Tests to assist in the staging of cutaneous squamous cell carcinoma: a generic protocol
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012773Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 29 August 2017see what's new
- Type:
-
- Diagnostic
- Stage:
-
- Protocol
- Cochrane Editorial Group:
-
Cochrane Skin Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
JDi was the contact person with the editorial base.
JDi co‐ordinated the contributions from the co‐authors and wrote the final draft of the protocol.
JDi, CD, NC, JDe, YT, and SB worked on the Methods sections.
JDi, AW, RM, and HW drafted the clinical sections of the Background and responded to the clinical comments of the referees.
JDi, JJD, YT, and CD responded to the methodology and statistics comments of the referees.
JDi, HW, RM, AW, CD, NC, JDe, YT, and SB contributed to writing the protocol.
CO was the consumer co‐author and checked the protocol for readability and clarity. She also ensured that the outcomes are relevant to consumers.
JDi is the guarantor of the final review.
Disclaimer
This project is supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group and Cochrane Programme Grant funding. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
NIHR Systematic Review Programme, UK.
-
National Institute for Health Research, UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group
Declarations of interest
Jacqueline Dinnes: nothing to declare.
Angela Webster: nothing to declare.
Rubeta N Matin: nothing to declare.
Susan E Bayliss: nothing to declare.
Naomi Chuchu: nothing to declare.
Yemisi Takwoingi: nothing to declare.
Clare Davenport: nothing to declare.
Pat Lawton: nothing to declare.
Kathie Godfrey: nothing to declare.
Colette O'Sullivan: nothing to declare.
Jonathan J Deeks: nothing to declare.
Hywel C Williams: nothing to declare.
Acknowledgements
We wish to thank the Cochrane DTA editorial base and colleagues, as well as all members of our Advisory Group (Jonathan Bowling, Colin Fleming, Matthew Gardiner, Abhilash Jain, Susan O’Connell, Pat Lawton, John Lear, Mariska Leeflang, Richard Motley, Paul Nathan, Julia Newton‐Bishop, Miranda Payne, Rachael Robinson, Simon Rodwell, Julia Schofield, Neil Shroff, Hamid Tehrani, Zoe Traill, and Fiona Walter).
The Cochrane Skin editorial base wishes to thank Michael Bigby who was the Key Editor for this protocol; Brian Stafford who was the consumer referee; and the clinical referees, Whitney High and Sabrina Newman. We also thank Kate Cahill, who copy‐edited the protocol.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 29 | Tests to assist in the staging of cutaneous squamous cell carcinoma: a generic protocol | Protocol | Jacqueline Dinnes, Rubeta N Matin, Angela C Webster, Pat Lawton, Naomi Chuchu, Susan E Bayliss, Yemisi Takwoingi, Clare Davenport, Kathie Godfrey, Colette O'Sullivan, Jonathan J Deeks, Hywel C Williams | |
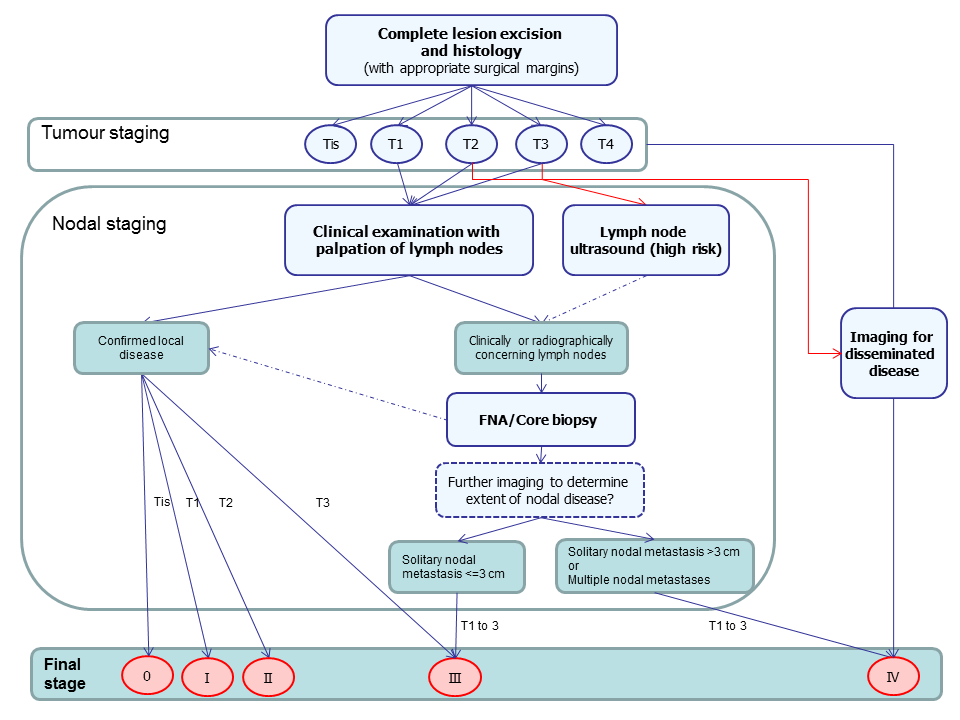
Summary of guideline recommendations for the staging of cutaneous ACC following primary diagnosis
| Term | Definition |
| Adjuvant therapy or treatment | A treatment given after the main treatment for cancer to reduce the risk of recurrence |
| Adnexal (in relation to the skin) | Structures in the skin including hair follicles and sebaceous glands |
| Biopsy | Removal of a sample of tissue from the body to assist in diagnosis or inform the choice of treatment of a disease |
| Computed tomography (CT) | Imaging technique in which the person lies on a table within a x‐ray gantry. The images are acquired using a spiral (helical) path and banks of detectors, allowing presentation of the internal organs and blood vessels in different projections including 3‐D views |
| Curettage | Surgical procedure to remove tissue or delineate borders of lesions via scraping |
| Cutaneous T cell lymphoma | A type of non‐Hodgkin lymphoma of the skin caused by uncontrolled growth of white blood cells |
| Dermal papilla | Small projections of the dermis into the overlying epidermis giving an undulating pattern and visible as "fingerprints" in hands and feet |
| Electrodessication | The use of high‐frequency electric currents to cut, destroy or cauterise tissue. It is performed with the use of a fine needle‐shaped instrument |
