Transfusiones plaquetarias profilácticas antes de la cirugía para pacientes con recuento plaquetario bajo
Resumen
Antecedentes
Los pacientes con trombocitopenia a menudo necesitan un procedimiento quirúrgico. Un recuento plaquetario bajo es una contraindicación relativa a la cirugía debido al riesgo de hemorragia. Las transfusiones de plaquetas se utilizan en la práctica clínica para prevenir y tratar las hemorragias en pacientes con trombocitopenia. La práctica actual en muchos países incluye la corrección de la trombocitopenia con transfusiones de plaquetas antes de la cirugía. También se utilizan alternativas a la transfusión plaquetaria antes de la cirugía.
Objetivos
Determinar la efectividad y la seguridad clínica de las transfusiones plaquetarias profilácticas antes de la cirugía para los pacientes con un recuento plaquetario bajo.
Métodos de búsqueda
Se buscó en las siguientes bases de datos principales: Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL; 2017, número 2), PubMed (solo publicaciones electrónicas), Ovid MEDLINE, Ovid Embase, la Transfusion Evidence Library y bases de datos de ensayos en curso hasta el 11 de diciembre de 2017.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios (ECA), así como los ensayos controlados no aleatorios y los estudios controlados tipo antes y después (CAD), que cumplieron con los criterios del Cochrane EPOC (Effective Practice and Organisation of Care) y que incluyeron la transfusión plaquetaria antes de la cirugía (cualquier dosis, en cualquier momento, única o múltiple) en pacientes con recuentos plaquetarios bajos. Se excluyeron los estudios en pacientes con un recuento plaquetario bajo que presentaban hemorragias activas.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos para la recolección de datos según Cochrane. Solo fue posible combinar los datos de dos resultados y el resto de los resultados se presentaron de forma narrativa.
Resultados principales
Se identificaron cinco ECA, todos realizados en pacientes adultos; no hubo estudios no aleatorios elegibles. Tres ensayos completados reclutaron a 180 adultos y dos ensayos en curso tienen como objetivo incluir a 627 participantes. Los ensayos completados se realizaron entre 2005 y 2009. Los dos ensayos en curso tienen programado completar el reclutamiento en octubre de 2019. Un ensayo comparó las transfusiones plaquetarias profilácticas con ninguna transfusión en pacientes con trombocitopenia en una unidad de cuidados intensivos (UCI). Dos ensayos pequeños, 108 participantes, compararon las transfusiones plaquetarias profilácticas con otros tratamientos alternativos en pacientes con enfermedades hepáticas. Un ensayo comparó la desmopresina con plasma fresco congelado o una unidad de transfusión de plaquetas, o ambos, antes de la cirugía. El segundo ensayo comparó la transfusión plaquetaria antes de la cirugía con dos tipos de miméticos de trombopoyetina: el romiplostim y el eltrombopag. Ninguno de los ensayos incluidos estuvo exento de sesgo metodológico. Ningún ensayo incluido comparó diferentes umbrales del recuento plaquetario para la administración de una transfusión plaquetaria profiláctica antes de la cirugía. Ninguno de los ensayos incluidos informó todos los resultados de la revisión y la calidad general por resultado informado fue muy baja.
Ninguno de los tres ensayos completados informó: mortalidad por todas las causas a los 90 días después de la cirugía; mortalidad secundaria a hemorragia, tromboembolia o infección; número de transfusiones de eritrocitos o plaquetas por participante; duración de la estancia hospitalaria; o calidad de vida.
Ninguno de los ensayos incluyó a niños ni a pacientes que necesitaran cirugía mayor o procedimientos quirúrgicos de urgencia.
Transfusión plaquetaria versus ninguna transfusión plaquetaria (un ensayo, 72 participantes)
No existe mucha seguridad con respecto a si la administración de una transfusión plaquetaria antes de la cirugía tuvo un efecto sobre la mortalidad por todas las causas en el transcurso de 30 días (un ensayo, 72 participantes; cociente de riesgos [CR] 0,78; intervalo de confianza [IC] del 95%: 0,41 a 1,45; evidencia de muy baja calidad). No existe mucha seguridad con respecto a si la administración de una transfusión plaquetaria antes de la cirugía tuvo un efecto sobre el riesgo de hemorragia grave (un ensayo, 64 participantes; CR 1,60; IC del 95%: 0,29 a 8,92; evidencia de muy baja calidad), o leve (un ensayo, 64 participantes; CR 1,29; IC del 95%: 0,90 a 1,85; evidencia de muy baja calidad). No ocurrieron eventos adversos graves en los brazos de estudio (un ensayo, 72 participantes, evidencia de muy baja calidad).
Transfusión plaquetaria versus alternativa a la transfusión plaquetaria (dos ensayos, 108 participantes)
No existe mucha seguridad con respecto a si la administración de una transfusión plaquetaria antes de la cirugía en comparación con una alternativa tiene algún efecto sobre el riesgo de hemorragia grave (dos ensayos, 108 participantes; ningún evento; evidencia de muy baja calidad), o leve (desmopresina: un ensayo, 36 participantes; CR 0,89; IC del 95%: 0,06 a 13,23; evidencia de muy baja calidad: miméticos de trombopoyetina: un ensayo, 65 participantes; ningún evento; evidencia de muy baja calidad). No existe mucha seguridad con respecto a si hubo una diferencia en los efectos adversos relacionados con la transfusión entre el grupo que recibió transfusión de plaquetas y el grupo de tratamiento alternativo (desmopresina: un ensayo, 36 participantes; CR 2,70; IC del 95%: 0,12 a 62,17; evidencia de muy baja calidad).
Conclusiones de los autores
Los resultados de esta revisión se basaron en tres ensayos pequeños de cirugía menor en adultos con trombocitopenia. No se encontró evidencia suficiente para recomendar la administración de transfusiones plaquetarias profilácticas antes del procedimiento en esta situación y existe falta de evidencia con respecto a si la transfusión dio lugar a una reducción de la hemorragia posoperatoria o la mortalidad por todas las causas. El escaso número de ensayos que cumplieron con los criterios de inclusión y la limitación en los resultados informados entre los ensayos impidió el metanálisis de la mayoría de los resultados. Se necesitan ensayos adicionales con poder estadístico adecuado en pacientes de todas las edades de las transfusiones plaquetarias profilácticas en comparación con ninguna transfusión y otros tratamientos alternativos, que consideren diferentes umbrales plaquetarios antes de los procedimientos quirúrgicos planificados y de urgencia. Los ensayos futuros deben incluir la cirugía mayor e informar sobre la hemorragia, los efectos adversos, la mortalidad (como un resultado a largo plazo) después de la cirugía, la duración de la estancia hospitalaria y las medidas de calidad de vida.
PICO
Resumen en términos sencillos
Transfusión plaquetaria antes de la cirugía para los pacientes con recuentos plaquetarios bajos
Pregunta de la revisión
Se procuró evaluar la seguridad y la efectividad clínica de la administración de transfusiones plaquetarias a los pacientes con un recuento plaquetario bajo que necesitan cirugía. Se incluyeron tres comparaciones: administración de transfusión plaquetaria versus ninguna transfusión plaquetaria; administración de transfusión plaquetaria versus un tratamiento alternativo (medicación que reduce el riesgo de hemorragia o aumenta el recuento plaquetario) o administración de transfusiones plaquetarias cuando el recuento plaquetario está por debajo de un número establecido (recuento plaquetario bajo p.ej., 20 × 109/l versus algo mayor p.ej., 50 × 109/l).
La población estudiada fue pacientes con un recuento plaquetario bajo de cualquier edad que requirieron cirugía. Se excluyeron los estudios en pacientes con un recuento plaquetario bajo que presentaban hemorragias activas.
Mensajes clave
No hubo evidencia suficiente para ayudar a guiar la administración de transfusiones plaquetarias antes de la cirugía en los pacientes con un recuento plaquetario bajo. No existe evidencia sobre los neonatos y los niños ni antes de una cirugía mayor.
¿Qué se estudió en esta revisión?
Las plaquetas son células diminutas en la sangre que forman coágulos para ayudar a interrumpir las hemorragias. Si un paciente con un recuento plaquetario bajo requiere cirugía está en mayor riesgo de hemorragia durante y después de la cirugía. Se utilizan varias estrategias para reducir el riesgo de hemorragia, que incluyen: la administración de transfusiones plaquetarias (inyección de plaquetas en la sangre) para aumentar el recuento plaquetario, la administración de medicación para aumentar al recuento plaquetario y la administración de medicación que reduce el riesgo de hemorragia. Las estrategias actuales no se basan en evidencia convincente, sino que se basan en la experiencia y el criterio clínico individual.
¿Cuáles son los principales resultados de esta revisión?
Se encontraron cinco ensayos controlados aleatorios (estudios clínicos en que los participantes se asignan al azar a uno de dos o más grupos de tratamiento) elegibles para inclusión en esta revisión. Dos de estos estudios todavía están en curso. Los tres ensayos completados eran pequeños, con un total combinado de 180 pacientes. Todos eran pacientes adultos que requerían cirugía menor o un procedimiento invasivo (un procedimiento que se realiza a través de la piel o una cavidad del cuerpo o una abertura anatómica). Dos ensayos se realizaron con pacientes adultos con enfermedades hepáticas y un ensayo se realizó con pacientes adultos en una unidad de cuidados intensivos. Un ensayo comparó la transfusión de plaquetas con ninguna transfusión. Un ensayo comparó la transfusión de plaquetas con fármacos que aumentaron el recuento plaquetario. Un ensayo comparó la transfusión de plaquetas con un fármaco que redujo el riesgo de hemorragia. No se encontraron estudios que compararan la administración de transfusiones de plaquetas con el uso de diferentes niveles de recuentos plaquetarios como una guía para la transfusión plaquetaria.
No hubo evidencia suficiente para determinar si las transfusiones plaquetarias afectaron el riesgo de muerte por cualquier causa, la hemorragia leve o grave relacionada con el procedimiento o el riesgo de un efecto secundario grave. La única evidencia disponible fue de muy baja calidad debido a que: las estimaciones fueron muy imprecisas, los estudios tuvieron riesgo de sesgo (los participantes y los médicos sabían qué tratamiento recibían) y la evidencia solo se relaciona con pacientes adultos con enfermedades hepáticas que requerían un procedimiento dental o biopsia hepática (muestra del hígado tomada para el análisis) o pacientes adultos en unidades de cuidados intensivos que requerían la inserción de un tubo para ayudar con la respiración (traqueotomía).
