Tests to assist in the staging of cutaneous squamous cell carcinoma: a generic protocol
Abstract
This is a protocol for a Cochrane Review (Diagnostic test accuracy). The objectives are as follows:
To determine the diagnostic accuracy of sentinel lymph node biopsy (SLNB) for the detection of nodal metastases (in the investigated nodal basin) for the staging of cutaneous squamous cell cancer (cSCC).
To determine the diagnostic accuracy of imaging tests, including ultrasound, computed tomography, magnetic resonance imaging and positron emission tomography, alone or in combination, for the detection of any metastasis for the staging of cutaneous squamous cell cancer.
Background
Cochrane Skin (Nottingham) in collaboration with the Test Evaluation Research Group in the Institute for Applied Health Research (TERG, Birmingham) are undertaking a series of Cochrane Diagnostic Test Accuracy (DTA) reviews on the diagnosis and staging of melanoma and keratinocyte skin cancers (basal cell and cutaneous squamous cell carcinoma) as part of the National Institute for Health Research (NIHR) Cochrane Systematic Reviews Programme. Appendix 1 shows the current content and structure of the programme.
As several reviews for each topic area will follow similar methodology, we have prepared generic protocols in order to avoid duplication of effort. This protocol concerns the evaluation of tests for the staging of cutaneous squamous cell carcinoma, including sentinel lymph node biopsy for detection of nodal metastases and imaging tests for the detection of any metastatic disease. A separate Cochrane protocol is available for the staging of cutaneous melanoma (Dinnes 2016) and for the diagnosis of melanoma (Dinnes 2015a) and of keratinocyte skin cancers (Dinnes 2015b). The Background and Methods sections of this protocol use some text that was originally published in the protocol for the evaluation of tests for the diagnosis of keratinocyte skin cancers (Dinnes 2015b) and the protocol for staging of melanoma skin cancer (Dinnes 2016).
Table 1 provides a glossary of terms used.
| Term | Definition |
| Adjuvant therapy or treatment | A treatment given after the main treatment for cancer to reduce the risk of recurrence |
| Adnexal (in relation to the skin) | Structures in the skin including hair follicles and sebaceous glands |
| Biopsy | Removal of a sample of tissue from the body to assist in diagnosis or inform the choice of treatment of a disease |
| Computed tomography (CT) | Imaging technique in which the person lies on a table within a x‐ray gantry. The images are acquired using a spiral (helical) path and banks of detectors, allowing presentation of the internal organs and blood vessels in different projections including 3‐D views |
| Curettage | Surgical procedure to remove tissue or delineate borders of lesions via scraping |
| Cutaneous T cell lymphoma | A type of non‐Hodgkin lymphoma of the skin caused by uncontrolled growth of white blood cells |
| Dermal papilla | Small projections of the dermis into the overlying epidermis giving an undulating pattern and visible as "fingerprints" in hands and feet |
| Electrodessication | The use of high‐frequency electric currents to cut, destroy or cauterise tissue. It is performed with the use of a fine needle‐shaped instrument |
| False negative | An individual who is truly positive for a disease, but whom a diagnostic test classifies as disease‐free |
| False positive | An individual who is truly disease‐free, but whom a diagnostic test classifies as having the disease |
| Follicular bulge | The portion of the hair follicle that contains the stem cells that give rise to skin cells. It contains the cells needed for wound repair, hair growth and development and tumour development |
| Histopathology | The study of tissue, usually obtained by biopsy or excision, for example under a microscope |
| Incidence | The number of new cases of a disease in a given time period |
| interfollicular epidermis | The part of the skin that lies in between the hair follicles |
| Local recurrence | Regrowth of a tumour in the area from which it was originally removed |
| Locoregional recurrence | Regrowth of a tumour in the area from which it was originally removed or in the regional lymph nodes (usually nearest to the original tumour site) |
| Lymph node dissection | Surgical removal or one or more lymph nodes in the absence of proven involvement with melanoma |
| Lymph node dissection (lymphadenectomy) | A surgical operation to remove one or more groups of lymph nodes |
| Lymphoscintigraphy | An imaging technique used to identify the lymph drainage basin, determine the number of sentinel nodes, differentiate sentinel nodes from subsequent nodes, locate the sentinel node in an unexpected location, and mark the sentinel node over the skin for biopsy. It requires the injection of a radio‐isotope into the skin around the biopsy scar and a scan some hours later to determine to which lymph nodes the tracer has travelled |
| Magnetic resonance imaging (MRI) | A type of scan which uses a magnetic field and radio waves to produce images of sections of the body |
| Meta‐analysis | A form of statistical analysis used to synthesise results from a collection of individual studies |
| Metastases/metastatic disease | Spread of cancer away from the primary site to somewhere else through the bloodstream or the lymphatic system |
| Micrometastases | Metastases so small that they can only be seen under a microscope |
| Morbidity | Detrimental effects on health |
| Mortality | Either (1) the condition of being subject to death; or (2) the death rate, which reflects the number of deaths per unit of population in relation to any specific region, age group, disease, treatment or other classification, usually expressed as deaths per 100, 1000, 10,000 or 100,000 people |
| Multidisciplinary team (MDT) | A team with members from different healthcare professions and specialties (e.g. urology, oncology, pathology, radiology, and nursing). Cancer care in the NHS uses this system to ensure that all relevant health professionals are engaged to discuss the best possible care for that patient |
| Oncology | The study of cancers. This term also refers to the medical specialty of cancer care, with particular reference to the use of radiotherapy or drugs to treat cancer. The medical specialty is often split into Clinical Oncology (doctors who use radiotherapy and drug treatment) and Medical Oncology (doctors who use drug treatment) |
| Palpation | Feeling with the fingers or hands as part of a clinical examination of the body |
| Perineural involvement | Spread or invasion of cancer to the nerves |
| Positron emission tomography (PET) | A nuclear medicine imaging technique whereby a radioactive glucose (usually 18FDG) is administered intravenously before a scan is conducted to create an image using colours to show where the FDG (or other radioactive tracer) has been taken up in the body |
| Prevalence | The proportion of a population found to have a condition |
| Prognostic factors / indicators | Specific characteristics of a cancer or the person who has it which might affect the patient’s prognosis |
| Radiotherapy | The use of radiation, usually high energy x‐rays, to control the growth of cancer cells |
| Recurrence | New cancer cells are detected following treatment. This can occur either at the site of the original tumour or at other sites in the body |
| Relapse | Where cancer starts to grow again after treatment |
| Sensitivity | In this context, the proportion of individuals with a disease who have that disease correctly identified by the study test |
| Sentinel lymph node biopsy | A radioactive tracer and blue dye are injected into the skin surrounding the primary lesion and the 'sentinel' lymph nodes to which the tracer drains are located by imaging (usually lymphoscintigraphy), and then removed and examined for nodal metastatic spread that cannot be detected clinically or on imaging |
| Specificity | The proportion of individuals without a disease who have the absence of disease correctly identified by the study test (i.e. the study test is negative) |
| Staging | Clinical description of the size and spread of a patient’s tumour, fitting into internationally agreed categories |
| Stem cells | Biological cells that can self‐renew and can differentiate into specialised cells; stem cells contribute to maintaining and protecting the skin and allowing hair regrowth |
| Subclinical (disease) | Disease that is usually asymptomatic and not easily observable, e.g. by clinical or physical examination |
| Systemic treatment | Treatment, usually given by mouth or by injection, that reaches and affects cancer cells throughout the body rather than targeting one specific area |
| Ultrasound | A type of scan in which high‐frequency sound waves are used to outline a part of the body |
Some of the definitions above have been obtained from the NICE Guideline for the management of melanoma (NICE 2015).
Target condition being diagnosed
Skin cancer is the most common form of human cancer (WHO 2017). Although melanoma skin cancer is the most dangerous form, most skin cancers, i.e. basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), arise from keratinocyte skin cells and are collectively called keratinocyte skin cancers. BCC can arise from multiple stem cell populations, including from the follicular bulge and interfollicular epidermis (Grachtchouk 2011), and almost always remains localised. Primary cSCC arises from the keratinising cells of the epidermis or its appendages. It is locally invasive with the potential to metastasise and spread to local lymph nodes and distant parts of the body. The term 'non‐melanoma skin cancers' has been applied in the past to loosely denote BCCs and cSCCs. We have instead opted to use the term ‘keratinocyte’, as to use a term to denote the commonest human cancer as something which it is not (i.e. 'non‐melanoma') is unusual, and furthermore ‘non‐melanoma skin cancer’ can also include other forms of skin cancer such as cutaneous T cell lymphoma and other adnexal skin cancers.
The overall worldwide incidence of skin cancer is difficult to estimate, as there is often no requirement for BCC or cSCC to be reported within most cancer registries (Lomas 2012). However, in 2003 the World Health Organization (WHO) estimated that between 2 and 3 million skin cancers occur globally each year (WHO 2003). BCC is estimated to account for around 80% (Madan 2010) and cSCC around 16% (Gordon 2013) to 20% (Rogers 2015) of skin cancer cases worldwide. A systematic review of incidence studies found the highest reported estimate of cSCC in Europe in Switzerland, at 28.9/100,000 person‐years (1997 data), with rates generally lower in Northern European countries (Lomas 2012). Incidence is higher in the USA and Australia, with rates in men of 60/100,000 person‐years reported in Alberta, 290/100,000 in Arizona and 387/100,000 in Australia (an increase from 166 cases per 100,000 in 1985 (Australian Institute of Health and Welfare 2016)). Based on data from 2000 to 2006, the annual incidence rates of cSCC in England, Scotland and Northern Ireland were 22.7 per 100,000, 27.0 per 100,000 and 30.6 per 100,000 person‐years respectively (Lomas 2012).
The number of deaths each year attributed to cSCC in the USA has been reported at between 3900 and 8800 (Karia 2013), compared to 9710 for melanoma (American Cancer Society 2014). Local recurrence and metastatic spread from cSCC at five years is estimated at 8% and 5% respectively, with a five‐year survival rate following the development of metastatic spread of only 25% to 40% (Rowe 1992).
People with cSCC often present with an ulcer or firm (indurated) papule, plaque, or nodule (Griffin 2016), often with an adherent crust and poorly defined margins (Madan 2010). Some lesions do not give rise to any symptoms, whereas others can cause itch, tenderness, pain or bleeding. Chronic ultraviolet light exposure through recreation or occupation is strongly linked to cSCC occurrence (Alam 2001) and it is therefore particularly common in people with fair skin and in less common genetic disorders of pigmentation such as albinism and xeroderma pigmentosum (Alam 2001). cSCC can arise in the absence of a precursor lesion or it can develop from pre‐existing actinic keratosis with an estimated annual risk of progression of less than 1% to 20% (Alam 2001). Bowens disease (squamous cell carcinoma in situ) is another precursor lesion which can lead to cSCC (estimated annual risk of progression 5% (Kao 1986)), especially if associated with human papilloma virus (HPV) in genital sites. Other recognised risk factors for cSCC development include immunosuppression, chronic wounds such as long‐standing venous ulcers, arsenic or radiation exposure, certain drug treatments such as voriconazole and BRAF inhibitors, and previous skin cancer history (Chowdri 1996; Baldursson 1993; Dabski 1986; Fasching 1989; Karagas 2015; Lister 1997; Maloney 1996; O'Gorman 2014). In transplant recipients, cSCC is the most common form of skin cancer; the risk of developing cSCC has been estimated at 65 to 253 times that of the general population (Hartevelt 1990; Jensen 1999).
