix (ECM) and connective tissue proteins such as collagen and elastin (Hahm 2011; Ladwig 2002; McCarty 2013; Nwomeh 1999; Velnar 2009). This protein breakdown is thought to be important in the early stages of the healing process because it facilitates movement of inflammatory cells into the injury site, which aids removal of unwanted material and bacteria. However, in the later stages of wound healing, protein breakdown is believed to be undesirable because the proteases damage newly formed tissue, preventing completion of healing (McCarty 2013; Velnar 2009).
Proteases are produced by an inflammatory process, which also inhibits the synthesis of chemicals that inhibit the action of metalloproteinases (tissue inhibitors of metalloproteinases; TIMPs). Some studies have measured the ratio of MMPs and TIMPs as a biomarker (McCarty 2013; Muller 2008), and it may be more valuable to consider the balance of proteases and their inhibitors as a biomarker (Löffek 2011; Yager 2007).
MMPs are divided into seven subtypes on the basis of their substrates and their domain structure (chemical components) (Lazaro 2016; Löffek 2011; Vihinen 2002): gelatinases (MMP‐2 and MMP‐9); collagenases (MMP‐1, MMP‐8, MMP‐13); stromelysins (MMP‐3, MMP‐10, MMP‐11); metalloelastase (MMP‐12); matrilysins (MMP‐7, MMP‐26); membrane‐type MMPs (MMP‐14, MMP‐15, MMP‐16, MMP‐17, MMP‐24, MMP‐25); and other MMPs (MMP‐19, MMP‐20, MMP‐23, MMP‐28). The main serine proteases are mast cell tryptase and chymase, plasmin, human neutrophil elastase (HNE), cathepsin G, urokinase‐type plasminogen activators (uPA) and tissue‐type plasminogen activators (t‐PA) (Grøndahl‐Hansen 1988). Other protease biomarkers include modular proteins that combine proteases with other biological species: for example, the families of A disintegrin and metalloproteinase (ADAMs) (Duffy 2009; Edwards 2008), and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) (Kelwick 2015). Chronic wounds also contain proteases associated with several types of bacteria (McCarty 2012; McCarty 2013; Percival 2012; Sibbald 2007; Suleman 2016). The activity level for each protease may be elevated at different stages of healing and there may also be different levels of activity in infected wounds (Serra 2016a), but there is currently insufficient evidence to differentiate proteases according to their function in the unhealed wound (Amato 2015; Lazaro 2016; Raffetto 2016; Serra 2017).
Protease activity can be measured in wound fluid using various biochemical tests. Laboratory‐based scientific studies have used several different techniques, including approaches that primarily detect MMP‐2 and MMP‐9 (gelatin zymography), and methods that detect enzyme activity using either chemical (e.g. quenched fluorescence substrate hydrolysis) or biological antibody‐based methods (enzyme‐linked immunosorbent assays (ELISAs)) (Harding 2011; McCarty 2013; Sittampalam 2017). A range of methods of obtaining wound fluid has been used and these vary with the type of protease measured (Cullen 2006; Quirk 2003; Trengove 1999; Yager 2007). There is also a commercial colorimetric indicator available, which is said to determine protease activity, giving a colour change if activity is elevated above a threshold; the test uses a weighted average of the activity of elastase and one or more MMPs (Gibson 2014; NICE 2016).
How the prognostic factor may be related to health outcomes
It has been suggested that, in chronic ulcers generally, non‐healing may be associated with prolonged high activity of proteases in the wound in the later stages of the wound healing process (Harding 2011; Hart 2002; McCarty 2013; Palolahti 1993).
Proteases are active in all phases of wound healing (haemostasis, inflammation, proliferation and remodelling) and are therefore thought to have a number of roles in the normal wound healing process (Patel 2016; Trengove 1999; Velnar 2009). It is thought that there is a burst of protease activity at the start of acute wound healing, and that in normally healing wounds, the activity peaks in the first few days and then declines to very low levels by one week, as healing progresses (Harding 2011; Nwomeh 1998).
However, in non‐healing wounds, it is thought that high protease activity may arise through two main routes (involving different types of proteases): relating to both the host cells (human) and to bacteria in the wound. It is thought that the two types of protease activity may reinforce each other (have a synergistic mechanism) (McCarty 2012; McCarty 2013; Percival 2012; Sibbald 2007; Suleman 2016). In the host, complex inflammatory mechanisms may result in proteases reaching higher levels and persisting for longer than in normally healing wounds (McCarty 2013; Trengove 1999). As previously noted, this persistent breakdown of proteins (proteolytic activity) is thought to damage newly formed tissue and to degrade growth factors, leading to non‐healing wounds (Cullen 2002a; Harding 2011; Wlaschek 1997; Yager 1997). Most chronic wounds are colonised with bacteria, though they are not necessarily infected. Bacterial proliferation and their formation into film‐like material (biofilms) in non‐healing wounds has been linked to chronic inflammation and then elevated protease levels. Infection refers to invasion of tissue by bacteria, leading to a clinically evident pathogenic inflammatory response and tissue damage (Percival 2012; Pugliese 2016; Sibbald 2007; Suleman 2016).
Limited evidence suggests correlations between elevated levels of MMPs and delayed healing in people with pressure ulcers (Ladwig 2002), or in foot ulcers of people with diabetes (Liu 2009), as well as in people with VLUs (Mwaura 2006; Serra 2013). It is possible that association of MMP level with delayed wound healing may be a general wound phenomenon, however differences between wound types have also been observed (Lazaro 2016; McCarty 2013).
For VLUs in particular, studies of protease activity in wound fluid have suggested that there are significantly higher levels of proteases in ulcer tissue compared with healthy tissue, and that these levels decrease following compression treatment in wounds that heal (Beidler 2008); other studies have reported higher levels in chronic wounds compared with acute wounds (Lazaro 2016; Trengove 1999; Wysocki 1993).
However, association between protease activity and non‐healing is not clear cut. Limited data from two industry‐sponsored studies found that only 28% of 162 (Serena 2011) and 23% of 139 (Gibson 2013) non‐healing wounds of mixed aetiology had high protease activity; one of these studies reported that 22% of 101 leg ulcers had elevated protease activity (Gibson 2013).
Wound fluid is a useful source of biomarkers, and its composition is broadly assumed to reflect the current clinical condition of a wound (Löffler 2013). Wound fluid can be obtained in a largely non‐invasive way, but to give reproducible and accurate measurement of biomarkers, the wound fluid has to be collected and processed reliably. There are several techniques for wound fluid sampling, including the use of occlusive dressings, entrapment of fluid in dressings, swabs, other techniques and devices. The duration of fluid collection can also be important (Löffler 2013).
Importance of evidence about prognostic factors
Biomarkers of this type may potentially be implicated in, or mediate, particular pathways to non‐healing. Studying the prognosis associated with protease biomarkers has a three‐fold purpose: first, it can help us understand mechanisms related to healing, including investigating the true causes of non‐healing. Second, it can allow identification of wounds at increased risk of non‐healing, which could allow selective treatment of these wounds according to the specific biomarker type and level. Third, biomarkers can be used to monitor a response to therapy (Riley 2013).
Targeted treatment of this type (the 'test‐and‐treat' or 'stratified medicine' approach) is important, especially if treatments are costly or have adverse effects, so that they are not used where they will not be effective (Hingorani 2013). It is likely that there is more than one pathway to non‐healing and other pathways will probably be represented by other biomarkers. The best approach may be to determine a set of biomarkers and treat selectively according to their activity levels.
A commercial test for protease activity is now available (Gibson 2014), and is intended for use at the point of care, in conjunction with protease‐modulating treatments (Barrett 2011; Harding 2011; Snyder 2011; Snyder 2013). This approach has mainly found application in diabetic foot ulcers (NICE 2016). A Cochrane Review on test‐and‐treat for healing in VLUs did not identify any studies (Norman 2016).
Why it is important to do this review?
VLUs are a common type of complex wound that have a negative impact on people's lives and incur high costs for health services and society. Leg ulcers are painful, sometimes malodorous, prone to infection and may severely affect the person's mobility and quality of life; in severe cases, there is a risk of limb amputation. There are a number of treatments for VLUs, but many ulcers prove hard to heal.
