Anticuerpos anti‐IL‐12/23p40 para el mantenimiento de la remisión en la enfermedad de Crohn
Appendices
Appendix 1. Appendix 1
Embase
1 random$.tw.
2 factorial$.tw.
3 (crossover$ or cross over$ or cross‐over$).tw.
4 placebo$.tw.
5 single blind.mp.
6 double blind.mp.
7 triple blind.mp.
8 (singl$ adj blind$).tw.
9 (double$ adj blind$).tw.
10 (tripl$ adj blind$).tw.
11 assign$.tw.
12 allocat$.tw.
13 crossover procedure/
14 double blind procedure/
15 single blind procedure/
16 triple blind procedure/
17 randomized controlled trial/
18 or/1‐17
19 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20 18 not 19
21 exp Crohn disease/ or crohn*.mp. or exp colon Crohn disease/
22 (inflammatory bowel disease* or IBD).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
23 21 or 22
24 ustekinumab.mp. or exp ustekinumab/
25 briakinumab.mp. or exp briakinumab/
26 (abt‐874 or "cnto 1275").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
27 24 or 25 or 26
28 "interleukin 12".mp. or exp interleukin 12/
29 "interleukin 23".mp. or exp interleukin 23/
30 (IL‐12 or IL‐23 or p40).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
31 28 or 29 or 30
32 exp monoclonal antibody/ or exp antibody/ or antibod*.mp.
33 31 and 32
34 anti‐IL‐12 23p40.mp.
35 27 or 33 or 34
36 20 and 23 and 35
MEDLINE
1 random$.tw.
2 factorial$.tw.
3 (crossover$ or cross over$ or cross‐over$).tw.
4 placebo$.tw.
5 single blind.mp.
6 double blind.mp.
7 triple blind.mp.
8 (singl$ adj blind$).tw.
9 (double$ adj blind$).tw.
10 (tripl$ adj blind$).tw.
11 assign$.tw.
12 allocat$.tw.
13 randomized controlled trial/
14 or/1‐13
15 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
16 14 not 15
17 exp Crohn disease/ or crohn*.mp.
18 ("inflammatory bowel disease*" or IBD).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
19 17 or 18
20 (ustekinumab or briakinumab or "CNTO 1275" or ABT‐874).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
21 interleukin 12.mp. or exp Interleukin‐12/
22 interleukin 23.mp. or exp Interleukin‐23/
23 (IL‐12 or IL‐23 or p40).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
24 21 or 22 or 23
25 antibod*.mp. or exp Antibodies/ or exp Antibodies, Monoclonal/
26 24 and 25
27 IL‐12 23p40.mp.
28 20 or 26 or 27
29 16 and 19 and 28
Cochrane Library (CENTRAL)
1 ustekinumab or briakinumab or ABT‐874 or CNTO 1275
2 interleukin 12 or interleukin 23 or IL‐12 or il‐23 or p40
3 antibod*
4 #2 and #3
5 anti‐il‐12/23p40
6 #1 or #4 or #5
7 #6 and (Crohn* or IBD or "inflammatory bowel disease*")
ClinicalTrials.gov, clinicaltrials.ifpma.org, and isrctn.com
(interleukin 12, interleukin‐12, IL‐12, interleukin 23, interleukin‐23, IL‐23, p40, ustekinumab, CNTO 1275, briakinumab and ABT‐874) were all searched with the search term: crohn*

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Ustekinumab versus placebo, Outcome 1 Failure to maintain clinical remission at 22 weeks.

Comparison 1 Ustekinumab versus placebo, Outcome 2 Failure to maintain clinical response at 22 weeks.