| False negative | An individual who is truly positive for a disease, but whom a diagnostic test classifies as disease‐free |
| False positive | An individual who is truly disease‐free, but whom a diagnostic test classifies as having the disease |
| Follicular bulge | The portion of the hair follicle that contains the stem cells that give rise to skin cells. It contains the cells needed for wound repair, hair growth and development and tumour development |
| Histopathology | The study of tissue, usually obtained by biopsy or excision, for example under a microscope |
| Incidence | The number of new cases of a disease in a given time period |
| interfollicular epidermis | The part of the skin that lies in between the hair follicles |
| Local recurrence | Regrowth of a tumour in the area from which it was originally removed |
| Locoregional recurrence | Regrowth of a tumour in the area from which it was originally removed or in the regional lymph nodes (usually nearest to the original tumour site) |
| Lymph node dissection | Surgical removal or one or more lymph nodes in the absence of proven involvement with melanoma |
| Lymph node dissection (lymphadenectomy) | A surgical operation to remove one or more groups of lymph nodes |
| Lymphoscintigraphy | An imaging technique used to identify the lymph drainage basin, determine the number of sentinel nodes, differentiate sentinel nodes from subsequent nodes, locate the sentinel node in an unexpected location, and mark the sentinel node over the skin for biopsy. It requires the injection of a radio‐isotope into the skin around the biopsy scar and a scan some hours later to determine to which lymph nodes the tracer has travelled |
| Magnetic resonance imaging (MRI) | A type of scan which uses a magnetic field and radio waves to produce images of sections of the body |
| Meta‐analysis | A form of statistical analysis used to synthesise results from a collection of individual studies |
| Metastases/metastatic disease | Spread of cancer away from the primary site to somewhere else through the bloodstream or the lymphatic system |
| Micrometastases | Metastases so small that they can only be seen under a microscope |
| Morbidity | Detrimental effects on health |
| Mortality | Either (1) the condition of being subject to death; or (2) the death rate, which reflects the number of deaths per unit of population in relation to any specific region, age group, disease, treatment or other classification, usually expressed as deaths per 100, 1000, 10,000 or 100,000 people |
| Multidisciplinary team (MDT) | A team with members from different healthcare professions and specialties (e.g. urology, oncology, pathology, radiology, and nursing). Cancer care in the NHS uses this system to ensure that all relevant health professionals are engaged to discuss the best possible care for that patient |
| Oncology | The study of cancers. This term also refers to the medical specialty of cancer care, with particular reference to the use of radiotherapy or drugs to treat cancer. The medical specialty is often split into Clinical Oncology (doctors who use radiotherapy and drug treatment) and Medical Oncology (doctors who use drug treatment) |
| Palpation | Feeling with the fingers or hands as part of a clinical examination of the body |
| Perineural involvement | Spread or invasion of cancer to the nerves |
| Positron emission tomography (PET) | A nuclear medicine imaging technique whereby a radioactive glucose (usually 18FDG) is administered intravenously before a scan is conducted to create an image using colours to show where the FDG (or other radioactive tracer) has been taken up in the body |
| Prevalence | The proportion of a population found to have a condition |
| Prognostic factors / indicators | Specific characteristics of a cancer or the person who has it which might affect the patient’s prognosis |
| Radiotherapy | The use of radiation, usually high energy x‐rays, to control the growth of cancer cells |
| Recurrence | New cancer cells are detected following treatment. This can occur either at the site of the original tumour or at other sites in the body |
| Relapse | Where cancer starts to grow again after treatment |