Two Cochrane Reviews investigated protease‐modulating matrix (PMM) dressings, but there was insufficient evidence on the modulation of protease activity. Westby 2016 examined the effects of PMM dressings for healing VLUs, and found it was unclear whether PMM dressings increased the probability of healing at 12 weeks, in comparison with non‐PMM dressings (risk ratio (RR) 1.28, 95% confidence interval (CI) 0.95 to 1.71; 4 trials; 192 participants). The study populations typically comprised people with difficult‐to‐heal wounds, but only one study reported the level of protease. A second review searched for evidence on 'test‐and‐treat' approaches for healing VLUs; for example, PMM treatment given selectively to wounds with elevated protease activity (Norman 2016). It found no eligible studies. This current review is the third part of this set of Cochrane Reviews on protease activity‐related treatment.
It is important to investigate whether elevated protease activity is a prognostic factor for healing. For practical use, the biomarker should be robust to adjustment for other factors such as age. This Cochrane Review was mainly exploratory in nature and its focus was on protease activity in general; it did not address associations of specific proteases, although we reported the actual proteases measured.
One literature review summarised clinical evidence on MMPs in chronic wound healing (Lazaro 2016). The authors reported studies that found correlations between MMP levels and various measures of healing, but did not give full quantitative data. They identified some studies investigating MMP thresholds for healing but these were in people with diabetic foot ulcers. The review did not examine the literature on proteases other than MMPs.
Objectives
To determine whether protease activity is an independent prognostic factor for wound healing in people with venous leg ulcers.
Methods
The methods used in this review have two underlying assumptions: first, that biomarkers are representative of wound processes and can be used to monitor wound healing. Second, that the removal of wound fluid does not interfere with the healing processes (Yager 2007). It is unclear how robust these assumptions are.
PICOTS system for this review (population, index, comparator, outcome(s), timing, setting)
The PICOTS summary for this review was:
| Population | Index (prognostic factor under study) | Outcomes | Timing | Setting |
| People with venous leg ulcers | Protease activity | Healing:
| Prognosis:
| Any |
We used the PICOTS system to formulate the review question, the objective and the inclusion criteria for the review (Debray 2017; Moons 2014), the comparator was not relevant in our review. Further details are given below.
Criteria for considering studies for this review
Types of studies
We included reports of prospective and retrospective longitudinal studies that investigated whether the prognostic factor, protease activity, was associated with healing of VLUs, as such studies can produce valid odds or hazard ratios of prognostic factors. We did not include studies that solely examined the validation of prediction models, but we did include prediction model development studies when they also reported associations of individual prognostic factors (protease activity) with the outcome under study (healing). We included randomised controlled trials (RCTs) analysed as cohort studies, provided interventions were taken into account in the analysis. We included case‐control studies only if there were no relevant eligible cohort studies for specific proteases. We included case‐control studies because the design can provide valid estimates of associations between a prognostic factor and outcomes provided that the follow‐up time is not too long. However, we acknowledge that case‐control studies often suffer from other deficiencies (Altman 2001): these were assessed in our risk of bias assessment. We did not include cross‐sectional studies or case reports because the association under study is inherently longitudinal. We included studies with any follow‐up period, given the prognostic nature of our objective.
We planned to consider evidence separately within the different phases of prognostic factor investigation: phase 1 (exploratory), and phase 2 (confirmatory) studies, which provide different levels of evidence (Hayden 2008; Riley 2013). Exploratory studies identify associations of many potential prognostic factors and outcomes. These studies measure associations between single factors and the outcome ('univariable' or 'univariate' associations), and provide the least conclusive information regarding the independence of a variable as a valid prognostic factor. Confirmatory studies aim to measure the independent effect of a prognostic factor on the relevant outcome while controlling for other factors. We planned to include all eligible studies in the review and, if possible, planned to restrict the results summary and any meta‐analyses to results from multivariable analyses. Where this was not possible, we included the results of univariable analyses, taking into account the phase of investigation in both the risk of bias assessment and GRADE rating on certainty of the summary estimates.
Targeted population
We included studies of people with a VLU, who were managed in any care setting and receiving any type of treatment. We expected the method of diagnosis of venous ulceration to vary, so accepted definitions as used in the included studies.
We included studies in people with VLUs alongside people with other types of wounds (e.g. arterial ulcers, pressure ulcers, diabetic foot ulcers) provided the results for people with venous ulcers were presented separately, or if most participants (at least 75%) had leg ulcers of venous aetiology. Where wounds were described only as leg ulcers without information as to aetiology, we assumed they were venous in origin because this is the most common type of leg ulcer.
We included studies that involved participants at any stage in their treatment pathway, and we recorded, where available, baseline data on the time since diagnosis and treatments previously given, together with ongoing treatments. We included studies that involved participants with any infection status at baseline and recorded any available data on this.
We excluded studies conducted solely in vitro and animal studies.
Types of prognostic factor
The prognostic factor was protease activity. Any type of protease or combination of proteases was eligible, either from the host or from bacteria, including:
-
MMPs: gelatinases (MMP‐2 and MMP‐9); collagenases (MMP‐1, MMP‐8 and MMP‐13); stromelysins (MMP‐3, MMP‐10 and MMP‐11); metalloelastase (MMP‐12); matrilysins (MMP‐7 and MMP‐26); membrane‐type MMPs (MMP‐14, MMP‐15, MMP‐16, MMP‐17, MMP‐24 and MMP‐25); and other MMPs (MMP‐19, MMP‐20, MMP‐23 and MMP‐28);
-
serine proteases: mast cell tryptases and chymases; human nucleophil elastase; cathepsin G; plasmin; uPA; t‐PA;
-
proteases combined with other biological species: ADAM; ADAMTS.
Where possible, we combined these three major categories in the analyses, taking into consideration cut‐off points used (see 'Data synthesis' section below).
We planned to include and report separately studies that investigated as biomarkers the ratio of proteases and their respective inhibitors (e.g. MMP‐2 (protease) and TIMP‐2 (inhibitor)).
The prognostic factor could be examined as a continuous or categorical variable and any cut‐off point was permitted.
We permitted any approach to obtaining samples from wounds, and any method of measurement of protease activity. We did not include measures of proteases in the blood or in tissue samples (biopsies).
We reported all unadjusted (simple) associations between the prognostic factor and healing and planned to report adjusted (multivariable) associations between the prognostic factor and healing, with details on any adjustment factors used, especially taking into consideration key adjustment factors of age and infection. No studies conducted multivariable analyses, and for some studies, we calculated unadjusted associations from raw data (e.g. mean and standard deviations of protease levels for healed and non‐healed wounds).
Types of outcomes
Primary outcomes
We planned to include the following primary outcomes:
-
time to healing (analysed by survival analysis);
-
proportion of people with ulcers completely healed, at any follow‐up duration.
We planned to consider subgroup analyses to explore the impact of follow‐up time. We accepted study authors' definitions of what constituted a healed wound.
We recorded study‐reported associations and extracted raw data to calculate univariate associations. Binary outcomes would preferably have been reported as time‐to‐event measures (survival), but failing that, we considered dichotomous summary data at the longest time point or the key time point specified in the study's methods section.
Secondary outcomes
-
Change in size of ulcer/rate of wound closure (e.g. centimetres squared per day).
If there were no ulcer healing data for a particular association, we planned to use data on the change (and percentage change) in ulcer size, with adjustment for baseline size. We did not contact study authors to request adjusted means when not presented. Where studies reported change in ulcer size without adjustment for baseline size, we analysed the results and assigned high risk of bias to the outcome measurement domain.
For continuous outcomes, we reported either continuous summary data or dichotomous data with any cut‐off point.
Search methods for identification of studies
We developed a search strategy based only on population terms and protease terms. We considered adding prognosis filters, but this review concerned only one type of biomarker and the number of records identified was manageable without the use of filters. Therefore, we followed the procedure recommended by Geersing 2012.
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (2018, Issue 2);
-
Ovid MEDLINE (1946 to February 2018);
-
Ovid Embase (1974 to February 2018);
-
CINAHL (1982 to February 2018).
The search strategies are in Appendix 1.
In addition, we handsearched the bibliographies of all included studies and of identified relevant systematic reviews.
Data collection
We collected and analysed data according to methods stated in the published protocol (Westby 2017), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and guidance from the Cochrane Prognosis Methods Group (Cochrane Prognosis Methods Group 2018; Riley 2007).
