Intervenciones para la prevención del síndrome de obstrucción intestinal distal (SOID) en la fibrosis quística
Appendices
Appendix 1. Glossary of terms
| Term | Explanation |
|---|---|
| anaphylactic reaction | a life‐threatening allergic reaction that may result in severe respiratory and/or cardiovascular distress and skin reactions; it is a medical emergency |
| autosomal recessive | a form of genetic inheritance in which two copies of a gene are required for a characteristic or condition to be carried to the offspring |
| caecum | this is the beginning of the large intestine and is connected to the end of the small intestine (known as the terminal ileum); it is located in the right lower quadrant of the abdomen |
| dyschezia | painful defecation |
| emesis | vomiting |
| intestinal perforation | a hole that forms in the intestine causing its contents to leak into the abdomen; this is a surgical emergency |
| mucosal erosions | the wearing away or abrasion of a surface or lining, e.g. gastric erosions relates to abrasion of the stomach lining |
| synonymous | terms or words that have the same meaning e.g. small is synonymous with petite |
| terminal ileum | the end of the small intestine, it is connected to the caecum (see above) |
| vomiting with electrolyte disturbance | severe vomiting that leads to important electrolytes e.g. sodium, potassium, calcium being lost from the body |
For definitions of statistical and methodological Cochrane terms (e.g. cross‐over trial, funnel plot, forest plot, heterogeneity, quasi‐randomised controlled trial) please see the Cochrane Community Glossary.
Appendix 2. Search strategies
| Database/Resource | Strategy |
|---|---|
| Cochrane Central Register of Controlled Trials (CENTRAL) | #1 Cystic Fibrosis [MeSH descriptor] #2 cystic fibrosis:ti,ab #3 fibrocystic near/10 disease near/10 pancreas #4 mucoviscidos*:ti,ab #5 cystic* near/10 fibros*:ti,ab #6 #1 or #2 or #3 or #4 or #5 #7 distal intestinal obstruction syndrome*:ti,ab #8 dios or mie:ti,ab #9 Intestinal Obstruction [MeSH descriptor] #10 meconium ileus equivalent:ti,ab #11 faecal near/3 (obstruction or impact*):ti,ab #12 Constipation [MeSH descriptor] #13 constipat*:ti,ab #14 laxative*:ti,ab #15 Laxatives [MeSH descriptor] #16 lactulose:ti,ab #17 Lactulose [MeSH descriptor] #18 (macrogol or polyethylene glycol*):ti,ab #19 Polyethylene Glycols [MeSH descriptor] #20 movicol:ti,ab #21 klean*:ti,ab #22 diatriozate:ti,ab #23 gastrografin:ti,ab #24 sennati:ti,ab #25 docusate:ti,ab #26 bicosulfate:ti,ab #27 acetylcysteine or fibrol:ti,ab #28 parvolex:ti,ab #29 fibre:ti,ab #30 picosulphate:ti,ab #31 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 #30 #32 #6 and #31 |
| MEDLINE Ovid (1946 onwards) | 1. Cystic Fibrosis/ 2. cystic fibrosis.tw. 3. (fibrocystic adj10 disease adj10 pancreas).tw. 4. mucoviscidos$.tw. 5. (cystic$ adj10 fibros$).tw. 6. 1 or 2 or 3 or 4 or 5 7. "distal intestinal obstruction syndrome*".tw. 8. (dios or mie).tw. 9. Intestinal Obstruction/ 10. meconium ileus equivalent.tw. 11. (faecal adj3 (obstruction or impact*)).tw. 12. Constipation/ 13. "constipat*".tw. 14. "laxative*".tw. 15. Laxatives/ 16. lactulose.tw. or Lactulose/ 17. (macrogol or polyethylene glycol*).tw. or Polyethylene Glycols/ 18. movicol.tw. 19. klean*.tw. 20. diatriozate.tw. 21. gastrografin.tw. 22. senna.tw. 23. docusate.tw. 24. bicosulfate.tw. 25. acetylcysteine or fibrol.tw. 26. parvolex.tw. 27. fibre.tw. 28. picosulphate.tw. 29. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 6 and 29 |