Ninguno de los estudios informó: muerte por cualquier causa a los 90 días después de la cirugía; muerte causada por hemorragia, infección o coágulo sanguíneo; número de eritrocitos (células que transportan oxígeno en la sangre) o transfusiones plaquetarias que recibió cada participante; duración de la estancia hospitalaria o calidad de vida.
Ninguno de los ensayos incluyó a niños ni a pacientes que necesitaran cirugía mayor o procedimientos quirúrgicos de urgencia.
¿Qué grado de actualización tiene esta evidencia?
Se buscaron estudios publicados hasta diciembre 2017.
Conclusiones de los autores
Summary of findings
| Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: no platelet transfusion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no platelet transfusion | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery | Study population | RR 0.78 | 72 | ⊕⊝⊝⊝ | — | |
| 405 per 1000 | 316 per 1000 | |||||
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery (surgical site bleeding requiring a second intervention or reoperation or surgical site bleeding that causes a haematoma or haemarthrosis of sufficient size to delay mobilisation or wound healing) | Study population | RR 1.60 | 64 | ⊕⊝⊝⊝ | — | |
| 61 per 1000 | 97 per 1000 | |||||
| The number of participants with minor procedure‐related bleeding within 7 days of surgery | Study population | RR 1.29 | 64 | ⊕⊝⊝⊝ | — | |
| 576 per 1000 | 743 per 1000 | |||||
| Serious adverse events (surgery‐related adverse effects within 30 days) | No events occurred in either study arm | 64 | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly adults in the intensive care unit were included in this trial (downgraded one level for indirectness). bThe confidence intervals included a serious risk of harm or benefit (downgraded two levels for imprecision). cThis is a subjective outcome and the trial was unblinded (downgraded one level for risk of bias). dThe confidence intervals included a risk of harm or benefit (downgraded one level for imprecision). | ||||||
| Prophylactic platelet transfusion prior to surgery versus alternative treatments | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: desmopressin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with desmopressin | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery (bleeding that required ≥ 2 units of whole blood/red blood cells within 24 hours of the bleeding) | No events in either study arm | 36 | ⊕⊝⊝⊝ | — | ||
| Number of participants with minor procedure‐related bleeding within 7 days of surgery | Study population | RR 0.89 | 36 | ⊕⊝⊝⊝ | — | |
| 59 per 1000 | 52 per 1000 | |||||
| Serious adverse events (transfusion‐related adverse effects within 24 hours of the transfusion) | Study population | RR 2.70 | 36 | ⊕⊝⊝⊝ | — | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOpen‐label trial (downgraded one level for risk of bias). bStudy only included adults with chronic liver disease (downgraded one level for indirectness). cConfidence intervals included a serious risk or benefit or treatment (downgraded one level for imprecision, as already downgraded one level for indirectness and risk of bias). | ||||||
| Different platelet count thresholds for administering a prophylactic platelet transfusion prior to surgery | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: TPO mimetic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TPO mimetic | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery | No bleeding in any of the study arms | 65 | ⊕⊝⊝⊝ | — | ||
| Number of participants with minor procedure‐related bleeding within 7 days of surgery | No bleeding occurred in any of the study arms | 65 | ⊕⊝⊝⊝ | — | ||
| Serious adverse events – not reported | — | — | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; TPO: thrombopoietin. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aStudy only included adults with chronic liver disease (downgraded one level for indirectness). bNo events occurred (downgraded two levels for imprecision). | ||||||
Antecedentes
Descripción de la afección
Las plaquetas son un componente esencial en la formación de un coágulo sanguíneo (BCSH 2003). Un recuento plaquetario bajo puede dar lugar a una variedad de síntomas de hemorragia como equimosis, hemorragias nasales y, con poca frecuencia, hemorragia potencialmente mortal o mortal.
La trombocitopenia se define como un recuento plaquetario menor de 150 × 109/l (BCSH 2003). Cuando es dilucional, asociada a una expansión del volumen sanguíneo, la disminución es leve y rara vez clínicamente significativa. La trombocitopenia grave se define como un recuento plaquetario menor de 50 × 109/l (BCSH 2003). La trombocitopenia puede ser causada por: una producción reducida de plaquetas en la médula ósea a menudo como resultado de la quimioterapia o de una neoplasia maligna hematológica (cáncer de la sangre) (Leguit 2010; Weinzierl 2013); un aumento en el consumo plaquetario como ocurre en la hemorragia o en la coagulación intravascular diseminada (Levi 2009); un aumento en la destrucción plaquetaria como la trombocitopenia inmune (TPI) (Neunert 2013; Pacheco 2011; Provan 2010); o una combinación de estos trastornos.
La trombocitopenia dilucional leve es frecuente en el embarazo (7% al 12% de los embarazos), pero la trombocitopenia grave es mucho menos frecuente (0,05% al 1% de los embarazos) y es un signo de complicaciones (Burrows 1990; Nisha 2012; Sainio 2000). Un recuento plaquetario menor de 150 × 109/l es muy frecuente en los pacientes con hepatopatía crónica (hasta el 76%) (Afdhal 2008) y en los pacientes en estado crítico (hasta el 68%) (Hui 2011). Un estudio grande del Reino Unido en pacientes en una unidad de cuidados intensivos (UCI) informó que el 9% desarrolló trombocitopenia grave (Stanworth 2013). La trombocitopenia también es frecuente en los pacientes con neoplasias malignas hematológicas (Leguit 2010; Weinzierl 2013) y la mayoría de las transfusiones plaquetarias se administran a los pacientes con trastornos hematológicos (Cameron 2007; Greeno 2007; Pendry 2011).
Los pacientes con trombocitopenia a menudo necesitan un procedimiento quirúrgico. Un recuento plaquetario bajo es una contraindicación relativa a la cirugía debido al riesgo de hemorragia (Estcourt 2017a; Kaufman 2015; NICE 2015). Las transfusiones plaquetarias son una de varias intervenciones utilizadas en la práctica clínica moderna para prevenir y tratar la hemorragia en los pacientes con trombocitopenia.
Descripción de la intervención
Los concentrados plaquetarios son el segundo componente sanguíneo utilizado con mayor frecuencia (Bolton‐Maggs 2016). En los EE.UU. se transfunden por año aproximadamente 2 200 000 unidades de plaquetas (Whitaker 2013). El 74% de las transfusiones plaquetarias se administran como profilaxis a los pacientes con trombocitopenia que no presentan hemorragias y el 15% se administra para prevenir la hemorragia antes de la cirugía o de un procedimiento en los pacientes con neoplasias malignas hematológicas. En muchos casos, las transfusiones de plaquetas se administran cuando los recuentos plaquetarios son mayores que los niveles desencadenantes recomendados (Estcourt 2012; Greeno 2007).
A diferencia de otros componentes de la sangre, las plaquetas se deben mantener en un agitador a temperatura ambiente, lo que limita el período máximo de almacenamiento de las unidades de plaquetas de cinco a siete días. Este hecho dificulta el manejo de la reserva plaquetaria por los hospitales (Fuller 2011).
La práctica actual en muchos países incluye la corrección de la trombocitopenia con transfusiones de plaquetas antes de la cirugía. Las guías a menudo recomiendan un umbral del recuento plaquetario de 50 × 109/l antes de una cirugía mayor y de 100 × 109/l antes de una cirugía que involucra el cerebro o los ojos (Estcourt 2017a; Kaufman 2015; NICE 2015). Las guías a menudo no entran en detalles adicionales acerca de los riesgos de diferentes tipos de cirugía. Alguna cirugía de bajo riesgo puede no requerir transfusiones plaquetarias en absoluto, otros procedimientos pueden ser de mayor riesgo y el riesgo también puede depender de las comorbilidades de los pacientes.
Las transfusiones plaquetarias no están libres de riesgos. En 2014, el 34% de todos los eventos adversos relacionados con la transfusión presentados al sistema de informe nacional del Reino Unido (Serious Hazards of Transfusion [SHOT]) se debieron a los componentes plaquetarios. Los eventos adversos más frecuentes causados por los componentes plaquetarios fueron las reacciones febriles y alérgicas (Birchall 2015). Aunque la mayoría de estas reacciones no son potencialmente mortales, pueden ser muy angustiantes para el paciente y la investigación y la exclusión de una causa más grave puede tomar mucho tiempo para los profesionales de la salud. Las secuelas más graves, pero menos frecuentes, incluyen: anafilaxia (reacción alérgica potencialmente mortal), infecciones transmitidas por la transfusión (ITT) y lesión pulmonar aguda relacionada con la transfusión (LPART) (Blumberg 2010; Chapman 2015; Kaufman 2015; Slichter 2007; Vlaar 2013). Las unidades de plaquetas se almacenan a temperatura ambiente en un agitador, lo que aumenta el riesgo de crecimiento bacteriano (1:2000 a 1:3000) (Jacobs 2011). En 2015 se informaron cuatro cuasi‐incidentes (tres en las plaquetas) entre 2011 y 2015 y un total de 37/44 transmisiones bacterianas por transfusión a receptores individuales (34 incidentes) fueron causadas por la transfusión de plaquetas (Bolton‐Maggs 2016).
Un estudio de cohortes multicéntrico prospectivo concluyó que en los pacientes con enfermedades graves, la transfusión de plaquetas, pero no de eritrocitos y plasma, es un factor de riesgo independiente de desarrollo de una infección nosocomial (Engele 2016).
Los agentes alternativos que podrían reemplazar o reducir las transfusiones plaquetarias podrían ser más efectivos que las transfusiones plaquetarias para controlar la hemorragia y tendrán un perfil diferente de efectos adversos. Las alternativas incluyen sustitutos plaquetarios artificiales, criosobrenadante, factor recombinante VIIa (rFVIIa, por sus siglas en inglés), fibrinógeno, factor recombinante XIII (rFXIII, por sus siglas en inglés), miméticos de trombopoyetina (TPO) y fármacos antifibrinolíticos.