Selection of studies
Two review authors independently assessed the titles and abstracts of retrieved records against the inclusion criteria. We obtained all potentially relevant studies in full and two review authors independently assessed these for eligibility. We resolved any disagreements at each stage through discussion and, where appropriate, we consulted a third review author. We did not contact the study authors to resolve the uncertainty. Where studies were reported in multiple publications/reports, we obtained all publications. Whilst we included a study only once in the review, we extracted data from all reports to ensure we obtained all available relevant data. We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. The study selection process is illustrated in a PRISMA diagram, see Figure 1. All studies excluded after full‐text assessment are listed in a Characteristics of excluded studies table with their reasons for exclusion.

Study flow diagram.
Data extraction and management
We collected all association data reported between the prognostic factor and outcomes, with details on any adjustment factors used. Where necessary, we extracted raw data that would allow calculation of association measures.
We extracted and recorded data from included studies using an Excel‐based data extraction sheet, which we piloted initially on a few studies. We based the Items extracted on the CHARMS guidance (Moons 2014). One review author extracted data, which was then checked by a second review author. We did not contact the study authors if key data were missing from reports, because the overall risk of bias was too high.
We extracted the following data for the prespecified prognostic factors and outcomes in this review. We collected outcome data as described in the 'Types of outcome measures' section:
-
country and setting in which study was conducted;
-
study design;
-
eligibility criteria;
-
participant details;
-
ulcer details, including duration of ulcer, ulcer size, number of ulcers and ulcer history, and ulcer severity (with the system used to classify this) as reported by the study authors;
-
treatment details, including compression and debridement (including type and frequency);
-
method of obtaining wound fluid for prognostic factor measurement, including duration of collection;
-
method of measurement of protease activity (e.g. Gelatin zymography);
-
details of each prognostic factor: type of protease/combination of factors, measurement of prognostic factor (including time of measurement), type of data (e.g. continuous/any cut‐off points and if so, whether they were predefined and what the justification was for that cut‐off point);
-
details of each outcome: measurement, type of data (e.g. continuous/any cut‐off points);
-
duration of follow‐up;
-
type of analysis: explanatory/confirmatory (including the presence of a predefined protocol and study registration); logistic regression/Cox regression;
-
any adjustment factors considered in the analysis;
-
association statistics for each prognostic factor for primary and secondary outcomes (e.g. odds ratios (ORs), hazard ratios (HRs) and their CIs/variances/standard errors);
-
loss to follow‐up and reasons.
We extracted minimal data where there were no association data reported.
Assessment of risk of bias in included studies
Two review authors independently appraised the included studies using a standardised approach. In the case of discrepancies, the review authors attempted to reach consensus; if necessary, a third review author resolved any disagreements. The review authors were not blinded to study authors, institution or journal of publication because this was not feasible.
We planned to assess risk of bias using an approach based on the Quality In Prognosis Studies (QUIPS) tool, which is appropriate for prognostic factor review questions (Hayden 2013; Hayden 2014), also drawing on ROBINS I tool (ROBINS‐I 2017), which assesses the risk of bias for non‐randomised intervention studies (Sterne 2016). However, because of the absence of multivariable analyses in all studies. we modified the approach (see below).
Original approach
Our original approach to risk of bias assessment is described fully in Appendix 2. This approach assesses risk of bias per study for each prognostic factor–outcome combination, considering six domains: study participation (selection bias), study attrition, prognostic factor measurement, outcome measurement, adjustment, and statistical analysis and reporting. To assess the adjustment bias domain, we identified key adjustment factors, both from review of the literature and in discussion with clinicians (Appendix 3).
Each domain is rated as having high, moderate or low risk of bias. We defined an all‐domain risk of bias per study for each prognostic factor–outcome combination, taking into account all the above domains, but especially focusing on the key domains of selection bias, study attrition, adjustment, and statistical analysis and reporting. As a guide, we assigned risk of bias as follows: low risk of bias if all (or all key) domains had low risk of bias; moderate risk of bias if there was high risk of bias for one key domain or moderate risk of bias for at least two key domains (with the rest as low risk of bias); high risk of bias if there was high risk of bias for at least two key domains. We planned to perform sensitivity analysis considering only studies at low all‐domain risk of bias (Hayden 2013).
Post‐hoc modified approach
In view of the lack of multivariable analysis in all the studies, we modified post‐hoc the QUIPS risk of bias assessment for domain 6 (statistical analysis and reporting) to avoid 'triple‐counting' the univariate features of the studies which are also addressed in the GRADE approach and in QUIPS domain 5 (adjustment factors) (see Appendix 2; Appendix 4). The GRADE approach to prognostic factor reviews assigns a moderate rating to phase 1 exploratory studies or other studies reporting univariable associations or having sufficient data to calculate associations. Domain 5 of QUIPS (adjustment) assigns high risk of bias if key adjustment factors were not taken into account in the design or the analysis. We modified the assessment of risk of bias for domain 6 so that if a well‐designed, appropriately analysed and well‐reported exploratory (univariate) study was described, a low risk of bias was assigned for this domain. For all other studies, we assigned moderate or high risk of bias to that domain, taking into account the aim of the study and the impact of interventions, as well as the other factors in Appendix 2.
Measures of association
We planned to extract all unadjusted and adjusted measures of association from included studies and to convert effect sizes, as necessary, to avoid possible selection bias, thus allowing us to use data from as many studies as possible.
We had anticipated that results from multivariable analyses would have been reported as ORs, RRs and HRs and, if so, we would have used ORs as the common measure of the association, using RRs and HRs to estimate ORs at a particular time point (Symons 2002). However, none of the included studies reported adjusted measures for binary healing outcomes, but some studies provided sufficient raw data to allow us to calculate associations.
-
Where both the healing outcomes and prognostic factors were reported as dichotomous variables (e.g. elevated and non‐elevated protease activities for complete healing), we formed 2 × 2 tables and analysed these data as the RR.
-
Where the prognostic factors were reported as continuous variables and individual participant data (IPD) were given and the outcome was dichotomous, we conducted a univariate regression analysis in STATA (STATA 2013).
-
Where there were summary data, we extracted the mean and standard deviation protease activity for each healing outcome state. To obtain an indication of possible associations between protease activity and healing, we conducted a t‐test to record associations between healing at the follow‐up time (as an independent variable) and protease levels at baseline (as the dependent variable) (i.e. we investigated the reverse association).
Where the outcomes were reported as continuous measures (e.g. change in ulcer size) and the prognostic factor was a dichotomous variable, we planned to analyse the regression coefficients with their standard errors or to conduct a simple t‐test. If both outcome and prognostic factor were continuous variables, we extracted (or calculated) Pearson correlation coefficients and P values and calculated CIs (Lowry 2018).
For consistency, we recalculated associations to be in the same direction, as necessary, with associations above 1 indicating better prognosis for positive binary outcomes (e.g. a healed wound). We did not contact study authors regarding missing or unusable data.
Unit of analysis issues
The prognostic factor (protease activity) and outcome (complete wound healing) were both considered at the ulcer level. A possible unit of analysis issue could have arisen when there was more than one ulcer per person and multivariable analysis was conducted with adjustment factors measured at the individual level (e.g. age). This represents clustered data and analyses should be conducted using hierarchical methods. Where studies included clustered data of this type, we planned to report this, noting whether data were analysed correctly, recording this as part of the risk of bias assessment. We planned to consider including such studies in any meta‐analysis, taking into account the associated risk of bias. No studies clearly included more than one wound per participant.
Dealing with missing data
We included studies that investigated the relationship between protease activity and healing regardless of whether there were missing data and even if limited evidence was provided about the size of the effect (e.g. if the factor was mentioned only as being ’non‐significant’ in the analyses). We did not contact study authors to attempt to retrieve any missing information.
Assessment of heterogeneity
We considered the clinical heterogeneity of included studies based on the population, measure of the prognostic factor, cut‐off points used, outcome measurement and methodological heterogeneity due to study design/potential biases. We planned to synthesise associations as appropriate within clinically relevant subgroups, grouping studies regardless of type of protease, duration of ulcer and presence of infection. We planned to examine the duration of ulcer and presence of infection in subgroup analyses where there was heterogeneity.
Assessment of reporting deficiencies
We planned to examine publication bias for each meta‐analysis, provided there were 10 or more studies, by visually examining asymmetry on funnel plots and testing for asymmetry at the 10% level, using Egger's test for HRs, and Peters' test for ORs (Debray 2018; Sterne 2011).