| Embase Ovid (1974 onwards) | 1. CYSTIC FIBROSIS/ 2. cystic fibrosis.tw. 3. (fibrocystic adj10 disease adj10 pancreas).tw. 4. mucoviscidos$.tw. 5. (cystic$ adj10 fibros$).tw. 6. 1 or 2 or 3 or 4 or 5 7. "distal intestinal obstruction syndrome*".tw. 8. (dios or mie).tw. 9. INTESTINE OBSTRUCTION/ 10. meconium ileus equivalent.tw. 11. (faecal adj3 (obstruction or impact*)).tw. 12. CONSTIPATION/ 13. "constipat*".tw. 14. "laxative*".tw. 15. LAXATIVE/ 16. lactulose.tw. or LACTULOSE/ 17. (macrogol or polyethylene glycol*).mp,hw. 18. movicol.tw. 19. klean*.tw. 20. diatriozate.tw. 21. gastrografin.tw. 22. senna.tw. 23. docusate.tw. 24. bicosulfate.tw. 25. acetylcysteine or fibrol.tw. 26. parvolex.tw. 27. fibre.tw. 28. picosulphate.tw. 29. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 6 and 29 |
| Clinicaltrials.gov | ADVANCED SEARCH Search 1 Search 2 |
| ISRCTN Registry | ADVANCED SEARCH Condition: cystic fibrosis |
| WHO ICTRP | BASIC SEARCHES Search 1: cystic fibrosis AND intestinal Search 2: cystic fibrosis AND constipation Search 3: cystic fibrosis AND faecal Search 4: cystic fibrosis AND meconium Search 5: mucoviscidose ADVANCED SEARCH Intervention: laxative OR laxatives OR lactulose OR macrogol OR polyethylene OR movicol OR klean OR diatriozate OR gastrografin OR senna OR docusate OR bicosulfate OR acetylcysteine OR fibrol OR parvolex OR picosulphate OR fibre Recruitment Status: All |
| Open Grey | (cystic fibrosis OR cf OR mucoviscidos*) AND (intestin* OR constipat* OR faecal OR meconium OR laxative* OR lactulose OR macrogol OR polyethylene OR movicol OR klean* OR diatriozate OR gastrografin OR senna OR docusate OR bicosulfate OR acetylcysteine OR fibrol OR parvolex OR picosulphate OR fibre) |
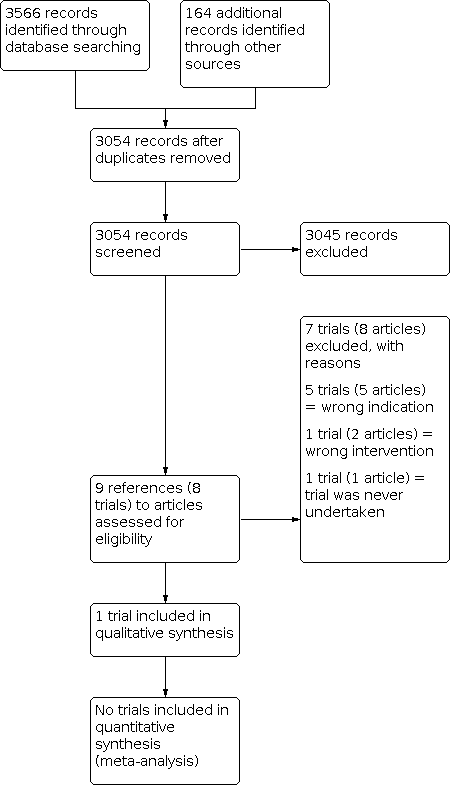
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| High‐dose pancreatic enzymes compared with low‐dose pancreatic enzymes for treating DIOS | ||||||
| Patient or population: children and adults with cystic fibrosis Settings: outpatient Intervention: high‐dose pancreatic enzymes Comparison: low‐dose pancreatic enzymes | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Low‐dose pancreatic enzymes | High‐dose pancreatic enzymes | |||||
| Time taken from start of treatment until the resolution of DIOS | Outcome not reported.
| N/A |
| |||
| Treatment failure rate | Outcome not reported. | N/A |
| |||
| Adherence | Outcome not reported. | N/A |
| |||
| Episodes of acute DIOS
Follow‐up: at 12 months | There was no difference between low‐dose and high‐dose pancreatic enzymes. | N/A | 20 (1 trial) | ⊕⊝⊝⊝
|
| |
| Adverse effects: abdominal mass
Follow‐up: at 12 months | There was no difference between low‐dose and high‐dose pancreatic enzymes. | N/A | 20 (1 trial) | ⊕⊝⊝⊝
|
| |
| Adverse effects: abdominal pain
Follow‐up: at 12 months | There was no difference between low‐dose and high‐dose pancreatic enzymes. | N/A | 20 (1 trial) | ⊕⊝⊝⊝
|
| |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a The method of measurement of episodes of acute DIOS (e.g. numbers, %, per person or total number of episodes) was not described in the trial. b The small number of participants in the trial (20) decreases the precision of the results. c The participants had a very limited age range (7.1 years to 23.2 years), making the evidence for these outcomes restricted to this group, and therefore increasing its indirectness. d The majority of the risk of bias domains were ranked as unclear (selection bias, attrition bias and reporting bias) as there was little information provided in the trial. eNo specific numerical data were provided for the results of these outcomes, therefore we cannot be sure of the significance or relevance of these results. f There was no washout period described in this cross‐over trial, therefore we cannot be sure whether there was a carry‐over effect of the treatments. | ||||||