A cSCC lesion remains locally invasive for a variable length of time, but it has the potential for spread to the regional lymph nodes or via the bloodstream to distant sites, especially in immunosuppressed individuals (Lansbury 2010). The histopathological factors established as of prognostic significance for cSCC are now part of the Royal College of Pathologists minimum data set for reporting (Royal College of Pathologists 2014). Indicators of high‐risk status include diameter greater than 2 cm (Clayman 2005; Rowe 1992); microscopic depth greater than 4 mm or extending beyond dermis (Breuninger 1990; Clayman 2005; Friedman 1985; Rowe 1992); poor differentiation (Rowe 1992); acantholytic, spindle and desmoplastic subtypes; perineural or lymph vascular involvement (Cottel 1982; Mendenhall 1989; Moore 2005). Additional factors potentially associated with poor prognosis include immunosuppression (Barksdale 1997); lesions situated in chronic wounds; Bowens disease; areas of radiation or thermal injury; non‐sun‐exposed sites; ear or lip (Afzelius 1980; Motley 2009; Rowe 1992).
The American Joint Committee on Cancer's TNM prognostic classification system (currently AJCC‐7) (American Joint Committee on Cancer 2010) is based on tumour size, degree of infiltration and presence of and extent of nodal metastasis and distant metastasis (Table 2). Those with nodal involvement are automatically assigned to stage III (and may be upstaged according to the number and location of diseased nodes or the presence of disseminated disease), while those with confirmed local disease are assigned a stage between 0 and III, according to histological tumour staging (tumour size and presence of high‐risk features including depth/invasion, anatomical location, and differentiation) (American Joint Committee on Cancer 2010).
| Classification | Criteriaa |
| T | |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 2 cm at largest horizontal width and < 2 high‐risk features |
| T2 | Tumor > 2 cm at largest horizontal width or any size with ≥ 2 high‐risk features |
| T3 | Infiltration of facial and cranial bones |
| T4 | Infiltration of skeletal bone or skull base |
| N | |
| N0 | No regional lymph node metastases |
| N1 | Solitary, ipsilateral lymph node metastasis, maximum diameter ≤ 3 cm |
| N2a | Solitary, ipsilateral lymph node metastasis, maximum diameter > 3 cm to max. 6 cm |
| N2b | Multiple, ipsilateral lymph node metastases, all with a maximum diameter ≤ 6 cm |
| N2c | Multiple, bilateral or contralateral lymph node metastases, all with a maximum diameter ≤ 6 cm |
| N3 | Lymph node metastasis, diameter > 6 cm |
| M | |
| M0 | No distant metastases |
| M1 | Distant metastases |
Table based on that reported in Edge 2010
AJCC: American Joint Committee on Cancer;
aHigh‐risk features include depth/invasion (> 2 mm thickness, Clark level ≥ IV, or perineural invasion), anatomical location (primary site on the ear or the non–hair‐bearing lip), and differentiation (poorly differentiated or undifferentiated).
The AJCC‐7 (American Joint Committee on Cancer 2010) classification system has been criticised for its development in head and neck lesions, lack of clinical validation beyond organ transplant recipients with cSCC and inability to distinguish sufficiently between low‐ and high‐risk T2 tumours (Jambusaria‐Pahlajani 2013; Schmitt 2014; SIGN 2014; Stratigos 2015). In order to more precisely identify tumours with a high risk of metastasis and death, Jambusaria‐Pahlajani 2013 has developed an alternative T‐staging system based on analysis of a group of 256 people with primary high‐risk cSCCs. Those risk factors found to be strong independent prognostic predictors of local recurrence, nodal metastasis, disease‐specific death, and all‐cause death on multivariate analysis included tumour diameter of 2 cm or more, poorly‐differentiated histological characteristics, perineural invasion, and tumour invasion beyond the subcutaneous fat, with bone invasion automatically upgrading the tumour to stage T3 (Table 3). When classified according to the new system, local recurrence, nodal metastasis, disease‐specific death, and all‐cause death occurred significantly more often in stage T2b rather than T2a, whereas according to the AJCC‐7 classification system these outcomes occurred in Stage 2 with no further differentiation (Jambusaria‐Pahlajani 2013). The new approach may better identify those at high risk of disease progression or later recurrence.
| Classification | Criteria a |
| Tis | Carcinoma in situ |
| T1 | 0 risk factors |
| T2a | 1 risk factor |
| T2b | 2 to 3 risk factors |
| T3 | 4 risk factors or bone invasion |
Alternative system proposed by Jambusaria‐Pahlajani 2013 and adapted in Schmitt 2014.
aRisk factors include tumour diameter of ≥ 2 cm, poorly‐differentiated histological characteristics, perineural invasion, and tumour invasion beyond the subcutaneous fat (excluding bone invasion, which automatically upgrades the tumour to alternative stage T3).
In terms of nodal disease, staging for cSCC primarily relies on clinical examination with palpation of the regional lymph nodes to identify any nodal involvement (Motley 2009; NCCN 2013; Stratigos 2015), which increases the risk of recurrence and mortality (survival rate of 30% at five years) (Stratigos 2015). Current staging systems do not account for the presence of micrometastatic disease as identified by sentinel lymph node biopsy (SLNB). However Schmitt 2014 has applied the AJCC‐7 system and the alternative system proposed above to a cohort of 130 people taken from the literature, to identify which was most closely associated with positive SLNB findings (micrometastasis). Using the AJCC‐7 classification, positive SLNB results were found in T2 tumours (in 13 of 116 patients (11.2%), but in none of those less than 2 cm in diameter) and T4 tumours (three of five; 60%); whereas for the alternative system positive SLNB results were found in those grouped as T2a (six of 85; 7.1%), T2b (five of 17; 29.4%) and T3 (three of six; 50%) (Schmitt 2014).
Treatment of cSCC
The British Association of Dermatology multi‐professional guideline (Motley 2009) strongly emphasises three important points about treatment of SCC: the lack of randomised evaluation of treatment for SCC; the variation in behaviour of SCC tumours; and the varied experience of those treating cSCC, with dermatologists primarily dealing with lower‐risk lesions, and higher‐risk, more aggressive tumours referred to plastic and maxillofacial surgeons. Furthermore, the guideline points to “the need for complete removal or treatment of the primary tumour, the possible presence of local ‘in transit’ metastases”, and “the tendency of metastases to spread by lymphatics to lymph nodes”. A 2010 Cochrane Review identified a single RCT in cSCC showing no evidence of benefit from adjuvant 13‐cis‐retinoic acid and interferon alpha after surgery (Lansbury 2010). Current practice therefore relies on evidence from observational studies, as reviewed, for example, by Lansbury 2013.
The standard treatment for SCC is usually surgical excision with predetermined margins (Motley 2009; Stratigos 2015); however, other locally destructive techniques can be employed as indicated, especially for smaller lesions. These include freezing (cryotherapy) or electrodessication and curettage; non‐surgical treatments including topical imiquimod; and photodynamic therapy (Motley 2009; Stratigos 2015). The European Dermatology Forum‐European Association of Dermato‐Oncology‐European Organization of Research and Treatment of Cancer (EDF–EADO–EORTC) consensus group have recommended standardised minimal surgical margins according to the presence of risk factors including vertical thickness, histological grade, subcutaneous invasion, perineural invasion, and tumour site (Bonerandi 2011). Mohs micrographic surgery, whereby horizontal sections of the tumour undergo histological analysis and re‐excisions are made until the margins are tumour‐free, can be considered where standard wider excision margins might lead to considerable functional impairment (Lansbury 2010; Motley 2009; Stratigos 2015). A systematic review and meta‐analysis of observational studies of treatments for cSCC suggest low recurrence rates for small, low‐risk lesions treated with cryotherapy or curettage and electrodesiccation (recurrence rates less than 2%) (Lansbury 2013). Results for Mohs microsurgery, surgical excision, or radiotherapy which are likely to have been evaluated in higher‐risk populations, showed pooled recurrence rates of 3%, 5.4% and 6.4% respectively, and with overlapping confidence intervals; the review authors advise caution when comparing results across treatments (Lansbury 2013).
Photodynamic therapy is currently not recommended for use in cSCC in the SIGN guideline, due to lack of evidence (SIGN 2014). Radiotherapy may also be an option for non‐surgical treatment of inoperable small cSCCs, with some evidence of good local control (Fort 2016). Electrochemotherapy (where chemotherapy is administered either intravenously or directly into the tumour, followed by brief and intense electric pulses around or directly into the tumour (NICE 2014)) may be used to control the progression of inoperable loco‐regional SCC recurrences (Stratigos 2015); however, evidence is scarce and based on mixed populations of people with BCC and cSCC (NICE 2014).
Intralesional 5‐Fluorouracil (5‐FU) alone may also be considered as a therapeutic option in invasive cSCC, although it is not commonly used in the UK and available evidence appears to be based on treatment of keratoacanthoma rather than invasive SCC (Good 2011; Kirby 2010).
As most cSCC is localised, elective lymph node dissection is generally not undertaken (Motley 2009) and the role of SLNB is yet to be established (Stratigos 2015). In the presence of clinically‐detectable nodal disease, regional lymph node dissection is undertaken (Stratigos 2015). Guidelines also recommend that adjuvant or post‐operative radiotherapy can be considered where there is substantial perineural involvement, when tissue margins are not tumour‐free after surgical excision, and in the presence of regional disease (NCCN 2013; SIGN 2014; Stratigos 2015). The National Comprehensive Care Network (NCCN) guideline highlights two small retrospective studies (Veness 2005; Givi 2011) suggesting that adding radiotherapy to lymph node dissection improves disease‐free survival (NCCN 2013). The evidence for systemic treatment in an adjuvant setting or for treatment of metastatic disease is scarce, with no available phase III trials (SIGN 2014). Chemotherapeutic agents that have been used include cisplatin or carboplatin, 5‐fluorouracil, bleomycin, methotrexate, adriamycin, taxanes, gemcitabine or ifosfomide (NCCN 2013; Stratigos 2015).
Although the prognosis for people with localised cSCC is generally very good, accurate diagnosis and staging to allow timely management may be important to reduce potentially significant morbidity, particularly if potentially effective new drugs to treat systemic disease become available.
Index test(s)
With such limited evidence for systemic therapies, interest in the staging of cSCC has largely focused on locoregional or nodal staging to determine whether lymph node dissection is required. The first step in this process is the identification of high‐risk patients on histopathology and the initial clinical examination and detailed history‐taking, including palpation of the regional lymph nodes followed up with fine‐needle aspiration cytology or core biopsy of enlarged lymph nodes. Lymph node ultrasound may also be used (Motley 2009; NCCN 2013; Stratigos 2015). SLNB is a technique that is increasingly used for nodal staging in a range of cancers but its role in cSCC has yet to be established.