Data synthesis
Synthesising data in prognostic factor studies requires us to recognise the many different ways of reporting and analysing results, and, where possible, to request alternative data or to transform results to a common format (see 'Measures of association' section). We planned to consider the impact of data transformations using sensitivity analyses.
We had expected that most studies would present data in the format of dichotomous outcome data and continuous prognostic factor data (e.g. protease activity), which may have been dichotomised or categorised using cut‐off values .
After carrying out any appropriate transformations, where possible we grouped together studies with similar prognostic factor cut‐off points/similar analytical approaches, and represented the data on forest plots. We had planned to conduct meta‐analyses if valid data were available assessing associations between individual prognostic factors and an outcome of interest for sufficiently homogeneous subgroups of studies. We defined 'sufficiently homogeneous' subgroups according to population, measures of the prognostic factor and outcome measurement. However, we considered all data to be at too high a risk of bias to pool. We had planned to include in the forest plots details of any adjustment factors considered in the study analyses, but instead gave other relevant details in the footnotes.
We planned to combine the data for all proteases, regardless of the source or type of protease mainly because of a lack of evidence to inform stratification. For prognostic factors analysed as dichotomous measures, we planned additionally to take cut‐off points into consideration as described above. We planned to analyse separately ratios of proteases and their inhibitors.
If meta‐analysis had been conducted, we would have analysed HRs and ORs separately (at similar follow‐up points). We also planned to transform the measures, where possible, so that a single analysis (OR) could have been conducted. We planned to conduct meta‐regression analysis if there were more than 10 studies providing sufficient data (Berkey 1995). We planned to conduct meta‐analyses using STATA (StataCorp version 14) with a random‐effects restricted maximum likelihood (REML) meta‐analysis model, which accounts for any between‐study heterogeneity in the prognostic effect (Cornell 2014; Riley 2010). Such heterogeneity is common in prognostic factor studies. Unless the heterogeneity (as assessed above) was too extensive for appropriate pooling, we planned to summarise the meta‐analysis by the pooled estimate (the average prognostic factor effect), the Hartung‐Knapp 95% CI, the estimate of Tau² (between‐study variance) and a 95% prediction interval for the prognostic effect in a single population (Riley 2011).
'Summary of findings' table and GRADE assessment
We used an approach modified from the GRADE framework (Guyatt 2011a) to assess the certainty of the summarised evidence for each prognostic factor–outcome combination (Hayden 2014; Huguet 2013; Iorio 2015). We rated the overall strength of evidence as high, moderate, low or very low considering the phase of prognostic study (confirmatory/explanatory or exploratory), the within‐study risk of bias, the directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Iorio 2015; Schünemann 2011a). We also considered two 'upgrading' factors, large effect and dose effect, although we noted that high risk of bias may artificially lead to large effects (see Appendix 4 for further details).
We did not present the main results of the review in formal 'Summary of findings' tables because the vast majority of the evidence was at high risk of bias and this, together with the study design, meant that the evidence was of very low certainty throughout. Instead we summarised the findings in the text. 'Summary of findings' tables present key information concerning the certainty of the evidence, the magnitude of the associations examined and the sum of the available data (Schünemann 2011b), and include an overall grading of the evidence. This defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest.
If it was not appropriate to combine results using a meta‐analysis (due to excess clinical heterogeneity), we planned to present the results qualitatively, considering the strength and consistency of results using the following schema:
-
strong evidence of effect: consistent findings (defined as greater than 75% of studies showing the same direction of effect) in multiple low risk of bias studies;
-
moderate evidence of effect: consistent findings in multiple high risk of bias or one study with low risk of bias (or both);
-
limited evidence of effect: one study available;
-
conflicting evidence of effect: inconsistent findings across studies;
-
no effect: no association between participant expectations and the outcome of interest.
We planned to calculate absolute risk differences for the effect of the prognostic factor using estimates of baseline risk from the literature where possible.
Subgroup and sensitivity analyses
We planned to use sensitivity analyses to explore the impact of study level all‐domain risk of bias, first restricting the analysis to studies rated as having low risk of bias, and if this was not feasible, to restrict to low or moderate risk of bias.
If there was heterogeneity, we planned to investigate it using the following prespecified subgroup analyses, provided there were at least two studies per subgroup:
-
baseline duration of ulcer (up to 24 weeks; 24 weeks or greater); duration may be a proxy for a non‐healing wound, in which protease activity may be associated differently with healing;
-
presence or absence of infection.
We did not plan to conduct subgroup analyses by type of protease because this would have introduced too high a level of complexity for this review, but we recorded the type of protease measured.
We planned to consider subgroup or sensitivity analyses to explore the impact of types of measurement approaches for assessing prognostic factors.
This Methods section was based on the exemplar Cochrane prognosis review protocol for prognostic factors (Hayden 2014) and the general protocol template of the Cochrane Prognosis Methods Group (Cochrane Prognosis Methods Group 2018). In conducting the review, we carried out a number of modifications to the methods, as described above.
Results
Description of studies
Results of the search
The search generated 1493 records and one of the review authors identified an additional paper separately: we obtained 153 full papers (Figure 1); some of these were reviews ordered for bibliographic checks and background material; we excluded 110 studies with reasons (Characteristics of excluded studies table). We included 19 studies described in 21 reports (see 'Included studies' for explanation). One study was placed in the Characteristics of studies awaiting classification section (Cullen 2009); this was a conference abstract and gave too little information.
Included studies
Studies are described in detail in the Characteristics of included studies table. In this table, we report two studies twice because they were RCTs with different risks of bias for each treatment group: we report each trial arm as a separate cohort (Moffatt 2014a; Moffatt 2014b; Serra 2015a; Serra 2015b).
Nineteen studies (involving 21 cohorts), with 646 participants, met the inclusion criteria for the review (Ahmad 2015; Cullen 2012; Frankova 2013; Gohel 2008; Grzela 2014; Harris 1995; Hoffman 1999; James 2003; Litwiniuk 2012; McDaniel 2017; Moffatt 2014a; Moffatt 2014b; Mwaura 2006; Raffetto 2015; Serra 2013; Serra 2015a; Serra 2015b; Smeets 2008; Trengove 1999; Trøstrup 2011; Wysocki 1999). The size of the cohorts was small, with a median (range) of 30 (7 to 80) participants. Two studies were reported only as conference abstracts or posters (Cullen 2012; Raffetto 2015).
Most included studies had a cohort study design and two were a type of case‐control study. Eleven studies reported a prospective longitudinal design (Ahmad 2015; Frankova 2013; Gohel 2008; Hoffman 1999; James 2003; Litwiniuk 2012; Mwaura 2006; Serra 2013; Trengove 1999; Trøstrup 2011; Wysocki 1999), of which three had 10 or fewer participants (Hoffman 1999; James 2003; Trøstrup 2011). There were six RCTs, two of which reported results separately for each arm (Moffatt 2014a; Moffatt 2014b; Serra 2015a; Serra 2015b); two of which reported relevant results for the study as a whole (Cullen 2012; McDaniel 2017); and two did not give any useable results (Grzela 2014; Smeets 2008). The remaining two studies had a type of case‐control design nested in a cross‐sectional study (Harris 1995; Raffetto 2015): we have interpreted these studies as having a longitudinal component that occurred before the study started.
Eight included studies did not provide sufficient useable results data in the published report (Frankova 2013; Grzela 2014; James 2003; Litwiniuk 2012; Smeets 2008; Trengove 1999; Trøstrup 2011; Wysocki 1999). One study reported association statistics only for an intermediate biomarker for both the prognostic factor and the outcome (James 2003); the other studies reported both the prognostic factor and the outcome as continuous variables, generally giving summary statistics for each, but no association statistics. To have useable data for the review, we would have had to request further analyses from the authors. Therefore, we included these studies for completeness, noted the lack of useable results and only extracted data for a minimal set of characteristics (see Characteristics of included studies table). We did not formally conduct 'Risk of bias' assessments and did not include these studies in the 'Risk of bias' figures (see 'Risk of bias' section).
The remaining 11 studies involving 13 cohorts are summarised in this section and analysed in the results section (Ahmad 2015; Cullen 2012; Gohel 2008; Harris 1995; Hoffman 1999; McDaniel 2017; Moffatt 2014a; Moffatt 2014b; Mwaura 2006; Raffetto 2015; Serra 2013; Serra 2015a; Serra 2015b). There were 522 participants in these studies, with a median of 40 participants (range 7 to 80).