De qué manera podría funcionar la intervención
Transfusiones plaquetarias
La premisa para la intervención previa al procedimiento con transfusiones plaquetarias es la siguiente: la trombocitopenia aumenta el riesgo de hemorragia, la transfusión plaquetaria corrige la trombocitopenia, un recuento plaquetario mayor previene la hemorragia y en general hay un beneficio para el paciente. Sin embargo, esta presunción es simplista.
En un ensayo controlado aleatorio (ECA) pequeño de 23 participantes con trombocitopenia que requirieron 35 procedimientos y la extracción de 84 dientes, las complicaciones hemorrágicas fueron mínimas sin apoyo de productos sanguíneos (Perdigão 2012).
Un estudio que incluyó a 1720 participantes con trombocitopenia sometidos a cirugía de injerto de derivación de arterias coronarias (IDAC) agrupó los datos de los pacientes individuales de un estudio piloto y seis ECA. La transfusión plaquetaria en comparación con ninguna transfusión plaquetaria se asoció con un aumento significativo de la mortalidad entre los pacientes sometidos a cirugía de IDAC (odds ratio [OR] 4,76; intervalo de confianza [IC] del 95%: 1,65 a 13,73; P = 0,009). Aunque los autores utilizaron el análisis de las puntuaciones de propensión, no está claro si el aumento en la mortalidad se debió a la transfusión plaquetaria o a que los pacientes que presentaban enfermedades más graves recibieron transfusiones plaquetarias (Spiess 2004).
Alternativas a las transfusiones plaquetarias
Las alternativas a la transfusión plaquetaria simulan los efectos de las plaquetas (sustitutos plaquetarios artificiales), estimulan la formación adicional de fibrina (criosobrenadante, rFVIIa y fibrinógeno), promueven la liberación de factores de von Willebrand y la función plaquetaria (desmopresina), aumentan la producción de plaquetas (miméticos de TPO), fortalecen la estructura del coágulo (rFXIII) o disminuyen la ruptura de los coágulos (antifibrinolíticos). Estos agentes procuran promover la hemostasia sin los efectos adversos asociados con las transfusiones plaquetarias. Su principal efecto adverso es la formación excesiva de coágulos y la trombosis.
En esta revisión se excluyeron los ensayos que evaluaron la administración de: rFVIIa; concentrado de fibrinógeno; rFXIII; concentrado del complejo de protrombina y desmopresina debido a que son el tema de otras Revisiones Cochrane que compararon estas intervenciones con un comparador activo en pacientes que requerían un procedimiento quirúrgico (Desborough 2017; Fabes 2013; Simpson 2012).
Sustitutos plaquetarios artificiales
Los sustitutos plaquetarios artificiales como las microesferas de albúmina humana recubiertas con fibrinógeno, las plaquetas liofilizadas, las membranas de plasma infusible y los liposomas con receptores plaquetarios insertados procuran reproducir los componentes activos de las plaquetas sin eventos adversos asociados (Desborough 2016). Las plaquetas artificiales todavía no se utilizan en la práctica clínica habitual, de manera que en la actualidad no se conocen sus costos ni sus eventos adversos.
Criosobrenadante
El criosobrenadante es una fuente de factores de coagulación y se puede administrar por vía intravenosa. Es un componente sanguíneo y se asocia con un riesgo pequeño de reacciones a las transfusiones e ITT.
Miméticos de trombopoyetina
La TPO es fabricada por el hígado y es el regulador clave de la producción plaquetaria de la médula ósea. Los miméticos de TPO se han utilizado en varios estadios de la enfermedad para promover el aumento de las células que producen las plaquetas (megacariopoyesis) y la producción de plaquetas en sí (trombopoyesis) (Kuter 2014). Los dos miméticos de TPO principales que se utilizan en la actualidad son romiplostim (inyección semanal) y eltrombopag (comprimido oral diario) y los dos se recomiendan por el National Institute for Health and Care Excellence (NICE) para uso en adultos con TPI y enfermedades graves que presentan un alto riesgo de hemorragia (NICE 2011; NICE 2013). Aunque una revisión sistemática encontró que estos agentes mejoraron los recuentos plaquetarios, no hubo evidencia de que redujeran el riesgo de hemorragia significativa en los pacientes con TPI (Zeng 2011). Los miméticos de TPO son más costosos que las transfusiones plaquetarias(Joint Formulary Committee 2016). La interleucina 6 y la interleucina 11 también pueden actuar como estimulantes de la trombopoyesis(Gordon 1995; Kurzrock 2011; Tsimberidou 2005). No forman parte de la práctica clínica habitual, de manera que actualmente no se conoce su costo.
Antifibrinolíticos
La fibrinólisis es el proceso por el cual los coágulos sanguíneos se desintegran después que se han formado. Los fármacos antifibrinolíticos bloquean este proceso, lo que da lugar a una mayor fuerza del coágulo. Los tres fármacos antifibrinolíticos utilizados con más frecuencia son el ácido tranexámico, la aprotinina y el ácido épsilon aminocaproico. Otras revisiones sistemáticas Cochrane han evaluado estos agentes en pacientes sometidos a procedimientos quirúrgicos (Henry 2011; McNicol 2016), o en pacientes con trastornos hematológicos (Estcourt 2016a).
Por qué es importante realizar esta revisión
Los pacientes con un recuento plaquetario bajo a menudo necesitan cirugía. Las guías actuales se basan principalmente en la opinión de los expertos en lugar de en evidencia convincente y con frecuencia no entran en detalles acerca de los riesgos de los diferentes tipos de cirugía ni definen un umbral específico del recuento plaquetario. Algunas cirugías de bajo riesgo, por ejemplo la extracción dental, pueden no requerir transfusiones plaquetarias en absoluto. Las transfusiones plaquetarias pueden causar efectos perjudiciales inmediatos y a más largo plazo y el retraso en el comienzo de los tratamientos que salvan vidas. Las alternativas a las plaquetas pueden ser más efectivas y más seguras. Por lo tanto, es necesario evaluar el beneficio probable de la transfusión plaquetaria y de sus alternativas, en diferentes procedimientos, en comparación con los riesgos conocidos.
En esta revisión, el objetivo era responder a las siguientes preguntas.
-
¿Los pacientes necesitan transfusiones plaquetarias profilácticas antes de determinados tipos de cirugía?
-
¿Si se necesitan transfusiones plaquetarias, qué umbral del recuento plaquetario se debe utilizar para realizar la transfusión plaquetaria profiláctica antes de la cirugía?
-
¿Las transfusiones plaquetarias profilácticas son superiores a otros tratamientos alternativos?
Objetivos
Determinar la efectividad y la seguridad clínica de las transfusiones plaquetarias profilácticas antes de la cirugía para los pacientes con un recuento plaquetario bajo.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron ECA, ensayos controlados no aleatorios y estudios controlados tipo antes y después (CAD), independientemente del idioma o el estado de publicación. Se excluyeron los estudios no controlados, los estudios transversales y los estudios de casos y controles.
Solo se incluyeron los ECA con asignación al azar grupal, los ensayos no aleatorios grupales y los CAD con al menos dos sitios de intervención y dos sitios control. En los estudios con solo una intervención o un sitio control, la intervención (o comparación) se ve completamente afectada por factores de confusión relacionados con el sitio de estudio, lo que dificulta atribuir cualquier diferencia observada a la intervención en lugar de a otras variables propias del sitio.
Se planificó que de haber datos suficientes para responder a las preguntas de esta revisión solo con el uso de los datos de los ECA, solo se informarían los datos de los ECA.
Tipos de participantes
Pacientes de todas las edades con un recuento plaquetario bajo que se iban a someter a cirugía que incluía procedimientos invasivos.
Se excluyeron los estudios en pacientes con un recuento plaquetario bajo que presentaban hemorragias activas debido a que recibirían transfusiones plaquetarias como parte del tratamiento de las hemorragias.
Tipos de intervenciones
Se compararon tres tipos de regímenes de transfusión plaquetaria.
-
Comparación 1: transfusión plaquetaria profiláctica antes de la cirugía versus ninguna transfusión plaquetaria profiláctica antes de la cirugía (placebo o ningún tratamiento).
-
Comparación 2: transfusión plaquetaria profiláctica antes de la cirugía versus tratamientos alternativos (criosobrenadante, antifibrinolíticos, miméticos de TPO). En esta revisión se excluyeron los ensayos que evaluaron la administración de rFVIIa; concentrado de fibrinógeno, rFXIII, concentrado del complejo de protrombina y desmopresina debido a que son el tema de otras Revisiones Cochrane que compararon estas intervenciones con un comparador activo en pacientes que requerían un procedimiento quirúrgico (Carless 2004; Fabes 2013; Simpson 2012).
-
Comparación 3: diferentes umbrales del recuento plaquetario para la administración de una transfusión plaquetaria profiláctica antes de la cirugía.
Se registró el tipo de componente plaquetario y la dosis del componente plaquetario recibido.
Tipos de medida de resultado
Resultados primarios
-
Mortalidad (todas las causas, secundaria a la hemorragia, secundaria a la tromboembolia y secundaria a la infección) en el transcurso de 30 días y 90 días desde la cirugía.
-
Número de participantes con hemorragia grave relacionada con el procedimiento en el transcurso de siete días desde la cirugía, definida como:
-
-
hemorragia del sitio quirúrgico que requiere una segunda intervención o nueva cirugía o hemorragia del sitio quirúrgico que causa un hematoma o hemartrosis de tamaño suficiente para retrasar la movilización o la cicatrización de la herida;
-
hemorragia de tamaño suficiente para causar el retraso de la cicatrización de la herida, o infección de la herida o hemorragia del sitio quirúrgico inesperada y que prolongó o causó inestabilidad hemodinámica (según lo definido en el estudio) y que se asoció con una disminución de 20 g/l en la hemoglobina (Hb);
-
hemorragia que requirió dos o más unidades de sangre entera/eritrocitos en el transcurso de las 24 horas después de la hemorragia;
-
hemorragia definida por el estudio sin detalles adicionales.
-
Resultados secundarios
-
Número de participantes con hemorragia leve relacionada con el procedimiento en el transcurso de siete días desde la cirugía (p.ej., hematoma, hemorragia prolongada en el sitio quirúrgico que no cumplió con la definición de hemorragia grave).
-
Número de transfusiones plaquetarias por participante y número de componentes plaquetarios por participante.