In terms of imaging tests other than ultrasound, the evidence for computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography‐computed tomography (PET‐CT) is unclear. The SIGN guideline suggests that evidence for further imaging such as MRI has to be extrapolated from evidence of its use in the management of other cancers such as squamous cell cancer of the head and neck (SIGN 2014).
Ultrasound
Ultrasound can be used as an alternative or adjunct to palpation for detection of enlarged lymph nodes, and is usually followed by fine‐needle aspiration cytology or core biopsy to confirm the presence of metastases. It has been highly recommended in the absence of clinically‐enlarged nodes (Bonerandi 2011; Motley 2009), particularly for people with tumours that have high‐risk characteristics (Jank 2003).
We found no systematic reviews of ultrasound in cSCC from our scoping searches.
Sentinel lymph node biopsy (SLNB)
For melanoma skin cancer, sentinel node biopsy is usually performed by a plastic surgeon, following wide local excision of the primary tumour (NICE 2015). A radioactive tracer and blue dye are injected into the skin surrounding the primary lesion and the 'sentinel' lymph nodes to which the tracer drains are located by imaging (usually lymphoscintigraphy) and then removed and examined for nodal metastatic spread that cannot be detected clinically or on imaging (NICE 2015). Although the SLNB result directly informs pathological staging of melanoma (Balch 2009), this is currently not the case for cSCC, and the prognostic and therapeutic role for SLNB remains unclear. Given that regional lymph node dissection would normally be undertaken in the presence of lymph node involvement, the accuracy of SLNB for detection of lymph node metastases according to primary tumour site is of clinical interest.
Previous systematic reviews of SLNB in cutaneous cSCC have found some limited data to suggest few false‐negative results and low morbidity from the procedure (Ahmed 2014; Allen 2015; Ross 2006), with further potentially eligible primary studies continuing to emerge (Gore 2016).
SLNB is useful only for the detection of locoregional disease via lymphatic spread, whereas the imaging‐based tests discussed below can also detect distant metastatic disease which occurs via lymphatic or haematogenous spread. Imaging tests are undertaken and interpreted by radiologists with decisions about patient management following imaging or SLNB made at multidisciplinary team meetings (MDTs) as discussed in the Clinical pathway section below.
Computed Tomography (CT; non‐contrast or contrast‐enhanced)
CT scans use X‐rays to take cross‐sectional images of the body, which can then be combined to create 3D images (Oncolink 2016b). The procedure involves small amounts of radiation according to the area of the body to be scanned (www.radiologyinfo.org/en/info.cfm?pg=safety‐xray), and can also be conducted using an intravenous contrast agent (contrast‐enhanced) to allow blood vessels or lymph nodes to be assessed.
We found no systematic reviews of CT use in cSCC from our scoping searches.
Magnetic Resonance Imaging (MRI; non‐contrast or contrast‐enhanced)
MRI scans use magnets and radiowaves rather than radiation to generate images, which are then computer‐processed to produce cross‐sectional 'slices' of the body. MRI scans are more expensive and take longer to carry out compared to CT scans (Oncolink 2016bc).
We found no systematic reviews of MRI for cSCC staging from our scoping searches.
18FDG ‐ Positron emission tomography (PET)
Positron emission tomography (PET) is a nuclear medicine technique whereby a radioactive glucose (usually 18FDG) is administered intravenously, and is then metabolised as part of the body’s normal function. The PET scanner detects the FDG and an image is created using colours to show where the FDG has been taken up; tumours take up more FDG than normal tissue, due to a higher rate of metabolism, with malignant masses generally being more 'active' than benign ones (Oncolink 2016d). PET can also be combined with CT to provide both functional and structural information. The use of PET in combination with CT will necessarily increase the radiation exposure of the patient (RPOP 2016).
We found no systematic reviews of PET for cSCC staging from our scoping searches. One small case series suggests that PET‐CT may be useful for nodal staging in high‐risk patients, e.g. those with chronic lymphocytic leukaemia (Tomaszewski 2014), while others suggest that management in most people with head‐and‐neck cSCC with regional metastasis does not change with the addition of PET‐CT (Supriya 2014).
Clinical pathway
The recommendations on the staging of cSCC following diagnosis as described in the UK (Motley 2009; SIGN 2014), Europe (Stratigos 2015), Australia (Cancer Council Australia 2008) and the USA (NCCN 2013) are summarised in Figure 1 and outlined below; however, it should be noted that practice may vary across and between countries.
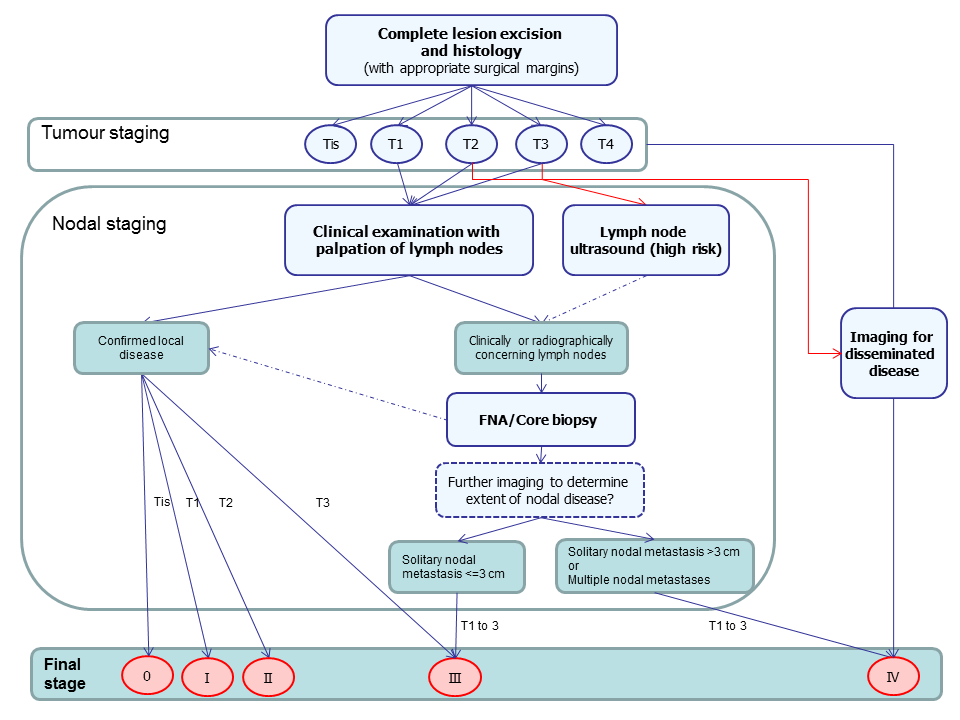
Summary of guideline recommendations for the staging of cutaneous ACC following primary diagnosis
Although cSCC can be locally very invasive, it is primarily a localised disease; the early identification of high‐risk tumours that are most likely to recur locally or to have nodal involvement is key to clinical management (Cancer Council Australia 2008; Motley 2009; SIGN 2014). In the UK National Health Service (NHS), all people with suspected cSCC are referred via the two‐week wait referral pathway to an appropriately‐trained specialist (London Cancer Alliance 2013). On histological confirmation of the presence of SCC and wide local excision of the primary lesion, patients undergo a full clinical examination of both the skin and regional lymph nodes with fine‐needle aspiration cytology or core biopsy of enlarged lymph nodes (Motley 2009; NCCN 2013; SIGN 2014; Stratigos 2015). The result of this examination and the histopathology results are used for initial staging of the tumour; those considered to be at high risk of metastatic spread may then undergo further staging investigations as determined by a multidisciplinary team (MDT). In the UK NHS, all people identified as being at high risk are considered at an MDT meeting (SIGN 2014). These teams may include dermatologists, surgeons (including plastic surgeons), oncologists, radiologists, specialist nurses, GPs with a special interest in skin cancer, physiotherapists, psychologists, lymphoedema services, occupational therapists, cosmetic camouflage advisers and histopathologists (NICE 2010).
High‐risk SCCs as defined by the National Institute for Health and Care Excellence (NICE) (and similarly in the SIGN 2014 guideline) are histologically “poorly differentiated, perineural invasion, depth greater than 4 mm or extending to subcutaneous tissue (Clark level 5)”, situated on “lip, ears, non‐sun‐exposed sites, e.g. penis, scrotum and soles of feet; in areas of previous injury, e.g. burns, irradiation and chronic ulcers” and with greater than 2 cm diameter, immunosuppression, or previously‐treated lesion (NICE 2010). Similar factors are considered in Australian guidelines with a recommendation for specialist referral for all cSCC of the central face, scalp, lip and ear due to higher risk of local recurrence and the possible need for specialist reconstruction techniques (Cancer Council Australia 2008).
Staging investigations that may be initiated by the MDT include:
-
SLNB (a technique that is increasingly used for nodal staging in a range of cancers but whose role in cSCC has yet to be established (SIGN 2014)); or
-
imaging tests, such as ultrasound, CT, MRI, or PET‐CT.
Ultrasound may be considered for nodal assessment in the absence of clinically‐enlarged nodes (Motley 2009; NCCN 2013; Stratigos 2015), especially in higher‐risk cases, such as SCC of the head and neck, to look at the different levels of nodes to determine whether lymph node dissection is feasible or not. In Australia , any clinical suspicion of lymph node involvement warrants investigation by CT or ultrasound, with diagnosis of nodal metastases confirmed by fine‐needle aspiration cytology (Cancer Council Australia 2008). The use of CT or MRI is generally based on clinical indication (NCCN 2013; SIGN 2014; Stratigos 2015), for example if perineural invasion is suspected (Cancer Council Australia 2008). In the elderly and in transplant recipients, whole‐body CT may be used in case of more aggressive disease or to identify any internal SCC. People with chronic lymphatic leukaemia (CLL) are another high‐risk group and a classic dilemma in SCC, as lymph nodes may be enlarged due to the CLL but the associated immunosuppression increases the incidence and rate of recurrence of SCC (Jennings 2010).
Follow‐up after treatment relies on skin self‐examination with "close medical examination" (Motley 2009) of those with high‐risk lesions (SIGN 2014), with imaging to detect metastatic spread undertaken on development of recurrence (SLNB is used only on primary presentation of skin cancer and is rarely employed for staging of recurrence, even for melanoma (Beasley 2017)).
Role of index test(s)
The assessment of people with cSCC lesions relies heavily on clinical examination, history and histological assessment; only a small percentage of them experience any disease spread (Rowe 1992), such that further disease staging is often not required. However, there is a small proportion of people with subclinical or disseminated disease in whom further investigations may be warranted (Rowe 1992). For people with melanoma, SLNB provides a means of identifying those without clinically palpable lymph nodes who may benefit from complete lymph node dissection, although the overall benefit to patients in terms of disease‐free or overall survival is as yet unclear (Sladden 2015). People with cSCC, however, do not generally undergo lymph node dissection unless they have clinically palpable lymph nodes (Motley 2009), as the prognostic significance of subclinical or micrometastatic disease is even less clear, primarily due to the small evidence base. There is some evidence, however, that micrometastatic disease can be identified in a significant proportion of those with high‐risk tumours who do not have clinically palpable lymph nodes (Gore 2016; Schmitt 2014).