| Sensitivity | In this context, the proportion of individuals with a disease who have that disease correctly identified by the study test |
| Sentinel lymph node biopsy | A radioactive tracer and blue dye are injected into the skin surrounding the primary lesion and the 'sentinel' lymph nodes to which the tracer drains are located by imaging (usually lymphoscintigraphy), and then removed and examined for nodal metastatic spread that cannot be detected clinically or on imaging |
| Specificity | The proportion of individuals without a disease who have the absence of disease correctly identified by the study test (i.e. the study test is negative) |
| Staging | Clinical description of the size and spread of a patient’s tumour, fitting into internationally agreed categories |
| Stem cells | Biological cells that can self‐renew and can differentiate into specialised cells; stem cells contribute to maintaining and protecting the skin and allowing hair regrowth |
| Subclinical (disease) | Disease that is usually asymptomatic and not easily observable, e.g. by clinical or physical examination |
| Systemic treatment | Treatment, usually given by mouth or by injection, that reaches and affects cancer cells throughout the body rather than targeting one specific area |
| Ultrasound | A type of scan in which high‐frequency sound waves are used to outline a part of the body |
| Some of the definitions above have been obtained from the NICE Guideline for the management of melanoma (NICE 2015). | |
| Classification | Criteriaa |
| T | |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 2 cm at largest horizontal width and < 2 high‐risk features |
| T2 | Tumor > 2 cm at largest horizontal width or any size with ≥ 2 high‐risk features |
| T3 | Infiltration of facial and cranial bones |
| T4 | Infiltration of skeletal bone or skull base |
| N | |
| N0 | No regional lymph node metastases |
| N1 | Solitary, ipsilateral lymph node metastasis, maximum diameter ≤ 3 cm |
| N2a | Solitary, ipsilateral lymph node metastasis, maximum diameter > 3 cm to max. 6 cm |
| N2b | Multiple, ipsilateral lymph node metastases, all with a maximum diameter ≤ 6 cm |
| N2c | Multiple, bilateral or contralateral lymph node metastases, all with a maximum diameter ≤ 6 cm |
| N3 | Lymph node metastasis, diameter > 6 cm |
| M | |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Table based on that reported in Edge 2010 AJCC: American Joint Committee on Cancer; aHigh‐risk features include depth/invasion (> 2 mm thickness, Clark level ≥ IV, or perineural invasion), anatomical location (primary site on the ear or the non–hair‐bearing lip), and differentiation (poorly differentiated or undifferentiated). | |
| Classification | Criteria a |
| Tis | Carcinoma in situ |
| T1 | 0 risk factors |
| T2a | 1 risk factor |
| T2b | 2 to 3 risk factors |
| T3 | 4 risk factors or bone invasion |
| Alternative system proposed by Jambusaria‐Pahlajani 2013 and adapted in Schmitt 2014. aRisk factors include tumour diameter of ≥ 2 cm, poorly‐differentiated histological characteristics, perineural invasion, and tumour invasion beyond the subcutaneous fat (excluding bone invasion, which automatically upgrades the tumour to alternative stage T3). | |
| Item | Response (delete as required) |
| PARTICIPANT SELECTION (1) ‐ RISK OF BIAS | |
| 1) Was a consecutive or random sample of participants or images enrolled? | Yes ‐ if paper states consecutive or random No – if paper describes other method of sampling Unclear – if participant sampling not described |
| 2) Was a case‐control design avoided? | Yes ‐ if consecutive or random or case‐control design clearly not used No – if study described as case‐control or describes sampling specific numbers of participants with particular diagnoses Unclear – if not described |
| 3) Did the study avoid inappropriate exclusions, e.g. needs examples of inappropriate exclusions in this context – for both melanoma and for cutaneous squamous cell carcinoma (cSCC) staging? | Yes ‐ if inappropriate exclusions were avoided No – if lesions were excluded that might affect test accuracy, e.g. indeterminate results or where disagreement between evaluators was observed Unclear – if not clearly reported |
| 4) For between‐person comparative (BPC) studies only (i.e. allocating different tests to different study participants such as randomised controlled trials (RCTs)): | |