Six studies were conducted in the UK (Ahmad 2015; Cullen 2012; Gohel 2008; Harris 1995; Hoffman 1999; Moffatt 2014a/Moffatt 2014b); two in Italy (Serra 2013; Serra 2015a/Serra 2015b); one in the USA (McDaniel 2017); one in Ireland (Mwaura 2006); and one abstract did not report the country (Raffetto 2015).
Of the longitudinal studies, two had a follow‐up time of substantially less than two months (four weeks: Cullen 2012; five weeks: Gohel 2008). The other studies ranged from eight weeks (Mwaura 2006; McDaniel 2017; Serra 2013) to 36 weeks (Hoffman 1999); one of these gave correlation coefficients between protease levels measured at four weeks and follow‐up at eight weeks (McDaniel 2017).
Participant characteristics
In 10 studies, all the participants had VLUs. In the remaining study, 56% of participants had VLU and 44% had mixed arterial‐venous leg ulcers (Moffatt 2014a/Moffatt 2014b). Three studies had similar numbers of males and females (Ahmad 2015; Gohel 2008; Moffatt 2014a/Moffatt 2014b); one was about two‐thirds male (McDaniel 2017); three studies had about twice as many females as males (Mwaura 2006; Serra 2013; Serra 2015a/Serra 2015b); and four did not report on sex (Cullen 2012; Harris 1995; Hoffman 1999; Raffetto 2015). Where reported, mean ages ranged from 51 to 75 years. Two studies reported that wounds were not infected (Gohel 2008; Harris 1995). The mean/median duration of wounds ranged from three months (Gohel 2008) to 14 years (Harris 1995).
Prognostic factors
The studies investigated the following protease biomarkers; some studies examined more than one biomarker:
-
MMP‐1 (Raffetto 2015);
-
MMP‐2 (Gohel 2008; Mwaura 2006; Raffetto 2015);
-
MMP‐3 (Raffetto 2015);
-
MMP‐7 (Raffetto 2015);
-
MMP‐8 (McDaniel 2017; Raffetto 2015);
-
MMP‐9 (Gohel 2008; Raffetto 2015; Serra 2013; Serra 2015a/Serra 2015b);
-
MMP‐10 (Raffetto 2015);
-
MMP‐12 (Raffetto 2015);
-
MMP‐13 (Raffetto 2015);
-
MMP‐unspecified (Harris 1995; Moffatt 2014a/Moffatt 2014b);
-
HNE (Cullen 2012; Hoffman 1999; McDaniel 2017);
-
uPA (Ahmad 2015).
No study clearly measured bacteria‐specific proteases and no studies reported ratios of biomarkers and their inhibitors.
Studies reported different methods for extracting wound fluid for analysis of protease activity: occlusive dressings (Ahmad 2015; Gohel 2008; Harris 1995; McDaniel 2017; Serra 2013); entrapment in dressings (Hoffman 1999; Mwaura 2006; Raffetto 2015), with two of these studies using dressings already used for treatment (Hoffman 1999; Mwaura 2006); swabs (Moffatt 2014a/Moffatt 2014b); and not stated (Cullen 2012; Serra 2015a/Serra 2015b).
Studies measured protease using ELISA techniques (Ahmad 2015; McDaniel 2017; Mwaura 2006; Raffetto 2015; Serra 2013; Serra 2015a/Serra 2015b); fluorogenic substrate assay (Cullen 2012); gelatin zymography (Gohel 2008); and other activity assays (Harris 1995; Hoffman 1999; McDaniel 2017; Moffatt 2014a/Moffatt 2014b). One study reported measurement only of pro‐MMP (and this was not cleaved to give the active form; Gohel 2008); another study (Ahmad 2015) measured total uPA including bound and complexed forms, with the active form level being below the level of the bioassay; Harris 1995 separately reported total, active and latent forms of MMP; three studies reported 'activity' (Cullen 2012; Hoffman 1999; Moffatt 2014a/Moffatt 2014b); one study appeared to measure total MMP (Mwaura 2006); and the other studies reported concentrations (McDaniel 2017; Raffetto 2015; Serra 2013; Serra 2015a/Serra 2015b), but it was unclear whether this meant total MMPs (active and pro‐MMP forms).
Two studies used a cut‐off point for the prognostic factor: Cullen 2012 used greater than 25 mU/110 µL for elastase (taken from their work in diagnostic studies (Serena 2011)) and Moffatt 2014a/Moffatt 2014b used an activity score of 5 or more to define high activity on a scale of 0 to 10 for MMPs (unspecified). The other studies reported the prognostic factor as a continuous measure, sometimes in relation to a standard (Ahmad 2015; Gohel 2008; Hoffman 1999), or per total protein in the sample (McDaniel 2017; Serra 2013; Serra 2015a/Serra 2015b), or as a concentration (Grzela 2014; Mwaura 2006; Raffetto 2015).
This variability in sampling, measurement techniques and use of standards meant that analyses of continuous outcomes were conducted using standardised mean differences.
Outcome measures
Studies measured healing in several ways: only three studies reported complete healing as a dichotomous outcome (Ahmad 2015; Hoffman 1999; Moffatt 2014a/Moffatt 2014b). Five studies reported partial healing (other dichotomous measures of 'healing'), compared with 'no healing,' defined as follows:
-
reduction in area of 30% or greater over four weeks versus no healing (Cullen 2012);
-
decreased size (greater than 20%), decrease in slough and development of healthy granulation tissue versus no healing (Mwaura 2006);
-
categorical healing: high healing (1 cm²/week or greater); low healing (less than 1 cm²/week) and no healing (Serra 2013);
-
granulating wounds versus non‐granulating/inflammatory wounds (Harris 1995; Raffetto 2015).
Two studies compared different degrees of healing:
-
high healing (1 cm²/week or greater) versus low healing (less than 1 cm²/week) (Serra 2013; Serra 2015a/Serra 2015b).
Three studies reported the outcome as a continuous measure: one measured the change in size (Gohel 2008), and the others the percentage change in size from baseline (Hoffman 1999; McDaniel 2017).
Interventions
Nine studies reported that the participants received compression therapy (Ahmad 2015; Cullen 2012; Gohel 2008; Hoffman 1999; McDaniel 2017; Moffatt 2014a/Moffatt 2014b; Mwaura 2006; Serra 2013; Serra 2015a/Serra 2015b). Two studies gave no information on compression interventions (Harris 1995; Raffetto 2015).
In addition to compression, two studies received vein surgery, as appropriate (Serra 2013; Serra 2015a/Serra 2015b). Six studies received other treatments, some of which were likely or possible protease‐modulating dressings:
-
likely protease modulating: Cullen 2012 (randomised to collagen/oxidised regenerated cellulose (ORC) matrix with or without silver);
-
possible protease modulating: Moffatt 2014a (oxyzyme or iodozyme plus basic treatment); Serra 2015a (doxycycline); McDaniel 2017 (oral n‐3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) plus silver dressing);
-
unlikely to be protease modulating: Gohel 2008 (non‐adherent); Hoffman 1999 (non‐adherent, wool padding, etc.); Moffatt 2014b (usual care continued); Serra 2015b ('most appropriate treatment' ‐ basic treatment).