Imaging tests are recommended at the discretion of the clinicians concerned, and are used for both regional lymph nodes and to identify any more distant disease. The current evidence base is poor, indicating a need to further define any potential role of ultrasound, CT, MRI and PET‐CT.
Alternative test(s)
When clinically‐palpable lymph nodes are identified, core‐needle biopsy or fine‐needle aspiration cytology of the lymph node may be undertaken to confirm the presence of macrometastases, i.e. metastases that are visible to the naked eye (Marsden 2010). Fine‐needle aspiration is a fairly simple procedure which allows a sample of cells to be taken from the lymph node with a fine needle (Hall 2013), while core‐needle biopsy uses a slightly larger needle with a hollow centre, allowing the removal of a core of tissue with the cell structure intact (Oncolink 2016a). Both procedures can be guided by simple palpation or, for more deep‐seated lesions, by image‐based techniques such as ultrasound (Bohelay 2015). Although the accuracy of core‐needle biopsy in comparison to fine‐needle aspiration has been identified as a key clinical question to be investigated by our advisory group, it is beyond the scope of these reviews, which focus on the detection of nonpalpable metastatic disease.
As with melanoma, biomarkers have potential more as indicators of prognosis in people with cSCC rather than as staging tools in themselves. The SIGN guideline identified only a small number of preliminary retrospective studies focusing on high‐risk tumours (SIGN 2014).
Rationale
Staging of cSCC relies predominantly on histopathological classification and clinical examination to identify nodal spread. Further investigations will be considered only in those people identified as having high‐risk cSCC; however, the evidence to support the use of additional investigations is relatively sparse.
Currently‐available systematic reviews of SLNB in cSCC suffer from being out of date, with searches covering periods up to 2006 (Ross 2006) and 2012 (Allen 2015), or restriction to certain patient groups, such as head and neck (Ahmed 2014). Although SLNB is not routinely used for cSCC, there is a need to keep a watching brief on the evidence in a developing field.
In terms of imaging, we found no systematic reviews in cSCC; nevertheless, imaging is used in certain high‐risk groups of patients and therefore any evidence to support or refute its use needs to be collected and systematically evaluated.
Our approach will allow any evidence for the accuracy of SLNB and of imaging tests in staging cSCC to be estimated. This generic protocol provides the methodology that we will use for our reviews of tests to assist in the staging of cSCC. We will appropriately tailor the Background sections for each individual test review.
Objectives
To determine the diagnostic accuracy of sentinel lymph node biopsy (SLNB) for the detection of nodal metastases (in the investigated nodal basin) for the staging of cutaneous squamous cell cancer (cSCC).
To determine the diagnostic accuracy of imaging tests, including ultrasound, computed tomography, magnetic resonance imaging and positron emission tomography, alone or in combination, for the detection of any metastasis for the staging of cutaneous squamous cell cancer.
Secondary objectives
To determine the diagnostic accuracy of imaging tests for the detection of nodal metastases in the staging of cutaneous squamous cell cancer.
To determine the diagnostic accuracy of imaging tests for the detection of distant metastases in the staging of cutaneous squamous cell cancer.
We will estimate these separately for those undergoing primary staging and those who have experienced a disease recurrence.
Sources of heterogeneity
We will consider a range of potential sources of heterogeneity for investigation in each individual test review. These may vary between reviews but may include the following.
i. Population characteristics
-
Primary tumour site (head and neck, trunk, limb, and other)
-
Primary staging versus mixed or unclear populations (i.e. including staging of recurrent disease)
ii. Index test characteristics
-
Differences in test positivity thresholds
iii. Reference standard characteristics
-
Reference standard used (histology, clinical or imaging‐based follow‐up)
iv. Study quality
-
Consecutive or random sample of participants recruited
-
Index test interpreted blinded to the result of any other index test
-
Presence of partial or differential verification bias (whereby only a sample of those subject to the index test are verified by the reference test or by the same reference test with selection dependent on the index test result)
-
Use of an adequate reference standard
-
Overall risk of bias
We anticipate that the volume of evidence retrieved will be small and will restrict our ability to formally investigate these sources of heterogeneity; however, data permitting, we will examine any impact on the effectiveness of each index test for the primary target condition and make recommendations for where further research might be required.
Methods
Criteria for considering studies for this review
Types of studies
We will include test accuracy studies that allow comparison of results of the index test with that of a reference standard, including the following:
-
prospective and retrospective studies;
-
studies where all participants receive a single index test and a reference standard;
-
studies where all participants receive more than one index test(s) (concurrently) and a reference standard;
-
studies where participants are allocated by any method to receive different index tests or combinations of index tests and all receive a reference standard (between‐person comparative studies (BPC));
-
studies that recruit series of participants unselected by true disease status; and
-
diagnostic case‐control studies that separately recruit diseased and non‐diseased groups (see Rutjes 2005).
We will exclude follow‐up or surveillance studies using repeated imaging tests to detect disease recurrence, as defining the most appropriate follow‐up schedule for cSCC is not an objective of these reviews. Given the lack of reliability in the interpretation of studies with very small sample sizes, we will apply a sample size restriction of at least five disease‐positive participants. As we anticipate a paucity of evidence meeting this criterion, if we find fewer than five studies meeting this criterion for any one test, we will include studies with fewer than five cases.
We will include studies reporting either lesion‐based or participant‐based analyses, but only those reporting data on a per‐patient basis in the primary analysis.
Participants
We will include studies in adults with cSCC at any primary site who are undergoing staging, either following primary presentation of disease or following recurrence of disease. We will include studies of mixed populations of participants or where we cannot determine the clinical pathway, and will examine any effect on test accuracy in subgroup analysis. We will exclude studies in which test results for participants with cSCC cannot be differentiated from those of participants with other diagnoses.
For studies of SLNB, outcomes must be presented for both sentinel lymph node‐positive and sentinel lymph node‐negative participants. For studies of imaging tests, we will include studies focusing on either sentinel lymph node‐positive or sentinel lymph node‐negative participants.
Index tests
We will undertake individual reviews for SLNB and for the following imaging tests, either alone or in combination:
-
ultrasound (with or without subsequent fine‐needle aspiration cytology or core biopsy)
-
CT (non‐contrast or contrast‐enhanced)
-
PET or PET‐CT (18FDG only)
-
MRI (non‐contrast or contrast‐enhanced)
SLNB studies may assess the effectiveness of methods of detection of SLNs, for example using different tracers or dyes or alternative imaging approaches. These will often compare approaches in terms of the number of diseased nodes identified, and we will exclude them unless an eligible reference standard, as described below, has been used.
Target conditions
The target condition for the SLNB review will necessarily be defined differently according to the result of the index test as follows:
-
For SLN‐positive participants, the presence of micrometastasis in the nodal basin investigated by the SLNB procedure
-
For SLN‐negative participants, the emergence of clinically‐detectable nodal disease or macrometastases in the nodal basin investigated by the SLNB procedure in the absence of evidence of distant metastases; the latter is in order to increase the likelihood that a nodal recurrence in SLN‐negative participants is more likely to be a false negative if there is no disease elsewhere in the body
-
In the event of inadequate data, we will drop the requirement to confirm the absence of distant metastases in SLN‐negative participants, and will consider the emergence of any nodal disease in the nodal basin investigated by the SLNB procedure a sufficient definition of a false negative result
The target conditions for the imaging test reviews are the detection of:
-
any metastases;
-
any nodal metastases;
-
any distant metastases.
The use of the same tests for the staging of cutaneous melanoma is the subject of a separate protocol (Dinnes 2016).
Reference standards
Acceptable reference standards include:
-
Histology of lymph node or distant specimens, with samples obtained by core biopsy, SLNB or lymph node dissection, for index test‐positive participants
-
Cytology of lymph node specimens, with samples obtained by core biopsy, or fine‐needle aspiration, for index test‐positive participants
-
Clinical or radiological follow‐up to identify nodal or distant recurrence of at least three months, for index test‐negative participants
-
Any combination of the above
Studies using cross‐sectional imaging‐based reference standards (i.e. a direct comparison of the index test versus an alternative reference standard imaging test) will not be eligible.
Search methods for identification of studies
The Information Specialist (SB) will carry out a comprehensive search for published and unpublished studies. As previously mentioned, a series of Cochrane Diagnostic Test Accuracy (DTA) reviews on the diagnosis and staging of melanoma and keratinocyte skin cancers is being carried out as part of a National Institute for Health Research (NIHR) Programme Grant.
Electronic searches
We have conducted a single large literature search for the programme grant, covering all conditions and tests. This allowed for the screening of search results for potentially relevant papers for all reviews at the same time. We formulated a MEDLINE scoping search combining disease‐related terms with terms related to the test names, using both text words and subject headings. As most records were related to the searches for tests for the staging of disease, we applied a filter using terms related to cancer staging and to accuracy indices to the staging test search, to try to eliminate irrelevant studies, e.g. those using imaging tests to assess treatment effectiveness. We screened a sample of 300 records that would be missed by applying this filter and adjusted the filter to make sure that we would not miss any potentially relevant studies. The final search filter (Appendix 2) reduces the overall numbers retrieved from MEDLINE by around 6000. We cross‐checked the final search result against the list of studies included in five systematic reviews; our search identified all but one of the studies, and this study is not indexed on MEDLINE. The Information Specialist, Susan Bayliss, has devised the search strategy, with input from the Information Specialist from Cochrane Skin, Elizabeth Doney. We used no additional limits.
We undertook further scoping searches to identify any relevant systematic reviews or health technology assessments. In addition to general bibliographic databases, we also accessed specialist databases with a focus on reviews of diagnostic test accuracy, such as ARIF.
We have now searched the following bibliographic databases, retrieving a total of 33,994 unique records:
Published studies
The Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library; the Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library; CRD Database of Abstracts of Reviews of Effects (DARE); CRD HTA (Health Technology Assessment) database; MEDLINE via OVID (from 1946); MEDLINE In‐Process & Other Non‐Indexed Citations via OVID; Embase via OVID (from 1980); and Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO from 1960 to the present.
Unpublished studies
Conference Proceedings Citation Index (CPCI) via Web of Science™ (from 1990); Zetoc (from 1993); and SCI Science Citation Index Expanded via Web of Science™ (from 1900), using the "Proceedings and Meetings Abstracts" Limit function.
Trials registers
The US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov); NIHR Clinical Research Network Portfolio Database (www.nihr.ac.uk/research‐and‐impact/nihr‐clinical‐research‐network‐portfolio/); and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
We aimed to identify all relevant studies, regardless of language or publication status (published, unpublished, in press, or in progress). We applied no date limits. Update searches will be time‐ and resource‐dependent.
Searching other resources
Due to time restrictions and the volume of evidence retrieved from the electronic searches, we will not conduct any handsearching of conference proceedings. By searching CENTRAL, we will retrieve relevant records identified by regular handsearching by Cochrane Skin. The handsearched conferences and journals are listed here: www.skin.cochrane.org/resources‐handsearchers.
We will include information about potentially relevant ongoing studies in the 'Characteristics of ongoing studies' tables. We will screen any relevant systematic reviews identified by the searches for their included primary studies, and we will include any that our searches have missed in the review. We will check the reference lists of all included papers, and subject experts within the author team will review the final list of included studies. We may use citation‐searching for key references when we consider it appropriate.