|
| Yes ‐ if same selection criteria were used for each index test No – if different selection criteria were used for each index test Unclear – if selection criteria per test were not described N/A – if only one index test was evaluated or all participants received all tests |
|
| Yes ‐ if adequate randomisation procedures are described No – if inadequate randomisation procedures are described Unclear – if the method of allocation to groups is not described (a description of ‘random’ or ‘randomised’ is insufficient) N/A – if only one index test was evaluated or all participants received all tests |
|
| Yes ‐ if appropriate methods of allocation concealment are described No – if appropriate methods of allocation concealment are not described Unclear – if the method of allocation concealment is not described (sufficient detail to allow a definite judgement is required) N/A – if only one index test was evaluated |
| Could the selection of participants have introduced bias? | |
| v FOR NON‐COMPARATIVE (NC) STUDIES | |
| If answers to all of questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk Unclear |
| v FOR BETWEEN‐PERSON COMPARATIVE STUDIES | |
| If answers to all of questions 1) and 2) and 3) and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘Unclear’: | Risk Unclear |
| PARTICIPANT SELECTION (1) ‐ CONCERNS REGARDING APPLICABILITY | |
| For sentinel lymph node biopsy and imaging tests: | |
| 1) Does the study report results for participants unselected by stage of disease or site of primary lesion, i.e. the study does not focus solely on those with a particular stage of disease such as AJCC I or melanoma <=1 mm in thickness? | Yes ‐ if an unrestricted group of participants have been included No ‐ if a selected group of study participants have been included, e.g. those with clinical stage I disease or only those with thin melanoma Unclear – if insufficient details are provided to determine the spectrum of included participants |
| 2) Did the study report data on a per‐patient rather than per‐lesion basis? | Yes – if a per‐patient analysis was reported No – if a per‐lesion analysis only was reported Unclear – if it is not possible to assess whether data are presented on a per‐patient or per‐lesion basis |
| For imaging tests only: | |
| 3) Does the study focus primarily on participants undergoing primary staging or those undergoing staging for disease recurrence? | Yes ‐ if at least 80% of study participants are undergoing primary staging following diagnosis of a primary cutaneous melanoma or staging of recurrence No ‐ if less than 80% of study participants are undergoing primary staging following diagnosis of a cutaneous melanoma or staging of recurrence Unclear – if insufficient details are provided to determine the proportion of patients undergoing primary staging versus those undergoing staging of recurrence |
| Is there concern that the included participants do not match the review question? | |
| If the answer to question 1) or 2) (and 3)) was ‘Yes’: | Concern is Low |
| If the answer to question 1) or 2) (and 3)) was ‘No’: | Concern is High |
| If the answer to question 1) or 2) (and 3)) was ‘Unclear’: | Concern is Unclear |
| INDEX TEST (2) ‐ RISK OF BIAS (to be completed per test evaluated) | |
| 1) Was the index test or testing strategy result interpreted without knowledge of the results of the reference standard? | Yes ‐ if index test described as interpreted without knowledge of reference standard result, or for prospective studies, if index test is always conducted and interpreted prior to the reference standard No – if index test described as interpreted in knowledge of reference standard result Unclear – if index test blinding is not described |
| 2) Was the diagnostic threshold at which the test was considered positive prespecified? | Yes ‐ if threshold was prespecified (i.e. prior to analysing study results) No ‐ if threshold was not prespecified Unclear ‐ if not possible to tell whether or not diagnostic threshold was prespecified |
| For imaging tests only: | |