Excluded studies
We excluded 110 studies from the review for the following main reasons (see Characteristics of excluded studies table): 13 studies had an ineligible population (Budzyn‐Napierala 2016; Cullen 2002b; Honda 2011; Huttunen 2005; Karatepe 2010; Kucukguven 2013; Mirastschijski 2002; Schultz 2004; Serra 2014; Shields 1994; Varelias 2002; Varelias 2006; Zamboni 2005); five did not assess a relevant prognostic factor (Cook 2009; Ibbotson 1994; Senet 2003; Stojadinovic 2014; Wlaschek 1997); 18 did not report an eligible healing outcome (Bernatchez 2012; Dalton 2005; Eming 2008; Failla 2008; Grinnell 1998; Huttunen 2000; Huttunen 2004; Impola 2005; Karim 2006; McInnes 2014; Moor 2009; Palolahti 1993; Rayment 2008; Schmid 1999; Schmidtchen 2000; Wysocki 1993; Wysocki 1996; Zillmer 2011); 38 studies where the study design was not longitudinal (Ahmad 2011; Amato 2015; Anon 2008; Ayuk 2016; Bogaczewicz 2004; Bohórquez‐Sierra 2006; Clark 2001; Cook 2000; Da Silva 2014; Derbyshire 2003; Duffy 2005; Fisher 1998; Gordon 1975; Herouy 2000a; Herouy 2000b; Ivins 2014; Körber 2006; Kucharzewski 2005; Ligi 2016; Lim 2010; McCarty 2012; McCarty 2013; Moore 2007; Nwomeh 1999; Ovington 2001; Phillips 2007; Raffetto 2014; Rayment 2009; Rogers 1999; Schmidtchen 2003; Serena 2016; Serra 2015c; Serra 2016a; Singh 2010; Tarlton 1999; Vahlquist 2000; Weckroth 1996; Widgerow 2011); and 34 studies where the samples were not obtained from wound fluids or were not obtained by an eligible method (Alexewicz 2007; Barros 2012; Caimi 2015; Eming 2006; Fernandez 2008; Gacka 2004; Grinnell 1992; Hasmann 2011; He 1999; Herouy 2004; Herrick 1997; Hoffman 1998; Lantis 2011; Lotti 1995; Mirshahi 1995; Nielsen 1992; Norgauer 2002Pirila 2007; Rechardt 2000; Saarialho‐Kere 1998; Salgado 2017; Serra 2016b; Serra 2017; Stacey 1993; Stacey 2000; Tauzin 2014; Turio 2002; Ulrich 2005; Vaalamo 1996; Vaalamo 1997; Vaalamo 1999; Van Bergen 1996; Weckroth 2004; Zeegelaar 1997).
One excluded study, Serena 2016, had similarities with two of the included studies (Harris 1995; Raffetto 2015), in that healing trajectories were analysed within a cross‐sectional study. However, the trajectories (healing and non‐healing) were derived from measurements of healing rate before the start of the study, rather than clinical assessment of the phase of the wound in the study. Therefore, we considered the study to be cross‐sectional and measuring diagnosis rather than prognosis. Additionally, Serena 2016 had only 32% VLUs and it would have been necessary to write to the authors.
Studies awaiting classification
We identified one study that is awaiting classification (Cullen 2009). This was a conference abstract and gave too little information.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
No study conducted multivariable analyses, but all 11 studies (13 cohorts) with useable results either had sufficient data to calculate univariate associations or reported correlations between protease measurements and a measure of healing. We did not report risk of bias assessments for studies with non‐useable results. However, all these studies were at high risk of bias due to inadequate analyses and lack of adjustment.
Figure 2 shows risk of bias judgements for each cohort. Judgements for each domain across studies are shown in Figure 3. These figures also included studies that did not have useable results and did not have risk of bias assessments, and these appear blank.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies with results. Studies without useable results do not have risk of bias assessments, and these appear blank.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study with results. Studies without useable results do not have risk of bias assessments, and these appear blank.
Overall, all studies (and all cohorts) except Mwaura 2006 were at high risk of bias (they had high risk of bias for at least two domains): this was commonly due to high risk of selection bias (five of 12 cohorts), outcome measurement bias (nine cohorts) and adjustment factor bias (11 cohorts). Mwaura 2006 was at moderate risk of bias overall.
Results
At the outset of this results section, we must stress that no reliable associations could be identified from the data presented here because all the evidence in this review was of very low certainty (see Certainty of the evidence section below). However, as this is a new type of review in the wounds field, we have presented the full set of (very low‐certainty) results in order to indicate possible factors that should inform future research and systematic review methodology in this area. We have not generally reported the numerical findings in the main text, but these can be seen in the forest plots as indicated.
Associations of protease activity (continuous data) with complete healing and partial healing
Healing as dichotomous data (versus no healing)
We used individual participant data (IPD) from one study including seven participants to conduct a univariate logistic regression analysis (Hoffman 1999). The OR per unit increase in elastase activity at baseline was 0.96 (95% CI 0.88 to 1.04). This was very low‐certainty evidence.
We used the data from each of six studies including 174 participants to carry out a 'reverse' t‐test of associations between healing and protease activity (Ahmad 2015; Harris 1995; Hoffman 1999; Mwaura 2006; Raffetto 2015; Serra 2013). The (theoretical) interpretation of this was that a negative SMD indicated that lower baseline levels of protease were associated with higher proportions of healing wounds at follow‐up (compared with non‐healing).
Results from all studies are shown on the same forest plot to visually display the findings and to allow possible influencing factors to be considered (Analysis 1.1; Figure 4).

Forest plot of comparison: 1 Protease activity continuous, outcome: 1.1 t‐test assocation of protease level and healing (dichotomous).
Two studies including 37 participants reported complete healing (versus no healing) (Ahmad 2015; Hoffman 1999). Both studies investigated serine proteases. This very low‐certainty evidence illustrated inconsistency in the point estimates regarding the direction of the association, high risk of bias and very wide CIs; in other words, it is completely unclear whether serine protease activity is associated with leg ulcer healing. Ahmad 2015 measured mainly bound and complexed forms of uPA protease and Hoffman 1999 appeared to measure the active form of neutrophil elastase.
Four studies including 137 participants reported partial healing (versus no healing) (Harris 1995; Mwaura 2006; Raffetto 2015; Serra 2013), with Harris 1995 comparing granulating/epithelialising ('healing') wounds with non‐granulating ('non‐healing') wounds in a type of case‐control study; Mwaura 2006 reporting a 'healing wound' as one with more than 20% decrease in wound size, decrease in slough and development of granulation tissue; and Serra 2013 comparing 'high healing' (1 cm²/week or greater decrease in size) and 'low healing' (less than 1 cm²/week) versus no healing. Raffetto 2015 reported protease levels for wounds in granulating versus inflammatory phases in a type of case‐control study, with protease levels given for a number of different proteases.
Raffetto 2015 (a conference abstract) suggested there may be two types of protease involved in wound healing, which they described as 'degrading' MMPs and 'reparative' MMPs. We explored this proposed differentiation of proteases in Analysis 1.1 (Figure 4). The degrading and reparative MMPs in Raffetto 2015 appeared to have different directions of association with healing, and the other studies may have supported this interpretation (Harris 1995; Mwaura 2006; Serra 2013). However, this was very low‐certainty evidence, dominated by a single study which identified healing and non‐healing wounds in the same cohort, and there may well be confounding and bias (Raffetto 2015).
Healing as dichotomous data ('high healing' versus 'low healing' chronic wounds)
Two studies including 95 participants examined the association of protease levels with 'high healing' versus 'low healing' chronic wounds (Serra 2013; Serra 2015a/Serra 2015b). Both studies defined 'high healing' wounds as those that had a change in wound size of 1 cm²/week or greater and 'low healing' wounds as those that had a change in wound size of less than 1 cm²/week. Findings are shown on a forest plot (Analysis 1.2; Figure 5). It was very uncertain whether there was a difference in protease activity between high‐ and low‐healing wounds.

Forest plot of comparison: 1 Protease activity continuous, outcome: 1.2 t‐test association of protease level and healing: high (≥ 1 cm²/week) versus low (< 1 cm²/week) healing.
Healing as continuous data
Three studies including 122 participants reported (or provided IPD to allow calculation of) correlation coefficients between protease levels and reduction or percentage reduction in ulcer size (i.e. healing); a positive correlation meant that as protease levels increased, the reduction in ulcer size increased (i.e. more healing) (Gohel 2008; Hoffman 1999; McDaniel 2017).
Results for all studies were represented on a forest plot, even though one had no numerical data (Analysis 1.3; Figure 6). Gohel 2008 reported narratively that there was "no relationship" between the initial levels (of proteases) and subsequent healing at five weeks (negative, but non‐significant correlation coefficients were reported for the change in protease levels versus the change in ulcer size between zero and five weeks, but these were cross‐sectional data only and therefore ineligible). McDaniel 2017, although measuring protease levels at baseline, only reported correlations between protease levels at four weeks and the percentage reduction in ulcer size between baseline and eight weeks; this study reported results for both arms of the RCT combined. Hoffman 1999 reported IPD for the seven participants included (four‐week protease levels and percentage reduction in size at 36 weeks). One of these data points appeared to be an outlier and we calculated the Pearson correlation coefficient and plotted a graph both with and without this outlier. This gave very different correlation coefficients (albeit with very wide CIs), and was thus an unstable finding; results from both calculations are shown on the forest plot. The evidence across all studies was of very low certainty showing wide CIs, high risk of bias and some inconsistency. Gohel 2008 also reported pro‐MMP levels, so was likely to represent indirect evidence for protease activity and McDaniel 2017 had, in effect, a very short follow‐up, and combined results for different intervention arms. It was possible that there was a difference in correlation coefficients for MMP‐8 and HNE in one study (McDaniel 2017), negative for MMP‐8 and no correlation for HNE, but the evidence was very uncertain.