Data collection and analysis
Selection of studies
Due to the volume of records retrieved, at least one review author (JDi or NC) has undertaken screening of the titles and abstracts, with any queries discussed and resolved by consensus. A pilot exercise independently screening 539 references from MEDLINE showed a good level of agreement (89% with a kappa of 0.77). So far, we have selected 822 records for full‐text review for the staging reviews. At least two review authors, including methodologists (JDi or NC) and clinical reviewers, using a study eligibility screening proforma based on prespecified inclusion criteria, will independently undertake subsequent assessment of potentially relevant full‐text articles for the staging reviews (Appendix 3). Where differences in opinion exist, a third review author, drawing on the clinical and methodological expertise in the team as appropriate to the content of the query (JDe, CD, HW, and RM) will help with resolution. We will compile a list of otherwise eligible studies for which insufficient data were presented to allow for the construction of a 2 x 2 contingency table, and we will contact study authors, asking them to provide the relevant data. We will describe the study selection process in an adapted PRISMA flowchart (Liberati 2009). At the full‐text inclusion stage, we will tag studies according to their target condition (melanoma or cSCC) and index test.
Data extraction and management
We will carry out data extraction using a predesigned and piloted data extraction form in Excel, to ensure that we collect relevant data. At least two review authors will independently extract details of the study design, participants, index test(s) or test combinations and criteria for index test positivity, reference standards, and data required to populate a 2 x 2 diagnostic contingency table for each index test. We will record where data are available at several index test thresholds. A third review author, drawing on clinical and methodological expertise in the team as appropriate to the content of the query, will resolve discrepancies.
We will try to contact authors of included studies where information is missing that we consider key to one or more of the assessments of the quality of an included study, investigation of heterogeneity, or completion of a 2 x 2 diagnostic contingency table. We will follow up studies published only as conference abstracts, to identify whether a full paper has been published. Where possible, we will contact the authors of conference abstracts published from 2015 to 2016 and ask whether full data are available. If we can identify no full paper, we will mark conference abstracts as 'pending' and revisit them. Experience of contacting authors for information about missing data in DTA reviews is limited. Where we seek missing data, we will therefore document the outcome of contact with the authors.
Dealing with multiple publications and companion papers
In the event of multiple reports of a primary study, we will examine all available data to determine the potential for overlapping populations and to identify a primary data source. Where we suspect overlapping study populations and are unable to identify a primary data source, we will contact study authors for clarification in the first instance. If contact with authors is unsuccessful, we will use the most complete and up‐to‐date data source available, thus avoiding the risk of double‐counting. We will examine the impact of inconsistencies in reporting of 2 x 2 data that remain unresolved in a sensitivity analysis.
Assessment of methodological quality
We will assess the applicability and risks of bias of included studies using the QUADAS‐2 checklist (Whiting 2011), which has been tailored to the review topic (see Table 4).
| Item | Response (delete as required) |
| PARTICIPANT SELECTION (1) ‐ RISK OF BIAS | |
| 1) Was a consecutive or random sample of participants or images enrolled? | Yes ‐ if paper states consecutive or random No – if paper describes other method of sampling Unclear – if participant sampling not described |
| 2) Was a case‐control design avoided? | Yes ‐ if consecutive or random or case‐control design clearly not used No – if study described as case‐control or describes sampling specific numbers of participants with particular diagnoses Unclear – if not described |
| 3) Did the study avoid inappropriate exclusions, e.g. needs examples of inappropriate exclusions in this context – for both melanoma and for cutaneous squamous cell carcinoma (cSCC) staging? | Yes ‐ if inappropriate exclusions were avoided No – if lesions were excluded that might affect test accuracy, e.g. indeterminate results or where disagreement between evaluators was observed Unclear – if not clearly reported |
| 4) For between‐person comparative (BPC) studies only (i.e. allocating different tests to different study participants such as randomised controlled trials (RCTs)): | |
|
| Yes ‐ if same selection criteria were used for each index test No – if different selection criteria were used for each index test Unclear – if selection criteria per test were not described N/A – if only one index test was evaluated or all participants received all tests |
|
| Yes ‐ if adequate randomisation procedures are described No – if inadequate randomisation procedures are described Unclear – if the method of allocation to groups is not described (a description of ‘random’ or ‘randomised’ is insufficient) N/A – if only one index test was evaluated or all participants received all tests |
|
| Yes ‐ if appropriate methods of allocation concealment are described No – if appropriate methods of allocation concealment are not described Unclear – if the method of allocation concealment is not described (sufficient detail to allow a definite judgement is required) N/A – if only one index test was evaluated |
| Could the selection of participants have introduced bias? | |
| v FOR NON‐COMPARATIVE (NC) STUDIES | |
| If answers to all of questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk Unclear |
| v FOR BETWEEN‐PERSON COMPARATIVE STUDIES | |
| If answers to all of questions 1) and 2) and 3) and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘Unclear’: | Risk Unclear |
| PARTICIPANT SELECTION (1) ‐ CONCERNS REGARDING APPLICABILITY | |
| For sentinel lymph node biopsy and imaging tests: | |
| 1) Does the study report results for participants unselected by stage of disease or site of primary lesion, i.e. the study does not focus solely on those with a particular stage of disease such as AJCC I or melanoma <=1 mm in thickness? | Yes ‐ if an unrestricted group of participants have been included No ‐ if a selected group of study participants have been included, e.g. those with clinical stage I disease or only those with thin melanoma Unclear – if insufficient details are provided to determine the spectrum of included participants |
| 2) Did the study report data on a per‐patient rather than per‐lesion basis? | Yes – if a per‐patient analysis was reported No – if a per‐lesion analysis only was reported Unclear – if it is not possible to assess whether data are presented on a per‐patient or per‐lesion basis |
| For imaging tests only: | |
| 3) Does the study focus primarily on participants undergoing primary staging or those undergoing staging for disease recurrence? | Yes ‐ if at least 80% of study participants are undergoing primary staging following diagnosis of a primary cutaneous melanoma or staging of recurrence No ‐ if less than 80% of study participants are undergoing primary staging following diagnosis of a cutaneous melanoma or staging of recurrence Unclear – if insufficient details are provided to determine the proportion of patients undergoing primary staging versus those undergoing staging of recurrence |
| Is there concern that the included participants do not match the review question? | |
| If the answer to question 1) or 2) (and 3)) was ‘Yes’: | Concern is Low |
| If the answer to question 1) or 2) (and 3)) was ‘No’: | Concern is High |
| If the answer to question 1) or 2) (and 3)) was ‘Unclear’: | Concern is Unclear |
| INDEX TEST (2) ‐ RISK OF BIAS (to be completed per test evaluated) | |
| 1) Was the index test or testing strategy result interpreted without knowledge of the results of the reference standard? | Yes ‐ if index test described as interpreted without knowledge of reference standard result, or for prospective studies, if index test is always conducted and interpreted prior to the reference standard No – if index test described as interpreted in knowledge of reference standard result Unclear – if index test blinding is not described |
| 2) Was the diagnostic threshold at which the test was considered positive prespecified? | Yes ‐ if threshold was prespecified (i.e. prior to analysing study results) No ‐ if threshold was not prespecified Unclear ‐ if not possible to tell whether or not diagnostic threshold was prespecified |
| For imaging tests only: | |
| 3) For studies reporting the accuracy of multiple diagnostic thresholds (tumour characteristic or parameter) for the same index test, was each threshold interpreted without knowledge of the results of the others? | Yes ‐ if thresholds were selected prospectively and each was interpreted by a different reader, or if study implements a retrospective (or no) cutoff No ‐ if study uses prospective threshold and report states reported by same reader Unclear ‐ if no mention of number of readers for each threshold or if pre‐specification of threshold not reported N/A ‐ multiple diagnostic thresholds not reported for the same index test |
| 4) For within‐person comparisons (WPC) of index tests or testing strategies (i.e. > 1 index test applied per participant), was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes ‐ if all index tests were described as interpreted without knowledge of the results of the others No ‐ if the index tests were described as interpreted in the knowledge of the results of the others Unclear – if it is not possible to tell whether knowledge of other index tests could have influenced test interpretation N/A – if only one index test was evaluated |
| Could the conduct or interpretation of the index test have introduced bias? | |
| v FOR NC and BPC STUDIES item 3) / 4) to be added | |
| If answers to questions 1) and 2) was ‘Yes’: | Risk is Low |
| If answers to either questions 1) or 2) was ‘No’: | Risk is High |
| If answers to either questions 1) or 2) was ‘Unclear’: | Risk is Unclear |
| v FOR WPC STUDIES | |
| If answers to all questions 1), 2) for any index test and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘Unclear’: | Risk is Unclear |
| INDEX TEST (2) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? This item applies equally to studies using objective and more subjective approaches to test interpretation. For SLNB studies, this requires description of the tracer threshold for identification of the SLN and the histological assessment. | Yes – if the criteria for diagnosis of the target disorder were reported in sufficient detail to allow replication No – if the criteria for diagnosis of the target disorder were not reported in sufficient detail to allow replication Unclear – if some but not sufficient information on criteria for diagnosis to allow replication were provided |
| 2) Was the test interpretation carried out by an experienced examiner? | Yes – if the test was interpreted by an experienced examiner as defined in the review protocol No – if the test was not interpreted by an experienced examiner (see above) Unclear – if the experience of the examiner(s) was not reported in sufficient detail to judge or if examiners described as 'Expert' with no further detail given |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | |
| If answers to questions 1) and 2) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 2) was ‘No’: | Concern is High |
| If answers to questions 1) or 2) was ‘Unclear’: | Concern is Unclear |
| REFERENCE STANDARD (3) ‐ RISK OF BIAS | |
| 1) Is the reference standard likely to correctly classify the target condition? | |
| a) DISEASE POSITIVE ‐ One or more of: ‐ Histological confirmation of metastases following lymph node dissection (or SLNB or core biopsy for imaging studies) ‐ Clinical/radiological follow up to identify clinically detectable disease in a mapped nodal basin (SLNB studies) ‐ Clinical/radiological follow up to identify any metastases (imaging studies) subsequently confirmed on histology | Yes – if all disease positive participants underwent one of the listed reference standards No – if a final diagnosis for any disease positive participant was reached without histopathology Unclear – if the method of final diagnosis was not reported for any disease positive participant |
| b) DISEASE NEGATIVE ‐ One or more of: ‐ Histological confirmation of absence of disease in a mapped nodal basin following lymph node dissection (or following SLNB for imaging studies) ‐ Clinical/radiological follow up of test negative participants | Yes – if at least 90% of disease negative participants underwent one of the listed reference standards No – if more than 10% of benign diagnoses were reached by concurrent imaging test Unclear – if the method of final diagnosis was not reported for any participant with benign or disease negative diagnosis |
| 2) Were the histology‐based reference standard results interpreted without knowledge of the results of the index test? | Yes – if the histopathologist was described as blinded to the index test result No – if the histopathologist was described as having knowledge of the index test result Unclear – if blinded histology interpretation was not clearly reported |
| 3) Were the reference standard results based on patient follow‐up interpreted without knowledge of the results of the index test? | Yes – if the clinician or radiologist was described as blinded to the index test result No – if the clinician or radiologist was described as having knowledge of the index test result Unclear – if blinded interpretation was not clearly reported |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| REFERENCE STANDARD (3) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Does the study use the same definition of disease positive as the primary review question or is it possible to fully disaggregate data such that data matching the review question can be extracted? | Yes – same definition of disease positive used, or patients can be disaggregated and regrouped according to review definition No – some patients cannot be disaggregated For SLNB review – disease positive includes participants with any nodal recurrence (not restricted to clinical recurrence in same nodal basin) For imaging reviews – participants with nodal versus distant recurrences cannot be disaggregated Unclear – definition of disease positive not clearly reported |
| For studies of imaging tests: | |
| 2) The result of another imaging test (without patient follow‐up to determine later emergence of disease) was not used as a reference standard | Yes – if imaging‐based diagnosis was not used as a reference standard for any participant No – if imaging‐based diagnosis was used as a reference standard for any participant Unclear – if not clearly reported |
| 3) Item on observer experience could be included? Is there concern that the target condition as defined by the reference standard does not match the review question? | |
| If answers to all questions 1), 2) and 3) was ‘Yes’: | Concern is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Concern is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Concern is Unclear |
| ***For teledermatology studies only: | |
| If answers to questions 1) and 3) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 3) was ‘No’: | Concern is High |
| If answers to questions 1) or 3) was ‘Unclear’: | Concern is Unclear |
| FLOW AND TIMING (4): RISK OF BIAS | |
| 1) Was there an appropriate interval between index test and reference standard? | |
|
| Yes – if study reports <= 1 month between index and histological reference standard No – if study reports > 1 month between index and histological reference standard Unclear – if study does not report interval between index and histological reference standard |
|
| Yes – if study reports a follow‐up visit within 6 months of application of the index test No – if study reports the first follow‐up visit beyond 6 months of the index test Unclear – if study does not report timing of follow‐up visits |
| 2) Did all participants receive the same reference standard? | Yes – if all participants underwent the same reference standard No – if more than one reference standard was used Unclear – if not clearly reported |
| 3) Were all participants included in the analysis? | Yes – if all participants were included in the analysis No – if some participants were excluded from the analysis Unclear – if not clearly reported |
| 4) For WITHIN‐PERSON COMPARISONS (WPC) of index tests: Was the interval between application of index tests <= 1 month? Could the participant flow have introduced bias? | Yes – if study reports <= 1 month between index tests No – if study reports > 1 month between index tests Unclear – if study does not report interval between index tests |
| v FOR NON‐COMPARATIVE and BPC STUDIES | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| v FOR WITHIN‐PERSON COMPARATIVE STUDIES (WPC) | |
| If answers to all questions 1), 2), 3), and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1), 2), 3), or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1), 2), 3), or 4) was ‘Unclear’: | Risk is Unclear |
Participant selection domain (1)
Selective recruitment of study participants can be a key influence on test accuracy. In general terms, all participants eligible to undergo a test should be included in a study, allowing for the intended use of that test within the context of the study.