| 3) For studies reporting the accuracy of multiple diagnostic thresholds (tumour characteristic or parameter) for the same index test, was each threshold interpreted without knowledge of the results of the others? | Yes ‐ if thresholds were selected prospectively and each was interpreted by a different reader, or if study implements a retrospective (or no) cutoff No ‐ if study uses prospective threshold and report states reported by same reader Unclear ‐ if no mention of number of readers for each threshold or if pre‐specification of threshold not reported N/A ‐ multiple diagnostic thresholds not reported for the same index test |
| 4) For within‐person comparisons (WPC) of index tests or testing strategies (i.e. > 1 index test applied per participant), was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes ‐ if all index tests were described as interpreted without knowledge of the results of the others No ‐ if the index tests were described as interpreted in the knowledge of the results of the others Unclear – if it is not possible to tell whether knowledge of other index tests could have influenced test interpretation N/A – if only one index test was evaluated |
| Could the conduct or interpretation of the index test have introduced bias? | |
| v FOR NC and BPC STUDIES item 3) / 4) to be added | |
| If answers to questions 1) and 2) was ‘Yes’: | Risk is Low |
| If answers to either questions 1) or 2) was ‘No’: | Risk is High |
| If answers to either questions 1) or 2) was ‘Unclear’: | Risk is Unclear |
| v FOR WPC STUDIES | |
| If answers to all questions 1), 2) for any index test and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘Unclear’: | Risk is Unclear |
| INDEX TEST (2) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? This item applies equally to studies using objective and more subjective approaches to test interpretation. For SLNB studies, this requires description of the tracer threshold for identification of the SLN and the histological assessment. | Yes – if the criteria for diagnosis of the target disorder were reported in sufficient detail to allow replication No – if the criteria for diagnosis of the target disorder were not reported in sufficient detail to allow replication Unclear – if some but not sufficient information on criteria for diagnosis to allow replication were provided |
| 2) Was the test interpretation carried out by an experienced examiner? | Yes – if the test was interpreted by an experienced examiner as defined in the review protocol No – if the test was not interpreted by an experienced examiner (see above) Unclear – if the experience of the examiner(s) was not reported in sufficient detail to judge or if examiners described as 'Expert' with no further detail given |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | |
| If answers to questions 1) and 2) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 2) was ‘No’: | Concern is High |
| If answers to questions 1) or 2) was ‘Unclear’: | Concern is Unclear |
| REFERENCE STANDARD (3) ‐ RISK OF BIAS | |
| 1) Is the reference standard likely to correctly classify the target condition? | |
| a) DISEASE POSITIVE ‐ One or more of: ‐ Histological confirmation of metastases following lymph node dissection (or SLNB or core biopsy for imaging studies) ‐ Clinical/radiological follow up to identify clinically detectable disease in a mapped nodal basin (SLNB studies) ‐ Clinical/radiological follow up to identify any metastases (imaging studies) subsequently confirmed on histology | Yes – if all disease positive participants underwent one of the listed reference standards No – if a final diagnosis for any disease positive participant was reached without histopathology Unclear – if the method of final diagnosis was not reported for any disease positive participant |
| b) DISEASE NEGATIVE ‐ One or more of: ‐ Histological confirmation of absence of disease in a mapped nodal basin following lymph node dissection (or following SLNB for imaging studies) ‐ Clinical/radiological follow up of test negative participants | Yes – if at least 90% of disease negative participants underwent one of the listed reference standards No – if more than 10% of benign diagnoses were reached by concurrent imaging test Unclear – if the method of final diagnosis was not reported for any participant with benign or disease negative diagnosis |