Forest plot of comparison: 1 Protease activity continuous, outcome: 1.3 Correlation coefficients: protease levels and change in size.
Associations of protease activity (dichotomous data) with healing and partial healing
Two studies (three cohorts) including 164 participants allowed calculation of an RR for the univariate association of elevated protease activity with healing: Moffatt 2014a/Moffatt 2014b reported complete healing at 12 weeks and Cullen 2012 defined healing as at least 30% reduction in area over four weeks. We did not pool the data because of the difference in healing definition, but show the results on the forest plot (Analysis 2.1; Figure 7), grouped according to the interventions: Cullen 2012 gave all participants an 'established' protease modulating matrix dressing (collagen/ORC with or without silver); in the intervention arm Moffatt 2014a participants received oxyzyme/iodozyme treatment, which was postulated to be protease modulating. In the control group of Moffatt 2014b the treatment was not protease modulating.

Forest plot of comparison: 2 Protease activity dichotomous, outcome: 2.1 Association of healing with elevated protease level; by protease modulating (PM) intervention.
There was a positive association between elevated protease levels and healing in Cullen 2012 and an indication of a negative (expected) association in the usual care group of Moffatt 2014b. Such a trend might be explained by interactions between the protease‐modulating treatment and protease levels. In non‐healing wounds with elevated protease levels, the protease‐modulating treatment may have selectively activated the healing process by removing excess protease, but in non‐healing wounds with normal protease levels, the causes of non‐healing will have been different and may not have been affected by protease‐modulating treatments. However, given the very low certainty of the evidence, there are other biases or confounding factors that may also explain any trend, including the nature and timing of action of the different proteases.
Certainty of the evidence (GRADE)
All the evidence was of very low certainty: the exploratory study design meant the GRADE rating started as moderate‐certainty evidence (Appendix 4), and all studies except Mwaura 2006 were at overall high risk of bias (high risk of bias for at least two domains) and so the evidence certainty was downgraded by two more levels, taking the rating to very low certainty. There was also imprecision in the findings and some inconsistency. Furthermore, we considered it likely that in these small observational studies the possible biases and confounding factors would have a large impact on the results, so limiting their reliability even more.
Discussion
Summary of findings
It was uncertain whether protease activity was a prognostic factor for wound healing in people with VLUs because the certainty of the evidence was very low. Of the 21 cohorts found from extensive searching, only 13 (522 participants) had data available for analysis. Of these 13 cohorts, none reported the time‐to‐healing outcome and only two recorded the proportion of people with ulcers completely healed, with other studies recording partial healing measures (such as 1 cm²/week or greater decrease in size) or change in ulcer size. There was marked clinical and methodological heterogeneity across studies, which precluded meta‐analysis and made narrative summary difficult. This heterogeneity included differences in the definition of healing, the type of protease and its measurement, the distribution of active and bound protease species, the types of treatment and the reporting of results. No study conducted multivariable analysis or provided IPD to allow us to do so, and all the included evidence was of very low certainty. This should be interpreted as complete uncertainty of the role of protease activity in venous ulcer healing rather than evidence of no association. No reliance can be placed on the validity of the findings.
However, this review can provide important insights for future research on the potential prognostic factors in wound healing generally and of the value of proteases for venous ulcer healing specifically. We also hope that this review presents an exemplar for future systematic reviews of biomarkers as prognostic factors in wound healing and allows reflection on the current clinical context of protease‐modulating dressing use. We enlarge on these aspects below.
Considerations for further prognosis research
The review was unable to answer the question of whether protease activity was an independent prognostic factor for the healing of VLUs. The review highlighted the heterogeneous nature of protease activity reporting, a lack of standardisation of protease measurement and a lack of standardisation of how the healing outcome was defined and measured. Proteases may have a role in defining the status of a chronic wound, but the limitations in study reporting mean that it is impossible to draw a robust conclusion about the role of proteases as biomarkers for leg ulcer healing. This remains an important question, and there is a need for well‐conducted, further research. Therefore, we propose a large prognostic factor research study that employs an appropriate study design and analytical approach, uses advanced methods for measuring protease activity, and takes account of confounding factors and treatments in its analysis. The following elements are important: study design, protease measurement and appropriate analysis.
Study design
A large prospective cohort study with adequate follow‐up times to measure complete healing as time‐to‐event is a minimum requirement for new primary prognosis studies in this field.
Protease measurement
The type of proteases should be selected carefully and advanced protease activity measurement methods should be used to allow investigation of specific proteases and focus on the active form of the enzymes, rather than protease bound to other molecules or surfaces.
The measurement of proteases has evolved over time and continues to evolve, and the studies in this review used numerous techniques ranging from radiolabelled collagen breakdown, to zymograms, ELISAs, Western blots and fluorogenic substrates. These tests have now been superseded by more advanced protein evaluations, which should be used in future research.
It is also important to consider the type of proteases that would be measured in future research. Proteases have many different biological functions and activity of one protease does not necessarily provide a complete wound signature that relates to the wound phenotype. For example, specific MMPs such as MMP‐2 and MMP‐9 have a particular role in type IV and V collagen proteolysis, but this varies according to the study or clinical context. In addition, the different temporal and spatial location of proteases in the wound will depend on which phase of healing (coagulation, inflammation, proliferation, synthesis, remodelling) or how inflamed or infected the tissue is, hence the value of proteases as simple biomarkers may be limited. This would suggest a whole‐system approach to future research, that, as a minimum, takes into account combinations of proteases and time‐dependent effects, but preferably also considers demographics, comorbidities, wound duration, location and bioburden as possible confounding or interacting factors, which in turn could allow investigation of mechanisms of wound healing.
Appropriate analysis
A future study should use multivariable analysis of survival data, with continuous factors analysed on their continuous scale, and potential non‐linear relationships also considered. The study should be sufficiently powered to take account of adjusting factors (Knofczynski 2008). IPD meta‐analyses may be needed to standardise definitions and reduce heterogeneity across studies.
Finally, there may be interactions between protease levels and protease‐modulating treatments, which distort any associations being studied. Future studies should take interactions into consideration in the analyses.
Considerations for prognosis reviews in wound care
In the context of systematic reviews of biomarker prognostic factor studies in wounds, serious consideration should be given to restricting the reviews to well‐conducted studies with multivariable analyses, and identifying the ensuing evidence gaps separately.
Current clinical context
In the current clinical context, a number of protease‐modulating dressings have been marketed and are used, based on very limited research that postulates that reducing protease activity could promote healing and that timing may also be important (Harding 2011; McCarty 2013). We have shown in this review that the evidence for protease biomarkers being prognostic for ulcer healing is of very low certainty. We have also shown in our PMM intervention review that it is unclear whether PMM dressings are more effective than other dressings in healing VLUs (Westby 2016). There is currently no study evidence on the effectiveness of a test‐and‐treat strategy, in which VLUs with elevated levels of protease are selectively treated with PMM dressings (Norman 2016).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies with results. Studies without useable results do not have risk of bias assessments, and these appear blank.
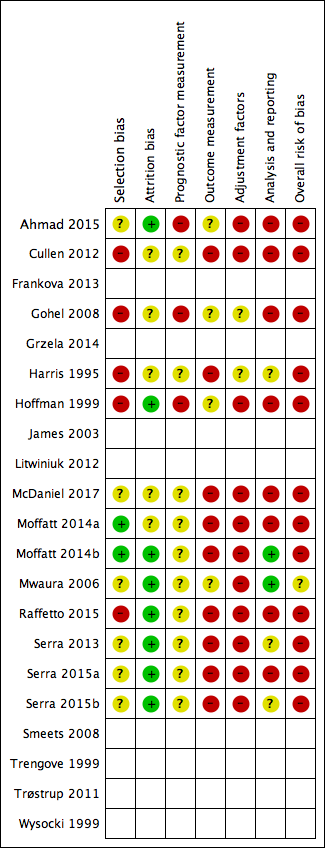
Risk of bias summary: review authors' judgements about each risk of bias item for each included study with results. Studies without useable results do not have risk of bias assessments, and these appear blank.