Inappropriate participant exclusions affecting the internal validity of a study of staging might include the exclusion of those with primary tumours at particular sites or exclusion of those with unsuccessfully‐mapped SLNs.
For SLNB studies, the applicability of a study’s results will be affected by the participant spectrum according to the clinical stage of disease (AJCC stage) and site of the primary tumour.
Imaging tests may be undertaken following diagnosis of the primary cSCC lesion or following disease recurrence, such that studies may include mixed populations of participants. Given the potential for variation in test accuracy according to participant spectrum and disease prevalence (Brenner 1997; Leeflang 2013; Mulherin 2002), the applicability of results will be affected by the proportion of participants undergoing primary staging versus staging for disease recurrence, as well as by the clinical stage of disease (AJCC stage or clinical nodal status) and site of the primary tumour.
Index test domain (2)
Given the subjectivity of test interpretation, particularly for imaging tests, the interpretation of the index test blinded to the result of the reference standard is a key means of reducing bias. For prospective studies, the index tests will by nature be interpreted before the result of the reference standard is known; however, retrospective studies will be susceptible to information bias, either if the person abstracting data from medical records is aware of individual patients’ final diagnoses, or if any reinterpretation of images is undertaken for the purposes of the study.
For imaging tests, studies reporting the accuracy of multiple diagnostic thresholds (different tumour characteristics or parameters) for the same index test will also be subject to information bias unless each characteristic was interpreted by a different reader. This would be an impractical and unlikely approach for most studies, but we include a quality item in order to highlight any studies where this occurs, to facilitate discussion.
In terms of applicability, despite the often subjective nature of test interpretation, it is important that study authors outline the particular characteristics that they considered to be indicative of the presence of disease, so that appropriate comparisons can be made between test evaluations and the test can be replicated in practice. For SLNB, a description of the tracer threshold for a “hot” versus a “cold” node will be required, as well as a description of the histology interpretation, such as the Royal College of Pathologists (RCP) requirements (Royal College of Pathologists 2014).
The experience of the observer will also impact on the applicability of study results. Detailed information on the experience and training of care providers is often lacking, such that a detailed analysis of the impact of examiner experience may not be possible. However to be considered ‘low concern’:
-
surgical members of the specialist skin cancer multidisciplinary team (SSMDT) should meet guideline recommendations, i.e. carrying out at least 15 inguinal or axillary lymph node dissections a year (NHS England 2014)
-
imaging tests should be interpreted by consultant radiologists
Reference standard domain (3)
In an ideal study, consecutively‐recruited participants should all undergo the same reference standard. In reality, both partial and differential verification biases are likely.
Partial verification bias will occur where histology (e.g. complete lymph node dissection) is the only reference standard used, and only those participants with a certain degree of suspicion of malignancy based on the result of the index test undergo verification, the others either being excluded from the study or being defined as disease‐negative without further assessment or follow‐up.
Differential verification bias will be present where other reference standards are used in addition to histological verification. Differential verification is inevitable in these reviews, because of the invasive nature of obtaining tissue samples for histological confirmation of presence/absence of malignancy. This is particularly true where complete lymph node dissection is the reference standard for detection of nodal metastases, as this will not be undertaken in those who have a negative SLNB. With imaging tests, histological confirmation would be impossible following a negative imaging result; however, those with borderline or indeterminate results are also unlikely to have subsequent histology. Any indeterminate results will be reviewed at the MDT and a decision made whether to repeat the imaging in three months, for example, or to image with a different modality to clarify. With borderline imaging, the finding is usually too small to be called a metastasis, making biopsy very unlikely for practical reasons.
Absence of disease in test‐negative participants will therefore be confirmed by another concurrent imaging test (which will have its own false‐negative (FN) and false‐positive (FP) rate), or preferably by clinical or radiological follow‐up. Ideally, a follow‐up‐based reference standard should be long enough to allow all present but ‘hidden’ cases of disease to become detectable (Naaktgeboren 2013); however, differentiating disease that was originally present but missed from newly‐emergent disease is problematic.
For the SLNB review, we will require studies to report the emergence of clinically‐detectable or macroscopic nodal disease in SLN‐negative participants in order to be included; therefore, we will judge all SLNB studies to have used an adequate reference standard. For the imaging reviews, we will define an adequate reference standard for imaging test‐negative participants as clinical or radiological follow‐up to detect any metastatic disease. We will rate studies that use a concurrently‐applied imaging test to determine final diagnosis of index test‐negative participants at high risk of bias.
A further challenge is the potential for incorporation bias, i.e. where the result of the index test is used to help determine the reference standard diagnosis. For both SLNB and imaging tests, only those with positive test results will undergo any procedure to allow histological confirmation (whether core biopsy, SLNB or complete lymph node dissection). In each case, the histopathologist will probably be aware that the index test was positive, and this knowledge will inform the pathology procedure.
There is also considerable potential for the clinicians or radiologists concerned with the clinical and/or radiological follow‐up of study participants to identify any subsequent emergence of nodal or distant disease to be aware of the original index test result and to use that to inform diagnostic decisions at the time of follow‐up.
Reference standard blinding is therefore extremely unlikely and its enforcement would significantly limit the generalisability of the study results. We will therefore assess the presence of blinded reference test interpretation (as it is a standard QUADAS‐2 item), but will not include it in our overall assessment of bias.
Flow and timing domain (4)
A period of one month has been defined as an appropriate interval (low risk of bias) between application of the index test and a histological reference standard (complete lymph node dissection or biopsy of possible distant metastases). Where the reference standard is follow‐up‐based, we have applied no restrictions on follow‐up timing.
Comparative domain
In the event that we identify comparative imaging test studies, we will add a comparative domain to the QUADAS‐2 checklist (Appendix 4). Questions reflect the possibility of selection bias (into the study and allocation to index test or testing strategies) and assessment of blinding of interpretation of each individual index test for within‐person comparisons. In addition, for within‐person test comparisons we have specified a maximum of one month between application of individual index tests, as intervals greater than this may be accompanied by changes in tumour characteristics. This is an arbitrary threshold, and in the event that a large proportion of included studies exceed this time period, we will undertake a sensitivity analysis to investigate the impact of this quality item on estimates of accuracy.
We will initially pilot the amended checklist tool on a small number of included full‐text articles. Ttwo review authors will independently rate each study on the four quality domains (patient selection, index test(s), reference standard, flow and timing). They will resolve any disagreements by consensus or by recourse to a third review author.
We will narratively summarise the results of quality assessment for all included studies at domain level, highlighting those domains that pose the greatest potential for risk of bias and concern about applicability for the body of evidence. We will supplement the narrative summary with summary graphics and tables as appropriate, to assist with the presentation of the results of quality assessment across included studies for important participant subgroups and by index test.
Statistical analysis and data synthesis
We anticipate a paucity of studies in this area, such that a only narrative review will be appropriate. However, if we find enough studies, we will apply the following methodology:
For the SLNB reviews, our primary analysis will focus on the detection of metastases in the investigated nodal basin. For the imaging test reviews, we will conduct separate analyses, firstly according to whether study participants are recruited on primary presentation of cSCC or with a disease recurrence, and secondly according to our primary and secondary objectives, i.e. detection of any metastasis (which must include both nodal and distant recurrence) and detection of nodal metastasis alone or detection of any distant metastasis, as defined under Target condition being diagnosed). SLNB is not used for staging of recurrence in skin cancer.
Studies may report test accuracy by lesion or by patient. Our unit of analysis for the primary analyses will be the patient, as study participants may have multiple metastatic sites at any one time, such that a 'by lesion' analysis may overestimate test accuracy. We will include data from studies that reported 'by lesion'‐level data in secondary analyses, such that by‐lesion and by‐patient data from different studies would be combined together, using by‐patient data in preference where both are reported within a study. The estimation of the accuracy metrics to be used in our reviews is detailed in Appendix 4.