Comparison 1 Ustekinumab versus placebo, Outcome 3 Failure to maintain clinical remission at 44 weeks.
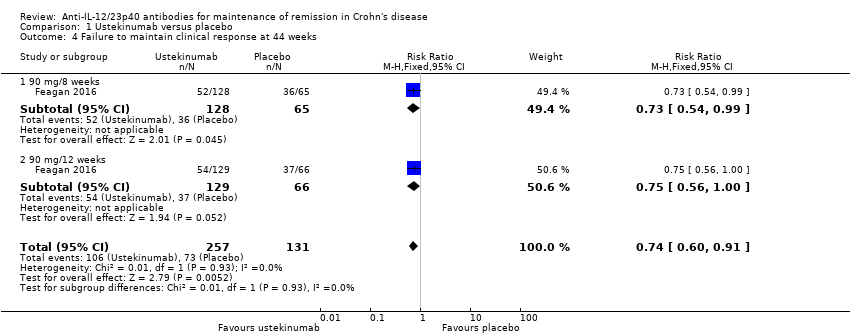
Comparison 1 Ustekinumab versus placebo, Outcome 4 Failure to maintain clinical response at 44 weeks.

Comparison 1 Ustekinumab versus placebo, Outcome 5 Adverse events.
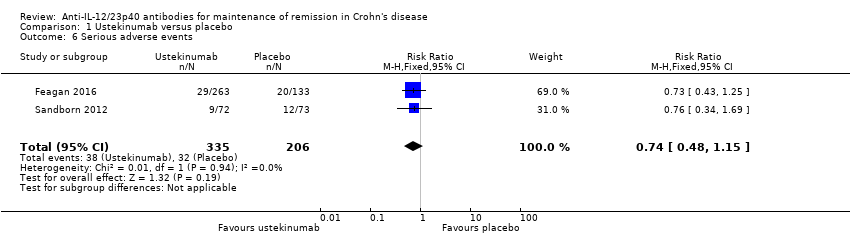
Comparison 1 Ustekinumab versus placebo, Outcome 6 Serious adverse events.

Comparison 1 Ustekinumab versus placebo, Outcome 7 Withdrawal due to adverse events.

Comparison 2 Briakinumab versus placebo, Outcome 1 Failure to maintain clinical remission at 24 weeks.

Comparison 2 Briakinumab versus placebo, Outcome 2 Failure to maintain clinical response at 24 weeks.

Comparison 2 Briakinumab versus placebo, Outcome 3 Adverse events.

Comparison 2 Briakinumab versus placebo, Outcome 4 Serious adverse events.