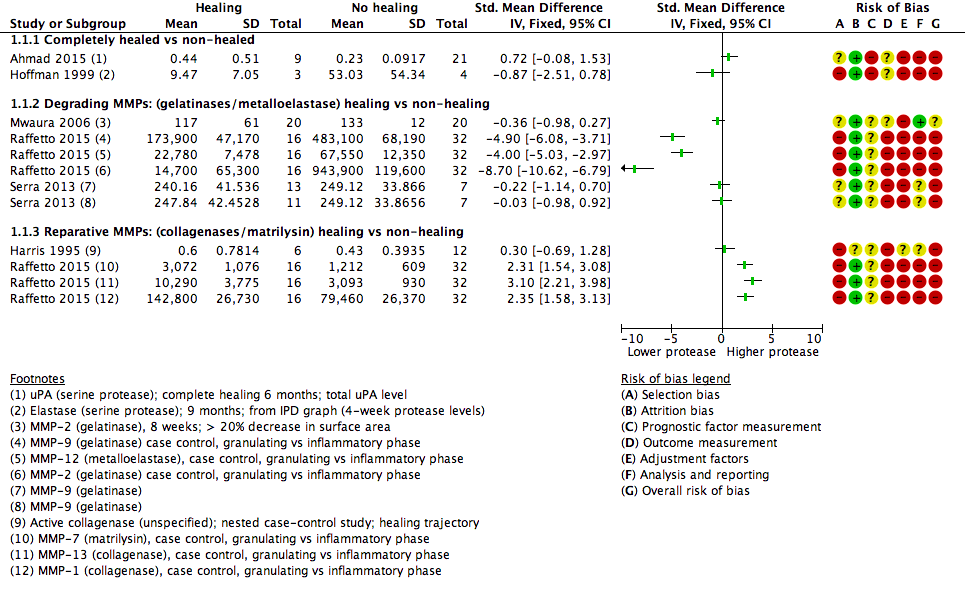
Forest plot of comparison: 1 Protease activity continuous, outcome: 1.1 t‐test assocation of protease level and healing (dichotomous).

Forest plot of comparison: 1 Protease activity continuous, outcome: 1.2 t‐test association of protease level and healing: high (≥ 1 cm²/week) versus low (< 1 cm²/week) healing.

Forest plot of comparison: 1 Protease activity continuous, outcome: 1.3 Correlation coefficients: protease levels and change in size.

Forest plot of comparison: 2 Protease activity dichotomous, outcome: 2.1 Association of healing with elevated protease level; by protease modulating (PM) intervention.

Prognostic factors for healing venous leg ulcers: participant characteristics. HR = hazard ratio; OR = odds ratio

Prognostic factors for venous leg ulcer healing: wound characteristics 1. HR = hazard ratio; OR = odds ratio
Prognostic factors for VLU healing: wound characteristics 2. HR = hazard ratio; OR = odds ratio
Prognostic factors for VLU healing: number of ulcers

Prognostic factors for venous leg ulcer healing: mobility and ankle flexion. HR = hazard ratio; OR = odds ratio

Prognostic factors for venous leg ulcer healing: bacteria and infection. HR = hazard ratio; OR = odds ratio; RR = risk ratio
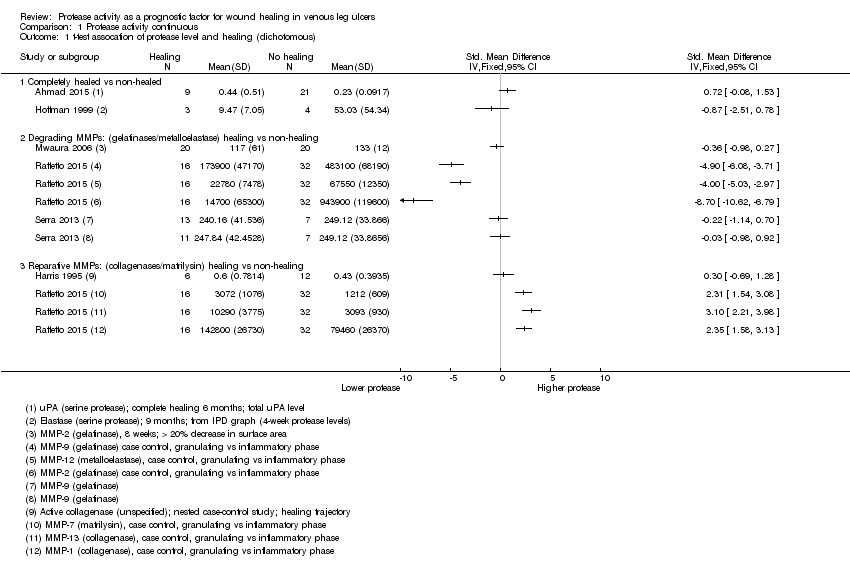
Comparison 1 Protease activity continuous, Outcome 1 t‐test assocation of protease level and healing (dichotomous).

Comparison 1 Protease activity continuous, Outcome 2 t‐test association of protease level and healing: high (≥ 1 cm²/week) vs low (< 1 cm²/week) healing.
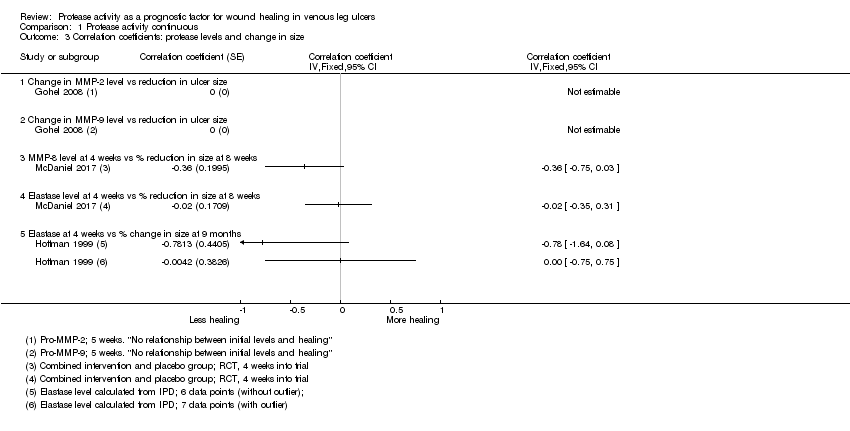
Comparison 1 Protease activity continuous, Outcome 3 Correlation coefficients: protease levels and change in size.

Comparison 2 Protease activity dichotomous, Outcome 1 Association of healing with elevated protease level; by protease modulating (PM) intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 t‐test assocation of protease level and healing (dichotomous) Show forest plot | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Completely healed vs non‐healed | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Degrading MMPs: (gelatinases/metalloelastase) healing vs non‐healing | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Reparative MMPs: (collagenases/matrilysin) healing vs non‐healing | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 t‐test association of protease level and healing: high (≥ 1 cm²/week) vs low (< 1 cm²/week) healing Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 With doxycycline treatment | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 With standard care treatment | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Treatment not reported (other than compression) | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Correlation coefficients: protease levels and change in size Show forest plot | 3 | Correlation coefficient (Fixed, 95% CI) | Totals not selected | |
| 3.1 Change in MMP‐2 level vs reduction in ulcer size | 1 | Correlation coefficient (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change in MMP‐9 level vs reduction in ulcer size | 1 | Correlation coefficient (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 MMP‐8 level at 4 weeks vs % reduction in size at 8 weeks | 1 | Correlation coefficient (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Elastase level at 4 weeks vs % reduction in size at 8 weeks | 1 | Correlation coefficient (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Elastase at 4 weeks vs % change in size at 9 months | 1 | Correlation coefficient (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Association of healing with elevated protease level; by protease modulating (PM) intervention Show forest plot | 3 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Elastase > 25 mU/100 μL vs ≤ 25 mU/100 μL + PM intervention | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 MMP (unspecified) level ≥ 5 (0‐10 scale) vs < 5 + possible PM intervention | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 MMP (unspecified) level ≥ 5 (0‐10 scale) vs < 5 + non‐PM intervention | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