-
Número de transfusiones de eritrocitos por participante y número de componentes de los eritrocitos por participante.
-
Proporción de participantes que requirieron intervenciones adicionales para interrumpir la hemorragia (quirúrgicas; médicas, p.ej., ácido tranexámico; otros productos sanguíneos, p.ej., plasma fresco congelado [PFC], crioprecipitado, fibrinógeno) en el transcurso de siete días desde la cirugía.
-
Evaluación de la calidad de vida medida con instrumentos validados.
-
Eventos adversos graves debido a:
-
transfusión (reacciones a las transfusiones, LPART, infección relacionada con la transfusión, sobrecarga circulatoria asociada con la transfusión, disnea relacionada con la transfusión) en el transcurso de 24 horas desde la transfusión;
-
cirugía (p.ej., retraso en la cicatrización de la herida, infección) en el transcurso de 30 días después de la cirugía.
-
-
Duración de la estancia hospitalaria y duración de la estancia en la UCI.
-
Tromboembolia venosa y arterial (incluida trombosis venosa profunda, embolia pulmonar, accidente cerebrovascular, infarto de miocardio).
Métodos de búsqueda para la identificación de los estudios
The Systematic Review Initiative's Information Specialist (CD) developed the search strategies in collaboration with the Cochrane Haematological Malignancies Group.
Búsquedas electrónicas
We searched the following databases.
Bibliographic databases
-
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, 2017, Issue 12) (Appendix 1).
-
MEDLINE (OvidSP, Epub Ahead of Print, In‐Process and other Non‐Indexed Citations, and 1946 to 11 December 2017) (Appendix 2).
-
PubMed (for e‐publications ahead or print only) (www.ncbi.nlm.nih.gov/pubmed) (Appendix 3).
-
Embase (OvidSP, 1974 to 11 December 2017) (Appendix 4).
-
CINAHL (EBSCOHost, 1937 to 11 December 2017) (Appendix 5).
-
Transfusion Evidence Library (www.transfusionevidencelibrary.com; 1950 to 11 December 2017 – this included a search of grey literature) (Appendix 6).
-
LILACS (1982 to present) (lilacs.bvsalud.org/en/) (Appendix 7).
-
Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (Thomson Reuters, 1990 to 11 December 2017) (Appendix 8).
Online databases of ongoing trials
We searched for ongoing studies to 11 December 2017 in the following databases:
-
ClinicalTrials.gov (clinicaltrials.gov) (Appendix 9);
-
World Health Organization (WHO) International Clinical Trials Registry Search Platform (ICTRP) (apps.who.int/trialsearch/AdvSearch.aspx) (Appendix 10).
We combined searches in MEDLINE and Embase with the recommended Cochrane RCT search filters (Lefebvre 2011), systematic review filters based on those of the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html), and CBA study filters based on those used in reviews of the Cochrane Effective Practice and Organisation of Care Group (EPOC 2015) (epoc.cochrane.org/). Searches in CINAHL were combined with the SIGN systematic review and RCT filter and an EPOC‐based filter. We did not limit searches by language, year of publication or publication type.
Once we identified studies for inclusion, we searched MEDLINE (OvidSP) for errata or retraction statements for the reports of these studies.
Búsqueda de otros recursos
We handsearched the reference lists of included studies and any relevant systematic reviews to identify further relevant studies. We made contact with lead authors of relevant studies to identify any unpublished material, missing data or information regarding ongoing studies.
Obtención y análisis de los datos
We summarised data in accordance with standard Cochrane methodologies. We planned to analyse data from different study designs separately.
Selección de los estudios
We selected studies with reference to the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The Systematic Review Initiative's Information Specialist (CD) initially screened all search hits for relevance against the eligibility criteria and discarded those that were clearly irrelevant. Thereafter, two review authors (LE, RM) independently screened all the remaining references for relevance against the full eligibility criteria. We retrieved full‐text papers for all references for which a decision on eligibility could not be made from only screening the title and abstract. If necessary, we requested additional information from study authors to assess the eligibility for inclusion of individual studies. The two review authors discussed the results of study selection and resolved any discrepancies between themselves, without the need for a third review author (MT). We reported the results of study selection using a PRISMA flow diagram (Moher 2009). We also recorded the reasons for excluding studies based on full‐text assessment and added those to the Characteristics of excluded studies table.
We collated multiple reports of one study so that the study, and not the report, was the unit of analysis
Extracción y manejo de los datos
Two review authors (RM, LE) independently extracted data as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), using standardised forms available in Covidence software (Covidence 2016). We planned to pilot two different data extraction forms for included RCTs and non‐RCTs separately, however we included only RCTs in this review. The two review authors came to consensus agreements without the need for a third review author (MT). The review authors were not be blinded to names of authors, institutions, journals or the study outcomes. They extracted the following information for each study.
Randomised controlled trials
-
Source: study ID, report ID, review author ID, date of extraction, ID of author checking extracted data, citation of paper, contact author's details.
-
General study information: publication type, study objectives, funding source, conflict of interest declared, other relevant study publication reviewed.
-
Study details and methods: location, country, clinical setting, number of centres, study design, total study duration, recruitment dates, length of follow‐up, power calculation, primary analysis (and definition), stopping rules, method of sequence generation, allocation concealment, blinding (of clinicians, participants and outcome assessors) and any concerns regarding bias.
-
Characteristics of interventions: number of study arms, description of experimental arm, description of control arm, type of platelet component (e.g. apheresis or pooled), dose of platelet component, thresholds of platelets transfusions, type of surgery.
-
Characteristics of participants: age, gender, primary diagnosis, surgery types procedure (minor, major, surgery to sensitive areas as ocular surgery or neurosurgery), platelet count, coagulation abnormalities, anticoagulant medications, antiplatelet medications.
-
Participant flow: total number screened for inclusion, total number recruited, total number excluded, total number allocated to each study arm, total number analysed (for review outcomes), number of allocated participants who received planned treatment, number of dropouts with reasons (percentage in each arm), protocol violations, missing data.
-
Method of data analyses.
-
Outcomes: mortality (all‐causes, secondary to bleeding, secondary to thromboembolism and secondary to infection) within 30 days and 90 days of surgery; number of participants with major procedure‐related bleeding within seven days of surgery; number of participants with minor procedure‐related bleeding within seven days of surgery; number of platelet transfusions per participant and number of platelet components per participant; number of red cell transfusions per participant and number of red cell components per participant; proportion of participants requiring additional interventions to stop bleeding within seven days of surgery; quality of life assessment using validated tools; serious adverse events due to transfusion (within 24 hours of the transfusion) or surgery (within 30 days after the operation); length of hospital stay and length of ICU stay, venous and arterial thromboembolism.
Non‐randomised controlled trials
There were no non‐RCTs eligible for inclusion in this review.
In future updates of this review, we plan to extract all the data collected for RCTs and additional information on the following.
-
Study design.
-
Method of selecting participants: sample source, sample size, participants eligibility criteria, number of participants at each follow‐up point, and the source of study control group and baseline differences between the two groups.
-
Confounding factors: baseline confounding factors and cointerventions that might lead potentially to bias are identified in the study and relevant confounding factors and cointerventions that could introduce bias after the starting of platelets transfusions; the comparability of groups on confounding factors.
-
Method of assigning the intervention.
-
Cointervention status: this is in order to document if any other cointerventions are considered in the study.
-
Method of data analysis: methods used to control for confounding and on multiple effect estimates (both unadjusted and adjusted estimates) as recommended in the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011).
Evaluación del riesgo de sesgo de los estudios incluidos
Randomised controlled trials
We assessed the risk of bias for all included RCTs using the Cochrane 'Risk of bias' tool according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Two review authors (LE, RM) independently assessed each element of potential bias listed below as 'high', 'low' or 'unclear' risk of bias. We reported a brief description of the judgement statements upon which the review authors assessed potential bias in the Characteristics of included studies table. We ensured that a consensus on the degree of risk of bias was met through comparison of the review authors' statements and, where necessary, through consultation with a third review author (SH). We used Cochrane's tool for assessing risk of bias, that included the following domains.
-
Selection bias: we described for each included study if and how the allocation sequence was generated and if allocation was adequately concealed prior to assignment. We described the method used to conceal the allocation sequence in detail and determine if intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment.
-
Performance bias: we described for each included study, where possible, if the study participants and personnel were adequately blinded from knowledge of which intervention a participant received. We judged studies as low risk of bias if they were blinded, or if judged that lack of blinding could not have had affected the results.
-
Detection bias: was blinding of the outcome assessors effective in preventing systematic differences in the way in which the outcomes were determined?
-
Incomplete outcome data: we described for each included study the attrition bias due to amount, nature or handling of incomplete outcome data. We also tried to evaluate whether intention‐to‐treat analysis were performed or could had been performed from published information.
-
Selective outcome reporting or reporting bias: we described for each included study the possibility of selective outcome reporting bias.
-
Other bias: was the study apparently free of other problems that could put it at risk of bias?
We summarised the risk of bias for each key outcome for each included study. We judged studies with at least one domain of high risk at high risk of bias overall.
Non‐randomised controlled trials
There were no non‐RCTs eligible for inclusion in this review.
In future updates of this review, we plan to use the ROBINS‐I tool to rate the quality of non‐randomised controlled trials (non‐RCTs) and CBAs (Sterne 2016). This tool is based on the Cochrane 'Risk of bias' tool for rating the quality of RCTs (Higgins 2011c). The tool covers seven domains and the quality of evidence is rated 'low,' 'moderate,' 'serious,' 'critical or no information,' and the response options were 'yes,' 'probably yes,' 'no,' 'probably no' and 'no information' (see Appendix 2 for a copy of the tool) and uses signalling questions for the assessment of:
-
bias due to confounding;
-
bias in the selection of participants;
-
bias in measurement of interventions;
-
bias due to departure from intended interventions;
-
bias due to missing data;
-
bias in measurement of outcomes;
-
bias in the selection of the reported result.
For 'low risk of bias,' the study was judged at low risk of bias on all the tool seven domains.
For 'moderate risk of bias,' the study was judged at low‐to‐moderate risk of bias on all tool seven domains.
For 'serious risk of bias ' the study is judged to be at serious risk of bias in at least one of the tool seven domains.
For 'critical risk of bias,' the study was judged at critical risk of bias in at least one domain of the tool seven domains.