| 2) Were the histology‐based reference standard results interpreted without knowledge of the results of the index test? | Yes – if the histopathologist was described as blinded to the index test result No – if the histopathologist was described as having knowledge of the index test result Unclear – if blinded histology interpretation was not clearly reported |
| 3) Were the reference standard results based on patient follow‐up interpreted without knowledge of the results of the index test? | Yes – if the clinician or radiologist was described as blinded to the index test result No – if the clinician or radiologist was described as having knowledge of the index test result Unclear – if blinded interpretation was not clearly reported |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| REFERENCE STANDARD (3) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Does the study use the same definition of disease positive as the primary review question or is it possible to fully disaggregate data such that data matching the review question can be extracted? | Yes – same definition of disease positive used, or patients can be disaggregated and regrouped according to review definition No – some patients cannot be disaggregated For SLNB review – disease positive includes participants with any nodal recurrence (not restricted to clinical recurrence in same nodal basin) For imaging reviews – participants with nodal versus distant recurrences cannot be disaggregated Unclear – definition of disease positive not clearly reported |
| For studies of imaging tests: | |
| 2) The result of another imaging test (without patient follow‐up to determine later emergence of disease) was not used as a reference standard | Yes – if imaging‐based diagnosis was not used as a reference standard for any participant No – if imaging‐based diagnosis was used as a reference standard for any participant Unclear – if not clearly reported |
| 3) Item on observer experience could be included? Is there concern that the target condition as defined by the reference standard does not match the review question? | |
| If answers to all questions 1), 2) and 3) was ‘Yes’: | Concern is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Concern is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Concern is Unclear |
| ***For teledermatology studies only: | |
| If answers to questions 1) and 3) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 3) was ‘No’: | Concern is High |
| If answers to questions 1) or 3) was ‘Unclear’: | Concern is Unclear |
| FLOW AND TIMING (4): RISK OF BIAS | |
| 1) Was there an appropriate interval between index test and reference standard? | |
|
| Yes – if study reports <= 1 month between index and histological reference standard No – if study reports > 1 month between index and histological reference standard Unclear – if study does not report interval between index and histological reference standard |
|
| Yes – if study reports a follow‐up visit within 6 months of application of the index test No – if study reports the first follow‐up visit beyond 6 months of the index test Unclear – if study does not report timing of follow‐up visits |
| 2) Did all participants receive the same reference standard? | Yes – if all participants underwent the same reference standard No – if more than one reference standard was used Unclear – if not clearly reported |
| 3) Were all participants included in the analysis? | Yes – if all participants were included in the analysis No – if some participants were excluded from the analysis Unclear – if not clearly reported |
| 4) For WITHIN‐PERSON COMPARISONS (WPC) of index tests: Was the interval between application of index tests <= 1 month? Could the participant flow have introduced bias? | Yes – if study reports <= 1 month between index tests No – if study reports > 1 month between index tests Unclear – if study does not report interval between index tests |
| v FOR NON‐COMPARATIVE and BPC STUDIES | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| v FOR WITHIN‐PERSON COMPARATIVE STUDIES (WPC) | |
| If answers to all questions 1), 2), 3), and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1), 2), 3), or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1), 2), 3), or 4) was ‘Unclear’: | Risk is Unclear |