For the SLNB review, both index test and reference standard positivity are defined histologically. In the absence of an additional suitable reference standard for SLNB test positivity, it will not be possible to estimate false positive cases, and specificity will always be 100%. We will therefore perform meta‐analysis only of sensitivities by using a univariate random‐effects logistic regression model. We will also estimate the pooled negative predictive value in a secondary analysis (the positive predictive value will not be possible to calculate due to false positives not being estimable). The definitions for each cell of the 2 x 2 contingency tables for the SLNB review are as follows:
-
TP = SLN‐positive (i.e. all participants with a positive SLN, regardless of any subsequent recurrence)
-
FP = not possible to estimate
-
FN = SLN‐negative participants who experience clinical emergence of disease in the same nodal basin, in the absence of disseminated disease
-
TN = SLN‐negative participants who do not experience clinical emergence of disease in same nodal basin
For the imaging test reviews, we will estimate sensitivity and specificity in the usual way. We will initially explore the data by plotting estimates of sensitivity and specificity on coupled forest plots and in receiver operating characteristic (ROC) space for each index test under consideration. We will use hierarchical models to perform meta‐analyses (Macaskill 2013). Where commonly‐used thresholds are reported, we will produce summary operating points (summary sensitivities and specificities) with 95% confidence and prediction regions, using the method of Reitsma 2005. Where different thresholds are used, we will fit a summary curve using a hierarchical summary ROC (HSROC) model (Rutter 2001). When few studies are available for meta‐analysis, we will simplify hierarchical models as appropriate, depending on whether the focus of inference is a summary point or summary curve (Takwoingi 2015). We anticipate that in many instances results from multiple thresholds within a single study may be reported. Where we need to select multiple thresholds for the review, we may use data from the same participants more than once in each analysis. For the analysis of summary curves, however, we will select standard or most commonly‐used thresholds from each study. Failing that, we will select one threshold at random from each study.
For the imaging tests review, if sufficient data are available we will perform both direct and indirect comparisons (the latter being required because we anticipate that comparative studies may be scarce (Takwoingi 2013)). To formally compare index tests, we will add a covariate for test type to the relevant hierarchical model. We will use likelihood ratio tests to assess the statistical significance of differences in test accuracy (sensitivity and specificity) for analyses of summary points and shape and accuracy for analyses of summary curves, by comparing models without the covariate terms with models containing the covariate terms.
We will conduct analyses using Review Manager 5 (RevMan 2014), the NLMIXED procedure in SAS, and the meqrlogit command in STATA 14.
Investigations of heterogeneity
We will initially examine heterogeneity between studies by visually inspecting the forest plots of sensitivity and specificity and summary ROC plots. Where a sufficient number of studies have assessed the same index test and the characteristics of interest (see Secondary objectives) were adequately reported to enable analyses, we will perform meta‐regression by adding the potential source of heterogeneity as a covariate to a hierarchical model. We will require at least five studies in each subgroup; we will only report heterogeneity analyses with fewer than five studies in each group when we can be convinced that models have achieved adequate convergence and that the distribution of studies across groups is adequate to provide valid estimates. Where factors to be investigated (e.g. AJCC stage of disease) could vary between participants within a study, we will rely on the inclusion criteria set out by the study authors (such as restriction to stage II, III, or IV melanoma), or use the results of any subgroup analyses within a study to examine the effect of that covariate. We will assess each of the factors listed under the secondary objectives where possible.
Sensitivity analyses
If enough studies (at least five) assess the same index test, we will perform sensitivity analyses, restricted according to:
-
those with direct test comparisons (where the period of application between the index tests was within one month);
-
where concerns around applicability for participant selection are low;
-
where there was low risk of bias for the index test; and
-
where there was low risk of bias for the reference standard
Assessment of reporting bias
Because of uncertainty about the determinants of publication bias for diagnostic accuracy studies and the inadequacy of tests for detecting funnel plot asymmetry (Deeks 2005), we will not perform tests to detect publication bias.

Summary of guideline recommendations for the staging of cutaneous ACC following primary diagnosis
| Term | Definition |
| Adjuvant therapy or treatment | A treatment given after the main treatment for cancer to reduce the risk of recurrence |
| Adnexal (in relation to the skin) | Structures in the skin including hair follicles and sebaceous glands |
| Biopsy | Removal of a sample of tissue from the body to assist in diagnosis or inform the choice of treatment of a disease |
| Computed tomography (CT) | Imaging technique in which the person lies on a table within a x‐ray gantry. The images are acquired using a spiral (helical) path and banks of detectors, allowing presentation of the internal organs and blood vessels in different projections including 3‐D views |
| Curettage | Surgical procedure to remove tissue or delineate borders of lesions via scraping |
| Cutaneous T cell lymphoma | A type of non‐Hodgkin lymphoma of the skin caused by uncontrolled growth of white blood cells |
| Dermal papilla | Small projections of the dermis into the overlying epidermis giving an undulating pattern and visible as "fingerprints" in hands and feet |
| Electrodessication | The use of high‐frequency electric currents to cut, destroy or cauterise tissue. It is performed with the use of a fine needle‐shaped instrument |
| False negative | An individual who is truly positive for a disease, but whom a diagnostic test classifies as disease‐free |
| False positive | An individual who is truly disease‐free, but whom a diagnostic test classifies as having the disease |
| Follicular bulge | The portion of the hair follicle that contains the stem cells that give rise to skin cells. It contains the cells needed for wound repair, hair growth and development and tumour development |
| Histopathology | The study of tissue, usually obtained by biopsy or excision, for example under a microscope |
| Incidence | The number of new cases of a disease in a given time period |
| interfollicular epidermis | The part of the skin that lies in between the hair follicles |
| Local recurrence | Regrowth of a tumour in the area from which it was originally removed |
| Locoregional recurrence | Regrowth of a tumour in the area from which it was originally removed or in the regional lymph nodes (usually nearest to the original tumour site) |
| Lymph node dissection | Surgical removal or one or more lymph nodes in the absence of proven involvement with melanoma |
| Lymph node dissection (lymphadenectomy) | A surgical operation to remove one or more groups of lymph nodes |
| Lymphoscintigraphy | An imaging technique used to identify the lymph drainage basin, determine the number of sentinel nodes, differentiate sentinel nodes from subsequent nodes, locate the sentinel node in an unexpected location, and mark the sentinel node over the skin for biopsy. It requires the injection of a radio‐isotope into the skin around the biopsy scar and a scan some hours later to determine to which lymph nodes the tracer has travelled |
| Magnetic resonance imaging (MRI) | A type of scan which uses a magnetic field and radio waves to produce images of sections of the body |
| Meta‐analysis | A form of statistical analysis used to synthesise results from a collection of individual studies |
| Metastases/metastatic disease | Spread of cancer away from the primary site to somewhere else through the bloodstream or the lymphatic system |
| Micrometastases | Metastases so small that they can only be seen under a microscope |
| Morbidity | Detrimental effects on health |
| Mortality | Either (1) the condition of being subject to death; or (2) the death rate, which reflects the number of deaths per unit of population in relation to any specific region, age group, disease, treatment or other classification, usually expressed as deaths per 100, 1000, 10,000 or 100,000 people |
| Multidisciplinary team (MDT) | A team with members from different healthcare professions and specialties (e.g. urology, oncology, pathology, radiology, and nursing). Cancer care in the NHS uses this system to ensure that all relevant health professionals are engaged to discuss the best possible care for that patient |
| Oncology | The study of cancers. This term also refers to the medical specialty of cancer care, with particular reference to the use of radiotherapy or drugs to treat cancer. The medical specialty is often split into Clinical Oncology (doctors who use radiotherapy and drug treatment) and Medical Oncology (doctors who use drug treatment) |
| Palpation | Feeling with the fingers or hands as part of a clinical examination of the body |
| Perineural involvement | Spread or invasion of cancer to the nerves |
| Positron emission tomography (PET) | A nuclear medicine imaging technique whereby a radioactive glucose (usually 18FDG) is administered intravenously before a scan is conducted to create an image using colours to show where the FDG (or other radioactive tracer) has been taken up in the body |
| Prevalence | The proportion of a population found to have a condition |
| Prognostic factors / indicators | Specific characteristics of a cancer or the person who has it which might affect the patient’s prognosis |
| Radiotherapy | The use of radiation, usually high energy x‐rays, to control the growth of cancer cells |
| Recurrence | New cancer cells are detected following treatment. This can occur either at the site of the original tumour or at other sites in the body |
| Relapse | Where cancer starts to grow again after treatment |
| Sensitivity | In this context, the proportion of individuals with a disease who have that disease correctly identified by the study test |
| Sentinel lymph node biopsy | A radioactive tracer and blue dye are injected into the skin surrounding the primary lesion and the 'sentinel' lymph nodes to which the tracer drains are located by imaging (usually lymphoscintigraphy), and then removed and examined for nodal metastatic spread that cannot be detected clinically or on imaging |
| Specificity | The proportion of individuals without a disease who have the absence of disease correctly identified by the study test (i.e. the study test is negative) |
| Staging | Clinical description of the size and spread of a patient’s tumour, fitting into internationally agreed categories |
| Stem cells | Biological cells that can self‐renew and can differentiate into specialised cells; stem cells contribute to maintaining and protecting the skin and allowing hair regrowth |
| Subclinical (disease) | Disease that is usually asymptomatic and not easily observable, e.g. by clinical or physical examination |
| Systemic treatment | Treatment, usually given by mouth or by injection, that reaches and affects cancer cells throughout the body rather than targeting one specific area |
| Ultrasound | A type of scan in which high‐frequency sound waves are used to outline a part of the body |
| Some of the definitions above have been obtained from the NICE Guideline for the management of melanoma (NICE 2015). | |
| Classification | Criteriaa |
| T | |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 2 cm at largest horizontal width and < 2 high‐risk features |
| T2 | Tumor > 2 cm at largest horizontal width or any size with ≥ 2 high‐risk features |
| T3 | Infiltration of facial and cranial bones |
| T4 | Infiltration of skeletal bone or skull base |
| N | |
| N0 | No regional lymph node metastases |
| N1 | Solitary, ipsilateral lymph node metastasis, maximum diameter ≤ 3 cm |
| N2a | Solitary, ipsilateral lymph node metastasis, maximum diameter > 3 cm to max. 6 cm |
| N2b | Multiple, ipsilateral lymph node metastases, all with a maximum diameter ≤ 6 cm |
| N2c | Multiple, bilateral or contralateral lymph node metastases, all with a maximum diameter ≤ 6 cm |
| N3 | Lymph node metastasis, diameter > 6 cm |
| M | |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Table based on that reported in Edge 2010 AJCC: American Joint Committee on Cancer; aHigh‐risk features include depth/invasion (> 2 mm thickness, Clark level ≥ IV, or perineural invasion), anatomical location (primary site on the ear or the non–hair‐bearing lip), and differentiation (poorly differentiated or undifferentiated). | |
| Classification | Criteria a |
| Tis | Carcinoma in situ |
| T1 | 0 risk factors |
| T2a | 1 risk factor |
| T2b | 2 to 3 risk factors |
| T3 | 4 risk factors or bone invasion |
| Alternative system proposed by Jambusaria‐Pahlajani 2013 and adapted in Schmitt 2014. aRisk factors include tumour diameter of ≥ 2 cm, poorly‐differentiated histological characteristics, perineural invasion, and tumour invasion beyond the subcutaneous fat (excluding bone invasion, which automatically upgrades the tumour to alternative stage T3). | |