For 'no information on bias,' when information in one or more key risk of bias domains were lacking.
Two review authors independently assessed each domain of potential bias listed and also tabulated a brief description of the judgement statements upon which they assessed potential bias in the Characteristics of included studies table. We ensured that a consensus on the degree of risk of bias was met through comparison of the review authors' statements and where necessary, through consultation with a third review author. We highlighted the highest quality evidence for each outcome.
We prespecified the following main potential confounding factors.
-
Primary diagnosis of participant (e.g. liver disease, critical illness, pregnancy).
-
Age: variability in the age of participants included, for example, paediatric (under 16 years) versus adult (older than 16 years) versus older adult (older than 60 years).
-
Gender: male to female ratio.
-
Previous severe bleeding (e.g. WHO grade 3 or 4 or equivalent).
Medidas del efecto del tratamiento
Randomised controlled trials
For dichotomous outcomes, we recorded the number of events and the total number of participants in both the treatment and control groups.
For continuous outcomes, we recorded the mean, standard deviation and total number of participants in both the treatment and control groups. For continuous outcomes using the same scale, we performed analyses using the mean difference (MD) with 95% CIs. If continuous outcomes were reported using different scales, we used standardised mean difference (SMD).
None of the studies reported hazard ratios (HR) and no HRs could be estimated using the available data. In future updates of this review, if available, we plan to extract and report HRs for time‐to‐event‐data (mortality or time in hospital) data. If HRs are not available, we will make every effort to estimate as accurately as possible the HR using the available data and a purpose‐built method based on the Parmar and Tierney approach (Parmar 1998; Tierney 2007). If sufficient studies provide HRs, we plan to use HRs in favour of RRs or MDs in a meta‐analysis, but for completeness, we will also perform a separate meta‐analysis of data from studies providing only RRs or MDs for the same outcome.
For dichotomous outcomes, we reported the pooled risk ratio (RR) with a 95% CI (Deeks 2011). No outcomes required an analysis using the Peto's odds ratio (OR). In future updates of this review, where the number of observed events is small (less than 5% of sample per group), and where trials have balanced treatment groups, we plan to report the Peto's OR with 95% CI (Deeks 2011).
There were no cluster‐randomised trials. In future updates of this review, for cluster‐randomised trials, we will extract and report direct estimates of the effect measure (e.g. RR with a 95% CI) from an analysis that accounts for the clustered design. We will obtain statistical advice to ensure the analysis is appropriate. If appropriate analyses are not available, we will make every effort to approximate the analysis following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d).
If data allowed, we undertook quantitative assessments using Review Manager 5 (Review Manager 2014).
Non‐randomised studies
There were no non‐randomised studies eligible for inclusion in this review.
In future updates of this review, for dichotomous outcomes, if available we will extract and report the RR with a 95% CI from statistical analyses adjusting for baseline differences (such as Poisson regressions or logistic regressions) or the ratio of risk ratios (i.e. the risk ratio postintervention/risk ratio preintervention). For continuous variables, if available we will extract and report the absolute change from a statistical analysis adjusting for baseline differences (such as regression models, mixed models or hierarchical models), or the relative change adjusted for baseline differences in the outcome measures (i.e. the absolute postintervention difference between the intervention and control groups, as well as the absolute preintervention difference between the intervention and control groups/the postintervention level in the control group) (EPOC 2015). If data allows, we will undertake quantitative assessments using Review Manager 5 (Review Manager 2014).
All studies
Where appropriate, we planned to report the number needed to treat for an additional beneficial outcome (NNTB) and harmful outcome (NNTH) with 95% CIs.
If we could not report the available data in any of the formats described above, we performed a narrative report, and if appropriate, we presented the data in tables.
Cuestiones relativas a la unidad de análisis
There were no unit of analysis issues in this review.
In future updates of this review if any unit of analysis issues arise, we will treat these in accordance with the advice given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). If participants are randomised more than once, we plan to contact the authors of the study to provide us with data associated with the initial randomisation. For studies with multiple treatment groups, we excluded subgroups that were considered irrelevant to the analysis. We tabulated all subgroups in the Characteristics of included studies table. When appropriate, we combined groups to create a single pair‐wise comparison. If this was not possible, we selected the most appropriate pair of interventions and excluded the others (Higgins 2011c).
Manejo de los datos faltantes
Where we identified data to be missing or unclear in published literature, we contacted study authors directly. We contacted the authors of all three completed studies for additional information, but no additional information was provided (Basu 2012; Stanca 2010; Veelo 2012).
Evaluación de la heterogeneidad
We planned to analyse the data in RCTs, non‐RCTs, and CBA studies separately. However, we only identified three RCTs for inclusion in this review.
We did not perform any meta‐analyses and, therefore, could not assess heterogeneity of the included RCTs (Deeks 2011).
In future updates of this review, if the clinical and methodological characteristics of individual studies are sufficiently homogeneous, we will combine the data and perform a meta‐analysis. We will assess the extent of heterogeneity by both visual inspection of forest plots and utilising statistical methods. We will assess statistical heterogeneity of treatment effects between studies using a Chi2 test with a significance level at P less than 0.1. We will use the I2 statistic to quantify the degree of potential heterogeneity and classify it as low if the I2 statistic is 50% or less, moderate if the I2 statistic is 50% to 80% or considerable if the I2 statistic is greater than 80%. We will use the random‐effects model for low‐to‐moderate heterogeneity. If statistical heterogeneity is considerable, we will not report the overall summary statistic. We will assess potential causes of heterogeneity by sensitivity and subgroup analyses (Deeks 2011).
Evaluación de los sesgos de notificación
We did not perform a formal assessment of publication bias because we did not perform any meta‐analyses (Sterne 2011).
Síntesis de los datos
We did not perform any meta‐analyses.
In future updates of this review, If studies are sufficiently homogenous in their study design, we will conduct a meta‐analysis according to the recommendations of Cochrane (Deeks 2011). We will not conduct meta‐analyses that include both RCTs and non‐RCTs. We will conduct separate meta‐analyses for each comparison. Different thresholds within the comparisons will only be grouped together if they were considered to be clinically similar.
Randomised controlled trials
For RCTs where meta‐analysis is feasible, we will use the random‐effects model for pooling the data. For binary outcomes, we will base the estimation of the between‐study variance on the Mantel‐Haenszel estimator. We will use the inverse‐variance method for continuous outcomes, outcomes that include data from cluster‐RCTs, or outcomes where HRs are available. If there is heterogeneity above 80%, and we identify a cause for the heterogeneity, we will explore this with subgroup analyses. If we cannot find a cause for the heterogeneity, then we will not perform a meta‐analysis, but comment on the results as a narrative with the results from all studies presented in tables.
Non‐randomised studies
We identified no non‐RCTs for inclusion in this review. In future updates, if meta‐analysis is feasible for non‐RCTs or CBA studies, we will analyse non‐RCTs and CBA studies separately. We will only analyse outcomes with adjusted effect estimates if these are adjusted for the same factors using the inverse‐variance method as recommended in the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011).
All studies
We used the random‐effects model for all analyses as we anticipated that true effects were related but were not the same for included studies. If we could not perform a meta‐analysis, we commented on the results as a narrative with the results from all studies presented in tables.
'Summary of findings' tables
We used the GRADE tool (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of evidence for each outcome. We presented 'Summary of findings' tables as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b).
We applied the GRADE approach to rate the quality of the evidence as 'high,' 'moderate,' 'low' or 'very low' using the five GRADE considerations.
-
Risk of bias: serious or very serious.
-
Inconsistency: serious or very serious.
-
Indirectness: serious or very serious.
-
Imprecision: serious or very serious.
-
Publication bias: likely or very likely.
The outcomes we included were listed below in order of most relevant endpoints for participants.
-
All‐cause mortality.
-
Mortality secondary to bleeding.
-
Mortality secondary to thromboembolism.
-
Mortality secondary to infection.
-
Number of participants with major procedure‐related bleeding within seven days of surgery.
-
Number of participants with minor procedure‐related bleeding within seven days of surgery.
-
Serious adverse events due to platelet transfusions.
Análisis de subgrupos e investigación de la heterogeneidad
There were insufficient data to perform any subgroup analyses. In future updates of this review, we plan to perform subgroup analyses for each of the following outcomes in order to assess the effect on heterogeneity.
-
Age of participant (neonate, infant, child, adult).
-
Type of surgery: minor or major (cardiac, eye, neurosurgery, dental, orthopaedic, liver, obstetric, gynaecological, plastic, gastrointestinal).
-
Underlying cause of thrombocytopenia (bone marrow failure due to disease or treatment, increased destruction of platelets or increased consumption of platelets).
-
Dose of platelet component.
-
Coexisting coagulopathy.
-
Coexisting platelet dysfunction (inherited or acquired).
Análisis de sensibilidad
We planned to assess the robustness of the results by performing the following sensitivity analyses when possible; however, no meta‐analyses were performed.
-
Including studies with a 'low risk of bias' (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation).
-
Including studies with less than a 20% dropout.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables for more details.
Results of the search
The search (conducted on 11 December 2017) identified 10,564 potentially relevant records (Figure 1). There were 8091 records after we removed duplicates. One reference was identified from other sources. We excluded 8032 references on the basis of the abstract and checked the eligibility of 60 full‐text papers. We identified five RCTs eligible for inclusion in this review, three completed RCTs (Basu 2012; Stanca 2010; Veelo 2012) and two ongoing RCTs (NTR5653; Rocha 2017).
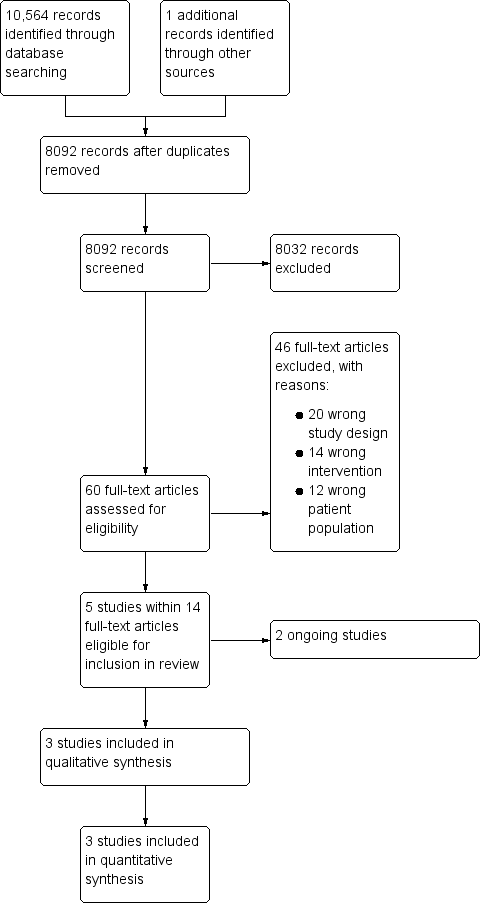
Study flow diagram.