Comparison 2 Briakinumab versus placebo, Outcome 5 Withdrawal due to adverse events.
| Ustekinumab compared to placebo for maintenance of remission in Crohn's disease | ||||||
| Patient or population: people with moderate to severe Crohn's disease in remission | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with ustekinumab | |||||
| Failure to maintain clinical remission Follow‐up: 22 weeks | 726 per 1000 | 581 per 1000 | RR 0.80 | 145 | ⊕⊕⊕⊝ | Clinical remission was defined as a CDAI < 150 points. |
| Failure to maintain clinical response Follow‐up: 22 weeks | 575 per 1000 | 305 per 1000 | RR 0.53 | 145 | ⊕⊕⊕⊝ | Clinical response was defined as a ≥ 100‐point decrease from the baseline CDAI score. |
| Failure to maintain clinical remission Follow‐up: 44 weeks | 641 per 1000 | 487 per 1000 (410 to 584) | RR 0.76 (0.64 to 0.91) | 388 (1 RCT) | ⊕⊕⊕⊝ | Clinical remission was defined as a CDAI < 150 points. |
| Failure to maintain clinical response Follow‐up: 44 weeks | 557 per 1000 | 412 per 1000 (334 to 507) | RR 0.74 (0.60 to 0.91) | 388 (1 RCT) | ⊕⊕⊕⊝ | Clinical response was defined as a decrease from baseline in the CDAI score of ≥ 100 points or a CDAI score < 150. |
| Adverse events Follow‐up: 44 weeks | 840 per 1000 | 789 per 1000 (731 to 865) | RR 0.94 (0.87 to 1.03) | 541 (2 RCTs) | ⊕⊕⊕⊕ | Commonly reported adverse events included worsening Crohn's disease, abdominal pain, nausea, arthralgia, and headache. |
| Serious adverse events Follow‐up: 44 weeks | 155 per 1000 | 115 per 1000 | RR 0.74 | 541 | ⊕⊕⊕⊝ | Commonly reported serious adverse events included malignant neoplasm, basal cell carcinoma, and injection site reactions. |
| Withdrawal due to adverse events Follow‐up: 44 weeks | 14 per 1000 | 68 per 1000 (8 to 572) | RR 4.93 (0.59 to 41.18) | 145 (1 RCT) | ⊕⊕⊝⊝ | Adverse events leading to withdrawal included worsening Crohn's disease |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to sparse data (95 events). | ||||||
| Briakinumab compared to placebo for maintenance of remission in Crohn's disease | ||||||
| Patient or population: people with moderate to severe Crohn's disease in remission | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with briakinumab | |||||
| Failure to maintain clinical remission Follow‐up: 24 weeks | 611 per 1000 | 513 per 1000 (354 to 733) | RR 0.84 (0.58 to 1.20) | 99 (1 RCT) | ⊕⊕⊝⊝ | Clinical remission was defined as a CDAI score < 150 points. |
| Failure to maintain clinical response Follow‐up: 24 weeks | 528 per 1000 | 338 per 1000 (211 to 538) | RR 0.64 (0.40 to 1.02) | 99 (1 RCT) | ⊕⊕⊝⊝ | Clinical response was defined as a decrease in CDAI score ≥ 100 points compared with week 0. |
| Adverse events Follow‐up: 24 weeks | 643 per 1000 | 656 per 1000 (431 to 996) | RR 1.02 (0.67 to 1.55) | 104 (1 RCT) | ⊕⊕⊝⊝ | Common adverse events included upper respiratory tract infection, nausea, abdominal pain, and headache. |
| Serious adverse events Follow‐up: 24 weeks | 71 per 1000 | 22 per 1000 (2 to 229) | RR 0.31 (0.03 to 3.21) | 104 (1 RCT) | ⊕⊕⊝⊝ | Serious adverse events included small bowel obstruction, deep vein thrombosis, and respiratory distress. |
| Withdrawals due to adverse events Follow‐up: 24 weeks | 0 per 1000 | 0 per 1000 (0 to 0) | RR 0.82 (0.04 to 16.34) | 104 (1 RCT) | ⊕⊕⊝⊝ | We were unable to calculate absolute effects. 2% of briakinumab participants withdrew due to an adverse event compared to none of the placebo participants. Adverse events leading to withdrawal were not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDAI: Crohn's Disease Activity Index; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to sparse data (54 events) and very wide confidence interval. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to maintain clinical remission at 22 weeks Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.02] |
| 2 Failure to maintain clinical response at 22 weeks Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.79] |
| 3 Failure to maintain clinical remission at 44 weeks Show forest plot | 1 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.64, 0.91] |
| 3.1 90 mg/8 weeks | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.94] |
| 3.2 90 mg/12 weeks | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.03] |
| 4 Failure to maintain clinical response at 44 weeks Show forest plot | 1 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.60, 0.91] |
| 4.1 90 mg/8 weeks | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.54, 0.99] |
| 4.2 90 mg/12 weeks | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.00] |
| 5 Adverse events Show forest plot | 2 | 541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.03] |
| 6 Serious adverse events Show forest plot | 2 | 541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.15] |
| 7 Withdrawal due to adverse events Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.93 [0.59, 41.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to maintain clinical remission at 24 weeks Show forest plot | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.20] |
| 1.1 200 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.02] |
| 1.2 Continued 400 mg | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.43, 1.24] |
| 1.3 700 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.38, 1.63] |
| 2 Failure to maintain clinical response at 24 weeks Show forest plot | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.40, 1.02] |
| 2.1 200 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.30, 1.78] |
| 2.2 Continued 400 mg | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.30, 1.16] |
| 2.3 700 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.60] |
| 3 Adverse events Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.55] |
| 4 Serious adverse events Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 3.21] |
| 5 Withdrawal due to adverse events Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.04, 16.34] |