| Item | Response (delete as required) |
| PARTICIPANT SELECTION (1) ‐ RISK OF BIAS | |
| 1) Was a consecutive or random sample of participants or images enrolled? | Yes ‐ if paper states consecutive or random No – if paper describes other method of sampling Unclear – if participant sampling not described |
| 2) Was a case‐control design avoided? | Yes ‐ if consecutive or random or case‐control design clearly not used No – if study described as case‐control or describes sampling specific numbers of participants with particular diagnoses Unclear – if not described |
| 3) Did the study avoid inappropriate exclusions, e.g. needs examples of inappropriate exclusions in this context – for both melanoma and for cutaneous squamous cell carcinoma (cSCC) staging? | Yes ‐ if inappropriate exclusions were avoided No – if lesions were excluded that might affect test accuracy, e.g. indeterminate results or where disagreement between evaluators was observed Unclear – if not clearly reported |
| 4) For between‐person comparative (BPC) studies only (i.e. allocating different tests to different study participants such as randomised controlled trials (RCTs)): | |
|
| Yes ‐ if same selection criteria were used for each index test No – if different selection criteria were used for each index test Unclear – if selection criteria per test were not described N/A – if only one index test was evaluated or all participants received all tests |
|
| Yes ‐ if adequate randomisation procedures are described No – if inadequate randomisation procedures are described Unclear – if the method of allocation to groups is not described (a description of ‘random’ or ‘randomised’ is insufficient) N/A – if only one index test was evaluated or all participants received all tests |
|
| Yes ‐ if appropriate methods of allocation concealment are described No – if appropriate methods of allocation concealment are not described Unclear – if the method of allocation concealment is not described (sufficient detail to allow a definite judgement is required) N/A – if only one index test was evaluated |
| Could the selection of participants have introduced bias? | |
| v FOR NON‐COMPARATIVE (NC) STUDIES | |
| If answers to all of questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk Unclear |
| v FOR BETWEEN‐PERSON COMPARATIVE STUDIES | |
| If answers to all of questions 1) and 2) and 3) and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘Unclear’: | Risk Unclear |
| PARTICIPANT SELECTION (1) ‐ CONCERNS REGARDING APPLICABILITY | |
| For sentinel lymph node biopsy and imaging tests: | |
| 1) Does the study report results for participants unselected by stage of disease or site of primary lesion, i.e. the study does not focus solely on those with a particular stage of disease such as AJCC I or melanoma <=1 mm in thickness? | Yes ‐ if an unrestricted group of participants have been included No ‐ if a selected group of study participants have been included, e.g. those with clinical stage I disease or only those with thin melanoma Unclear – if insufficient details are provided to determine the spectrum of included participants |
| 2) Did the study report data on a per‐patient rather than per‐lesion basis? | Yes – if a per‐patient analysis was reported No – if a per‐lesion analysis only was reported Unclear – if it is not possible to assess whether data are presented on a per‐patient or per‐lesion basis |
| For imaging tests only: | |
| 3) Does the study focus primarily on participants undergoing primary staging or those undergoing staging for disease recurrence? | Yes ‐ if at least 80% of study participants are undergoing primary staging following diagnosis of a primary cutaneous melanoma or staging of recurrence No ‐ if less than 80% of study participants are undergoing primary staging following diagnosis of a cutaneous melanoma or staging of recurrence Unclear – if insufficient details are provided to determine the proportion of patients undergoing primary staging versus those undergoing staging of recurrence |
| Is there concern that the included participants do not match the review question? | |
| If the answer to question 1) or 2) (and 3)) was ‘Yes’: | Concern is Low |
| If the answer to question 1) or 2) (and 3)) was ‘No’: | Concern is High |
| If the answer to question 1) or 2) (and 3)) was ‘Unclear’: | Concern is Unclear |
| INDEX TEST (2) ‐ RISK OF BIAS (to be completed per test evaluated) | |
| 1) Was the index test or testing strategy result interpreted without knowledge of the results of the reference standard? | Yes ‐ if index test described as interpreted without knowledge of reference standard result, or for prospective studies, if index test is always conducted and interpreted prior to the reference standard No – if index test described as interpreted in knowledge of reference standard result Unclear – if index test blinding is not described |
| 2) Was the diagnostic threshold at which the test was considered positive prespecified? | Yes ‐ if threshold was prespecified (i.e. prior to analysing study results) No ‐ if threshold was not prespecified Unclear ‐ if not possible to tell whether or not diagnostic threshold was prespecified |
| For imaging tests only: | |
| 3) For studies reporting the accuracy of multiple diagnostic thresholds (tumour characteristic or parameter) for the same index test, was each threshold interpreted without knowledge of the results of the others? | Yes ‐ if thresholds were selected prospectively and each was interpreted by a different reader, or if study implements a retrospective (or no) cutoff No ‐ if study uses prospective threshold and report states reported by same reader Unclear ‐ if no mention of number of readers for each threshold or if pre‐specification of threshold not reported N/A ‐ multiple diagnostic thresholds not reported for the same index test |
| 4) For within‐person comparisons (WPC) of index tests or testing strategies (i.e. > 1 index test applied per participant), was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes ‐ if all index tests were described as interpreted without knowledge of the results of the others No ‐ if the index tests were described as interpreted in the knowledge of the results of the others Unclear – if it is not possible to tell whether knowledge of other index tests could have influenced test interpretation N/A – if only one index test was evaluated |
| Could the conduct or interpretation of the index test have introduced bias? | |
| v FOR NC and BPC STUDIES item 3) / 4) to be added | |
| If answers to questions 1) and 2) was ‘Yes’: | Risk is Low |
| If answers to either questions 1) or 2) was ‘No’: | Risk is High |
| If answers to either questions 1) or 2) was ‘Unclear’: | Risk is Unclear |
| v FOR WPC STUDIES | |
| If answers to all questions 1), 2) for any index test and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘Unclear’: | Risk is Unclear |
| INDEX TEST (2) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? This item applies equally to studies using objective and more subjective approaches to test interpretation. For SLNB studies, this requires description of the tracer threshold for identification of the SLN and the histological assessment. | Yes – if the criteria for diagnosis of the target disorder were reported in sufficient detail to allow replication No – if the criteria for diagnosis of the target disorder were not reported in sufficient detail to allow replication Unclear – if some but not sufficient information on criteria for diagnosis to allow replication were provided |
| 2) Was the test interpretation carried out by an experienced examiner? | Yes – if the test was interpreted by an experienced examiner as defined in the review protocol No – if the test was not interpreted by an experienced examiner (see above) Unclear – if the experience of the examiner(s) was not reported in sufficient detail to judge or if examiners described as 'Expert' with no further detail given |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | |
| If answers to questions 1) and 2) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 2) was ‘No’: | Concern is High |
| If answers to questions 1) or 2) was ‘Unclear’: | Concern is Unclear |
| REFERENCE STANDARD (3) ‐ RISK OF BIAS | |
| 1) Is the reference standard likely to correctly classify the target condition? | |
| a) DISEASE POSITIVE ‐ One or more of: ‐ Histological confirmation of metastases following lymph node dissection (or SLNB or core biopsy for imaging studies) ‐ Clinical/radiological follow up to identify clinically detectable disease in a mapped nodal basin (SLNB studies) ‐ Clinical/radiological follow up to identify any metastases (imaging studies) subsequently confirmed on histology | Yes – if all disease positive participants underwent one of the listed reference standards No – if a final diagnosis for any disease positive participant was reached without histopathology Unclear – if the method of final diagnosis was not reported for any disease positive participant |
| b) DISEASE NEGATIVE ‐ One or more of: ‐ Histological confirmation of absence of disease in a mapped nodal basin following lymph node dissection (or following SLNB for imaging studies) ‐ Clinical/radiological follow up of test negative participants | Yes – if at least 90% of disease negative participants underwent one of the listed reference standards No – if more than 10% of benign diagnoses were reached by concurrent imaging test Unclear – if the method of final diagnosis was not reported for any participant with benign or disease negative diagnosis |
| 2) Were the histology‐based reference standard results interpreted without knowledge of the results of the index test? | Yes – if the histopathologist was described as blinded to the index test result No – if the histopathologist was described as having knowledge of the index test result Unclear – if blinded histology interpretation was not clearly reported |
| 3) Were the reference standard results based on patient follow‐up interpreted without knowledge of the results of the index test? | Yes – if the clinician or radiologist was described as blinded to the index test result No – if the clinician or radiologist was described as having knowledge of the index test result Unclear – if blinded interpretation was not clearly reported |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| REFERENCE STANDARD (3) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Does the study use the same definition of disease positive as the primary review question or is it possible to fully disaggregate data such that data matching the review question can be extracted? | Yes – same definition of disease positive used, or patients can be disaggregated and regrouped according to review definition No – some patients cannot be disaggregated For SLNB review – disease positive includes participants with any nodal recurrence (not restricted to clinical recurrence in same nodal basin) For imaging reviews – participants with nodal versus distant recurrences cannot be disaggregated Unclear – definition of disease positive not clearly reported |
| For studies of imaging tests: | |
| 2) The result of another imaging test (without patient follow‐up to determine later emergence of disease) was not used as a reference standard | Yes – if imaging‐based diagnosis was not used as a reference standard for any participant No – if imaging‐based diagnosis was used as a reference standard for any participant Unclear – if not clearly reported |
| 3) Item on observer experience could be included? Is there concern that the target condition as defined by the reference standard does not match the review question? | |
| If answers to all questions 1), 2) and 3) was ‘Yes’: | Concern is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Concern is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Concern is Unclear |
| ***For teledermatology studies only: | |
| If answers to questions 1) and 3) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 3) was ‘No’: | Concern is High |
| If answers to questions 1) or 3) was ‘Unclear’: | Concern is Unclear |
| FLOW AND TIMING (4): RISK OF BIAS | |
| 1) Was there an appropriate interval between index test and reference standard? | |
|
| Yes – if study reports <= 1 month between index and histological reference standard No – if study reports > 1 month between index and histological reference standard Unclear – if study does not report interval between index and histological reference standard |
|
| Yes – if study reports a follow‐up visit within 6 months of application of the index test No – if study reports the first follow‐up visit beyond 6 months of the index test Unclear – if study does not report timing of follow‐up visits |
| 2) Did all participants receive the same reference standard? | Yes – if all participants underwent the same reference standard No – if more than one reference standard was used Unclear – if not clearly reported |
| 3) Were all participants included in the analysis? | Yes – if all participants were included in the analysis No – if some participants were excluded from the analysis Unclear – if not clearly reported |
| 4) For WITHIN‐PERSON COMPARISONS (WPC) of index tests: Was the interval between application of index tests <= 1 month? Could the participant flow have introduced bias? | Yes – if study reports <= 1 month between index tests No – if study reports > 1 month between index tests Unclear – if study does not report interval between index tests |
| v FOR NON‐COMPARATIVE and BPC STUDIES | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| v FOR WITHIN‐PERSON COMPARATIVE STUDIES (WPC) | |
| If answers to all questions 1), 2), 3), and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1), 2), 3), or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1), 2), 3), or 4) was ‘Unclear’: | Risk is Unclear |