Included studies
See Characteristics of included studies table for full details of the included studies.
Of the three completed RCTs, two trials were published in full (Stanca 2010; Veelo 2012), and one was only available as an abstract (Basu 2012).
Study design and setting
All included trials were single‐centre parallel‐group RCTs. Two trials were conducted in the USA (Basu 2012; Stanca 2010), and one was conducted in the Netherlands (Veelo 2012).
Trials comparing platelet transfusions with no transfusion before the surgical procedure
One trial (72 participants) compared platelet transfusion to no platelet transfusion before a surgical procedure (Veelo 2012). It included people requiring mechanical ventilation who had mild coagulation disorders (defined as prothrombin time (PT) 14.7 seconds to 20.0 seconds, or platelet count 40 × 109/L to 100 × 109/L, or active treatment with aspirin (acetylsalicylic acid), or any combination) who required a percutaneous dilational tracheotomy (PDT). Eight participants did not undergo the tracheotomy after randomisation. About 60% of all participants had a prolonged PT and just over a third had a low platelet count and another third had received aspirin. There was more than one coagulation disorder was present in about 20% of the participants.
Study interventions
Veelo 2012 randomised participants into two groups. Group A received one unit of FFP if the PT was between 14.7 seconds and 18 seconds and two units of FFP when the PT was between 18 seconds and 20 seconds, as well as five units of buffy coat prepared platelets if they had a low platelet count or had received aspirin. Group B received neither plasma nor platelets; however, in the event of bleeding during or after the procedure, immediate transfusion of FFP or platelets was available.
Study outcomes
The primary outcomes were volume of blood loss during the PDT procedure, severity of intratracheal bleeding and time until no blood was visible in tracheal aspirates. Veelo 2012 assessed bleeding during and within the first 12 hours after the PDT procedure. Bleeding was defined as "minor" for blood loss of less than 100 g from the site of the procedure that could be controlled by applying local pressure and did not require the transfusion of red blood cells. "Major" bleeding was defined as the presence of blood in the airways which required repeated suction or emergency surgery or red blood cell transfusion, or both.
Trials comparing platelet transfusion to alternative treatments before the surgical procedure
Two trials (108 participants) compared platelet transfusions to different alternative treatments (Basu 2012; Stanca 2010). Both included adults with liver disease, and excluded people with bleeding disorders, hepatocellular carcinoma and treatment with antiplatelet medications. Participants in Basu 2012 had severe thrombocytopenia (no further details). Stanca 2010 included people with moderate coagulopathy (defined as platelet count of 30 × 10 9/L to 50 × 10 9/L) (22 participants), or an international normalised ratio (INR) of 2.0 to 3.0, or both.
In Basu 2012, the procedure was percutaneous liver biopsy, and in Stanca 2010, the procedure was dental extractions.
Study interventions
Basu 2012 randomly assigned participants to three groups: group A received seven units of platelets the night before the procedure; group B received romiplostim 500 μg subcutaneously given once two weeks before the procedure and group C received eltrombopag 57 mg per day by mouth for two weeks before the procedure.
Stanca 2010 randomised participants were to two groups: group A received intranasal desmopressin (one spray in each nostril, total of 300 μg) and group B received FFP 10 mL/kg if the INR was 2.0 to 3.0 or one single unit of donor platelets when the platelet count was between 30 × 109/L and 50 × 109/L.
Study outcomes
Basu 2012 reported on the incidence of bleeding and haematoma after the procedure, and adverse effects and cost effectiveness. Stanca 2010 reported incidence of bleeding and adverse effects at 24 hours to 48 hours after dental extraction, and a cost comparison between the two interventions.
Excluded studies
We excluded 46 studies for the following reasons:
-
wrong study design: 20 studies (Abdelfatah 2016; Al‐Zaabi 2014; Barrera 1996; Cai 2014; Chantarangkul 2013; Chen 2011; Embrey 2006; Fayed 2014; Hibbert 2013; Khair 2015; Napolitano 2017; NCT02200419; NCT02987712; NCT03011827; NCT00549484; Park 2016; Pereboom 2009; Perek 2016; Ray 1997; Tripodi 2013);
-
wrong intervention: 14 studies (Afdhal 2012; De Pietri 2016; EudraCT 2007‐005851‐40; Karkouti 2016; Kultufan 2006; NCT00678587; NCT01919840; NCT02042898; NCT02352181; NCT02990273; Perl 2016; Pietri 2014; Tripodi 2013; Smart 2017);
-
wrong patient population: 12 studies (Backos 1999; Harding 1975; Hess 2015; Holcomb 2015; NCT00521664; NCT01291290; NCT01402739; NCT02074436; NCT03022253; Simon 1982; Simon 1984; Tanaka 2014).
Ongoing studies
We found two ongoing clinical trials (NTR5653; Rocha 2017). See Characteristics of ongoing studies for further details.
NTR5653
This multicentre RCT is currently enrolling participants and aims to complete recruitment by October 2019 (NTR5653). The study is being conducted in the Netherlands and plans to enrol 462 adults on the ICU with a platelet count of 50 × 109/L or less who require insertion of a central venous catheter. Participants will be randomised to receive or not receive a platelet transfusion.
Rocha 2017
The POCKET trial (point‐of‐care versus standard coagulation tests versus restrictive strategy to guide transfusion in chronic liver failure patients requiring central venous line) is a single‐centre, double‐blind RCT that is currently enrolling participants and aims to complete recruitment by December 2018 (Rocha 2017). The study is being conducted in Brazil and plans to enrol 165 adults with chronic liver disease, randomised to receive transfusion of FFP, platelets or cryoprecipitate, or any combination guided by the use of standard tests of coagulation (INR greater than 1.5, activated partial thromboplastin time (aPPT) greater than 50 seconds or platelet count less than 50 × 109/L) versus thromboelastometry (ROTEM) versus standard tests of coagulation with a restrictive threshold (INR greater than 5, platelets less than 25 × 109/L) prior to central venous catheterisation.
Risk of bias in included studies
Allocation
Sequence generation
We rated two trials at low risk of bias for sequence generation because they used a computer‐generated technique (Stanca 2010; Veelo 2012).
We rated Basu 2012 at unclear risk of bias because it was only available as an abstract and there were no further details available.
Allocation concealment
We rated two trials at low risk of bias for allocation concealment as both trials used opaque sealed envelopes to allocate the treatment to participants (Stanca 2010; Veelo 2012).
We rated Basu 2012 at unclear risk of bias due to lack of relevant information in the published abstract.
Blinding
We rated Basu 2012 at low risk of performance and detection bias because it was described as a double‐blind trial with no further information available.
We rated two trials at high risk of bias for both performance and detection bias because they were both open‐label trials (Stanca 2010; Veelo 2012).
Incomplete outcome data
We rated one trial at low risk of attrition bias because all participants who received platelet transfusions were included in the analysis (Veelo 2012).
We rated two trials at unclear risk of attrition bias (Basu 2012; Stanca 2010). In Basu 2012, the trial was only published as an abstract, and they did not report the number of participants who were randomised and included in the analyses. Stanca 2010 did not include all randomised participants in the analysis of outcome data. The study enrolled 43 participants, but the analysis included only 36 participants (19% dropout). The reasons for excluding these participants were clearly provided: two participants assigned to the blood transfusion group withdrew their consent; five participants were not included in the analysis because their blood results prior to the procedure showed they no longer met the trial eligibility criteria. Although this was a significant dropout, it was unclear what effect this would have on the study's findings, as most of the exclusions were because participants were no longer eligible.
Selective reporting
We rated two trials at unclear risk of selective reporting (Basu 2012; Stanca 2010). Basu 2012 was only available as an abstract, and there was no trial registration. The only outcome listed on the trial registration (NCT00816127) for Stanca 2010 was: "Necessity of rescue blood transfusion in patients who received DDAVP [desmopressin] or blood transfusion prior to dental extraction." There were no other secondary outcomes listed. Although this outcome was reported, there was no protocol available to assess if other outcomes were planned.
We rated Veelo 2012 at "high" risk of selective reporting, because not all the outcomes stated in the trial registration (ISRCTN31808827) were reported in the published paper such as the amount of blood products used during and after the procedure (not reported for intervention group). Some other outcomes were classified as the trial's primary outcome measures in the trial registration (ISRCTN31808827), but were reported as secondary outcomes in the published paper for example the volume of blood loss during the surgical procedure, the intensity of intra‐tracheal bleeding, time until no blood was visible in tracheal aspirates.
Other potential sources of bias
We rated Stanca 2010 at low risk of other potential sources of bias, because baseline characteristics were balanced for the two groups, and there were no obvious sources of bias.
We rated two trials at unclear risk of other potential sources of bias (Basu 2012; Veelo 2012). Basu 2012 did not report adequate baseline information for the three study groups. The Veelo 2012 trial was stopped early due to increased resistance to recruitment and low rate of bleeding in either trial arm, planned recruitment was 152 participants but only 64 participants were included in the analysis.
Effects of interventions
See: Summary of findings for the main comparison Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery; Summary of findings 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments; Summary of findings 3 Different platelet count thresholds for administering a prophylactic platelet transfusion prior to surgery
Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery
Only one trial compared prophylactic platelet transfusions versus no prophylactic platelet transfusions (Veelo 2012) (summary of findings Table for the main comparison).
Primary outcomes
Mortality
All‐cause mortality within 30 days of the surgery
There was no evidence of a difference in the risk of death due to any cause between participants having their coagulopathy corrected (platelets, plasma, or both) and participants who did not (1 trial, 72 participants; RR 0.78, 95% CI 0.41 to 1.45; very‐low quality evidence; Analysis 1.1).
Mortality secondary to bleeding; mortality secondary to thromboembolism and mortality secondary to infection (within 30 days of surgery)
The trial did not report these outcomes.
All‐cause mortality; mortality secondary to bleeding; mortality secondary to thromboembolism and mortality secondary to infection (within 90 days of the surgery)
The trial did not report these outcomes.
Number of participants with major procedure‐related bleeding within seven days of surgery
Veelo 2012 defined major bleeding as the presence of blood in the airways requiring repeated suction post procedure, emergency surgery, transfusion of packed red cells, or a combination of these.
There was no evidence of a difference on the incidence of procedure‐related bleeding between participants having their coagulopathy corrected (platelets, plasma, or both) and participants who did not (1 trial, 72 participants; RR 1.60, 95% CI 0.29 to 8.92; very‐low quality evidence; Analysis 1.2).
Secondary outcomes
Number of participants with minor procedure‐related bleeding within seven days of surgery
Veelo 2012 defined minor bleeding as blood loss less than 100 g that could be controlled with the application of local pressure and did not require re‐exploration or transfusion of packed red cells. There was no evidence of a difference in the risk of minor bleeding during or for 12 hours after the procedure for participants having their coagulopathy corrected (platelets, plasma, or both) and participants who did not (1 trial, 64 participants; RR 1.29, 95% CI 0.90 to 1.85; Analysis 1.3).
Number of platelet transfusions per participant and number of platelet components per participant
Twenty‐three participants received a platelet transfusion (five buffy coat units) prior to the procedure in the intervention arm and no participants received a platelet transfusion in the comparator arm. It was not clear whether any participant in the intervention arm received a platelet transfusion during or after the procedure.
Number of red cell transfusions per participant and number of red cell components per participant
No participants in either treatment arm received a red cell transfusion during or after the procedure (1 trial, 64 participants; no events).
Proportion of participants requiring additional interventions to stop bleeding (surgical; medical, e.g. tranexamic acid; other blood products, e.g. fresh frozen plasma, cryoprecipitate, fibrinogen) within seven days of surgery
There was no evidence of a difference in the risk of requiring additional procedures to stop bleeding between participants having their coagulopathy corrected (platelets, plasma or both) and participants who did not (1 trial, 64 participants; RR 0.35, 95% CI 0.01 to 8.38; Analysis 1.4). Bleeding occurred directly after skin incision and before opening the airway in one participant in the "no correction" group who was on active aspirin treatment, this was treated with suturing and application of local compression.
Quality of life assessment
The trial did not report this outcome.
Serious adverse events
Serious adverse events due to transfusion
The trial did not report this outcome.
Serious adverse events due to surgery
No participants in either treatment arm developed a serious adverse event due to the procedure (1 trial, 64 participants; no events; very‐low quality evidence).
Length of hospital stay and length of intensive care unit stay
The trial did not report length of hospital stay.
There was no evidence of a difference in the length of ICU stay (median: 15 days (range 8 to 29) with coagulopathy correction versus median 21 days (range 14 to 26); P = 0.21 (analysis by study authors)).
Venous and arterial thromboembolism (including deep vein thrombosis, pulmonary embolism, stroke, myocardial infarction)
The trial did not report these outcomes.
Prophylactic platelet transfusion prior to surgery versus alternative treatments
Two trials compared platelet transfusions to other alternative treatments before surgery (Basu 2012; Stanca 2010). Stanca 2010 compared platelet transfusion or plasma transfusion or both to internasal desmopressin, whereas Basu 2012 compared platelet transfusion to TPO mimetics (romiplostim and eltrombopag) (summary of findings Table 2; summary of findings Table 3).
Primary outcomes
Mortality
All‐cause mortality; mortality secondary to bleeding; mortality secondary to thromboembolism and mortality secondary to infection (within 30 days of the surgery)
The trials did not report mortality within 30 days.
All‐cause mortality; mortality secondary to bleeding; mortality secondary to thromboembolism and mortality secondary to infection (within 90 days of the surgery)
The trials did not report mortality within 90 days.
Number of participants with major procedure‐related bleeding within seven days of surgery
Platelet transfusion versus desmopressin
No participants had major procedure‐related bleeding (1 trial, 36 participants; no events; very‐low quality evidence).
Platelet transfusion versus thrombopoietin mimetic
No participants had major procedure‐related bleeding (1 trial, 65 participants; no events; very‐low quality evidence).
Secondary outcomes
Number of participants with minor procedure‐related bleeding within seven days of surgery
Platelet transfusion versus desmopressin
There was no evidence of a difference in minor procedure‐related bleeding (1 trial, 36 participants, 0.89, 95% CI 0.06 to 13.23; very‐low quality evidence; Analysis 2.1).
Platelet transfusion versus thrombopoietin mimetic
No participants had minor procedure‐related bleeding (1 trial, 65 participants; no events; very‐low quality evidence).
Number of platelet transfusions per participant and number of platelet components per participant
The trials did not report these outcomes.
Number of red cell transfusions per participant and number of red cell components per participant
Platelet transfusion versus desmopressin
In Stanca 2010, no participants required red cells transfusions to treat bleeding post dental extraction (1 trial, 36 participants; no events).
Platelet transfusion versus thrombopoietin mimetic
In Basu 2012, no participants required red cells transfusions to treat bleeding post liver biopsy (1 trial, 65 participants; no events).
Proportion of participants requiring additional interventions to stop bleeding
Platelet transfusion versus desmopressin
In Stanca 2010, four participants reused desmopressin at home, the amount of bleeding prior to repeat use of desmopressin administration was not measured, in comparison only one participant in the transfusion group had bleeding after the procedure and required an additional transfusion. There was no evidence of a difference between the two groups for this outcome (1 trial, 36 participants; RR 0.22, 95% CI 0.03 to 1.81; Analysis 2.2).
Platelet transfusion versus thrombopoietin mimetic
In Basu 2012, no bleeding occurred in either study arm, therefore no treatments were required to stop bleeding (1 trial, 65 participants; no events).
Quality of life assessment
The trials did not report this outcome.
Serious adverse events within 30 days after the operation
Platelet transfusion versus desmopressin
There was no evidence of a difference in the risk of serious adverse reaction related to transfusion (1 trial, 36 participants; RR 2.70, 95% CI 0.12 to 62.17, very‐low quality evidence; Analysis 2.3).
The trials did not report serious adverse events related to surgery.
Platelet transfusion versus thrombopoietin mimetic
The trials did not report serious adverse events related to surgery or transfusion.
Length of hospital stay and length of intensive care unit stay
The trials did not report these outcomes.
Venous and arterial thromboembolism (including deep vein thrombosis, pulmonary embolism, stroke, myocardial infarction)
The trials did not report these outcomes.
Different platelet count thresholds for administering a prophylactic platelet transfusion prior to surgery
We found no trials comparing different platelet count thresholds for platelet transfusion.
Discusión
Resumen de los resultados principales
El objetivo de esta revisión era evaluar la eficacia y la seguridad de las transfusiones plaquetarias profilácticas en los pacientes con recuentos plaquetarios bajos antes de la cirugía (incluidos los procedimientos invasivos).
Se incluyeron tres ensayos completados en esta revisión; dos ensayos todavía están en curso, pero aún no están reclutando participantes. Los tres ensayos completados fueron ECA pequeños de centro único (180 participantes en total) (Basu 2012; Stanca 2010; Veelo 2012). Los ensayos en curso son un ECA multicéntrico y un ECA de centro único que planean reclutar a más de 600 adultos (tabla "Características de los estudios en curso"), que es una cifra mayor que el número combinado de participantes incluidos en los tres ensayos completados. Los tres ensayos se realizaron entre 2005 y 2009. Dos ensayos incluyeron a pacientes con trastornos hepáticos crónicos (Basu 2012; Stanca 2010), mientras que un ensayo se realizó en una UCI con pacientes con diversas morbilidades (Veelo 2012).
Ninguno de los ensayos incluidos reclutó a niños ni incluyó procedimientos quirúrgicos mayores o cirugías de urgencia.
Transfusión plaquetaria profiláctica antes de la cirugía versus ninguna transfusión plaquetaria profiláctica antes de la cirugía
Un ensayo pequeño en pacientes de la UCI que requirieron una traqueotomía (72 asignados al azar, 64 sometidos a una traqueotomía) comparó las transfusiones plaquetarias versus ninguna transfusión plaquetaria antes del procedimiento quirúrgico (Veelo 2012).
-
No se informaron casos de muerte en el transcurso de los 30 días desde la cirugía.
-
No existe mucha seguridad con respecto a si hubo una diferencia en el riesgo de hemorragia grave entre los dos grupos durante o después del procedimiento quirúrgico.
-
No existe seguridad con respecto a si hubo diferencias en el riesgo de hemorragia leve entre los dos grupos durante o después del procedimiento quirúrgico.
-
No existe mucha seguridad con respecto a si hubo una diferencia en el número de participantes que requirieron intervenciones quirúrgicas adicionales para interrumpir la hemorragia.
-
Ninguno de los participantes presentó eventos adversos graves relacionados con el procedimiento.
-
No existe mucha seguridad con respecto a si hubo una diferencia en la duración de la estancia en la UCI.
El ensayo no informó la calidad de vida ni la duración de la estancia hospitalaria.
Transfusión plaquetaria profiláctica antes de la cirugía versus tratamientos alternativos
Dos ensayos pequeños en pacientes con enfermedades hepáticas compararon la transfusión plaquetaria versus tratamientos alternativos antes del procedimiento quirúrgico (Basu 2012; Stanca 2010). Basu 2012 (65 participantes) comparó la transfusión plaquetaria con dos tipos de análogos de TPO (romiplostim, eltrombopag) en pacientes que requerían una biopsia hepática. Stanca 2010 comparó la transfusión plaquetaria con desmopresina en pacientes que requerían procedimientos dentales.
-
No existe mucha seguridad con respecto a si hubo una diferencia en el riesgo de hemorragia grave entre los dos grupos durante o después del procedimiento quirúrgico.
-
No existe seguridad con respecto a si hubo diferencias en el riesgo de hemorragia leve entre los dos grupos durante o después del procedimiento quirúrgico.
-
No existe mucha seguridad con respecto a si hubo diferencias en el número de participantes que requirieron transfusiones de sangre para interrumpir la hemorragia después de la cirugía.
-
Existen muchas dudas con respecto a si hubo diferencias en el número de participantes que presentaron eventos adversos graves relacionados con la transfusión.
Ningún ensayo informó la mortalidad, la calidad de vida ni la duración de la estancia hospitalaria.
Diferentes umbrales del recuento plaquetario para la administración de una transfusión plaquetaria profiláctica antes de la cirugía
No se encontraron ensayos que compararan diferentes umbrales del recuento plaquetario.
Compleción y aplicabilidad general de las pruebas
Las conclusiones que se pueden extraer después de esta revisión sistemática fueron muy limitadas debido a la inclusión de solo tres ECA pequeños (180 asignados al azar). No hubo evidencia suficiente para determinar el efecto de la transfusión plaquetaria antes de la cirugía sobre los resultados primarios mortalidad por todas las causas o hemorragia grave relacionada con el procedimiento. No hubo datos suficientes para determinar el efecto sobre la hemorragia leve relacionada con el procedimiento o cualquier otro resultado secundario.
Hay dos ECA en curso (se anticipa que se reclutarán 627 participantes), que están programados para completar el reclutamiento en octubre de 2019 (NTR5653; Rocha 2017). Uno compara la transfusión plaquetaria versus ninguna transfusión plaquetaria antes de la inserción de una vía central (462 participantes) (NTR5653), el otro ensayo compara un algoritmo de transfusión restrictivo versus liberal versus basado en tromboelastografía (165 participantes) antes de la inserción de una vía central (Rocha 2017).
La evidencia disponible sobre la efectividad y la seguridad de las transfusiones plaquetarias profilácticas solo está relacionada con pacientes adultos y la mayor parte de la evidencia proviene de pacientes con hepatopatía crónica. Ninguno de los ensayos incluidos o en curso reclutó a niños ni incluyó procedimientos quirúrgicos mayores o cirugías de urgencia.
Esta revisión excluyó los estudios que compararon un algoritmo de transfusión basado en un umbral del recuento plaquetario versus en tromboelastografía, o diferentes algoritmos de transfusión basados en tromboelastografía, y varios estudios que se excluyeron en el estadio de texto completo evaluaron esta comparación (ver tabla "Características de los estudios excluidos"), incluido un estudio completado en pacientes con enfermedad hepática (De Pietri 2016). La tromboelastografía se ha indicado como una alternativa al recuento plaquetario para evaluar la necesidad de una transfusión plaquetaria mediante la evaluación de la velocidad y la fuerza de la formación de coágulos sanguíneos in vitro (Bolliger 2012).
Calidad de la evidencia
En términos generales, la calidad de la evidencia se consideró muy baja según la metodología GRADE a través de los diferentes resultados debido al alto riesgo de sesgo (estudios no cegados); la imprecisión de las estimaciones y la imposibilidad para generalizar la evidencia (los estudios solo incluyeron a adultos con enfermedad hepática o a adultos en la UCI) (Resumen de los hallazgos, tabla 1; Resumen de los hallazgos, tabla 2; Resumen de los hallazgos, tabla 3).
La calidad de la evidencia según los criterios GRADE se consideró muy baja para:
-
mortalidad por todas las causas;
-
número de participantes con hemorragia grave relacionada con el procedimiento en el transcurso de siete días desde la cirugía;
-
número de participantes con hemorragia leve relacionada con el procedimiento en el transcurso de siete días desde la cirugía;
-
eventos adversos graves.
No fue posible evaluar la calidad de la evidencia para: la mortalidad secundaria a la hemorragia, la mortalidad secundaria a la tromboembolia ni la mortalidad secundaria a la infección debido a que ninguno de los ensayos informó estos resultados.
Los tres ensayos incluidos en esta revisión fueron ECA; sin embargo, ninguno de los ensayos estuvo exento de sesgo metodológico. Ver Figura 2 para el resumen visual de la evaluación del riesgo de sesgo sobre cada dominio. Dos ensayos tuvieron riesgo de sesgo de realización y de detección, lo que se debió a la naturaleza de la intervención de "transfusión plaquetaria" (Stanca 2010; Veelo 2012). La mayoría de los dominios de la herramienta de "riesgo de sesgo" de uno de los ensayos incluidos tuvo riesgo incierto de sesgo debido a que el ensayo solo estuvo disponible en forma de resumen (Basu 2012).
Sesgos potenciales en el proceso de revisión
Se realizó una búsqueda exhaustiva en múltiples bases de datos y registros de ensayos clínicos para obtener todos los estudios relevantes. La búsqueda no se limitó por el idioma ni el estado de publicación. Hay dos estudios en curso. Dos autores de la revisión seleccionaron de forma cuidadosa todos los artículos identificados mediante la búsqueda y realizaron la extracción de datos y las evaluaciones de la calidad. Se predeterminaron todos los resultados antes de realizar la búsqueda. No fue posible realizar un metanálisis ni evaluar el sesgo de publicación debido a que la revisión solo incluyó tres estudios y hubo diferencias clínicas significativas entre los estudios (que compararon diferentes intervenciones).
Acuerdos y desacuerdos con otros estudios o revisiones
Hasta donde se sabe esta revisión es la primera en evaluar de forma sistemática la evidencia relacionada con la transfusión plaquetaria profiláctica antes de la cirugía para los pacientes con trombocitopenia. Dos revisiones han evaluado la evidencia sobre las transfusiones plaquetarias profilácticas antes de punciones lumbares y de anestesia epidural (Estcourt 2016b) y antes de la inserción de una vía central (Basu 2012).Ninguna de estas revisiones incluyó estudios no aleatorios. Estcourt 2015 identificó uno de los estudios en curso identificados en esta revisión (Rocha 2017), y Estcourt 2016b no identificó estudios relevantes. Estas otras revisiones destacan la falta de evidencia con respecto a esta pregunta.

Study flow diagram.
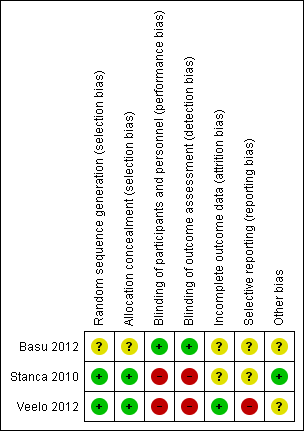
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 1 All‐cause mortality within 30 days of surgery.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 2 Number of participants with major procedure‐related bleeding within 7 days of surgery.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 3 Number of participants with minor procedure‐related bleeding within 7 days of surgery.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 4 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery.

Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 1 Number of participants with minor procedure‐related bleeding within 7 days of surgery.

Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 2 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery.
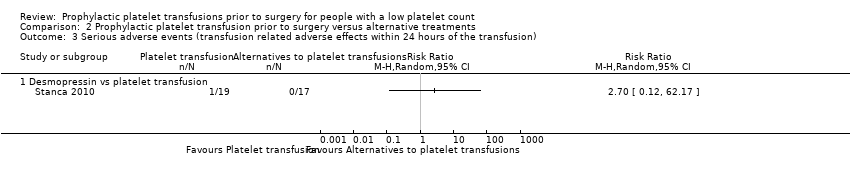
Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 3 Serious adverse events (transfusion related adverse effects within 24 hours of the transfusion).
| Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: no platelet transfusion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no platelet transfusion | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery | Study population | RR 0.78 | 72 | ⊕⊝⊝⊝ | — | |
| 405 per 1000 | 316 per 1000 | |||||
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery (surgical site bleeding requiring a second intervention or reoperation or surgical site bleeding that causes a haematoma or haemarthrosis of sufficient size to delay mobilisation or wound healing) | Study population | RR 1.60 | 64 | ⊕⊝⊝⊝ | — | |
| 61 per 1000 | 97 per 1000 | |||||
| The number of participants with minor procedure‐related bleeding within 7 days of surgery | Study population | RR 1.29 | 64 | ⊕⊝⊝⊝ | — | |
| 576 per 1000 | 743 per 1000 | |||||
| Serious adverse events (surgery‐related adverse effects within 30 days) | No events occurred in either study arm | 64 | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly adults in the intensive care unit were included in this trial (downgraded one level for indirectness). bThe confidence intervals included a serious risk of harm or benefit (downgraded two levels for imprecision). cThis is a subjective outcome and the trial was unblinded (downgraded one level for risk of bias). dThe confidence intervals included a risk of harm or benefit (downgraded one level for imprecision). | ||||||
| Prophylactic platelet transfusion prior to surgery versus alternative treatments | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: desmopressin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with desmopressin | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery (bleeding that required ≥ 2 units of whole blood/red blood cells within 24 hours of the bleeding) | No events in either study arm | 36 | ⊕⊝⊝⊝ | — | ||
| Number of participants with minor procedure‐related bleeding within 7 days of surgery | Study population | RR 0.89 | 36 | ⊕⊝⊝⊝ | — | |
| 59 per 1000 | 52 per 1000 | |||||
| Serious adverse events (transfusion‐related adverse effects within 24 hours of the transfusion) | Study population | RR 2.70 | 36 | ⊕⊝⊝⊝ | — | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOpen‐label trial (downgraded one level for risk of bias). bStudy only included adults with chronic liver disease (downgraded one level for indirectness). cConfidence intervals included a serious risk or benefit or treatment (downgraded one level for imprecision, as already downgraded one level for indirectness and risk of bias). | ||||||
| Different platelet count thresholds for administering a prophylactic platelet transfusion prior to surgery | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: TPO mimetic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TPO mimetic | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery | No bleeding in any of the study arms | 65 | ⊕⊝⊝⊝ | — | ||
| Number of participants with minor procedure‐related bleeding within 7 days of surgery | No bleeding occurred in any of the study arms | 65 | ⊕⊝⊝⊝ | — | ||
| Serious adverse events – not reported | — | — | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; TPO: thrombopoietin. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aStudy only included adults with chronic liver disease (downgraded one level for indirectness). bNo events occurred (downgraded two levels for imprecision). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality within 30 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Number of participants with major procedure‐related bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number of participants with minor procedure‐related bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with minor procedure‐related bleeding within 7 days of surgery Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Thrombopoietin mimetics vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events (transfusion related adverse effects within 24 hours of the transfusion) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

